Abstract
An apparently rare Neisseria meningitidis isolate containing one copy of a Neisseria gonorrhoeae 16S rRNA gene is described herein. This isolate was identified as N. meningitidis by biochemical identification methods but generated a positive signal with Gen-Probe Aptima assays for the detection of Neisseria gonorrhoeae. Direct 16S rRNA gene sequencing of the purified isolate revealed mixed bases in signature regions that allow for discrimination between N. meningitidis and N. gonorrhoeae. The mixed bases were resolved by sequencing individually PCR-amplified single copies of the genomic 16S rRNA gene. A total of 121 discrete sequences were obtained; 92 (76%) were N. meningitidis sequences, and 29 (24%) were N. gonorrhoeae sequences. Based on the ratio of species-specific sequences, the N. meningitidis strain seems to have replaced one of its four intrinsic 16S rRNA genes with the gonococcal gene. Fluorescence in situ hybridization (FISH) probes specific for meningococcal and gonococcal rRNA were used to demonstrate the expression of the rRNA genes. Interestingly, the clinical isolate described here expresses both N. meningitidis and N. gonorrhoeae 16S rRNA genes, as shown by positive FISH signals with both probes. This explains why the probes for N. gonorrhoeae in the Gen-Probe Aptima assays cross-react with this N. meningitidis isolate. The N. meningitidis isolate described must have obtained N. gonorrhoeae-specific DNA through interspecies recombination.
INTRODUCTION
Species of the genus Neisseria are well-known to be naturally transformable. Numerous studies describe lateral gene transfer between Neisseria spp. (1–3). Recombination appears to be frequent among Neisseria spp. that are closely related. Isolates of a human pathogen of the group, Neisseria meningitidis, have been shown to contain foreign gene sequences that were first identified in commensal strains, such as Neisseria lactamica or Neisseria cinerea (4–8). These data are based on multilocus sequence typing (MLST) of housekeeping genes (9–14) and multilocus enzyme electrophoresis (15). Conversely, very few recombinations between meningococci and gonococci have been reported, even though the two are very closely related and even could be considered a single species based on DNA-DNA hybridization findings (16, 17). Those two species historically were thought to be isolated populations (18). However, N. meningitidis is quite frequently described as the causative agent of urogenital infections (19–24), and there are numerous examples of N. meningitidis and Neisseria gonorrhoeae being coisolated either from the pharynx (25–27) or from urogenital sites (28–31). Consequently, Hodge et al. discovered a Neisseria strain that phenotypically resembles N. meningitidis but reacts with serological tests intended for gonococcal confirmation (32). A more-detailed study was able to show that this isolate is a meningococcus that has acquired a gonococcal protein IB (PIB) porin (33).
One common conclusion of these studies is that the boundaries between Neisseria spp. often are fuzzy and difficult to resolve due to the high degrees of relatedness and interspecies recombination. Many sequences need to be analyzed in concatenated data sets to improve the level of resolution (34). Likewise, confirmation of species allocations based on nucleic acid characteristics at at least two loci was recommended (35).
The biological concept of species has been discussed in great detail over the past years, with taxonomists using a variety of methods to define a bacterial species. One of the most widely used classification tools for cultured and uncultured bacteria is the 16S rRNA gene. It is present in all bacteria and contains conserved and variable sequence regions, allowing classification of both distant and closely related species. Previously, 16S rRNA gene typing proved to be very sensitive and specific in identifying N. meningitidis strains (36, 37). Despite the presence of four rRNA operons (38, 39), it was long thought that all 16S rRNA gene copies of N. meningitidis have identical sequences (40), whereas a more detailed analysis demonstrated low levels of variation among N. meningitidis isolates (41). However, that study was still able to distinguish N. meningitidis strains from N. gonorrhoeae based on 16S rRNA gene sequences. Smith et al. (42) performed extensive sequence analysis of a number of Neisseria spp. (N. meningitidis and N. gonorrhoeae strains as well as commensal Neisseria isolates) based on multiple genes, including 16S rRNA genes. High levels of variation and diversity among the strains analyzed were described, pointing to a high level of recombination, especially within the N. lactamica group. In that publication, no recombination events were reported for N. gonorrhoeae, which formed a distinct branch in all gene sequences analyzed.
In addition to its function as a taxonomic tool in the bacterial world, rRNA can be used to detect and to identify bacterial species. Diagnostic tests such as the Gen-Probe AccuProbe culture identification tests and the Aptima line of amplified assays take advantage of the high rRNA copy numbers in bacterial cells, resulting in very high sensitivity for detection. The Aptima Combo 2 and Aptima GC assays for the detection of gonococci are characterized by specificities of 97.5% to 100% for urogenital samples, depending on the type of sample (43). Cross-reactivity with N. meningitidis was reported recently by Tabrizi et al. (44), whereas a follow-up study did not confirm those initial data (45).
Here, we describe an isolate that was characterized as N. meningitidis based on phenotypic and serological characteristics but was identified as positive for N. gonorrhoeae with Aptima assays. Amplification and sequencing of single copies of its 16S rRNA gene revealed that this isolate contains 16S rRNA genes from both N. meningitidis and N. gonorrhoeae, which led to the hypothesis that the isolate laterally acquired a gonococcal 16S rRNA gene. We were able to show with fluorescence in situ hybridization (FISH) that both meningococcal and gonococcal rRNA genes are expressed in this isolate.
(Part of this work, including Fig. 2, was presented at the European Congress of Clinical Microbiology and Infectious Diseases, 2011.)
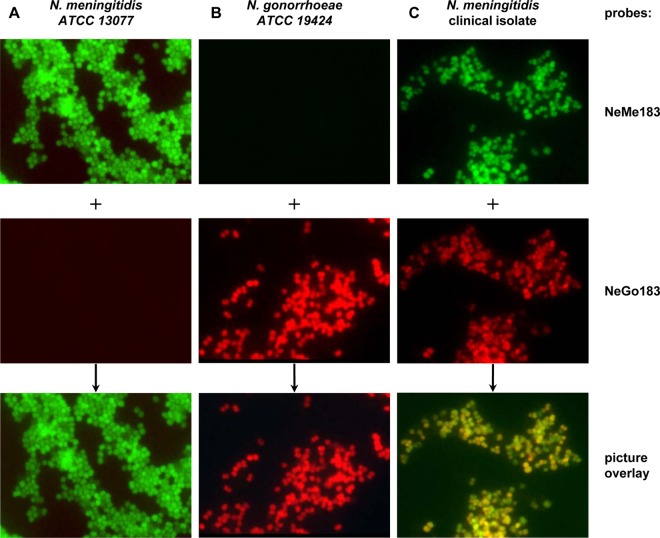
Fig 2.
FISH results for the detection of N. gonorrhoeae-specific and N. meningitidis-specific rRNA. The probes used were NeMe183 (specific for N. meningitidis and labeled with fluorescein isothiocyanate [green]) (first row) and NeGo183 (specific for N. gonorrhoeae and labeled with Cy3 [red]) (second row); the probes were used in conjunction as competitors to each other. The overlay of red and green signals results in a yellow color (third row). The specificities of the two probes were determined with a panel of 5 N. meningitidis control strains and 5 N. gonorrhoeae control strains. (A) One N. meningitidis control strain. (B) One N. gonorrhoeae control strain. (C) Probe signals detected with both probes in single cells of clinical isolate CI5240. All images were recorded with an exposure time of 40 ms.
MATERIALS AND METHODS
Isolation, cultivation, and identification.
The isolate described herein was isolated from a urethral swab sample on Thayer-Martin agar and subsequently cultured on chocolate agar. Probe specificity for FISH was verified with 5 strains of both N. meningitidis and N. gonorrhoeae (strains ATCC 13077, ATCC 13090, ATCC 35560, ATCC 35562, and ATCC 43744 for N. meningitidis and ATCC 19424, ATCC 27633, ATCC 31426, ATCC 49226, and ATCC 53420 for N. gonorrhoeae). The strains were cultured on chocolate agar (Hardy Diagnostics, Santa Maria, CA) at 37°C in 5% CO2.
Biochemical identification.
The clinical isolate was characterized by biochemical methods. Both the Vitek system (bioMérieux, Marcy l'Etoile, France) and Biolog MicroStation system (Biolog, Inc., Hayward, CA) were used for identification according to the manufacturer's instructions.
DNA probe assay identification.
Patient samples as well as pure cultures were characterized by Gen-Probe Aptima GC and Aptima Combo 2 assays for the detection of N. gonorrhoeae. Bacterial cells from either cultures or urethral swab samples were lysed and assayed according to the manufacturer's instructions.
Serological identification.
Single colonies of this isolate were tested with specific antibodies for serological classification. Serological tests were performed with the following Becton Dickinson Neisseria meningitidis antisera: X, Y, Z, Z′ (E), W135 (W), Poly (against serogroups A, B, and C), and Poly 2 (against serogroups X, Y, and Z). All tests were performed and recorded according to the manufacturer's instructions. The specificities of the sera were confirmed with strains affiliated with known N. meningitidis serogroups, i.e., serogroups A (ATCC 13077), X (ATCC 35560), Z (ATCC 35562), and W (ATCC 43744), and Neisseria gonorrhoeae (ATCC 31426).
Sequence analysis. (i) PCR.
The 16S rRNA gene was amplified from whole cells. A cell suspension from a single colony was prepared in specimen transport medium (Gen-Probe) and diluted 1:100 in sterile water. One microliter of this dilution was mixed with 49 μl of PCR master mix containing 25 μl 2× HotStarTaq master mix (Qiagen, Valencia, CA) and 400 nM concentrations of each forward and reverse primer. The primers used for amplification of the 16S rRNA gene were the universal bacterial primers 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′). The 16S rRNA gene was amplified with an initial denaturation step at 94°C for 10 min, followed by 35 cycles of denaturation (94°C for 45 s), annealing (55°C for 45 s), and elongation (72°C for 1 min), with a final elongation step at 72°C for 10 min.
The 23S rRNA gene was amplified with the universal primers 129F (5′-CYG AAT GGG GVA ACC-3′) and 2241R (5′-ACC GCC CCA GTH AAA CT-3′), using the following protocol. After an initial denaturation step at 94°C for 15 min, the 23S rRNA gene was amplified with 35 cycles of denaturation (94°C for 1 min), annealing (57°C for 1 min), and elongation (72°C for 2 min), with a final elongation step at 72°C for 10 min. Positive (Escherichia coli cell suspension) and negative (water only) controls were included.
(ii) Single-copy 16S rRNA gene PCR.
DNA was isolated using the DNeasy Blood & Tissue minikit (Qiagen, Valencia, CA) and was fragmented with the restriction enzyme BbsI, according to the manufacturer's instructions. BbsI was chosen since it does not have a restriction site within the Neisseria 16S rRNA gene. An analysis of published genomes for both N. meningitidis and N. gonorrhoeae revealed that the average size of all fragments generated by BbsI digestion is approximately 1,600 bp and the fragments containing 16S rRNA genes are approximately 5 kb long. Restriction digestion with BbsI generates 1,350 to 1,400 fragments from whole-genome Neisseria DNA.
The fragmented DNA was diluted to generate suspensions containing approximately 25 to 50 fragments/μl. Statistically, a maximum of one 16S rRNA gene fragment is present when 1 μl of this dilution is used in a PCR. The PCR cycle number was increased to 45 for better sensitivity due to the single target molecule. For a higher specificity, and to avoid contaminated negative-control samples during PCR, a Neisseria genus-specific forward primer (NeissPCR2F, 5′-CGG GTG AGT AAC ATA TCG G-3′) was used in conjunction with the universal 1492R reverse primer. Due to the risk of contaminating the Neisseria-specific single-copy PCRs, no positive-control reactions were performed concurrently for this set of experiments. All PCR products were analyzed on an agarose gel and purified using Zymo DNA Clean & Concentrator-5 columns (Zymo Research, Irvine) for further analysis.
(iii) Direct sequencing.
Purified PCR products were used for direct sequencing. Two microliters of template was mixed with 8 μl of BigDye Terminator v3.1 ready reaction mix (Applied Biosystems) and 3.2 pmol of one primer, in a total reaction volume of 20 μl. The 16S rRNA gene was sequenced with the following four primers: 27F or NeissPCR2F (for single-copy PCR products), BacA519+ (5′-CAG CAG CCG CGG TAA TAC-3′), EcoA806− (5′-CTA CCA GGG TAT CTA ATC-3′), and 1492R. The 23S rRNA gene was sequenced with the following five primers: 129F, EubB801+ (5′-GAT AGC TGG TTC TCC CCG AAA-3′), EubB1059+ (5′-GTT GGC TTA GAA GCA GCC A-3′), EubB1601+ (5′-GTA CCC CAA ACC GAC ACA GGT-3′), and 2241R.
Sequencing reactions consisted of 25 cycles of denaturation (96°C for 30 s), annealing (50°C for 15 s), and elongation (60°C for 4 min). The reaction products were purified with a DyeEx 2.0 spin kit (Qiagen), dried in a SpeedVac concentrator for 20 min, and resuspended in 10 μl Hi-Di formamide (Applied Biosystems). Samples were denatured at 96°C for 1 min, cooled on ice, and analyzed with an ABI 3100 genetic analyzer (Applied Biosystems). The sequences obtained were manually proofread and annotated with Gene Codes Sequencher 4.7 software.
(iv) Cloning and sequencing.
Purified PCR products were ligated into the vector pCR2.1 and transformed into E. coli Top10 cells using the TOPO TA cloning kit (Life Technologies, Carlsbad, CA), according to the manufacturer's instructions. Overnight cultures from clones were used to isolate plasmids (QIAprep spin miniprep kit; Qiagen). Four microliters of the purified plasmids was used for sequencing reactions (see above) with primer 27F.
FISH.
A single colony of a Neisseria culture was resuspended in phosphate-buffered saline (PBS) and fixed by addition of 3 volumes of a 4% paraformaldehyde solution and incubation at 4°C for 3 h (46). Fixed cells were washed once with PBS and stored in a 50% ethanol-PBS mixture at −20°C. Sufficient amounts of cells were transferred onto gelatin-coated glass slides, dried, and dehydrated by serial immersion of the slides in increasing ethanol concentrations (47). FISH was carried out with a formamide concentration of 30%, as described previously (48). In brief, immobilized cells were incubated for 2 h at 46°C in hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.2], 0.01% SDS, 30% formamide) containing 1 μM labeled probe and then were washed by immersion in wash buffer (0.1 M NaCl, 20 mM Tris-HCl [pH 7.2], 0.01% SDS, 0.5 mM EDTA) for 15 min at 48°C. The following probes were used in conjunction for hybridization of N. meningitidis and N. gonorrhoeae: NeMe183-FAM, 5′-CCT GCT TTC TCT CTC AAG A-3′; NeGo183-Cy3, 5′-CCT GCT TTC CCT CTC AAG A-3′ (48). The probes acted as competitors for each other to ensure specificity and to distinguish the single-base mismatch.
RESULTS
The phenotypic identity of clinical isolate CI5240 is N. meningitidis.
Three single colonies of the isolate were selected for phenotypic identification with the bioMérieux Vitek system (bioMérieux) and the Biolog MicroStation system (Biolog, Inc.). All three colonies were identified as N. meningitidis with 98 to 99% confidence values with both systems.
Clinical isolate CI5240 reacts positively with the Aptima series of tests.
Pure cultures picked from single colonies of the clinical isolate CI5240, identified as N. meningitidis, tested positive for Neisseria gonorrhoeae with the Gen-Probe Aptima Combo 2 and Aptima GC assays.
N. meningitidis CI5240 is classified in serogroup Z.
Serological testing confirmed the identity of CI5240 as Neisseria meningitidis. A detailed analysis with several antisera proved that this isolate is categorized as N. meningitidis serogroup Z. Pure cultures demonstrated strong agglutination reactions with antisera for N. meningitidis groups X, Y, and Z as well as with a specific antiserum for serogroup Z.
N. meningitidis CI5240 contains at least one copy of the N. gonorrhoeae 16S rRNA gene.
To confirm the identity of the N. meningitidis clinical isolate, we sequenced the 16S rRNA gene, which is commonly used for phylogenetic classification of bacterial strains. Also, since the Aptima Combo 2 and Aptima GC assays both target 16S rRNA for capture and detection, the sequence obtained should provide clarification on why the isolate tested positive for N. gonorrhoeae. Interestingly, direct sequencing of PCR products produced double peaks, which indicates the presence of at least two types of 16S rRNA gene sequences in the reaction mixture. These mixed bases are in signature positions used to discriminate between N. meningitidis and N. gonorrhoeae, suggesting the presence of both N. gonorrhoeae and N. meningitidis 16S rRNA gene sequences. The two peaks for all mixed bases found were base transitions (A to G or C to T).
We cloned the PCR products to isolate the different sequence types and retrieved almost-full-length high-quality sequences for 44 clones. Thirty-three sequences matched one of two sequence types that were clearly affiliated with either N. gonorrhoeae or N. meningitidis. Eleven chimeric sequences, with nine different chimeric patterns in the signature regions, also were detected (data not shown).
It is highly unlikely that all of these sequences represent individual 16S rRNA genes. Members of the genus Neisseria contain four rRNA operons. Therefore, the 11 different sequence types detected (nine chimeric types and two species-specific types) cannot be explained by operon-to-operon variations. The appearance of chimeric sequences obviously can be explained by PCR and sequencing bias (49, 50), but it is very unlikely that this would have resulted in chimeras in 25% of the clones analyzed. However, these numerous sequence types could have been generated by heteroduplex formation during PCR cycling. When transformed into E. coli, the host's intrinsic MutHLS-mediated mismatch repair system randomly repairs mismatched bases on both strands, creating random chimeras that are detected when the plasmid inserts are sequenced (51–53).
To overcome this PCR and cloning bias, a single-copy PCR method was developed using fragmented genomic DNA dilutions. The PCR products were again sequenced directly. Two sequence types were obtained from 119 unambiguous sequences with this method. Of those, 91 (76%) were N. meningitidis specific, and 28 (24%) were N. gonorrhoeae specific. The two sequence types are deposited under GenBank no. KC561932 and KC561933. Their affiliations with N. meningitidis (accession no. KC561933) and N. gonorrhoeae (accession no. KC561932) are shown in the dendrogram in Fig. 1.
Fig 1.
Dendrogram showing the affiliations of the two 16S rRNA gene sequence types found in the clinical isolate described. The two sequence types are highlighted in bold and are clustered with N. meningitidis (GenBank accession no. KC561933) and N. gonorrhoeae (GenBank accession no. KC561932) sequences. Outgroups are not shown. The scale bar represents 5% distance between sequences.
The presence of those two types of species-specific sequences supports the hypothesis that the gonococcal 16S rRNA gene was laterally transferred and integrated into the genome of the clinical isolate described herein. Furthermore, based on the relative ratio of N. meningitidis-specific sequences to N. gonorrhoeae-specific sequences, one of the intrinsic 16S rRNA genes was most likely replaced with the gonococcal gene.
The 23S rRNA gene sequence data for N. meningitidis CI5240 do not suggest mixed template sequences.
The analysis described above clearly demonstrated the presence of 16S rRNA gene sequences from both N. meningitidis and N. gonorrhoeae, but it was unknown if strain CI5240 horizontally acquired only a gonococcal 16S rRNA gene or if it integrated a full rRNA operon. For this reason, its 23S rRNA gene was amplified with the universal primers 129F and 2241R (54) and sequenced. No mixed bases or mismatches with known N. meningitidis strains were observed (data not shown), suggesting that the whole-genome template DNA (containing all four 23S rRNA genes) did not have sequence variabilities. A comparison of publicly available GenBank sequences for meningococci and gonococci did not allow clear discrimination of the two species based on 23S rRNA gene sequences, due to the presence of many intraspecies differences and very few interspecies differences, which makes this gene unsuitable for further analysis.
No similar 16S rRNA gene sequence heterogeneity can be found in publicly available N. meningitidis or N. gonorrhoeae sequences.
In addition to the 16S rRNA gene sequences derived from the clinical isolate described herein, we analyzed a total of 836 N. meningitidis and 5 N. gonorrhoeae 16S rRNA gene sequences from GenBank as well as 48 N. gonorrhoeae sequences from in-house clinical isolates. All sequences were at least 1,350 bp long.
Interestingly, we observed very high levels of intraspecies heterogeneity within the 16S rRNA gene sequences for both species. Among the 53 N. gonorrhoeae sequences analyzed, we identified 13 different alleles. Likewise, 105 different alleles were observed for all N. meningitidis sequences. For this study, an allele was defined when at least three different strains of the 889 strains analyzed showed the same mismatch to a reference sequence. Mismatches that were less frequent could be caused by PCR and/or sequencing errors and therefore were omitted from the analysis. We found 45 positions in the almost-full-length 16S rRNA gene sequences analyzed that met the criteria for an allele. Of those 45 positions, only 11 were clear signature regions in which all N. meningitidis sequences differed from all N. gonorrhoeae sequences, allowing differentiation between the two species.
None of the 889 sequences displayed rRNA gene sequences or mixed bases similar to those of the clinical isolate described herein. Based on the GenBank sequences analyzed, none of the 836 N. meningitidis strains would have resulted in a false-positive cross-reaction with the N. gonorrhoeae-specific probes in the Gen-Probe Aptima assays.
N. meningitidis CI5240 expresses the foreign gonococcal 16S rRNA gene.
To confirm that both 16S rRNA gene sequences were derived from a pure culture of N. meningitidis CI5240 and to determine the expression patterns of both rRNA genes, fluorescence in situ hybridization (FISH) was performed in order to achieve single-cell resolution. Two probes were used for the detection of both N. meningitidis-specific (NeMe183, labeled with fluorescein) and N. gonorrhoeae-specific (NeGo183, labeled with Cy3) rRNA types (slight modification of the method described by Poppert et al. [48]). We confirmed the specificities of both probes with five N. meningitidis and five N. gonorrhoeae strains obtained from the ATCC. Interestingly, both N. meningitidis-specific and N. gonorrhoeae-specific rRNA sequences are expressed in this clinical isolate, as shown in Fig. 2. Not all cells of the isolate expressed both sequence types equally. This can be seen by the different intensities of green and red fluorescence, as well as by the color resulting from the overlay of red and green. Equal signal intensities from the red and green fluorescently labeled probes would result in a yellow color, whereas the green (N. meningitidis-specific) signals prevailed over the red (N. gonorrhoeae-specific) signals in many single cells, suggesting that the N. meningitidis type of rRNA was expressed in greater amounts. Although not quantitative in nature, this finding corresponds well with the number of N. meningitidis versus N. gonorrhoeae 16S rRNA genes present in the strain.
DISCUSSION
The discrepancy between Aptima assay and conventional identification results is explained by horizontal gene transfer.
The clinical isolate described here has been identified with Gen-Probe Aptima assays for the detection of N. gonorrhoeae and phenotypic identification using automated systems. It was identified as N. gonorrhoeae by positive Aptima Combo 2 and Aptima GC test results, whereas the phenotypic identification was N. meningitidis. The discrepancy in those test results led us to investigate this isolate in further detail. Since the target sequence of the Gen-Probe Aptima assays is bacterial 16S rRNA, we investigated the specificity of the assay through sequence analysis of the 16S rRNA gene.
During this analysis, it was determined that the Neisseria strain possesses a copy of a N. gonorrhoeae 16S rRNA gene. Sequencing of 16S rRNA gene PCR products from a single gene copy using dilutions of fragmented genomic DNA revealed 76% pure N. meningitidis and 24% pure N. gonorrhoeae sequences, an approximate ratio of 3:1. The presence of the N. gonorrhoeae gene can be explained by lateral gene transfer between the two species. Furthermore, considering the fact that the genomes of all Neisseria spp. contain four rRNA operons, it seems that this isolate also replaced one of its own 16S rRNA genes with the foreign gene, according to the ratio of individual meningococcal 16S rRNA gene sequences to gonococcal 16S rRNA gene sequences obtained in this study.
Since Gen-Probe Aptima assays target 16S rRNA rather than genomic DNA, it was important to determine if the isolate expresses gonococcal RNA. We were able to prove the purity of this isolate as well as the expression of gonococcal rRNA by in situ hybridization using specific probes for the two sequence types (Fig. 2).
Interoperon variability of 16S rRNA has been described previously.
Numerous publications over the past 2 decades described 16S rRNA gene sequence variability when comparing the individual genes from each rRNA operon of a single bacterial strain. Variability in the small rRNA subunit of representative bacteria has been shown across several bacterial phyla (55–60). Clear determination of species affiliations was still possible in all of those studies. The frequency of this variability might even be underestimated, considering the fact that direct amplification and sequencing often do not deliver the necessary level of resolution to reveal intrastrain rRNA gene sequence variability, due to the fact that base-calling algorithms often cannot detect mixed bases when minor alleles account for only a small percentage of the target sequence (61, 62). Single copies must be separated first to show sequence variations. Expression of the different rRNA gene sequences has not been investigated in these studies.
Lateral gene transfer of rRNA genes between Neisseria spp. is an apparently uncommon event.
Horizontal gene transfer between Neisseria spp. has been described previously. It is well-known that this genus is naturally competent (6, 63, 64). Many mosaic sequences from N. meningitidis or N. gonorrhoeae and other commensal nonpathogenic members of this genus have been found (5, 65–69). To our knowledge, none of these studies described rRNA gene transfer between two Neisseria species. In order to explore the frequency of interspecies rRNA gene exchange between gonococci and meningococci, all N. meningitidis and N. gonorrhoeae 16S rRNA gene sequences publicly available at the time of the study were investigated. GenBank sequences might not be entirely representative of all sequences circulating clinically; therefore, the possibility of false-positive Aptima assay results cannot be ruled out. However, it is still interesting to see that none of the almost 900 sequences analyzed featured anomalies similar to those of the isolate described herein, such as mixed bases in signature regions or a chimeric 16S rRNA gene sequence that would point toward mixed genes in the genome. A detailed analysis of the binding regions of all publicly available N. meningitidis rRNA gene sequences for the Aptima assay probe proved that none of the corresponding isolates would have resulted in a positive reaction with the N. gonorrhoeae probe (data not shown).
It was noted that most base differences between the two species are also present in sequences from different strains of the same species. Discrimination between N. meningitidis and N. gonorrhoeae using 16S rRNA gene sequences is still possible but must be based on the 11 signature regions described herein.
To our knowledge, this is the first description of a N. meningitidis strain possessing and also expressing gonococcal 16S rRNA. It is generally assumed that highly conserved genes that are already present in a host's genome, such as the 16S rRNA gene, are not easily transferable between different species (70, 71). It should be mentioned that there are few examples of lateral gene transfer of 16S rRNA genes between different strains of the same species, e.g., Bartonella henselae (72), Vibrio parahaemolyticus (73), and Campylobacter hyointestinalis (58), or between different species of the same genus, e.g., Veillonella spp. (74). These are rare examples of lateral rRNA gene transfer, which has not yet been described between N. meningitidis and N. gonorrhoeae.
Summary.
Based on the results of this study, N. meningitidis isolate CI5240 appears to have obtained N. gonorrhoeae-specific DNA containing the 16S rRNA gene through interspecies recombination. Based on the observed 1:3 ratio of gonococcal sequences to meningococcal sequences and on the fact that the genomes of Neisseria spp. contain four rRNA operons, the N. meningitidis isolate described seems to have replaced one of its four intrinsic 16S rRNA genes with the gonococcal gene. We have also shown that this gonococcal 16S rRNA gene is expressed, which explains why the probe for N. gonorrhoeae in the Gen-Probe Aptima assays cross-reacts with this N. meningitidis isolate. Lateral gene transfer of gonococcal rRNA genes is a potential source of false-positive N. gonorrhoeae results with the Aptima Combo 2 assay. Additional studies will be required to determine if other clinical isolates of N. meningitidis were incorrectly identified using 16S rRNA probe-based assays as a result of similar lateral gene transfer and integration events. To our knowledge, this is the first description of a Neisseria meningitidis isolate that has acquired a gonococcal rRNA gene, has replaced one of its own intrinsic 16S rRNA genes with the gonococcal one, and even expresses the acquired 16S rRNA.
ACKNOWLEDGMENTS
This work was financially supported by Gen-Probe Incorporated (now Hologic). At the time of the study, all the authors were employees of Gen-Probe, the company that manufactures and sells the Aptima Combo 2 and Aptima GC assays.
We thank JoAnn Hayduk-Kramer of the New Jersey Department of Health for providing the Neisseria meningitidis isolate described in this work.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1.Gibbs CP, Meyer TF. 1996. Genome plasticity in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 145:173–179 [DOI] [PubMed] [Google Scholar]
- 2.Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59:376–385 [DOI] [PubMed] [Google Scholar]
- 3.Linz B, Schenker M, Zhu P, Achtman M. 2000. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol. Microbiol. 36:1049–1058 [DOI] [PubMed] [Google Scholar]
- 4.Dunning Hotopp JC, Grifantini R, Kumar N, Tzeng YL, Fouts D, Frigimelica E, Draghi M, Giuliani MM, Rappuoli R, Stephens DS, Grandi G, Tettelin H. 2006. Comparative genomics of Neisseria meningitidis: core genome, islands of horizontal transfer and pathogen-specific genes. Microbiology 152:3733–3749 [DOI] [PubMed] [Google Scholar]
- 5.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marri PR, Paniscus M, Weyand NJ, Rendon MA, Calton CM, Hernandez DR, Higashi DL, Sodergren E, Weinstock GM, Rounsley SD, So M. 2010. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 5:e11835. 10.1371/journal.pone.0011835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feil EJ, Enright MC, Spratt BG. 2000. Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between Neisseria meningitidis and Streptococcus pneumoniae. Res. Microbiol. 151:465–469 [DOI] [PubMed] [Google Scholar]
- 8.Feil EJ, Maiden MC, Achtman M, Spratt BG. 1999. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol. Biol. Evol. 16:1496–1502 [DOI] [PubMed] [Google Scholar]
- 9.Bennett JS, Jolley KA, Sparling PF, Saunders NJ, Hart CA, Feavers IM, Maiden MC. 2007. Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 5:35. 10.1186/1741-7007-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakubu DE, Abadi FJ, Pennington TH. 1999. Molecular typing methods for Neisseria meningitidis. J. Med. Microbiol. 48:1055–1064 [DOI] [PubMed] [Google Scholar]
- 11.Didelot X, Urwin R, Maiden MC, Falush D. 2009. Genealogical typing of Neisseria meningitidis. Microbiology 155:3176–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urwin R, Maiden MC. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479–487 [DOI] [PubMed] [Google Scholar]
- 13.Jolley KA, Brehony C, Maiden MC. 2007. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol. Rev. 31:89–96 [DOI] [PubMed] [Google Scholar]
- 14.Jolley KA, Maiden MC. 2006. AgdbNet: antigen sequence database software for bacterial typing. BMC Bioinformatics 7:314. 10.1186/1471-2105-7-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caugant DA. 2001. Global trends in meningococcal disease. Methods Mol. Med. 67:273–292 [DOI] [PubMed] [Google Scholar]
- 16.Guibourdenche M, Popoff MY, Riou JY. 1986. Deoxyribonucleic acid relatedness among Neisseria gonorrhoeae, N. meningitidis, N. lactamica, N. cinerea and “Neisseria polysaccharea.” Ann. Inst. Pasteur Microbiol. 137B:177–185 [DOI] [PubMed] [Google Scholar]
- 17.Kingsbury DT. 1967. Deoxyribonucleic acid homologies among species of the genus Neisseria. J. Bacteriol. 94:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez JA, de la Fuente L, Berron S, O'Rourke M, Smith NH, Zhou J, Spratt BG. 1993. Ecological separation and genetic isolation of Neisseria gonorrhoeae and Neisseria meningitidis. Curr. Biol. 3:567–572 [DOI] [PubMed] [Google Scholar]
- 19.Faur YC, Wilson ME, May PS. 1981. Isolation of N. meningitidis from patients in a gonorrhea screen program: a four-year survey in New York City. Am. J. Public Health 71:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson AP, Wolff J, Atia W. 1989. Acute urethritis due to Neisseria meningitidis group A acquired by orogenital contact: case report. Genitourin. Med. 65:122–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck A, Fluker JL, Platt DJ. 1974. Neisseria meningitidis in urogenital infection. Br. J. Vener. Dis. 50:367–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanemitsu N, Hayashi I, Satoh N, Hayakawa T, Mitsuya H, Hayase Y, Hiramoto K, Kojima M. 2003. Acute urethritis caused by Neisseria meningitidis. Int. J. Urol. 10:346–347 [DOI] [PubMed] [Google Scholar]
- 23.Katz AR, Chasnoff R, Komeya A, Lee MV. 2011. Neisseria meningitidis urethritis: a case report highlighting clinical similarities to and epidemiological differences from gonococcal urethritis. Sex. Transm. Dis. 38:439–441 [DOI] [PubMed] [Google Scholar]
- 24.Urra E, Alkorta M, Sota M, Alcala B, Martinez I, Barron J, Cisterna R. 2005. Orogenital transmission of Neisseria meningitidis serogroup C confirmed by genotyping techniques. Eur. J. Clin. Microbiol. Infect. Dis. 24:51–53 [DOI] [PubMed] [Google Scholar]
- 25.Unemo M, Golparian D, Potocnik M, Jeverica S. 2012. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 17(25):pii=20200 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20200 [PubMed] [Google Scholar]
- 26.Young H, Bain SS. 1983. Neisserial colonisation of the pharynx. Br. J. Vener. Dis. 59:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, Garland S, Chen MY. 2011. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J. Clin. Microbiol. 49:4304–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judson FN, Ehret JM, Eickhoff TC. 1978. Anogenital infection with Neisseria meningitidis in homosexual men. J. Infect. Dis. 137:458–463 [DOI] [PubMed] [Google Scholar]
- 29.Quarto M, Barbuti S, Germinario C, Vena GA, Foti C. 1991. Urethritis caused by Neisseria meningitidis: a case report. Eur. J. Epidemiol. 7:699–701 [DOI] [PubMed] [Google Scholar]
- 30.Hagman M, Danielsson D. 1989. Increased adherence to vaginal epithelial cells and phagocytic killing of gonococci and urogenital meningococci associated with heat modifiable proteins. APMIS 97:839–844 [DOI] [PubMed] [Google Scholar]
- 31.Hagman M, Forslin L, Moi H, Danielsson D. 1991. Neisseria meningitidis in specimens from urogenital sites: is increased awareness necessary? Sex. Transm. Dis. 18:228–232 [DOI] [PubMed] [Google Scholar]
- 32.Hodge DS, Ashton FE, Terro R, Ali AS. 1987. Organism resembling Neisseria gonorrhoeae and Neisseria meningitidis. J. Clin. Microbiol. 25:1546–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vazquez JA, Berron S, O'Rourke M, Carpenter G, Feil E, Smith NH, Spratt BG. 1995. Interspecies recombination in nature: a meningococcus that has acquired a gonococcal PIB porin. Mol. Microbiol. 15:1001–1007 [DOI] [PubMed] [Google Scholar]
- 34.Hanage WP, Fraser C, Spratt BG. 2005. Fuzzy species among recombinogenic bacteria. BMC Biol. 3:6. 10.1186/1741-7007-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer HM, Mallinson H, Wood RL, Herring AJ. 2003. Evaluation of the specificities of five DNA amplification methods for the detection of Neisseria gonorrhoeae. J. Clin. Microbiol. 41:835–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popovic T, Sacchi CT, Reeves MW, Whitney AM, Mayer LW, Noble CA, Ajello GW, Mostashari F, Bendana N, Lingappa J, Hajjeh R, Rosenstein NE. 2000. Neisseria meningitidis serogroup W135 isolates associated with the ET-37 complex. Emerg. Infect. Dis. 6:428–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer LW, Reeves MW, Al-Hamdan N, Sacchi CT, Taha MK, Ajello GW, Schmink SE, Noble CA, Tondella ML, Whitney AM, Al-Mazrou Y, Al-Jefri M, Mishkhis A, Sabban S, Caugant DA, Lingappa J, Rosenstein NE, Popovic T. 2002. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electrophoretic type-37 complex. J. Infect. Dis. 185:1596–1605 [DOI] [PubMed] [Google Scholar]
- 38.Klappenbach JA, Dunbar JM, Schmidt TM. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 66:1328–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klappenbach JA, Saxman PR, Cole JR, Schmidt TM. 2001. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 29:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turova TP. 2003. Copy number of ribosomal operons in prokaryotes and its effect on phylogenic analyses. Mikrobiologiia 72:437–452 (In Russian.) [PubMed] [Google Scholar]
- 41.Sacchi CT, Whitney AM, Reeves MW, Mayer LW, Popovic T. 2002. Sequence diversity of Neisseria meningitidis 16S rRNA genes and use of 16S rRNA gene sequencing as a molecular subtyping tool. J. Clin. Microbiol. 40:4520–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith NH, Holmes EC, Donovan GM, Carpenter GA, Spratt BG. 1999. Networks and groups within the genus Neisseria: analysis of argF, recA, rho, and 16S rRNA sequences from human Neisseria species. Mol. Biol. Evol. 16:773–783 [DOI] [PubMed] [Google Scholar]
- 43.Lowe P, O'Loughlin P, Evans K, White M, Bartley PB, Vohra R. 2006. Comparison of the Gen-Probe APTIMA Combo 2 assay to the AMPLICOR CT/NG assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine samples from Australian men and women. J. Clin. Microbiol. 44:2619–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll SO, Garland SM, Tapsall J. 2011. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J. Clin. Microbiol. 49:3610–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golparian D, Tabrizi SN, Unemo M. 2013. Analytical specificity and sensitivity of the APTIMA Combo 2 and APTIMA GC assays for detection of commensal Neisseria species and Neisseria gonorrhoeae on the Gen-Probe Panther instrument. Sex. Transm. Dis. 40:175–178 [DOI] [PubMed] [Google Scholar]
- 46.Amann R, Snaidr J, Wagner M, Ludwig W, Schleifer KH. 1996. In situ visualization of high genetic diversity in a natural microbial community. J. Bacteriol. 178:3496–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amann RI, Krumholz L, Stahl DA. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poppert S, Essig A, Stoehr B, Steingruber A, Wirths B, Juretschko S, Reischl U, Wellinghausen N. 2005. Rapid diagnosis of bacterial meningitis by real-time PCR and fluorescence in situ hybridization. J. Clin. Microbiol. 43:3390–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanagawa T. 2003. Bias and artifacts in multitemplate polymerase chain reactions (PCR). J. Biosci. Bioeng. 96:317–323 [DOI] [PubMed] [Google Scholar]
- 50.Keohavong P, Thilly WG. 1989. Fidelity of DNA polymerases in DNA amplification. Proc. Natl. Acad. Sci. U. S. A. 86:9253–9257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurata S, Kanagawa T, Magariyama Y, Takatsu K, Yamada K, Yokomaku T, Kamagata Y. 2004. Reevaluation and reduction of a PCR bias caused by reannealing of templates. Appl. Environ. Microbiol. 70:7545–7549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu X, Wu L, Huang H, McDonel PE, Palumbo AV, Tiedje JM, Zhou J. 2001. Evaluation of PCR-generated chimeras, mutations, and heteroduplexes with 16S rRNA gene-based cloning. Appl. Environ. Microbiol. 67:880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speksnijder AG, Kowalchuk GA, De Jong S, Kline E, Stephen JR, Laanbroek HJ. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunt DE, Klepac-Ceraj V, Acinas SG, Gautier C, Bertilsson S, Polz MF. 2006. Evaluation of 23S rRNA PCR primers for use in phylogenetic studies of bacterial diversity. Appl. Environ. Microbiol. 72:2221–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueda K, Seki T, Kudo T, Yoshida T, Kataoka M. 1999. Two distinct mechanisms cause heterogeneity of 16S rRNA. J. Bacteriol. 181:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulick S, Moccia C, Didelot X, Falush D, Kraft C, Suerbaum S. 2008. Mosaic DNA imports with interspersions of recipient sequence after natural transformation of Helicobacter pylori. PLoS One 3:e3797. 10.1371/journal.pone.0003797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cilia V, Lafay B, Christen R. 1996. Sequence heterogeneities among 16S ribosomal RNA sequences, and their effect on phylogenetic analyses at the species level. Mol. Biol. Evol. 13:451–461 [DOI] [PubMed] [Google Scholar]
- 58.Harrington CS, On SL. 1999. Extensive 16S rRNA gene sequence diversity in Campylobacter hyointestinalis strains: taxonomic and applied implications. Int. J. Syst. Bacteriol. 49:1171–1175 [DOI] [PubMed] [Google Scholar]
- 59.Ninet B, Monod M, Emler S, Pawlowski J, Metral C, Rohner P, Auckenthaler R, Hirschel B. 1996. Two different 16S rRNA genes in a mycobacterial strain. J. Clin. Microbiol. 34:2531–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reischl U, Feldmann K, Naumann L, Gaugler BJ, Ninet B, Hirschel B, Emler S. 1998. 16S rRNA sequence diversity in Mycobacterium celatum strains caused by presence of two different copies of 16S rRNA gene. J. Clin. Microbiol. 36:1761–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korch C, Drabkin H. 1999. Improved DNA sequencing accuracy and detection of heterozygous alleles using manganese citrate and different fluorescent dye terminators. Genome Res. 9:588–595 [PMC free article] [PubMed] [Google Scholar]
- 62.Davidson CJ, Zeringer E, Champion KJ, Gauthier MP, Wang F, Boonyaratanakornkit J, Jones JR, Schreiber E. 2012. Improving the limit of detection for Sanger sequencing: a comparison of methodologies for KRAS variant detection. Biotechniques 53:182–188 [DOI] [PubMed] [Google Scholar]
- 63.Joseph B, Schwarz RF, Linke B, Blom J, Becker A, Claus H, Goesmann A, Frosch M, Muller T, Vogel U, Schoen C. 2011. Virulence evolution of the human pathogen Neisseria meningitidis by recombination in the core and accessory genome. PLoS One 6:e18441. 10.1371/journal.pone.0018441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoen C, Weber-Lehmann J, Blom J, Joseph B, Goesmann A, Strittmatter A, Frosch M. 2011. Whole-genome sequence of the transformable Neisseria meningitidis serogroup A strain WUE2594. J. Bacteriol. 193:2064–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 49:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tapsall JW, Ray S, Limnios A. 2010. Characteristics and population dynamics of mosaic penA allele-containing Neisseria gonorrhoeae isolates collected in Sydney, Australia, in 2007-2008. Antimicrob. Agents Chemother. 54:554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiden MC. 1993. Population genetics of a transformable bacterium: the influence of horizontal genetic exchange on the biology of Neisseria meningitidis. FEMS Microbiol. Lett. 112:243–250 [DOI] [PubMed] [Google Scholar]
- 68.Rokbi B, Maitre-Wilmotte G, Mazarin V, Fourrichon L, Lissolo L, Quentin-Millet MJ. 1995. Variable sequences in a mosaic-like domain of meningococcal tbp2 encode immunoreactive epitopes. FEMS Microbiol. Lett. 132:277–283 [DOI] [PubMed] [Google Scholar]
- 69.Taha MK. 2002. Molecular detection and characterization of Neisseria meningitidis. Expert Rev. Mol. Diagn. 2:143–150 [DOI] [PubMed] [Google Scholar]
- 70.Hugenholtz P, Goebel BM, Pace NR. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765–4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rappe MS, Giovannoni SJ. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369–394 [DOI] [PubMed] [Google Scholar]
- 72.Viezens J, Arvand M. 2008. Simultaneous presence of two different copies of the 16S rRNA gene in Bartonella henselae. Microbiology 154:2881–2886 [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Escalona N, Romero J, Espejo RT. 2005. Polymorphism and gene conversion of the 16S rRNA genes in the multiple rRNA operons of Vibrio parahaemolyticus. FEMS Microbiol. Lett. 246:213–219 [DOI] [PubMed] [Google Scholar]
- 74.Michon AL, Aujoulat F, Roudiere L, Soulier O, Zorgniotti I, Jumas-Bilak E, Marchandin H. 2010. Intragenomic and intraspecific heterogeneity in rrs may surpass interspecific variability in a natural population of Veillonella. Microbiology 156:2080–2091 [DOI] [PubMed] [Google Scholar]