Abstract
Thyroid nodules are a common clinical problem with the widespread use of ultrasonography. Fine needle aspiration (FNA) is the mainstay for diagnosing a thyroid malignancy. There have been several guidelines on when to perform FNA in thyroid nodules. This review is based on several published recommendations and helps physicians easily understand the factors favoring FNA.
Keywords: Thyroid; Ultrasonography; Biopsy, fine-needle; Indications; Thyroid malignancy; Thyroid nodule
INTRODUCTION
With the increase in worldwide use of neck ultrasonography (US), the detection of thyroid nodules has rapidly increased to up to 67% of patients who undergo neck US [1]. Among them, only 4% to 7% of patients have palpable nodules on physical examination [2]. In South Korea, the yearly incidence has rapidly increased from 6.3 per 100,000 in 1999 to 47.5 per 100,000 in 2009 [3].
Fine needle aspiration (FNA) is the standard tool for detecting thyroid cancer and has led to a decrease in the number of thyroid surgeries and an increase in cancer detected during thyroid surgery [4-6]. The overall incidence of thyroid cancer is about 9.2% to 13% in patients with thyroid nodules who undergo FNA [7-9]. Also, the high detection rate of US makes FNA for all US-detected nodules impractical, if not impossible. Therefore, deciding which nodules should be biopsied is an important medical issue to ensure that no clinically significant thyroid cancers are missed. There are several guidelines for the indications of FNA in thyroid nodules [10-14]. This work demonstrates these guidelines and compares their merits.
CONSIDERATION FACTORS TO PERFORM FNAs
Size
Thyroid nodule size itself is not a predictive factor of malignancy [7,9-11]. Nevertheless, most guidelines recommend FNA for nodules larger than 10 mm [11,13,15], unless patients have high risk factors [15] or suspicious US features [13]. High risk factors include a history of thyroid cancer in one or more first-degree relatives, a history of exposure to external beam radiation or ionizing radiation in childhood or adolescence, prior hemithyroidectomy with discovery of thyroid cancer, fluorodeoxyglucose (18F) avidity on positron emission tomography scanning, multiple endocrine neoplasia (MEN) 2/familial medullary thyroid cancer-associated RET proto-oncogene mutation, or calcitonin levels >100 pg/mL [15]. Based on the revised American Thyroid Association (ATA) guidelines, FNA is recommended in thyroid nodules larger than 5 mm with suspicious US features in high risk patients. This guideline does not recommend FNA in thyroid nodules smaller than 5 mm. The size criterion for FNA is slightly different between the ATA and the American Association of Clinical Endocrinologists/Associazione Medici Endocrinologi (AACE/AME) guidelines. The AACE/AME guidelines suggests that FNA be performed regardless of lesion size when patients have a history of neck irradiation, a family history of medullary thyroid cancer or MEN2, extracapsular growth, or metastatic cervical lymph nodes [13]. The Korean Society of Thyroid Radiology (KSThR) has an even more strict size criteria [14]. They recommend FNA in thyroid nodules larger than 5 mm with suspicious US features, even when a patient does not have high risk factors. They also recommend selective FNA in thyroid nodules smaller than 5 mm according to risk factors and according to the performing radiologist's experience.
This diversity in size criteria stems from the uncertainty regarding the clinical meaning of early diagnosis for tiny thyroid cancers in patients at low risk. The rationale behind the recommendations for FNA in larger nodules is based on a high rate of false positives and nondiagnostic cytology in thyroid nodules smaller than 5 mm [16,17].
Number of nodules
Traditionally, single nodules are considered to be at more risk for malignancy than multiple nodules. However, patients with multiple thyroid nodules do not have a decreased likelihood of thyroid cancer when compared to patients with solitary nodules [7,18]. The incidence of thyroid cancer in patients with multiple nodules is the same as that in patients with a solitary nodule, although the cancer rate per nodule decreases in patients with multiple nodules [19]. Thus, an FNA operator should focus on the US features of each individual nodule to decide whether or not to perform FNA.
Interval growth
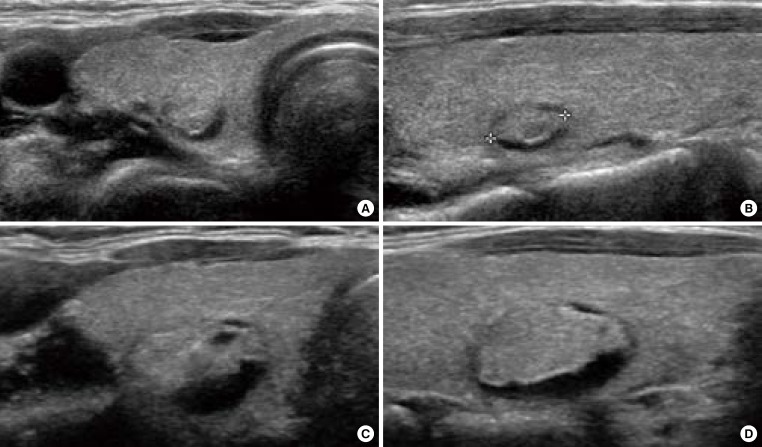
Nodule growth is defined when a nodule shows more than a 50% increase in volume or a 20% increase in at least two nodule dimensions with a minimal increase of 2 mm in solid or in the solid portion of mixed nodules [15] in order to minimize interobserver bias of each measurement (Fig. 1) [20]. Although the growth itself is not a pathognomonic feature of malignancy [21] and the risk of malignancy is very low in a thyroid nodule with benign cytology [13], repeated FNA is usually indicated in nodules that increase in size [14,15].
Fig. 1.
Ultrasonography (US) of growing benign mass in a 40-year-old woman who underwent fine needle aspiration twice. (A) Transverse and (B) longitudinal US images demonstrate a 0.7-cm-sized isoechoic nodule with the cystic portion of the posterior portion of the nodule in the right thyroid gland. (C, D) After 4 years, the nodule was enlarged. The cytology was benign, twice.
US features
Internal content of nodules
Depending on the components of the internal part of the thyroid nodule, nodules can be classified into cystic, mixed (both solid and cystic components), and solid nodules [22-24]. In cases where microcysts are aggregated in mixed nodules, the nodule is further defined as spongiform [25].
An anechoic cyst is definitely benign and can contain hyperechoic spots with comet tail artifacts. The comet tail artifacts are related to microcrystals inside colloid cysts which should be differentiated from the microcalcifications of malignant nodules.
Solidity itself is not considered a suspicious US feature. However, several guidelines recommend FNA in solid nodules larger than 10 mm and mixed echoic nodules larger than 15 mm with some variation in the defining size depending on the guidelines used [11,15].
Echogenicity
Traditionally, hypoechogenicity is a well-known US feature related to malignancy, and most thyroid carcinomas are hypoechoic compared with surrounding thyroid parenchyma [10,26,27]. Hypoechogenicity is a very sensitive sign of malignancy but is less specific than other features. Marked hypoechogenicity is defined as decreased echogenicity less than that of the adjacent strap muscle [10] and is a specific sign of thyroid cancer.
The ATA and AACE/AME consider hypoechogenicity as a suspicious US feature [13,15]. In comparison, the KSThR considers marked hypoechogenicity as a suspicious US feature [14].
Calcification
Microcalcification is a very specific US finding that suggests thyroid malignancy [11,13,15,22,24]. It is defined as a prominent echogenic focus with or without posterior shadowing. Unlike other reports [11,13,15,22,24], the KSThR considers both microcalcification and macrocalcification as suspicious US features [14].
Margin
The analysis of nodule margins is very subjective, resulting in very low interobserver agreement [28-32]. Microlobulated, irregular, infiltrative, or speculated margins have been considered as suspicious US features.
Shape
A taller than wide shape was first described by Kim et al. [10], after which it has been considered a suspicious US feature [13-15]. Although the ATA guidelines suggests that a taller than wide shape on transverse view alone is a suspicious US feature, a recent study demonstrates that a taller than wide shape in either the transverse or longitudinal plane can be accurate and sensitive for predicting thyroid malignancy [33].
Vascularity
Intranodular and chaotic vascularity have been considered as suspicious US features [13,15]. Recently, Moon et al. [34] published a study with color Doppler US performed on 1,083 nodules and showed that there was actually more blood flow distribution in benign nodules than in malignant nodules, and that vascularity was not helpful in differentiating benign and malignant nodules. The KSThR does not consider vascular pattern as a suspicious US feature [14].
Combinations of several suspicious US features
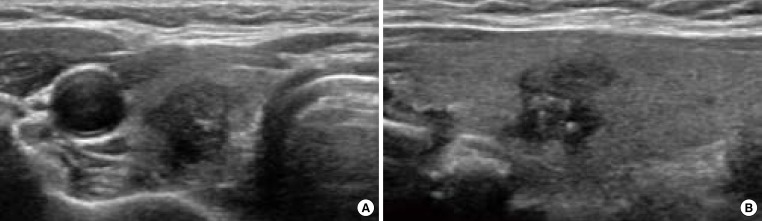
All of the US features explained thus far cannot alone diagnose thyroid nodules. The risk of malignancy increases as the number of suspicious US features increases (Fig. 2) [13,24].
Fig. 2.
Ultrasonography (US) of papillary thyroid cancer in a 60-year-old woman who underwent surgery. (A) Transverse and (B) longitudinal US images demonstrate a 1-cm-sized hypoechoic taller than wide nodule with an irregular margin and internal microcalcifications in the right thyroid gland.
CONCLUSIONS
Current guidelines vary on how to differentiate thyroid nodules into benign or malignant nodules through US. Reported guidelines show substantial overlap in what defines benign and malignant nodules, and what might be considered benign by one recommendation might be considered malignant by another. Another issue is that US assessment is subjective and operator-dependent. Therefore, US performers should continuously compare their readings with confirmed diagnostic results to maintain and improve proficient diagnostic abilities.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hamberger B, Gharib H, Melton LJ, 3rd, Goellner JR, Zinsmeister AR. Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and cost of care. Am J Med. 1982;73:381–384. [PubMed] [Google Scholar]
- 6.Mittendorf EA, Tamarkin SW, McHenry CR. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery. 2002;132:648–653. doi: 10.1067/msy.2002.127549. [DOI] [PubMed] [Google Scholar]
- 7.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 8.Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 9.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, Shong YK. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 11.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 13.Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR, Jr, Zeiger MA, Zini M AACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, Kwak JY, Lee JH, Lee YH, Na DG, Park JS, Park SW Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferri EL, Sipos J. Should all patients with subcentimeter thyroid nodules undergo fine-needle aspiration biopsy and preoperative neck ultrasonography to define the extent of tumor invasion? Thyroid. 2008;18:597–602. doi: 10.1089/thy.2008.0100. [DOI] [PubMed] [Google Scholar]
- 17.Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol. 2012;19:52–59. doi: 10.1245/s10434-011-1813-1. [DOI] [PubMed] [Google Scholar]
- 18.Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, Mandel SJ. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700. doi: 10.7326/0003-4819-133-9-200011070-00011. [DOI] [PubMed] [Google Scholar]
- 19.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD, Jr, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 20.Brauer VF, Eder P, Miehle K, Wiesner TD, Hasenclever H, Paschke R. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid. 2005;15:1169–1175. doi: 10.1089/thy.2005.15.1169. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma K, Kobayashi S, Shingu K, Hama Y, Yokoyama S, Fujimori M, Amano J. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167:102–105. doi: 10.1080/110241501750070538. [DOI] [PubMed] [Google Scholar]
- 22.Kwak JY, Jung I, Baek JH, Baek SM, Choi N, Choi YJ, Jung SL, Kim EK, Kim JA, Kim JH, Kim KS, Lee JH, Moon HJ, Moon WJ, Park JS, Ryu JH, Shin JH, Son EJ, Sung JY, Na DG Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol. 2013;14:110–117. doi: 10.3348/kjr.2013.14.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MJ, Hong SW, Chung WY, Kwak JY, Kim MJ, Kim EK. Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: emphasis on correlation with sonographic findings. Yonsei Med J. 2011;52:838–844. doi: 10.3349/ymj.2011.52.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 25.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology. Benign and malignant thyroid nodules: US differentiation: multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 26.Kim KE, Kim EK, Yoon JH, Han KH, Moon HJ, Kwak JY. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg. 2013;37:385–391. doi: 10.1007/s00268-012-1826-3. [DOI] [PubMed] [Google Scholar]
- 27.Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253:854–860. doi: 10.1148/radiol.2533090471. [DOI] [PubMed] [Google Scholar]
- 28.Park SJ, Park SH, Choi YJ, Kim DW, Son EJ, Lee HS, Yoon JH, Kim EK, Moon HJ, Kwak JY. Interobserver variability and diagnostic performance in US assessment of thyroid nodule according to size. Ultraschall Med. 2012;33:E186–E190. doi: 10.1055/s-0032-1325404. [DOI] [PubMed] [Google Scholar]
- 29.Kim HG, Kwak JY, Kim EK, Choi SH, Moon HJ. Man to man training: can it help improve the diagnostic performances and interobserver variabilities of thyroid ultrasonography in residents? Eur J Radiol. 2012;81:e352–e356. doi: 10.1016/j.ejrad.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR Am J Roentgenol. 2009;193:W416–W423. doi: 10.2214/AJR.09.2541. [DOI] [PubMed] [Google Scholar]
- 31.Choi SH, Kim EK, Kwak JY, Kim MJ, Son EJ. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid. 2010;20:167–172. doi: 10.1089/thy.2008.0354. [DOI] [PubMed] [Google Scholar]
- 32.Park CS, Kim SH, Jung SL, Kang BJ, Kim JY, Choi JJ, Sung MS, Yim HW, Jeong SH. Observer variability in the sonographic evaluation of thyroid nodules. J Clin Ultrasound. 2010;38:287–293. doi: 10.1002/jcu.20689. [DOI] [PubMed] [Google Scholar]
- 33.Moon HJ, Kwak JY, Kim EK, Kim MJ. A taller-than-wide shape in thyroid nodules in transverse and longitudinal ultrasonographic planes and the prediction of malignancy. Thyroid. 2011;21:1249–1253. doi: 10.1089/thy.2010.0372. [DOI] [PubMed] [Google Scholar]
- 34.Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–269. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]