Abstract
Primary hyperparathyroidism occurs as a result of isolated parathyroid adenoma in 80% to 85% of all cases. A 99mtechnetium (99mTc) sestamibi scan or neck ultrasonography is used to localize the neoplasm prior to surgical intervention. A 53-year-old female was referred for the exclusion of metabolic bone disease. She presented with low back pain that had persisted for the past 6 months and elevated serum alkaline phosphatase (1,253 IU/L). Four years previously, she had been diagnosed at a local hospital with a 2.3-cm thyroid nodule, which was determined to be pathologically benign. Radiofrequency ablation was performed at the same hospital because the nodule was still growing during the follow-up period 2 years before the visit to our hospital, and the procedure was unsuccessful in reducing the size of the nodule. The results of the laboratory tests in our hospital were as follows: serum calcium, 14.6 mg/dL; phosphorus, 3.5 mg/dL; and intact parathyroid hormone (iPTH), 1,911 pg/mL. Neck ultrasonography and 99mTc sestamibi scan detected a 5-cm parathyroid neoplasm in the left lower lobe of the patient's thyroid; left parathyroidectomy was performed. This case indicated that thyroid ultrasonographers and pathologists need to be experienced enough to differentiate a parathyroid neoplasm from a thyroid nodule; 99mTc sestamibi scan, serum calcium, and iPTH levels can help to establish the diagnosis of parathyroid neoplasm.
Keywords: Parathyroid neoplasms, Thyroid nodule, Thyroid ultrasonography
INTRODUCTION
Primary hyperparathyroidism occurs as a result of isolated parathyroid adenoma in 80% to 85% of all cases. The hyperparathyroidism can present with no symptoms or with nonspecific phenomena, such as systemic weakness, fatigue, neuromuscular phenomena, anxiety, and cognitive function disorder [1]. In addition, hyperparathyroidism is often detected when it provokes insulin resistance, hyperglycemia, dyslipidemia, high blood pressure, bone density reduction, and renal calculus. To diagnose hyperparathyroidism, measurement of the serum calcium and parathyroid hormone should be performed; to localize the neoplasm, 99mtechnetium (99mTc) sestamibi scan or neck ultrasonography should be conducted prior to any surgical procedure. As neck ultrasonography is often used to make the differential diagnosis, parathyroid adenoma is sometimes mistaken for a thyroid nodule, resulting in the wrongful use of fine needle aspiration cytology [2]. Even pathology departments sometimes fail to distinguish thyroid tissue from parathyroid tissue, as they make the determination based on the histology of the cells in the sample obtained by fine needle aspiration; they may miss the diagnosis of parathyroid adenoma when there is no clinical information about existing parathyroid disease [3]. The first choice of treatment for parathyroid adenoma is surgical excision, and when done by a skilled surgeon, the full recovery rate is 95% to 98%, which is comparatively high, while the rate of side effects is 1% to 3% [1].
Radiofrequency ablation has been used for the treatment of solid cancers, such as liver cancer, but it is now also being used to treat thyroid nodules and thyroid cancer. This type of treatment is mostly used for nodules that cause cosmetic problems or autonomic and functional nodules accompanied by neck symptoms, and the treatment is sometimes indicated for thyroid cancer recurrence in the neck or to treat patients who are not suited for surgery at the time of lymph node recurrence [4]. A number of side effects can result from the application of radiofrequency ablation to the thyroid, and reported side effects include voice change, nodule rupture, and subsequent abscess formation, hypothyroidism, brachial plexus damage, hematoma, skin burn, and neck pain [5]. However, many local clinics use radiofrequency ablation to remove thyroid nodules without regard for aforementioned indications.
We experienced a case in which a thyroid nodule was diagnosed as a parathyroid adenoma and was subsequently treated with radiofrequency ablation, which resulted in a delay of the proper diagnosis and treatment by 4 years; the case is reported here.
CASE REPORT
A 53-year-old female presented to our hospital complaining of a 6-month history of backache. Judging from the blood testing results, she had hypercalcemia, and alkaline phosphatase (ALP) was increased to 1,253 U/L (normal range, 35 to 130); the patient was sent to the endocrinology department. Her past history was significant for a surgical procedure for fibrocystoma of her right humerus 20 years ago, and she had donated one of her kidneys to a relative 7 years previously. She had taken atorvastatin 10 mg, amlodipine 5 mg, and omega-3 fatty acid 1,000 mg daily for the past 3 years to treat high blood pressure and dyslipidemia. The patient had been misdiagnosed with a thyroid nodule and hypothyroidism at another hospital 4 years previously and had immediately been prescribed levothyroxine 50 µg/day. At that time, the thyroid ultrasonography revealed two nodules that were 0.3 and 0.5 cm in diameter on her right lobe and another nodule that was 2.3×1.3 cm in size that was projected backwards. She had fine needle aspiration cytology for the nodule on the left lobe, and this had revealed a benign nodule. She had been followed up every 6 months by neck ultrasonography. Two years later, when the nodule on the left lobe of the thyroid had increased in size to 2.9×1.7 cm, they carried out radiofrequency ablation. However, the thyroid nodule remained the same size even after the radiofrequency ablation, so they monitored its size every 6 months for 2 more years (Fig. 1). During that time, the patient began experiencing backache and visited the orthopedics department of our hospital where she was suspected of having metabolic bone disease due to the high serum ALP value.
Fig. 1.
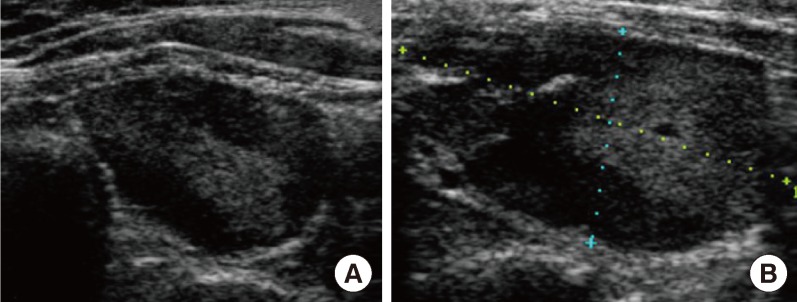
Ultrasonographic findings of a parathyroid adenoma at a local hospital before radiofrequency ablation. (A) Ultrasonographic image of the parathyroid mass before radiofrequency ablation. An isoechoic ovoid mass with a peripheral hypoechoic area in the lower portion of the left and infrathyroid area. (B) Ultrasonographic image on the sixth day after radiofrequency ablation of the parathyroid adenoma.
The patient presented with generalized weakness and fatigue without other hypercalcemic symptoms. The peripheral blood examination was as follows: leukocyte count, 7,020/mm3; hemoglobin, 9.3 g/dL; platelet count, 249,000/mm3; prothrombin time (PT), 14.4 seconds (normal range, 11.9 to 14.3); activated partial thromboplastin time (aPTT), 31.3 seconds (normal range, 29.1 to 43.5); serum iron, 65 µg/dL (normal range, 40 to 160); total iron binding capacity (TIBC), 258 µg/dL (normal range, 230 to 430); transferrin saturation, 25%; and ferritin, 158.6 ng/mL (normal range, 13 to 150). Serum chemistry values were also obtained: blood urea nitrogen, 26.9 mg/dL; creatinine, 1.98 mg/dL; glucose, 110 mg/dL; ALP, 1,011 U/L; aspartate aminotransferase, 23 U/L; and alanine aminotransferase, 11 U/L. Serum calcium was 14.6 mg/dL, phosphate was 3.5 mg/dL, and ionized calcium was 7.2 mg/dL. Serum protein and serum albumin were 6.9 and 3.7 g/dL, respectively. The results of the serum electrolyte test revealed that sodium was 135.7 mmol/L, potassium was 4.3 mmol/L, chloride was 104.0 mmol/L, and serum osmotic pressure was 286 mOsm/kg. According to the serum lipid examination, the total cholesterol was 146 mg/dL, triglyceride was 95 mg/dL, high density lipoprotein was 33 mg/dL, and low density lipoprotein was 79 mg/dL. A thyroid function test found T3 was 108.9 ng/dL (normal range, 80 to 200), thyroid stimulating hormone was 0.40 mIU/L (normal range, 0.27 to 4.2), and free T4 was 0.74 ng/dL (normal range, 0.93 to 1.70). Under dual energy X-ray absorptiometry-based bone density examination, the T-score of the lumbar region was -1.4, while the T-scores of the neck were -1.2 on the left and -1.8 on the right. Except for the fifth lumbar vertebra, which had spondylolisthesis, nothing remarkable was found on magnetic resonance imaging.
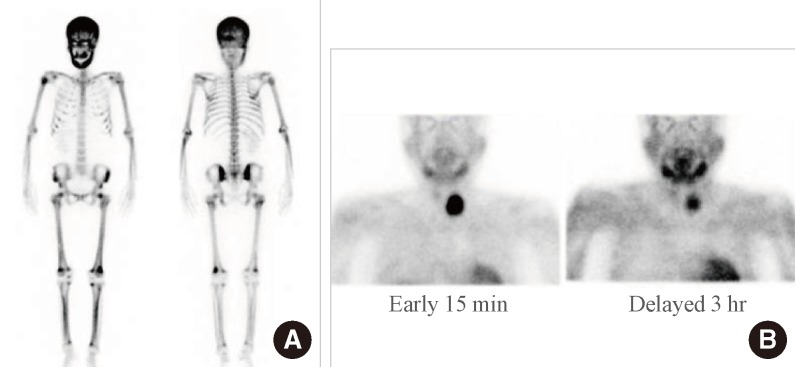
According to further blood tests, 25-hydroxyvitamin D3 was 9.6 ng/mL (normal range, 4.8 to 52.8), and 1,25-dihydroxyvitamin D3 was 10.5 pg/mL (normal range, 19.6 to 54.3), while the intact parathyroid hormone (iPTH) was 1,911 pg/mL (normal range, 15 to 65). When we performed bone scanning with 99mTc, the amount of cortical bone radioactive isotope uptake increased very high (Fig. 2A). Because we suspected hyperparathyroidism, we carried out a 99mTc sestamibi scan and suspected the lesion that had been previously diagnosed as a left thyroid nodule was likely a parathyroid adenoma (Fig. 2B). Using thyroid Doppler ultrasonography, we discovered a hypervascular adenoma that was 5-cm-sized solid mass with similar echo of surrounding thyroid tissue in the center, and slightly low echo in outer part in the inferior site of left thyroid gland (Fig. 3A), while on the right thyroid parenchyma we discovered two thyroid nodules, 0.3 and 0.5 cm each in diameter, that the patient had had 4 years before. We performed neck computed tomography and found there was no neck lymph node metastastasis and local invasion of other organs (Fig. 3B).
Fig. 2.
Nuclear images of the patient. (A) 99mTechnetium hydroxymethane diphosphonate bone scan showing generalized, increased radiotracer uptake in the entire skeleton, especially with hot uptake in the skull and facial bones, which was suggestive of a metabolic bone disease caused by hyperparathyroidism. (B) 99mTechnetium sestamibi scan showing persistent, focal radiotracer uptake in the left lobe of the thyroid on a delayed 3-hour image, which was suggestive of a parathyroid adenoma.
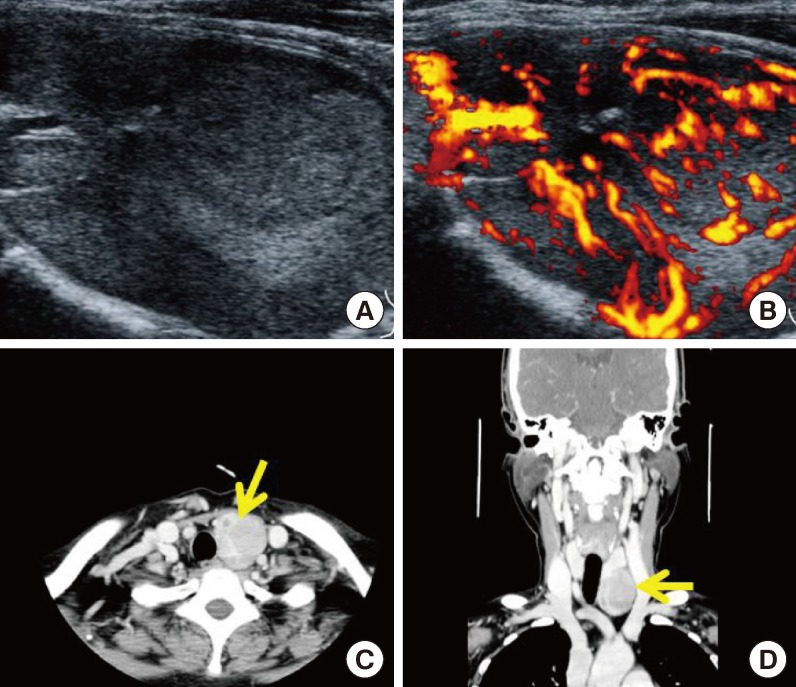
Fig. 3.
Radiologic findings of the parathyroid mass in our hospital. (A, B) Two-dimensional color Doppler ultrasonography showed a large, 5-cm hypervascular mass below the left thyroid, suggestive of a parathyroid adenoma or exophytic thyroid tumor. (C, D) Computed tomography images of the pharynx revealed a large mass (arrow) below the left thyroid, suggestive of a parathyroid adenoma or exophytic thyroid tumor.
The patient received both hydration and diuretic treatments until she underwent a surgical operation to control the hypercalcemia; she also had pamidronate 90 mg intravenous injections twice a week. On the 11th day after hydration and diuretic treatment, her condition had improved; serum calcium was reduced to 11.6 mg/dL, and creatinine improved to 1.11 mg/dL. On the 12th day, the patient had left parathyroid excision and was diagnosed with a parathyroid adenoma (Fig. 4). Following the surgical procedure, iPTH normalized to 42.5 pg/mL, but calcium and vitamin D were prescribed for 9 months to cure the hypocalcemia that had developed due to hungry bone syndrome. It has been a year since the surgical operation, and her calcium, phosphate, ALP, and iPTH all remain at normal levels.
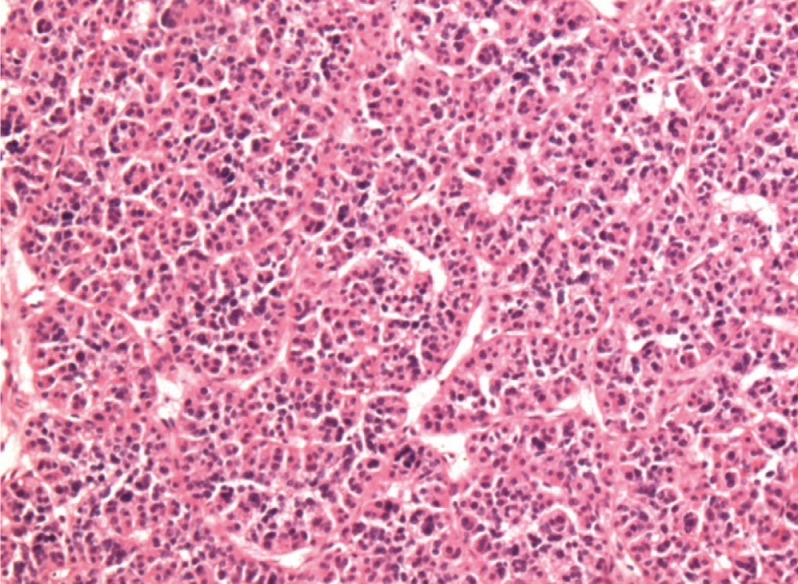
Fig. 4.
Postoperative pathology of the parathyroid mass consisting mainly of chief cells with occasional groups of oxyphil cells and water clear cells. Chief cells were solid sheet-like and had an acinar, follicle-like arrangement that corresponded to the appearance seen in parathyroid adenoma (H&E stain, ×100).
DISCUSSION
The 99mTc sestamibi scan method has long been used to localize parathyroid adenomas for patients with hyperthyroidism. The sensitivity and specificity of this examination method is 90%, which is a comparatively high diagnostic rate that is superior to neck ultrasonography in detecting ectopic adenoma in the mediastinum [6,7]. Due to the recent popularity of thyroid ultrasonography, neck ultrasonography equipment has been in widespread use. The convenience of use, technological developments, and upgrades in practitioner's skills, as well as a higher accuracy in detection, have led to its popularity as a diagnostic tool. In most cases, neck ultrasonography hardly trace the normal parathyroid tissue because it is located behind the thyroid tissue. If it is discovered by ultrasonography, the parathyroid gland is small (below 4 mm) and shows low echogenicity [6]. Neck ultrasonography sensitivity differs from reporter to reporter; it is reportedly 60% on average with a range from 34% to 92%. This sensitivity is lower than the 99mTc sestamibi scan, but the specificity is comparatively high (92% to 97%) [6]. Parathyroid adenomas are generally round or oval shaped upon neck ultrasonography, and they are clearly divided from the surrounding tissue and observed as a homogeneous solid mass of low echogenicity [6]. A usual size of parathyroid adenoma is less than 2 cm, and if it is bigger than that, the adenoma sometimes looks tubular. On ultrasonography, it also looks like a cyst that contains liquid material; but internal calcification is very rare [2]. The disadvantage of ultrasonography in parathyroid adenoma diagnosis is that it is difficult to localize the parathyroid adenoma when it has an ectopic location behind the mediastinum, esophagus or larynx, when it is located inside the thyroid, or if the patient has had previous neck surgery [8]. Another disadvantage of neck ultrasonography is that this investigation has a wide range of sensitivity, which makes the results dependent on the patient's body shape, the performance of the ultrasonographer, the parathyroid adenoma size or location, and any associated underlying thyroid disease. More significantly, the test results are dependent on the technique and experience of the inspector [6].
Krausz et al. [9] used neck ultrasonography to search for lesion locations in 77 patients who were diagnosed with parathyroid adenoma prior to the surgical operation. According to their study, the lesion was found for 81% of the patients without thyroid disease, but this rate was reduced to 53% for patients with nodular thyroid disease. It may be difficult to distinguish patients with thyroid nodules from those with parathyroid adenoma from neck ultrasonography results alone. In this patient's case, her doctor also misdiagnosed parathyroid adenoma for one of the thyroid nodules, resulting in a delay of diagnosis.
Another reason why this patient was misdiagnosed as having a thyroid nodule was that the fine needle aspiration cytology that was performed suggested a benign thyroid nodule. Under pathology examination, parathyroid cells are generally smaller than thyroid cells, have less cytoplasm and have much chromatin gathered inside in a dot shape, but this is not a critical feature [3]. In addition, although colloids or macrophage are generally observed in thyroid tissue, they are also often seen in parathyroid tissue, so it is difficult to rely entirely on fine needle aspirate-based cytology examination for accurate diagnosis. Kwak et al. [2] performed fine needle aspiration cytology on incidentally identified parathyroid adenomas, and 14 of the 24 total parathyroid adenomas tested falsely negative. Therefore, it is very difficult to diagnose parathyroid adenoma using only fine needle cytology without the available clinical information.
Should neck ultrasonography fail to distinguish parathyroid adenoma from thyroid nodule, 99mTc sestamibi scanning is a useful investigation to perform, but this method is usually only available at hospitals with nuclear medical facilities. The alternative for hospitals without nuclear medical facilities is to measure the iPTH value through fine needle aspiration examination or the parathyroid immunohistochemical test. Abraham et al. [10] used the collection from fine needle aspiration for the examination of 32 parathyroid adenoma patients and 13 thyroid nodule patients to measure iPTH. In parathyroid adenoma, this value was 22,060±6,653 pg/mL, and with thyroid nodules, it was 9.0±1.0 pg/mL; the difference was significant. They reported 91% and 95% for the examination's sensitivity and specificity, respectively. Mansoor et al. [11] used immunochemical staining with fine needle examination collection and found 98% sensitivity and 96.1% specificity for distinguishing parathyroid adenoma from thyroid tissue. This patient had received fine needle examination only once before at the initial diagnosis 4 years previously, but if she had undergone a pathology examination when the size of the lesion increased or before the radiofrequency ablation, there may have been less possibility of a misdiagnosis. If the initial neck ultrasonography had been performed by an experienced radiologist or the pathology examination had been carried out by a highly experienced pathologist, the diagnosis could have been discovered much earlier. This patient had only nonspecific hypercalcemic symptoms, such as systemic weakness and fatigue, without other associated hyperparathyroidism symptoms or signs, which delayed the diagnosis even further. She had undergone blood examinations regularly for the past 4 years because of high blood pressure, dyslipidemia, having a single kidney, and hypothyroidism; if the serum calcium had been checked even once during that period, it would have helped in the early detection of her disease. Physicans who perform neck ultrasonography should always have a high index of suspicion not only for thyroid nodule but also for parathyroid adenoma when a thyroid nodule is found. Relatively inexperienced physicians should ask an experienced expert to investigate the thyroid nodule if it does not have a typical shape or is deviated from the normal position. In cases when the adenoma gets bigger or a surgical intervention like radiofrequency ablation is needed, it is recommended that the pathology examination should be performed again. The clinician who investigates the thyroid disease must check and report if the patient has any signs or symptoms associated with hypercalcemia. If there are suspicions of a parathyroid adenoma on the basis of neck ultrasonography, physicians should examine the serum calcium and serum iPTH or carry out a 99mTc sestamibi scan to identify hyperparathyroidism.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med. 2011;365:2389–2397. doi: 10.1056/NEJMcp1106636. [DOI] [PubMed] [Google Scholar]
- 2.Kwak JY, Kim EK, Moon HJ, Kim MJ, Ahn SS, Son EJ, Sohn YM. Parathyroid incidentalomas detected on routine ultrasound-directed fine-needle aspiration biopsy in patients referred for thyroid nodules and the role of parathyroid hormone analysis in the samples. Thyroid. 2009;19:743–748. doi: 10.1089/thy.2008.0263. [DOI] [PubMed] [Google Scholar]
- 3.Lieu D. Cytopathologist-performed ultrasound-guided fineneedle aspiration of parathyroid lesions. Diagn Cytopathol. 2010;38:327–332. doi: 10.1002/dc.21203. [DOI] [PubMed] [Google Scholar]
- 4.Ha EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2011;18:310–314. doi: 10.1097/MED.0b013e32834a9168. [DOI] [PubMed] [Google Scholar]
- 5.Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, Baek SM, Kim YS, Shin JH, Park JS, Kim DW, Kim JH, Kim EK, Jung SL, Na DG Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342. doi: 10.1148/radiol.11110416. [DOI] [PubMed] [Google Scholar]
- 6.Khati N, Adamson T, Johnson KS, Hill MC. Ultrasound of the thyroid and parathyroid glands. Ultrasound Q. 2003;19:162–176. doi: 10.1097/00013644-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nichols KJ, Tomas MB, Tronco GG, Rini JN, Kunjummen BD, Heller KS, Sznyter LA, Palestro CJ. Preoperative parathyroid scintigraphic lesion localization: accuracy of various types of readings. Radiology. 2008;248:221–232. doi: 10.1148/radiol.2481071066. [DOI] [PubMed] [Google Scholar]
- 8.Giron J, Ouhayoun E, Dahan M, Berjaud J, Esquerre JP, Senac JP, Railhac JJ. Imaging of hyperparathyroidism: US, CT, MRI and MIBI scintigraphy. Eur J Radiol. 1996;21:167–173. doi: 10.1016/0720-048X(95)00711-X. [DOI] [PubMed] [Google Scholar]
- 9.Krausz Y, Lebensart PD, Klein M, Weininger J, Blachar A, Chisin R, Shiloni E. Preoperative localization of parathyroid adenoma in patients with concomitant thyroid nodular disease. World J Surg. 2000;24:1573–1578. doi: 10.1007/s002680010280. [DOI] [PubMed] [Google Scholar]
- 10.Abraham D, Sharma PK, Bentz J, Gault PM, Neumayer L, McClain DA. Utility of ultrasound-guided fine-needle aspiration of parathyroid adenomas for localization before minimally invasive parathyroidectomy. Endocr Pract. 2007;13:333–337. doi: 10.4158/EP.13.4.333. [DOI] [PubMed] [Google Scholar]
- 11.Mansoor I, Zalles C, Zahid F, Gossage K, Levenson RM, Rimm DL. Fine-needle aspiration of follicular adenoma versus parathyroid adenoma: the utility of multispectral imaging in differentiating lesions with subtle cytomorphologic differences. Cancer. 2008;114:22–26. doi: 10.1002/cncr.23252. [DOI] [PubMed] [Google Scholar]