Abstract
Ornithobacterium rhinotracheale is a Gram-negative bacterium associated with respiratory diseases in many avian species, with worldwide distribution, and it causes significant economic loss to the poultry industry. In this study, the isolation and characterization of O. rhinotracheale small-colony variants (SCVs) are described for the first time. O. rhinotracheale isolates (n = 27) were recovered from tracheal samples (n = 321) collected from different avian species with clinical signs of respiratory disease. Of the 27 O. rhinotracheale isolates, 21 (77.8%) showed SCVs in their primary cultures. Five O. rhinotracheale SCV isolates showed high levels of stability and were chosen for further characterization with their wild-type (WT) isolates. Stable O. rhinotracheale SCVs were oxidase negative, while their WT isolates were positive. Growth curves for stable O. rhinotracheale SCVs indicated lower growth rates and longer lag phases than for their WT isolates. Furthermore, it was possible to increase the efficacy of the broth medium in supporting the growth of O. rhinotracheale WT isolates by supplementing it with 5% fetal bovine serum (FBS) and 2% IsoVitaleX Enrichment. Antibiotic susceptibility tests showed that O. rhinotracheale SCVs had higher MIC values than their WT isolates. This study suggests that successful antibiotic treatment of respiratory diseases associated with O. rhinotracheale must take into consideration the resistance patterns of O. rhinotracheale SCVs. Intracellular persistence in murine RAW 264.7 macrophages revealed that O. rhinotracheale SCV28 had higher survival rates than its WT isolate. Finally, small-colony variants may be important contributors to the pathogenesis of O. rhinotracheale.
INTRODUCTION
Small-colony variants (SCVs) represent a naturally occurring, slow-growing, auxotrophic subpopulation of bacteria with distinctive phenotypic and pathogenic traits (1). Phenotypically, SCVs have low growth rates and atypical colony morphology (SCVs grow in pinpoint colonies nearly one-tenth the size of their wild-type [WT] counterparts). They also typically have deficiencies or reductions in biochemical reaction activities (2). The biochemical basis of this phenotypic abnormality is auxotrophism caused by mutations of genes involved in the biosynthesis of thiamine, menadione, hemin, or thymidine (3). Two main domains of auxotrophic SCVs have been defined, i.e., SCVs that are deficient in electron transport and SCVs that are deficient in thymidine biosynthesis. However, SCVs for which the auxotrophism cannot be defined also have been described (1). Clinically, SCVs are more resistant to some classes of antibiotics than are their wild-type counterparts (1, 3, 4). They are often associated with persistent or recurrent infections (5, 6). Furthermore, a recent study on Enterococcus faecalis SCVs isolated from chickens indicates that SCVs have higher infection potency than their WT isolates (7).
SCVs have been described for a wide range of bacterial genera and species, including Staphylococcus aureus (8), Pseudomonas aeruginosa (9), Salmonella serovars (10), Escherichia coli (11), Neisseria gonorrhoeae (12), and Enterococcus spp. (7, 13). However, their uncommon morphological and physiological properties cause them to be frequently undetected or misidentified by standard clinical microbiology procedures (1).
Ornithobacterium rhinotracheale is a Gram-negative bacterium of the family Flavobacteriaceae in rRNA superfamily V (14). The pathogen was relatively recently discovered and is distributed worldwide in chickens and turkeys, as well as in other avian species (15–17). O. rhinotracheale is associated with respiratory diseases and causes significant economic loss to the poultry industry (17–19).
In the present study, we report for the first time the isolation of O. rhinotracheale SCVs. Furthermore, the O. rhinotracheale SCV isolates obtained were characterized and compared with wild-type isolates with regard to auxotrophism, biochemical profiles, growth curves, hemagglutinating activity, antimicrobial susceptibility, autolytic activity, intracellular persistence, and phylogenetic patterns.
MATERIALS AND METHODS
Sample collection.
Between December 2011 and August 2012, tracheal samples (n = 321) from different avian sources (chicken, n = 246; duck, n = 42; turkey, n = 1; pigeon, n = 4; goose, n = 19; pheasant, n = 9) with clinical signs of respiratory disease were submitted to the poultry diagnostic laboratories at China Agricultural University (Beijing, China), Animal Health Directorate (Beijing, China), and Shandong Agricultural University (Shandong, China). The tracheas were aseptically removed, put in separate plastic bags, and kept at −20°C until tested.
Isolation and identification of O. rhinotracheale WT isolates and SCVs.
Tracheal swabs were streaked onto tryptic soy agar (BBL; Becton, Dickinson and Co., Sparks, MD) supplemented with 5% sheep blood (i.e., sheep blood agar [SBA]), with or without gentamicin (10 μg/ml), and were incubated for 24 to 48 h under microaerobic conditions in candle jars at 37°C (17, 20). After incubation, all colonies that were pinpoint/small, circular, opaque, and gray to gray-white were chosen for further identification (15, 21). Colonies suspected to be O. rhinotracheale were subcultured on SBA (under the same environmental conditions), tentatively identified on the basis of routine phenotypic and biochemical traits, and genetically confirmed by PCR and DNA sequencing (17).
During the primary course of cultivation of O. rhinotracheale isolates, most of the pure cultures of O. rhinotracheale isolates showed a vast difference in colony size after 48 h of incubation. This difference goes beyond size variance and discriminates all isolates into two main phenotypically different subpopulations, i.e., large colonies (∼1 to 3 mm in diameter) and pinpoint small colonies (up to 10 times smaller). If the large colonies are cultured on SBA, they grow typical, uniformly large O. rhinotracheale colonies. In contrast, small colonies generally produce both types. To further investigate this observation, one colony from each type was picked and passaged on SBA several times, to assess stability. All O. rhinotracheale isolates (WT isolates and SCVs) then underwent sequence analysis of the complete 16S rRNA gene (22). Furthermore, all of the stable SCVs and the WT isolates were chosen for further studies, including Gram staining, scanning electron microscopy (SEM), autolysis assay, growth curve analysis, determination of biochemical characteristics, hemagglutination assay (HA), auxotrophism test, antimicrobial susceptibility test, and intracellular persistence assay. The O. rhinotracheale isolates with small colonies were termed SCVs if they maintained their phenotype on SBA for at least 10 passages, while the O. rhinotracheale isolates with typical colonies were termed wild-type (WT) isolates. Cryopreservation of the O. rhinotracheale isolates was done by swabbing the pure cultures directly from the SBA and storing the samples at −80°C in cryoprotection medium composed of trypticase soy broth (TSB) supplemented with 25% glycerol (23).
PCR and sequence analysis.
Primary identification was conducted by extracting the DNA from the suspected colonies using the conventional boiling method. Subsequently, PCR was conducted using specific O. rhinotracheale primers (which amplify a 784-bp region of the 16S rRNA), as described previously (24). Following O. rhinotracheale PCR, identification was confirmed by single-strand sequencing of the amplified fragments, and comparison with the published GenBank sequences was performed.
For sequence analysis, the complete 16S rRNA genes from all O. rhinotracheale isolates (WT isolates and SCVs) were amplified and sequenced as described previously (22). The PCR products were purified and sequenced on an ABI 3730 automated sequencer at Beijing Sunbiotech, Inc. (Beijing, China). The chromatographs obtained were verified by eye and aligned using the program MUSCLE (25), as implemented in MEGA5 (26). The program DnaSP v.5 (27) was used to compute haplotype diversity (h) (28) and nucleotide diversity (π) (29). Consequently, phylogenetic analysis for the discrimination of clades was performed with the neighbor-joining method (28) using the Tamura 3-parameter substitution model (30), as implemented in MEGA5 (26). The phylogenetic results obtained were verified by bootstrap tests (31), as well as Bayesian statistics in MrBayes 3.1 (32).
Biochemical characteristics.
Pairs of O. rhinotracheale isolates (stable SCVs and their WT counterparts) were compared with respect to their biochemical profiles using API-20NE identification strips (bioMérieux, France), according to the manufacturer's instructions (15, 21). Moreover, isolates were tested for growth on MacConkey agar, catalase activity, and oxidase activity. Two methods were used to test oxidase activity, i.e., the oxidase reagent purchased with the API-20NE identification strips (bioMérieux, France) and oxidase disks (Sigma, India) to confirm the results.
Bacterial growth curves and enrichment on broth medium.
Growth kinetics for all stable O. rhinotracheale SCVs and WT isolates were determined as described previously, with modification (13, 33). Single loopfuls of 48-h pure O. rhinotracheale cultures were inoculated in 225-ml Falcon tubes containing 20 ml of TSB (BBL; Becton, Dickinson and Co., Sparks, MD) supplemented with 5% fetal bovine serum (FBS). The tubes were then sealed tightly and incubated for 24 h at 37°C with shaking (200 rpm). After incubation, 1-ml culture samples were transferred to new tubes containing 40 ml TSB supplemented with 5% FBS and were incubated under the same environmental conditions (initial optical density at 600 nm [OD600] values were adjusted to be less than 0.1). Bacterial growth was assessed every 3 h, by measuring the OD600 of the culture with a Multiskan-MK3 microplate reader (Thermo Labsystems). Growth curves were constructed in triplicate. Additionally, the purity and stability (switching and reverting between SCV and WT forms) of the cultures were checked on SBA.
Furthermore, bacterial growth was determined with the standard plate counting method. Thus, serial 10-fold dilutions of bacterial cultures were made in phosphate-buffered saline (PBS), 100-μl aliquots were spread on SBA in triplicate, and the plates were then incubated to quantify the viable CFU.
Hemagglutination assay.
The hemagglutinating activity of all stable O. rhinotracheale SCVs and WT isolates was tested with fresh chicken, pigeon, and rabbit erythrocytes, as described previously (15, 34). Briefly, fresh bacterial suspensions of the O. rhinotracheale isolates were prepared from 48-h cultures on SBA; they were harvested in 1.5 ml of PBS (pH 7.0), adjusted to an OD600 value of 1.5, and washed 3 times with PBS. Serial dilutions of each O. rhinotracheale isolate were then prepared in 0.85% NaCl. The hemagglutinating activity was determined with 50-μl volumes of relevant fresh 1% erythrocyte suspension. Wells containing only suspensions of erythrocytes served as negative controls. The hemagglutination titer was the reciprocal of the highest dilution of antigen showing complete agglutination of the erythrocytes after 40 min of incubation at room temperature. Each test was performed in triplicate, and the hemagglutination assay (HA) results were regarded as positive when erythrocyte suspensions were agglutinated in the three repetitions.
Auxotrophism assay.
Stable O. rhinotracheale SCV isolates underwent single auxotrophism tests as described previously (5). Briefly, colonies were taken from 48-h cultures and suspended in 1 ml TSB, 50-μl aliquots were swabbed on SBA, and 10-mm disks (Sigma, India) loaded with 20 μl of thymidine at 100 μg/ml (Sigma), hemin at 100 μg/ml (Sigma), or menadione at 25 μg/ml (Sigma) were laid on the top of the SBA plates. An isolate was considered auxotrophic if a zone of WT colonies or enhanced growth surrounding the disks, compared to the periphery, was detected following 24 to 48 h of incubation at 37°C under microaerobic conditions (13).
Antimicrobial susceptibility testing.
The in vitro susceptibility of stable O. rhinotracheale SCVs and wild-type isolates was determined by the agar disc diffusion method with SBA (15). The antibiotics tested included aminoglycosides (kanamycin, 30 μg; gentamicin, 10 μg), β-lactams (penicillin G, 10 IU; amoxicillin, 10 μg; ampicillin, 10 μg; cefuroxime sodium, 30 μg; cefotaxime, 30 μg), macrolides (clarithromycin, 15 μg; erythromycin, 15 μg), tetracyclines (doxycycline, 30 μg; tetracycline, 30 μg), norfloxacin (10 μg), polymyxin B (300 IU), sulfamethoxazole-trimethoprim (23.75 μg and 1.25 μg), clindamycin (20 μg), and chloramphenicol (30 μg). Results were read after 24 and 48 h of incubation at 37°C under microaerobic conditions, according to the National Committee for Clinical Laboratory Standards procedure for fastidious Gram-negative organisms (35).
Subsequently, pairs of O. rhinotracheale isolates (stable O. rhinotracheale SCVs and their WT counterparts) that showed different patterns of susceptibility to certain antibiotics were further tested to identify the MIC values for those antibiotics, as described previously (36). Therefore, O. rhinotracheale isolates were grown on TSB supplemented with 5% FBS and 2% IsoVitaleX Enrichment at 37°C under microaerobic conditions (see below), and standard microdilution procedures were conducted using the same medium and conditions to determine MIC values.
Autolysis assay.
The Triton X-100-induced autolysis assay was performed as described previously (37). Briefly, 48-h cultures of stable O. rhinotracheale SCVs and WT isolates were subcultured on TSB and incubated for 5 h at 37°C with shaking (220 rpm), and the OD600 was adjusted to 1.0. The cells were then pelleted, washed twice with ice-cold PBS, and subsequently resuspended in PBS containing 0.04% Triton X-100. Suspensions were then incubated with shaking (220 rpm) at 37°C. OD600 readings were taken at 0 min, at 15 min, and then at 30-min intervals up to 4 h. Triton X-100-induced autolysis was measured as a percentage of the initial OD600 value.
Preparation of O. rhinotracheale isolates for the assay.
Before testing, stable O. rhinotracheale SCVs and WT isolates were grown on TSB supplemented with 5% serum and 2% IsoVitaleX Enrichment at 37°C under microaerobic conditions, with shaking (220 rpm), to the early exponential phase. CFU/ml values were enumerated using SBA plates, and stability was observed. For the intracellular persistence assay, bacterial suspensions were centrifuged at 8,000 rpm for 5 min, and the cell pellets were washed two times with PBS. Washed cells were then suspended in invasion medium (Dulbecco's modified Eagle's medium [DMEM]) and adjusted to cell densities that corresponded to a multiplicity of infection (MOI) of 100 CFU of O. rhinotracheale per cell for each isolate (38).
Intracellular persistence assay.
To investigate the intracellular persistence of O. rhinotracheale SCVs and WT isolates, an intracellular survival assay was conducted as described previously, with modifications (38). Briefly, confluent monolayers of murine RAW 264.7 macrophages were seeded in 24-well tissue culture plates. Before testing, the medium was removed from the wells and 1 ml of invasion medium (MOI of 100 CFU/cell) was added to each well except for the control. The plates were reincubated for 2 h, the medium was removed, and 1 ml of DMEM containing chloramphenicol at 40 μg/ml was added to each well to kill extracellular bacteria. The medium was then removed, the cells were washed three times with PBS, 1 ml of DMEM with 2% FBS was added to each well, and the plates were incubated for 3, 24, and 48 h. At these time points, the monolayers were washed 3 times with PBS, followed by addition of 500 μl of ice-cold sterile water to disrupt the cells and to release the intracellular bacteria. After this, serial dilutions were made in PBS, and the numbers of intracellular CFU were determined by plating 100-μl aliquots on SBA in duplicate. Each test was done in triplicate. Statistical analysis of the differences in CFU between O. rhinotracheale SCV28 and its WT isolate after 3, 24, and 48 h of incubation was performed using Student's t test (P < 0.05).
RESULTS
Isolation of O. rhinotracheale.
A total of 27 O. rhinotracheale isolates were recovered from the collected samples; 26 of the isolates were obtained from chickens and one O. rhinotracheale isolate was obtained from a turkey (Table 1). In contrast, no O. rhinotracheale could be isolated from the other avian species. Moreover, with the exception of O. rhinotracheale isolates 21, 22, and 23, all O. rhinotracheale isolates were picked from SBA supplemented with gentamicin.
Table 1.
Bacterial isolates in this study
| Isolate | Origin of isolate, age, and location | SCVa | Geographical region | Accession no.b |
|---|---|---|---|---|
| Ornithobacterium rhinotracheale isolates | ||||
| 1 | Chicken hen, 150 days, trachea | − | Hebei Province | KC454287 |
| 3 | Chicken hen, 157 days, trachea | + | Hebei Province | KC454288, KC454289 |
| 4 | − | KC454290 | ||
| 5 | − | |||
| 6 | Chicken broiler, 10 days, trachea | + | Shandong Province | KC454291 |
| 7 | Chicken hen, 30 days, trachea | + | Shandong Province | KC454292 |
| 8 | Chicken broiler, 37 days, trachea | + | Hebei Province | KC454293, KC454294 |
| 9 | + | |||
| 10 | Chicken hen, 300 days, trachea | + | Hebei Province | KC454295 |
| 11 | + | KC454296, KC454297 | ||
| 12 | − | |||
| 13 | Chicken broiler, 45 days, trachea | + | Hebei Province | KC454298 |
| 14 | Turkey, trachea | − | Hebei Province | KC454299 |
| 15 | Chicken hen, 70 days, trachea | + | Shandong Province | KC454300 |
| 16 | + | KC454301 | ||
| 17 | Chicken hen, 21 days, trachea | + | Shandong Province | KC454302 |
| 18 | + | KC454303 | ||
| 19 | Domestic chicken, 70 days, trachea | + | Shandong Province | KC454304 |
| 20 | Chicken hen, 67 days, trachea | − | Shandong Province | KC454305 |
| 21 | Chicken hen, 50 days, trachea | + | Shandong Province | KC454306 |
| 22 | + | KC454307 | ||
| 23 | + | KC454308 | ||
| 24 | Chicken hen, 70 days, trachea | + | Shandong Province | KC454309 |
| 25 | + | KC454310 | ||
| 26 | Chicken hen, 60 days, trachea | + | Shandong Province | KC454311 |
| 27 | Chicken hen, 240 days, trachea | + | Shandong Province | KC454312 |
| 28 | Chicken breeder, 70 days, trachea | + | Shandong Province | KC454313 |
| Non-O. rhinotracheale isolates | ||||
| Riemerella columbina RC29 | Chicken hen, 50 days, trachea | NA | Hebei Province | KC454317 |
| Coenonia anatina CA39 | Duck broiler, 25 days, trachea | NA | Shandong Province | KC454314 |
| Riemerella anatipestifer RA37 | Duck broiler, 31 days, trachea | NA | Shandong Province | KC454315 |
| Riemerella anatipestifer RA38 | Duck broiler, 37 days, trachea | NA | Shandong Province | KC454316 |
−, no SCV recovered; +, SCV recovered; NA, not applicable.
GenBank accession number of the 16S rRNA gene.
Cultivation and morphological characteristics of O. rhinotracheale WT isolates and SCVs.
The best results for isolation of O. rhinotracheale were achieved by picking pinpoint colonies on SBA (with or without gentamicin) after incubation for 24 h at 37°C under microaerobic conditions (39). Furthermore, unique characteristics of the O. rhinotracheale colonies were their paraffinic structure and their poor adherence to agar (40).
After 48 h of incubation on SBA, 21 of the 27 O. rhinotracheale isolates showed heterogeneous populations of both typical O. rhinotracheale colonies (∼1 to 3 mm, circular, opaque, and gray to gray-white) and potential O. rhinotracheale SCV pinpoint colonies (Fig. 1A). In addition, the stability of these potential O. rhinotracheale SCVs was checked by passaging every isolate on SBA for 10 generations. Five O. rhinotracheale SCVs (SCV3, SCV13, SCV16, SCV21, and SCV28) showed high stability and were chosen for further studies.
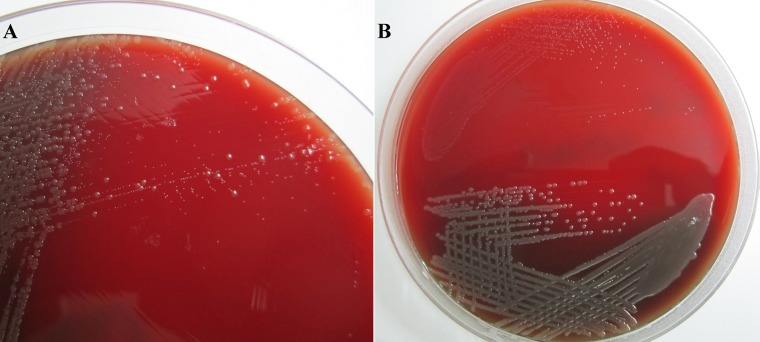
Fig 1.
(A) Primary plate with a mixed culture of pinpoint small-colony variants and typical normal-sized wild-type colonies of Ornithobacterium rhinotracheale. (B) Colony morphology of a stable O. rhinotracheale SCV (top) and its WT isolate (bottom). Note the weak hemolytic activity of the wild-type isolate.
The characteristics of these stable O. rhinotracheale SCV isolates included slow growth (requiring up to 48 h of incubation at 37°C to be detected), tiny pinpoint (approximately 10 times smaller than their WT isolates), less-opaque, gray-white colonies on SBA, and failure to present any type of hemolytic activity after 48 h (Fig. 1B and Table 2). No differences between O. rhinotracheale SCVs and their WT isolates were detected under the light microscope; both types showed Gram-negative, pleomorphic, rod-shaped bacteria. In contrast, SEM observations showed major differences in the amounts of intercellular substances, with O. rhinotracheale WT isolates showing larger amounts than the SCV isolates (Fig. 2A to D).
Table 2.
Colony diameters for stable O. rhinotracheale SCVs and their wild-type isolates on SBA
| Incubation time (h) | Colony diameters (mm) for the SCV or WT isolatea: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCV3 | WT3 | SCV13 | WT13 | SCV16 | WT16 | SCV21 | WT21 | SCV28 | WT28 | |
| 24 | —b | 0.6 | — | 0.3 | 0.1 | 0.4 | — | 0.3 | — | 0.1 |
| 48 | 0.2 | 1.9 | 0.1 | 1.5 | 0.2 | 2.4 | 0.2 | 1.8 | 0.1 | 0.6 |
| 72 | 0.3 | 3.4 | 0.3 | 2.5 | 0.5 | 4 | 0.3 | 3.2 | 0.2 | 1.1 |
Values are means of 30 measured colonies for each isolate.
—, no growth detected at the measured time point.
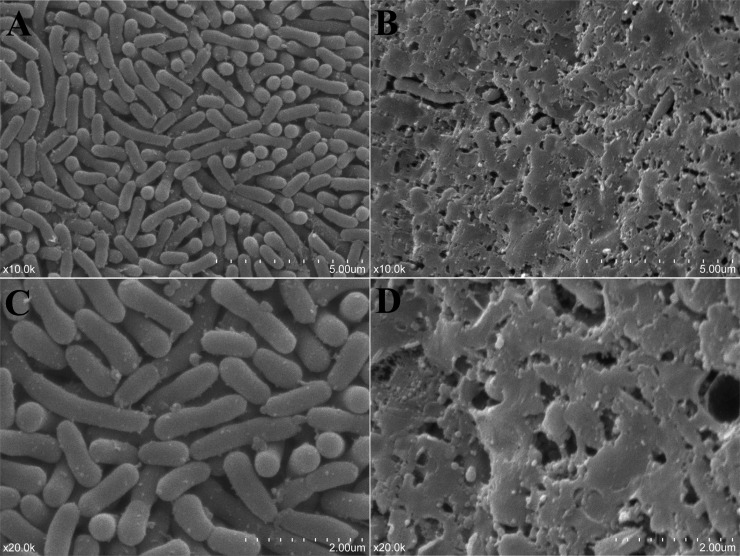
Fig 2.
Scanning electronic microscopy (SEM) photographs of a stable O. rhinotracheale SCV (A and C) and its wild-type isolate (B and D) on SBA after 48 h of incubation. The O. rhinotracheale WT isolate showed larger amounts of intercellular substances than did its SCV. Note the pleomorphic and rod-shaped bacteria. Magnification, ×10,000 (A and B) or ×20,000 (C and D).
Furthermore, auxotrophism assays for stable O. rhinotracheale SCV isolates on SBA revealed enhanced growth rates only around thymidine discs for SCV16 and around menadione discs for SCV3. In contrast, isolates SCV13, SCV21, and SCV28 showed no differences in growth rates around any of the impregnated discs.
Biochemical characteristics.
The results with API-20NE strips were assessed initially after 24 h of incubation at 30°C and did not change after 48 h. All five stable O. rhinotracheale SCV isolates gave identical API-20NE codes (code 0020000). In contrast, the WT isolates showed two different codes; isolates WT3, WT13, WT21, and WT28 gave code 0020004 and WT16 gave code 0220004. All five pairs of isolates gave positive results in the β-galactosidase assay. In contrast to their WT isolates, all O. rhinotracheale SCV isolates were oxidase negative. Additionally, only WT16 was positive for urease. Furthermore, all of the tested isolates were catalase negative and did not grow on MacConkey agar.
Bacterial growth curves.
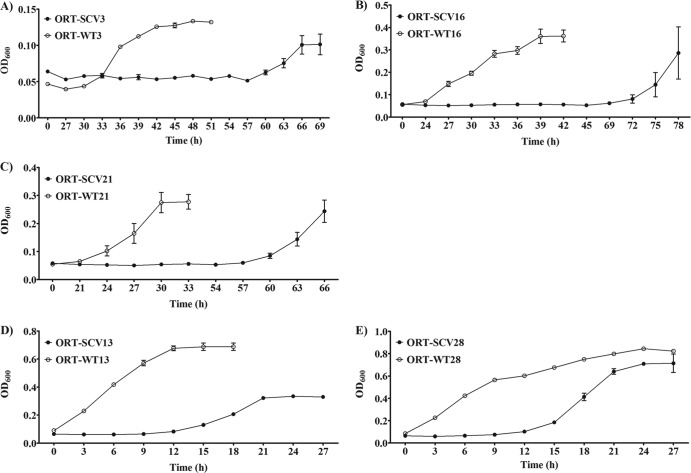
Growth curves for the stable O. rhinotracheale SCVs and their WT isolates were drawn (Fig. 3A to E). The five tested pairs of O. rhinotracheale isolates showed two different patterns in growth on TSB supplemented with FBS. Whereas the WT isolates with the first pattern (WT3, WT16, and WT21) showed prolonged lag phases (up to 27 h) and lower exponential-phase peaks (OD600 of 0.361 for WT16), the WT isolates with the second pattern (WT13 and WT28) had very short lag phases and higher exponential-phase peaks (OD600 of 0.844 for WT28). Furthermore, the SCV isolates of both patterns showed extended lag phases, compared with the WT isolates. The plate counting method revealed that SCV13 grew nearly 4 times more slowly than its WT counterpart (the generation time for SCV13 was 205 min, compared to 54 min for WT13). Moreover, O. rhinotracheale SCVs and their WT isolates showed high levels of stability on TSB with 5% FBS. It should be noted that the efficacy of the TSB and other enrichment broth media (brain-heart infusion medium and Mueller-Hinton broth) in the enrichment and cultivation of O. rhinotracheale isolates was significantly enhanced by providing the broth with both 5% FBS and 2% IsoVitaleX Enrichment (BBL; Becton, Dickinson and Co., Sparks, MD) (data not shown).
Fig 3.
Growth curves for the five stable O. rhinotracheale (ORT) SCVs in TSB supplemented with 5% FBS, in comparison with their wild-type isolates.
Hemagglutination assay.
No differences in HA activity between stable O. rhinotracheale SCVs and the WT isolates were detected. Furthermore, except for isolate 21 (SCV21 and WT21) with pigeon erythrocytes, all tested O. rhinotracheale isolates showed HA activity with the tested erythrocytes. Thus, all five pairs of O. rhinotracheale isolates tested demonstrated HA activity with chicken and rabbit erythrocytes.
Antimicrobial susceptibility testing.
Similar patterns of antibiotic susceptibility were recorded for the stable O. rhinotracheale SCVs and their WT isolates with clindamycin, kanamycin, tetracycline, ampicillin, chloramphenicol, cefotaxime, doxycycline, erythromycin, and penicillin G (data not shown). In contrast, all O. rhinotracheale SCV isolates were resistant to norfloxacin, unlike their WT isolates, which were susceptible to that drug. Furthermore, variable differences between some O. rhinotracheale SCVs and their WT isolates were recorded for amoxicillin, sulfamethoxazole, clarithromycin, polymyxin B, cefuroxime sodium, and gentamicin. O. rhinotracheale SCVs showed higher MIC values than their WT isolates (Table 3).
Table 3.
MICs of selected antibiotics for stable O. rhinotracheale SCVs and their wild-type isolates
| Antibiotic | Antibiotic MICs (μg/ml) for the SCV or WT isolatea: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCV3 | WT3 | SCV13 | WT13 | SCV16 | WT16 | SCV21 | WT21 | SCV28 | WT28 | |
| Amoxicillin | 4 | 4 | 8 | 8 | 8 | 4 | 4 | 4 | 32 | 8 |
| Gentamicin | 128 | 128 | >256 | 256 | 128 | 64 | >256 | 8 | 256 | 256 |
| Sulfamethoxazole | 128 | 128 | 256 | 256 | >256 | 128 | 256 | 4 | 256 | 16 |
| Clarithromycin | 32 | 8 | 128 | 16 | 8 | 4 | 64 | 64 | 8 | 8 |
| Polymyxin B | 64 | 64 | 256 | 8 | 64 | 64 | 64 | 8 | 256 | 8 |
| Cefuroxime sodium | 64 | 16 | 16 | 16 | 128 | 8 | 8 | 2 | 64 | 4 |
| Norfloxacin | 32 | 8 | 64 | 16 | 32 | 2 | 32 | 8 | 64 | 2 |
Values are median MICs from three tests.
Autolysis assay.
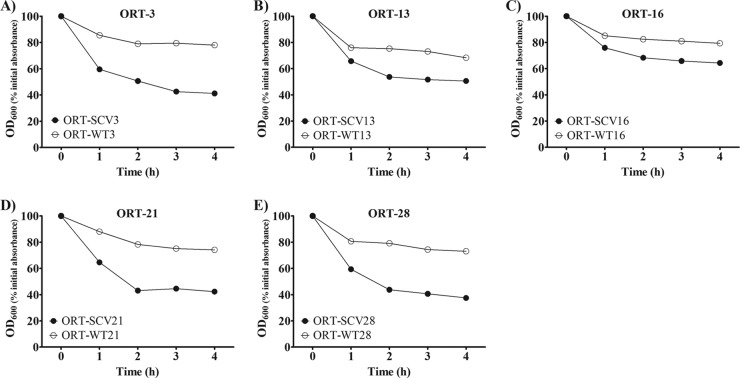
Two patterns were observed for the autolytic activity of the five pairs of O. rhinotracheale isolates (Fig. 4A to E). All of the stable O. rhinotracheale SCV isolates showed higher autolytic activity, as indicated by a decrease in the OD600 of about 53% within 4 h, compared to 25% for the WT isolates.
Fig 4.
Triton X-100-induced autolysis for all stable O. rhinotracheale (ORT) SCVs and their WT isolates. Each test was done three times, and mean results are presented.
Intracellular persistence assay.
One of the five pairs of O. rhinotracheale isolates tested was investigated for intracellular persistence using murine RAW 264.7 macrophages at three time points, i.e., 3, 24, and 24 h (Fig. 5). WT28 showed higher CFU values than did its SCV counterpart after 3 h of incubation, indicating greater bacterial uptake by the macrophages for WT28. In contrast, the next two time points showed higher CFU values for SCV28 than for its WT isolate, which showed noticeable CFU decreases after 48 h of incubation. Furthermore, reversion was observed only in the SCV28 cultures after 24 and 48 h of incubation (24.5% and 33% of the enumerated CFU, respectively). Lower switching rates were observed for the WT28 cultures (8.6% of the enumerated CFU after 48 h of incubation).
Fig 5.

O. rhinotracheale (ORT) SCV28 (CFU/ml) recovered from RAW 264.7 cells at three time points, compared with its WT isolate (MOI of 100 CFU/ml). ∗, significant difference (P < 0.05).
Phylogenetic analysis.
In the entire data set (n = 27), 15 haplotypes were identified, and all nucleotide sites were monomorphic for SCV and WT forms of the same isolate. These 15 haplotypes clustered into two main well-supported clades. Clade 1 consisted of 23 isolates that clustered into three subclades and clade 2 was formed by four isolates (Fig. 6). The five stable SCVs did not show a phylogenetic pattern. Data set diversity indices revealed high levels of haplotype diversity in O. rhinotracheale (h = 0.9373) and low nucleotide diversity (π = 0.00496). The average number of nucleotide differences (k) was 6.76923. Phylogeny inferred from 16S rRNA findings showed independent patterns for geographical region and host.
Fig 6.
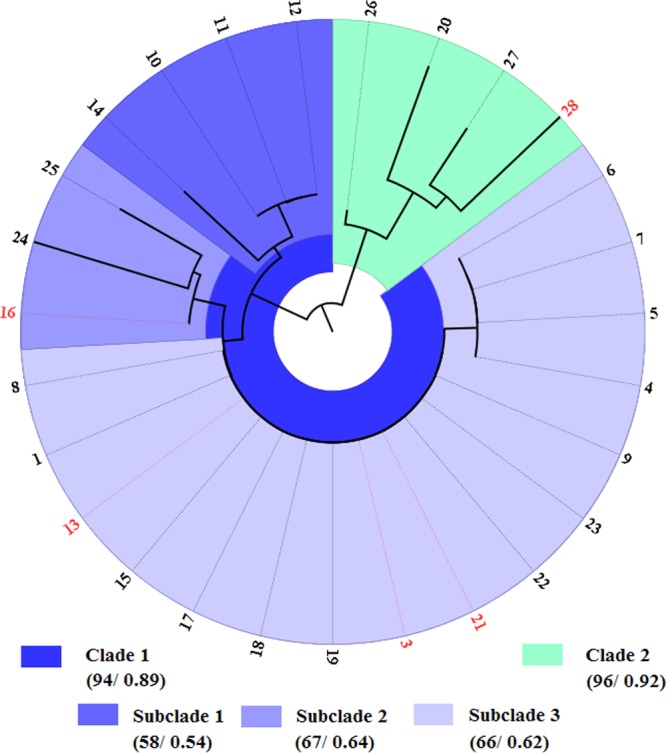
Phylogenetic relationships of the 27 isolates of Ornithobacterium rhinotracheale inferred from 16S rRNA gene sequence analysis of a 1,366-bp sequence. The five pairs of O. rhinotracheale isolates (stable ORT-SCV isolates and their WTs) are marked in red. The first and second values in the key are the bootstrap and Bayesian values, respectively. Branch length, represented by the black lines, corresponds to the number of substitutions.
DISCUSSION
In this study, we report for the first time the isolation and characterization of small-colony variants of O. rhinotracheale from chickens with clinical signs of respiratory disease. O. rhinotracheale, which is considered an endemic pathogen associated with respiratory diseases in domestic poultry, causes significant economic loss in the poultry industry, with worldwide distribution in almost all avian species (16, 17, 19).
All of the O. rhinotracheale isolates gave a clear band at 784 bp by using the PCR with O. rhinotracheale-specific primers under the recommended conditions, but the primer pair also amplified fragments of Riemerella anatipestifer RA37 and RA38 (500 bp), Riemerella columbina RC29 (1,200 bp), and Coenonia anatina CA39 (200 bp). That finding is in agreement with a previous study that noted that false-positive results were recorded using this set of primers (41). Therefore, it is recommended that the identification of O. rhinotracheale be confirmed by sequencing the amplified fragment.
Forming small-colony variants appears to be a natural phenomenon characteristic of the O. rhinotracheale isolates; 21 (77.8%) of 27 isolates of O. rhinotracheale proved to have SCVs in their primary cultures. A typical O. rhinotracheale WT isolate appeared to be very stable and showed uniform colony size on SBA. However, different O. rhinotracheale WT isolates showed different colony sizes (Table 2). This is in accordance with a previous study that reported that different O. rhinotracheale isolates had significant differences in their colony sizes (15). In contrast to the WT isolates, the stability of O. rhinotracheale SCVs varied greatly, and 16 O. rhinotracheale SCV isolates showed partial reversion upon subculturing on SBA. Notably, five O. rhinotracheale SCV isolates proved to maintain their phenotype and did not revert in 10 generations. However, subcultures on SBA from the stocks of these five frozen stable O. rhinotracheale SCVs revealed partial reversion and were stable again after subculturing on SBA. This is in agreement with a previous study on Enterococcus faecium SCVs that reported that reversion of SCVs was observed after freezing and thawing (13).
With respect to the biochemical profiles, tested O. rhinotracheale isolates (SCV and WT isolates) showed poor positive rates using API-20NE identification strips, in agreement with previous studies that reported low positive rates for biochemical reactions (15, 41). Two codes were obtained for O. rhinotracheale WT isolates (codes 0020004 and 0220004), in accordance with previous studies that reported that these two codes are prevalent and are found for about 99.5% of O. rhinotracheale isolates (17, 20, 21). Moreover, it was possible to discriminate between the O. rhinotracheale SCVs and their WT isolates by using API-20NE strips, because O. rhinotracheale SCVs gave one code (code 0020000). All of the O. rhinotracheale SCV isolates tested were oxidase negative. Additionally, WT16 was positive and SCV16 was negative for urease, in accordance with previous studies that reported that SCV bacteria have reductions in biochemical reaction activities (1, 3).
One of the major problems in enriching and testing O. rhinotracheale is that isolates do not grow equally on different broth media. Therefore, preparing and optimizing a broth medium that can support the growth of O. rhinotracheale isolates should be highlighted (15, 17, 20, 42–44). This can be clearly seen in the results presented; three of the tested O. rhinotracheale WT isolates showed longer lag phases and lower exponential-phase peaks than did the other two isolates. Furthermore, O. rhinotracheale SCVs showed extremely low growth rates, compared to their WT isolates, and the length of their lag phases correlated with the lag phases of their WT isolates. This is in agreement with studies of other SCV bacteria that indicated that SCVs show low growth rates on broth media, compared to their WT isolates (13, 38).
Supplementing the broth medium with 5% FBS and 2% IsoVitaleX Enrichment (BBL; Becton, Dickinson and Co., Sparks, MD) proved to have a great impact on the growth rates of O. rhinotracheale isolates. Hence, such media can be of great use in future studies on O. rhinotracheale. However, the stability of O. rhinotracheale isolates (SCVs and WT isolates) was greatly affected with this medium, and high rates of reversion and switching were observed.
The results presented indicate that O. rhinotracheale SCV isolates have higher MIC values for antibiotics than do their WT isolates. These findings support previous studies that indicated that SCVs were more resistant to antibiotics than were their WT counterparts (1, 3). However, one study on Enterococcus faecium SCVs reported identical antibiotic resistance profiles for the SCVs and their WT isolates (13). Challenges in the treatment of respiratory diseases associated with O. rhinotracheale could be partially explained by the ability of O. rhinotracheale to exist as SCVs.
The Triton X-100-induced autolysis assay revealed decreases in the autolytic activity of the O. rhinotracheale WT isolates, compared to their SCVs (Fig. 4A to E); this could be explained by the accumulation of cell wall material and the abundance of intercellular substances observed in the O. rhinotracheale WT isolates, which covered the bacterial cells and might protect them from the effects of Triton X-100 (Fig. 2A to D). A previous study reported that S. aureus SCVs and their WT isolates differed in the amounts of intercellular substances (45). In that study, however, intercellular substances were more abundant in the SCVs.
Previous studies reported that SCVs are phenotypes adapted for intracellular persistence (1, 2, 46, 47). In our study, the results of the intracellular persistence assay indicated that SCV28 had the ability to survive intracellularly for a prolonged time, compared to its WT isolate (Fig. 5). One explanation for the improved intracellular survival of SCVs is their decreased overall metabolism and dampened production of cytotoxins, which may downregulate the induction of cell lysis or apoptosis (1, 48). These data support the hypothesis that the SCV phenotype might be one of the survival strategies that bacteria use for optimal internalization and survival in the host (1).
In contrast to the phenotypic and antibiotic test results, all of the O. rhinotracheale SCVs and their O. rhinotracheale WT isolates showed identical 16S rRNA sequences. This is in accordance with a previous study on E. faecalis SCVs that reported identical 16S rRNA sequences for SCVs and their wild-type isolates (7). This finding indicates that other phylogenetic methods should be used to discriminate between the O. rhinotracheale SCVs and their WT isolates.
To the best of our knowledge, this is the first study to report the isolation and characterization of small-colony variants of Ornithobacterium rhinotracheale. Low growth rates, negative oxidase results, the presence in mixed cultures with the wild-type phenotype, and the strong tendency to exhibit unstable phenotypes explain why O. rhinotracheale SCVs are misidentified and overlooked in routine veterinary laboratory diagnostic procedures. The results presented indicate that O. rhinotracheale SCV isolates possess higher resistance to antibiotics than do their wild-type isolates, which emphasizes that successful antibiotic treatment of respiratory diseases associated with O. rhinotracheale needs to take into consideration the resistance patterns of O. rhinotracheale SCVs. In general, there is little understanding of the pathophysiology of SCVs and their clinical importance in veterinary medicine. Therefore, further studies are needed for better understanding of the pathogenic role and underlying mechanisms of the SCV phenotype. O. rhinotracheale can be a model for that purpose, considering the high probability of isolation of SCVs from cultures.
ACKNOWLEDGMENTS
This research was supported by China Ministry of Science and Technology Project 973 (grant 2012CB518702), the China Ministry of Education (grant 313054), and the National Natural Science Foundation of China (grant 31272623).
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305 [DOI] [PubMed] [Google Scholar]
- 2.von Eiff C, Heilmann C, Proctor R, Woltz C, Peters G, Gotz F. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melter O, Radojevic B. 2010. Small colony variants of Staphylococcus aureus: review. Folia Microbiol. (Praha) 55:548–558 [DOI] [PubMed] [Google Scholar]
- 4.Proctor RA, Kahl B, von Eiff C, Vaudaux PE, Lew DP, Peters G. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl 1):S68–S74 [DOI] [PubMed] [Google Scholar]
- 5.Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029 [DOI] [PubMed] [Google Scholar]
- 6.Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95–102 [DOI] [PubMed] [Google Scholar]
- 7.Petersen A, Chadfield MS, Christensen JP, Christensen H, Bisgaard M. 2008. Characterization of small-colony variants of Enterococcus faecalis isolated from chickens with amyloid arthropathy. J. Clin. Microbiol. 46:2686–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen J. 1957. Biosynthesis of hematin compounds in a hemin requiring strain of Micrococcus pyogenes var. aureus. I. The significance of coenzyme A for the terminal synthesis of catalase. J. Bacteriol. 73:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan LE, Kwan S. 1981. Aminoglycoside-resistant mutants of Pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob. Agents Chemother. 19:958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiemann DA. 1995. An unstable small-colony variant of a noninvasive mutant of Salmonella typhimurium is highly invasive for MDCK cells. FEMS Microbiol. Lett. 130:45–49 [DOI] [PubMed] [Google Scholar]
- 11.Colwell CA. 1946. Small colony variants of Escherichia coli. J. Bacteriol. 52:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raven C. 1934. Dissociation of the gonococcus. J. Infect. Dis. 55:328–339 [Google Scholar]
- 13.Gröbner S, Beck J, Schaller M, Autenrieth IB, Schulte B. 2012. Characterization of an Enterococcus faecium small-colony variant isolated from blood culture. Int. J. Med. Microbiol. 302:40–44 [DOI] [PubMed] [Google Scholar]
- 14.Vandamme P, Segers P, Vancanneyt M, Van Hove K, Mutters R, Hommez J, Dewhirst F, Paster B, Kersters K, Falsen E, Devriese LA, Bisgaard M, Hinz K-H, Mannheim W. 1994. Ornithobacterium rhinotracheale gen. nov., sp. nov., isolated from the avian respiratory tract. Int. J. Syst. Bacteriol. 44:24–37 [DOI] [PubMed] [Google Scholar]
- 15.Tsai H-J, Huang C-W. 2006. Phenotypic and molecular characterization of isolates of Ornithobacterium rhinotracheale from chickens and pigeons in Taiwan. Avian Dis. 50:502–507 [DOI] [PubMed] [Google Scholar]
- 16.Moreno B, Chacon G, Villa A, Fernandez A, Vela AI, Fernandez-Garayzabal JF, Ferre S, Gracia E. 2009. Nervous signs associated with otitis and cranial osteomyelitis and with Ornithobacterium rhinotracheale infection in red-legged partridges (Alectoris rufa). Avian Pathol. 38:341–347 [DOI] [PubMed] [Google Scholar]
- 17.van Empel PCMV, Hafez HM. 1999. Ornithobacterium rhinotracheale: a review. Avian Pathol. 28:217–227 [DOI] [PubMed] [Google Scholar]
- 18.Tabatabai LB, Zimmerli MK, Zehr ES, Briggs RE, Tatum FM. 2010. Ornithobacterium rhinotracheale North American field isolates express a hemolysin-like protein. Avian Dis. 54:994–1001 [DOI] [PubMed] [Google Scholar]
- 19.Hafez HM, Lierz M. 2010. Ornithobacterium rhinotracheale in nestling falcons. Avian Dis. 54:161–163 [DOI] [PubMed] [Google Scholar]
- 20.Hafez HM. 2002. Diagnosis of Ornithobacterium rhinotracheale. Int. J. Poult. Sci. 1:114–118 [Google Scholar]
- 21.van Empel P, van den Bosch H, Loeffen P, Storm P. 1997. Identification and serotyping of Ornithobacterium rhinotracheale. J. Clin. Microbiol. 35:418–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amonsin A, Wellehan JF, Li LL, Vandamme P, Lindeman C, Edman M, Robinson RA, Kapur V. 1997. Molecular epidemiology of Ornithobacterium rhinotracheale. J. Clin. Microbiol. 35:2894–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhardt P, Murray RGE, Wood WA, Krieg NR. (ed). 1994. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC [Google Scholar]
- 24.Hung AL, Alvarado A. 2001. Phenotypic and molecular characterization of isolates of Ornithobacterium rhinotracheale from Peru. Avian Dis. 45:999–1005 [PubMed] [Google Scholar]
- 25.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452 [DOI] [PubMed] [Google Scholar]
- 28.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 29.Nei M, Tajima F. 1981. DNA polymorphism detectable by restriction endonucleases. Genetics 97:145–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 9:678–687 [DOI] [PubMed] [Google Scholar]
- 31.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- 32.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 33.Jansen R, Chansiripornchai N, Gaastra W, van Putten JP. 2004. Characterization of plasmid pOR1 from Ornithobacterium rhinotracheale and construction of a shuttle plasmid. Appl. Environ. Microbiol. 70:5853–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vega V, Zepeda A, Ramirez S, Morales V, Fernandez P, de Oca RM, Guerra-Infante FM, de Jesus de Haro-Cruz M, Blackall PJ, Soriano EV. 2008. Hemagglutinating activity of serovar reference strains of Ornithobacterium rhinotracheale. J. Vet. Diagn. Invest. 20:353–355 [DOI] [PubMed] [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—second edition. NCCLS document M31-A2 National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 36.Soriano VE, Vera NA, Salado CR, Fernández RP, Blackall PJ. 2003. In vitro susceptibility of Ornithobacterium rhinotracheale to several antimicrobial drugs. Avian Dis. 47:476–480 [DOI] [PubMed] [Google Scholar]
- 37.Rowe SE, Mahon V, Smith SG, O'Gara JP. 2011. A novel role for SarX in Staphylococcus epidermidis biofilm regulation. Microbiology 157:1042–1049 [DOI] [PubMed] [Google Scholar]
- 38.Atalla H, Gyles C, Jacob CL, Moisan H, Malouin F, Mallard B. 2008. Characterization of a Staphylococcus aureus small colony variant (SCV) associated with persistent bovine mastitis. Foodborne Pathog. Dis. 5:785–799 [DOI] [PubMed] [Google Scholar]
- 39.Odor EM, Salem M, Pope CR, Sample B, Primm M, Vance K, Murphy M. 1997. Isolation and identification of Ornithobacterium rhinotracheale from commercial broiler flocks on the Delmarva Peninsula. Avian Dis. 41:257–260 [PubMed] [Google Scholar]
- 40.Roepke DC, Back A, Shaw DP, Nagaraja KV, Sprenger SJ, Halvorson DA. 1998. Isolation and identification of Ornithobacterium rhinotracheale from commercial turkey flocks in the upper Midwest. Avian Dis. 42:219–221 [PubMed] [Google Scholar]
- 41.Canal CW, Leão JA, Rocha SLS, Macagnan M, Lima-Rosa CAV, Oliveira SD, Back A. 2005. Isolation and characterization of Ornithobacterium rhinotracheale from chickens in Brazil. Res. Vet. Sci. 78:225–230 [DOI] [PubMed] [Google Scholar]
- 42.Leroy-Setrin S, Flaujac G, Thénaisy K, Chaslus-Dancla E. 1998. Genetic diversity of Ornithobacterium rhinotracheale strains isolated from poultry in France. Lett. Appl. Microbiol. 26:189–193 [DOI] [PubMed] [Google Scholar]
- 43.Hafez HM, Sting R. 1999. Investigations on different Ornithobacterium rhinotracheale “ORT” isolates. Avian Dis. 43:1–7 [PubMed] [Google Scholar]
- 44.Ak S, Turan N. 2001. Antimicrobial susceptibility of Ornithobacterium rhinotracheale isolated from broiler chickens in Turkey. Vet. Arhiv. 71:121–127 [Google Scholar]
- 45.Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, Peters G. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balwit JM, van Langevelde P, Vann JM, Proctor RA. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033–1037 [DOI] [PubMed] [Google Scholar]
- 47.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Löffler B. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202:1031–1040 [DOI] [PubMed] [Google Scholar]
- 48.Vaudaux P, Kelley WL, Lew DP. 2006. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin. Infect. Dis. 43:968–970 [DOI] [PubMed] [Google Scholar]