Abstract
Here, we describe the capacity of Bacillus anthracis peptidoglycan (BaPGN) to trigger an antimicrobial response in human white blood cells (WBCs). Analysis of freshly isolated human blood cells found that monocytes and neutrophils, but not B and T cells, were highly responsive to BaPGN and produced a variety of cytokines and chemokines. This BaPGN-induced response was suppressed by anthrax lethal toxin (LT) and edema toxin (ET), with the most pronounced effect on human monocytes, and this corresponded with the higher levels of anthrax toxin receptor 1 (ANTXR1) in these cells than in neutrophils. The supernatant from BaPGN-treated cells altered the growth of B. anthracis Sterne, and this effect was blocked by LT, but not by ET. An FtsX mutant of B. anthracis known to be resistant to the antimicrobial effects of interferon-inducible Glu-Leu-Arg (ELR)-negative CXC chemokines was not affected by the BaPGN-induced antimicrobial effects. Collectively, these findings describe a system in which BaPGN triggers expression of antimicrobial factors in human WBCs and reveal a distinctive role, not shared with ET, in LT's capacity to suppress this response.
INTRODUCTION
Bacteremia is a hallmark of late-stage anthrax (1, 2), with up to 1 × 108 organisms per milliliter of blood present during advanced stages of disease (3, 4). At this stage of disease, treatments, including antibiotic therapy, are largely ineffective. Thus, the ability of Bacillus anthracis to survive and grow in the bloodstream is important in the progression of life-threatening anthrax disease. Rapid growth in the bloodstream is likely to result in the release of B. anthracis-derived factors that can be detected by the host immune system; however, the reasons why the host does not respond to these factors by reducing bacteremia are not completely understood. Indeed, neutrophils, macrophages, transferrin, and CXC chemokines have all been shown to limit the growth of B. anthracis, and these cells and factors should present a formidable defense against the pathogen (5–8). Despite the remarkable bacteremia present in anthrax, the relationships between the activation of host defenses and their suppression during the systemic phase of B. anthracis infection have not been fully elucidated.
Anthrax toxin (AT) is a tripartite toxin composed of protective antigen (PA), lethal factor (LF), and edema factor (EF) that disrupts both innate and humoral immune responses (9, 10) and likely supports the survival of B. anthracis during systemic growth. Once delivered into the cell via PA, LF blocks mitogen-activated protein kinase (MAPK) signaling through proteolytic cleavage of MAPKKs and has recently been shown to activate the rat inflammasome by cleaving NLRP1 (11). EF generates high levels of cyclic AMP (cAMP) as an adenylate cyclase and has been found to modulate multiple intracellular signaling pathways (12). AT intoxicates many different cell types, and a substantial number of studies have described the immunosuppressive effects of lethal toxin (LT) and edema toxin (ET) (10, 13). Both innate and humoral immune responses are crippled by AT, with effects such as blocking cytokine production, reducing neutrophil chemotaxis, altering T and B cell responses, and inducing anergy in NKT cells (9, 14–19).
In contrast to studies on AT's immunosuppressive effects, little is known about the B. anthracis-derived factors that are detected by the host and trigger what might otherwise be protective responses. Peptidoglycan is a major component of the Gram-positive cell wall and is composed of chains of repeating N-acetylglucosamine β-(1,4) N-acetylmuramic disaccharides cross-linked by short peptide chains. During cell division, peptidoglycan is disassembled, and fragments are either shed from the organism or captured for recycling into the cell walls of daughter cells. In the case of B. anthracis, peptidoglycan (BaPGN) is released during growth in blood and has been shown to be a pathogen-associated molecular pattern (PAMP) (20). Further, we have shown that highly purified BaPGN activates the p38 MAPK pathway in monocytes (20, 21), and BaPGN polymers are taken up by mononuclear cells, causing monocytes to produce interleukin 8 (IL-8), tumor necrosis factor alpha (TNF-α), IL-6, and IL-1β (20, 22). Thus, BaPGN is detected by monocytes and, upon phagocytosis, triggers a response that could assist in clearing the organism from the host.
The fact that AT has the capacity to suppress immune responses has been established through numerous studies (10, 15–17), yet the specificity of this response in regard to stimulatory factors produced by B. anthracis has not been described. For example, despite extensive studies in past years, there is no formal experimental evidence that AT suppresses responses to specific B. anthracis PAMPs either in vivo or in vitro. Moreover, the specific immune cells that might need to be targeted by AT in order to suppress responses to a B. anthracis PAMP have not been determined. Whether suppression of responses to a B. anthracis PAMP requires the actions of both LT and ET or if one of the two toxins alone can sufficiently alter the response is also not known. Thus, there are several gaps in our knowledge in this area, but perhaps the most critical gap is the limited understanding of these interactions in primary human cells, making it difficult to know how findings apply to the clinically relevant form of the disease in humans. The experiments reported here addressed these areas and examined the impacts of LT and ET on human blood cell responses to a B. anthracis PAMP, BaPGN. The results of this study indicate human monocytes and neutrophils respond to BaPGN, and this response is most effectively quelled by LT through the targeting of monocytes via anthrax toxin receptor 1 (ANTXR1) interactions by PA. The data also show that the inflammatory response to BaPGN includes the production of antimicrobial factors capable of suppressing the growth of B. anthracis and that LT, but not ET, is capable of preventing this antimicrobial response.
MATERIALS AND METHODS
Ethics statement.
Whole blood and buffy coats were obtained from volunteer donors or from the Oklahoma Blood Institute with the approval of the University of Oklahoma Health Sciences Center (OUHSC) Institutional Review Board. Written informed consent was obtained from all of the volunteer donors after proper information was provided prior to enrollment in the study.
Bacterial strains, culture conditions and reagents.
B. anthracis strain Sterne 7702 (pXO1+ pXO2−) was used to perform the antimicrobial assays in this study (23). Cultures were grown at 37°C with shaking in brain heart infusion (BHI) medium (Becton, Dickinson, Sparks, MD). A B. anthracis Sterne ΔftsX mutant strain (a kind gift from Scott Stibitz, FDA) was grown and maintained as described previously by Crawford et al. (24). The following materials were used in this study. B. anthracis EF, LF, and PA were purchased from List Biological Laboratories (Campbell, CA). B. anthracis Sterne BaPGN was isolated as described previously (20, 21). Fluorochrome-conjugated antibodies to human proteins and other flow cytometry-related items were purchased from the following vendors: anti-human CD3 (phycoerythrin [PE]-Alexa Fluor 610) and CD19 (allophycocyanin [APC]-Alexa Fluor 750) and Pacific blue-conjugated streptavidin from Invitrogen, Carlsbad, CA; anti-human CD16b (fluorescein isothiocyanate [FITC]) from GeneTex, Irvine, CA; anti-human CD14 (peridinin chlorophyll protein [PerCP]-Cy5.5), IL-1α (FITC), IL-1β (PE), TNF-α (PE-Cy7), IL-6 (Alexa Fluor 700), and brefeldin A from eBioscience, San Diego, CA; biotin-conjugated anti-human CD16b from Exbio, San Diego, CA; anti-human MIP-1β (PE-Cy7) from BD Pharmingen, San Diego, CA; anti-human IL-8 (APC) from BioLegend, San Diego, CA; anti-human GRO-α (PE), MIP-1α (APC), and anthrax toxin receptor 2 (ANTXR2/CMG2) and APC-conjugated streptavidin from R&D Systems, Minneapolis, MN; anti-human TEM8/ANTXR1 from Abcam, Cambridge, MA; and recombinant human GRO-α (CXCL1), CXCL10 (IP-10), and MCP1 (CCL2) from PeproTech, Rocky Hill, NJ. Human cytokine antibody arrays were obtained from R&D Systems (Minneapolis, MN). Histopaque-1077, Histopaque-1119, human IgG, and heparin were purchased from Sigma-Aldrich (St. Louis, MO). RPMI 1640 medium with l-glutamine, penicillin-streptomycin, and fetal bovine serum (FBS) was purchased from ATCC (Manassas, VA). BD Vacutainer ACD blood collection tubes were purchased from BD (Franklin Lakes, NJ).
Blood sample collection and isolation and culture of human WBCs.
Whole blood and buffy coat were obtained with the approval of the University of Oklahoma Health Sciences Center Institutional Review Board. Whole blood (50 ml) was drawn from healthy adult volunteers (aged 20 to 45 years) with proper informed consent. Whole blood was collected by drawing from the antecubital vein into heparinized blood collection tubes (BD, Franklin Lakes, NJ). Buffy coats were obtained from the Oklahoma Blood Institute (Oklahoma City, OK). Both the whole blood and buffy coat were diluted in complete RPMI 1640 medium with l-glutamine and penicillin-streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin; Invitrogen, Carlsbad, CA). White blood cells (WBCs), including peripheral blood mononuclear cells (PBMC) (monocytes, B cells, and T cells), and granulocytes (neutrophils), were isolated from either whole blood or buffy coat using density gradient centrifugation with Histopaque 1077 and 1119 according to the manufacturer's manual (Sigma-Aldrich, St. Louis, MO). Cell viability was checked by trypan blue staining, and the leukocytes were counted with a hemocytometer.
Human cytokine antibody array assay.
After separation by centrifugation, WBCs were washed twice with complete RPMI 1640 medium, and the cells were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum and penicillin-streptomycin. WBCs (5 × 106 to 10 × 106 cells per ml) were treated with ET (25 pmol PA plus 25 pmol EF), LT (25 pmol PA plus 25 pmol LF), and/or BaPGN (10 μg/ml) in a final volume of 2 ml. The treated cells were then incubated in non-tissue culture 6-well plates for 6 h in a humidified atmosphere of 5% CO2 in air at 37°C. After incubation, 750 μl of the culture supernatant was collected by centrifugation for 10 min at 500 × g and stored at −80°C. To detect secreted cytokines and chemokines in the WBC culture supernatant, a Human Cytokine Array (R&D Systems, Minneapolis, MN) that simultaneously measures the levels of 36 human cytokines and chemokines was used according to the manufacturer's instructions. Briefly, WBC culture supernatants were mixed with a cocktail of biotinylated detection antibodies and incubated on an array membrane containing 36 anti-human cytokine capture antibodies in duplicate. Then, streptavidin-horseradish peroxidase and chemiluminescent detection reagents were added. The signals produced were proportional to the amounts of the cytokines bound. After detection on X-ray film, the signals were quantified by scanning the film on a transmission-mode scanner and analyzing the array image file with AlphaView software (AlphaInnotech/ProteinSimple, Santa Clara, CA) image analysis software. The signals (pixel density) of the pair of duplicate spots representing each cytokine were then averaged.
Flow cytometric analysis of human WBCs.
Separated WBCs (5 × 106 cells per ml) were treated with anthrax components as detailed in the figure legends. For intracellular cytokine and chemokine staining, WBCs were maintained in the presence of an intracellular protein transport inhibitor, brefeldin A (3 μg/ml), during exposure to BaPGN and AT and during the staining process. After treatment, the WBCs were collected by centrifugation for 10 min at 500 × g. The cells were resuspended in 100 μl of fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] with 3% FBS and 0.1% sodium azide) and incubated with purified human IgG (0.1 mg/ml) for 10 min on ice to block Fc receptors. To detect individual cell populations, cells were stained for 20 min on ice with fluorochrome-conjugated surface antibodies against CD3, CD14, CD16b, and CD19 as described previously (21). For experiments examining the ANTXR levels, the WBCs were also incubated with anti-human ANTXR1/TEM8 (2 μg/ml) or anti-human ANTXR2/CMG2 (250 ng/ml) antibodies for 1 h at 4°C. After surface staining, the cells were washed with FACS buffer and fixed with 1% formaldehyde. For intracellular staining, samples were fixed overnight and then permeabilized with 0.5% saponin in FACS buffer. The cells were then incubated with specific human intracellular cytokine/chemokine antibodies. Flow cytometry was performed with a BD LSR II (BD Biosciences, San Jose, CA) flow cytometer collecting 200,000 events per sample. The data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Determination of PA binding in human WBCs.
Heparinized human peripheral blood was diluted 1:3 with Dulbecco's minimal essential medium (DMEM). One milliliter of the diluted blood was distributed into individual wells of a non-tissue culture 24-well plate and exposed at 37°C to 40 nM Alexa Fluor 647-conjugated PA. After treatment, the cells were collected by centrifugation for 10 min at 500 × g. The cells were resuspended in 100 μl of FACS buffer and purified human IgG (0.1 mg/ml) as an isotype control, following by a 10-min incubation on ice. The cells were then stained with anti-human CD14-PE and anti-human CD16b-FITC for 20 min on ice. Following surface staining, red blood cells were lysed by a brief exposure to a buffer consisting of 155 mM ammonium chloride, 10 mM KHCO3, and 0.1 mM EDTA. The cells were then washed with FACS buffer and fixed with 1% formaldehyde. The relative amount of PA binding to monocytes and neutrophils was determined by using a FACSCalibur (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo (Tree Star, Inc., Ashland, OR) software.
Antimicrobial assays and microscopic imaging.
Purified human peripheral white blood cells were resuspended in RPMI 1640 medium plus 10% FBS without any antibiotics and treated with BaPGN alone and in combination with different anthrax toxin components, such as BaPGN plus AT, BaPGN plus ET, and BaPGN plus LT, at the above-mentioned concentration. After overnight treatment for 12 to 15 h, the culture supernatants were filter sterilized with 0.2-μm cellulose acetate filters (VWR International, San Dimas, CA) and collected to determine its antimicrobial effects. Cultures of B. anthracis Sterne 7702 and its ΔftsX mutant were grown overnight and subcultured to an optical density at 600 nm (OD600) of 0.7. For antimicrobial assay of the different BaPGN-treated WBC supernatants and recombinant human cytokines/chemokines (rhGRO-α, rhCXCL10, and rhMCP1), mid-log-phase B. anthracis and ΔftsX mutant cultures were diluted in the RPMI 1640-plus-10% FBS medium at a ratio of 1:1,000. Twenty-five microliters of bacteria was mixed with 175 μl of different BaPGN-treated WBC supernatants and different concentrations of rhGRO-α, rhCXCL10, and rhMCP1 to an OD600 of 0.1 in the wells of a 100-well Honeycomb 2 microplate and incubated for 24 h in a Bioscreen plate reader (Bioscreen C, Piscataway, NJ) with constant shaking at 37°C. Experiments were performed in triplicate with at least three different independent WBC supernatant preparations, and the mean absorbance was determined. After 24 h of growth, bacterial cultures were harvested and pictures were taken using an IX51 Olympus microscope with a DP70 camera. At least 10 random images were photographed for each treatment from three independent experiments.
Statistical analysis.
The data shown for cytokine array blotting, cytokine/chemokine flow cytometry, and PA receptor and PA binding analysis experiments represent averages of three independent experiments with three individual volunteers. Experiments requiring statistical analysis were performed in triplicate, as indicated, and analyzed by using the statistics module of GraphPad Prism (La Jolla, CA). Significance was determined by using a standard two-tailed Student's t test, with a P value of <0.05 considered significant.
RESULTS
Anthrax toxin suppresses cytokine/chemokine expression in human WBCs treated with BaPGN.
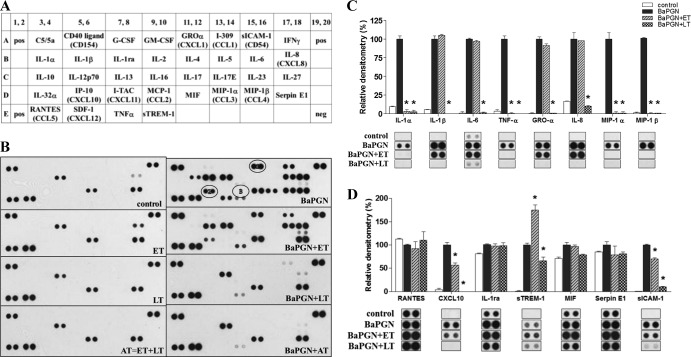
To assess the response of human WBCs to BaPGN and the impact of AT on that response, freshly isolated human WBCs were exposed to ET (25 pmol EF plus 25 pmol PA), LT (25 pmol LF plus 25 pmol PA), or AT (ET plus LT) in the presence and absence of 20 μg BaPGN. Controls included WBCs treated with PA, EF, and LF alone. Following treatment for 6 h, supernatants were collected and analyzed for secreted cytokines and chemokines by using a capture membrane array. The results of the 6-h treatment are shown in Fig. 1. Thirty-six cytokines and chemokines (Fig. 1A shows the template) were examined under each of the experimental conditions, and their relative levels were quantified by densitometry (Fig. 1C and D).
Fig 1.
AT-mediated suppression of cytokines/chemokines in BaPGN-treated WBCs after 6 h. Fresh human WBCs were treated with BaPGN (10 μg/ml) for 6 h in the presence or absence of ET (25 pmol EF and 25 pmol PA), LT (25 pmol LF and 25 pmol PA), or AT (25 pmol EF, 25 pmol LF, and 25 pmol PA). (A and B) Antibody array depicting cytokines/chemokines secreted by WBCs under each condition. The array shown is representative of 3 experiments. Circled 1, GRO-α; circled 2, CXCL10; circled 3, MCP1. (C and D) Relative abundances of the cytokines/chemokines induced by BaPGN and modulated by ET and LT, as determined by densitometry (n = 3). The error bars indicate the standard errors of the mean (SEM). The asterisks indicate a significant (P < 0.05) decrease compared to BaPGN alone.
While treatment with LT or AT (Fig. 1) or with the individual proteins of anthrax toxin (data not shown) had no discernible impact on WBCs, exposure to BaPGN alone induced the secretion of 4 cytokines (IL-1α, IL-1β, IL-6, and TNF-α), 5 chemokines (GRO-α [CXCL1], MIP-1α [CCL3], MIP-1β [CCL4], IL-8 [CXCL8], and CXCL10 [IP-10]), and two soluble receptors (sICAM-1 and sTREM-1). ET alone induced IL-8 production, which was the only detectable effect of the toxin; however, this was likely offset when LT was included with ET due to LF's capacity to disrupt IL-8 expression (15, 25, 26). Therefore, in the 6-h treatment, BaPGN induced extensive cytokine/chemokine secretion by human WBCs while AT, LT, and ET did not. Compared to treatment with BaPGN alone, treatment with BaPGN plus LT or AT resulted in a decrease in cytokine and chemokine secretion. The secretion of each BaPGN-induced factor was abrogated by the addition of LT or AT. LT reduced the levels of sTREM-1 and sICAM-1, but not completely below the levels of detection. All other BaPGN-induced cytokines and chemokines were reduced to below the levels of detection following addition of LT. In contrast, ET had a more limited effect, suppressing the BaPGN-mediated induction of 2 cytokines (IL-1α and TNF-α) and 2 chemokines (MIP-1α and MIP-1β). In the presence of BaPGN, ET increased levels of sTREM. These data indicate that BaPGN induces a potent inflammatory response in human WBCs and that this response can be suppressed by AT, LT, and, to a lesser extent, ET.
Anthrax toxin suppresses BaPGN-induced responses in monocytes and neutrophils.
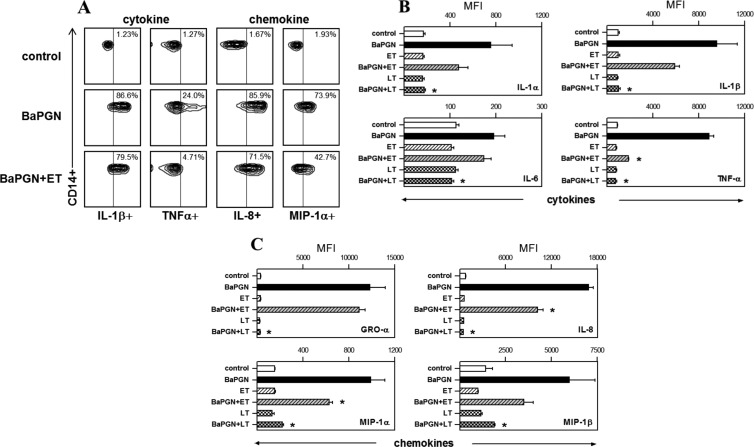
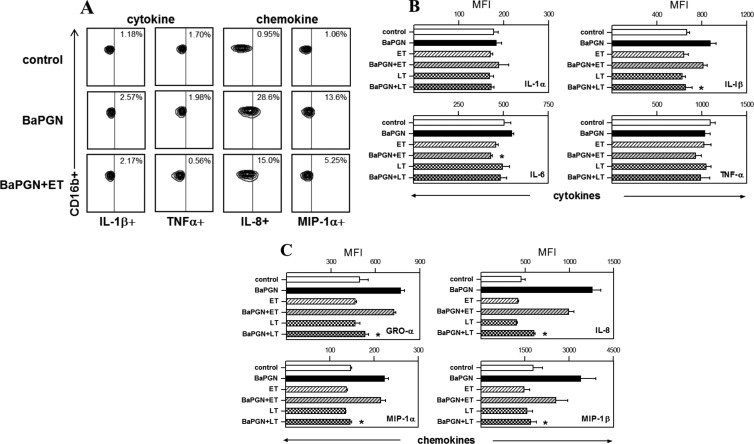
To identify the human cell types responsible for BaPGN-induced cytokine/chemokine production, WBCs were treated with BaPGN as described above and then immunostained for surface markers unique to monocytes (CD14), neutrophils (CD16b), B cells (CD19), and T cells (CD3). Using a multilabeling approach, each cell type was also analyzed for production of representative cytokines (IL-1α, IL-1β, IL-6, and TNF-α) and chemokines (GRO-α, MIP-1α, MIP-1β, and IL-8). We found that monocytes and neutrophils, but not T cells or B cells, produced the cytokines/chemokines detected on the membrane array shown in Fig. 1; therefore, only monocyte and neutrophil data are presented in Fig. 2 and 3. In response to BaPGN exposure, monocytes produced all eight of the cytokines and chemokines (Fig. 2) included in this analysis. Following BaPGN treatment of WBCs, neutrophils did not produce detectable cytokines but produced all four of the chemokines (Fig. 3).
Fig 2.
Immunosuppressive effects of AT on BaPGN-treated human monocytes. Fresh human WBCs were treated with BaPGN, ET, or LT alone or BaPGN plus ET or LT, as for Fig. 1. To identify the major cell types, the WBCs were stained with antibodies to cell surface markers: CD14 (monocytes), CD16b (neutrophils), CD19 (B lymphocytes), and CD3 (T lymphocytes). The cells were then stained for intracellular cytokines/chemokines, and flow cytometry was used to analyze cytokine/chemokine production for each cell type. (A) Representative contour plots showing cytokine (IL-1β and TNF-α) and chemokine (IL-8 and MIP-1α) staining intensities (horizontal axis) in monocytes. The numbers in the upper right corners indicate the percentages of cells expressing the relevant surface marker (CD14) as well as the cytokine or chemokine. (B and C) Intracellular levels of cytokines IL-1α, IL-1β, IL-6, and TNF-α (B) and chemokines GRO-α, IL-8, MIP-1α, and MIP-1β (C) in monocytes, quantified as the MFI. The data shown are the averages of three separate experiments, each using WBCs from a different donor. The error bars indicate SEM. The asterisks indicate a significant (P < 0.05) decrease in MFI compared to BaPGN treatment alone.
Fig 3.
Immunosuppressive effects of AT on BaPGN-treated human neutrophils. Fresh human WBCs were treated with BaPGN, ET, or LT alone or BaPGN plus ET or LT, as for Fig. 1, and then stained with antibodies to intracellular cytokines/chemokines and to cell surface markers: CD14 (monocytes), CD16b (neutrophils), CD19 (B lymphocytes), and CD3 (T lymphocytes). Cytokine/chemokine profiles for each cell type were then analyzed by flow cytometry. (A) Representative contour plots showing the staining intensities of cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) in neutrophils. The numbers in the upper right corners indicate the percentages of cells in the samples that stained positive for the relevant cytokine or chemokine. (B and C) Intracellular levels of cytokines IL-1α, IL-1β, IL-6, and TNF-α (B) and chemokines GRO-α, IL-8, MIP-1α, and MIP-1β (C) in neutrophils, quantified as MFI. The data shown are the averages of three separate experiments, each using WBCs from a different donor. The error bars indicate SEM. The asterisks indicate a significant (P < 0.05) decrease compared to treatment with BaPGN alone.
To determine whether AT modulates BaPGN-induced cytokine or chemokine production by certain cell types in human blood, WBCs were treated with LT or ET alone or with BaPGN and analyzed as described above. In monocytes, treatment of WBCs with BaPGN plus LT resulted in significantly lower levels of every analyzed cytokine and chemokine compared to BaPGN treatment alone (Fig. 2) (P < 0.05), while treatment with BaPGN plus ET resulted in lower levels of TNF-α, MIP-1α, and IL-8. In neutrophils, LT, but not ET, suppressed BaPGN-induced chemokine production (Fig. 3). Importantly, cell death was not detected under any of the conditions tested (data not shown), indicating the observed effects were not due to reduced cell viability. Collectively, these data demonstrate that LT suppresses the expression of BaPGN-induced cytokines and chemokines in monocytes and neutrophils and that LT is a more potent immune suppressor than ET in this system.
Anthrax toxin preferentially targets monocytes in human blood.
We hypothesized that AT and LT suppress cytokine and chemokine production preferentially in targeted monocytes, because cytokines such as IL-1α, IL-1β, IL-6, and TNF-α were completely suppressed in BaPGN-treated monocytes, but not in neutrophils. For ET and LT to target host cells, the receptor binding protein, PA, must first interact with one of two related host cell surface receptors, ANTXR1/TEM8 (27) or ANTXR2/CMG2 (28). Therefore, we analyzed the expression and levels of ANTXR1 and ANTXR2 on different cell types in human WBCs to determine if these levels might correspond to different sensitivities to AT. WBCs were stained with antibodies against cell-type-specific surface markers and with antibodies against ANTXR1 or ANTXR2 and then analyzed by flow cytometry in order to assess whether the cell type expressed the receptors. Although both ANTXR1 and ANTXR2 were expressed on monocytes and neutrophils, monocytes expressed higher levels of ANTXR1 than neutrophils, B cells, or T cells (Fig. 4A and B), as indicated by the measured mean fluorescence intensity (MFI). Monocytes and neutrophils showed similar levels of ANTXR2 expression (Fig. 4C and D). We next compared PA binding to monocytes and neutrophils in human peripheral blood. Alexa Fluor 647-labeled PA was added to human peripheral blood, and flow cytometry was used to detect PA binding to monocytes and neutrophils, as determined by the intensity of Alexa Fluor 647 staining on cells with high CD14 (monocytes) and high CD16b (neutrophils) staining. As shown in Fig. 4E and F, both monocytes and neutrophils were bound by PA in human blood; however, substantially more PA was found to interact with monocytes than with neutrophils. Binding of labeled PA to monocytes and neutrophils was blocked by preincubation with an equal amount of unlabeled PA, confirming that the interaction of labeled PA with monocytes and neutrophils was specific (Fig. 4E and F). Therefore, although monocytes and neutrophils express similar levels of ANTXR2, PA preferentially binds monocytes, which were found to express higher levels of ANTXR1.
Fig 4.
Cell types targeted by anthrax toxin in human blood. (A to D) Fresh human WBCs were stained with antibodies against ANTXR1 (A and B) or ANTXR2 (C and D) and cell surface markers CD14 (monocytes), CD16b (neutrophils), CD19 (B lymphocytes), and CD3 (T lymphocytes). Flow cytometry was then used to analyze the expression levels of the receptors on individual cell types. The bar graphs (A and C) indicate ANTXR1 and ANTXR2 expression levels for each cell type, quantified as MFI (n = 3). Representative histograms (B and D) show ANTXR1 and ANTRX2 expression levels for monocytes and neutrophils. The results shown are representative of three different experiments. The error bars indicate SEM. *, P < 0.05 compared with monocytes. (E and F) Human peripheral blood was incubated with fluorescently labeled PA at 37°C for 1 or 2 h and then stained with antibodies recognizing CD14 (monocytes) and CD16b (neutrophils). Flow cytometry was used to analyze the intensity of PA staining on monocytes and neutrophils. A control sample was incubated with unlabeled PA for 1 h prior to the addition of labeled PA. A graph depicting the amounts of PA bound to monocytes and neutrophils, as indicated by MFI (E), and a representative histogram (F) are shown. The results shown are representative of three different experiments. The error bars indicate SEM. For each condition, the MFI for neutrophils was significantly lower than that for monocytes (P < 0.05 for each pairwise comparison). The asterisks indicate a significant (P < 0.05) decrease compared to 1 h of treatment with labeled PA.
BaPGN-induced factors suppress growth of B. anthracis strain Sterne.
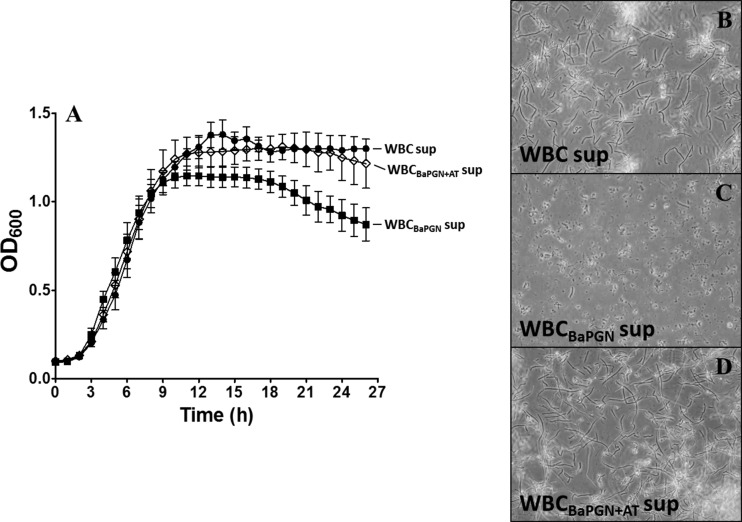
Previous work by Crawford and colleagues found that interferon-inducible CXC chemokines could suppress the growth of B. anthracis (6, 29). We noted that CXCL10 was induced in WBCs exposed to BaPGN, raising the possibility that chemokines and perhaps other factors released in response to BaPGN could have an inhibitory effect on the growth of B. anthracis. Surprisingly, when we tested the supernatants from the 6-h treatment, there was no detectable effect on B. anthracis growth, despite the extensive inflammatory response. However, supernatants collected from the 15-h time point following BaPGN treatment revealed a potent antimicrobial effect. As shown in Fig. 5, addition of the supernatant from BaPGN-treated WBCs reduced the overall growth of B. anthracis, with a notable reduction in growth as the culture reached stationary phase. Under the conditions used for these experiments, we found the lag and exponential phases of growth were not impacted by the supernatant from BaPGN-treated WBCs; however, as growth approached stationary phase, the optical density of the culture began to decline compared to control cells. This effect appeared to be due to an overall reduction in the total number of organisms, as the images in Fig. 5C show substantially fewer bacilli in the cultures of B. anthracis treated with the supernatant from BaPGN-exposed WBCs. Supernatants collected from WBCs treated with AT in the presence of BaPGN had no effect on the growth of B. anthracis (Fig. 5A and C), indicating that AT blocked the production of antimicrobial factors in human WBCs responding to BaPGN. Similar to the results following 6 h of AT treatment, the toxin had no effect on cell viability.
Fig 5.
Anthrax toxin suppresses the antimicrobial effects of supernatants from BaPGN-treated WBCs. WBCs were treated with BaPGN alone and in combination with AT as described in Materials and Methods for 15 h. (A) Filter-sterilized supernatants from WBCs alone (untreated control) (WBC sup), WBCs treated with BaPGN (WBCBaPGN sup), and WBCs treated with BaPGN plus AT (WBCBaPGN+AT sup) were then exposed to mid-log-phase B. anthracis Sterne 7702 vegetative cells. Bacterial growth curves were recorded in a Bioscreen C plate reader for at least three independent WBC supernatants, and the mean absorbances were plotted in GraphPad Prism software. BaPGN-treated (■) WBCs released factors that markedly reduced bacterial growth during the early stationary phase in comparison to the untreated control (●), which was suppressed by anthrax toxin (♢) when it was combined with BaPGN. The error bars indicate SEM. (B, C, and D) Bacterial cultures from WBC supernatants treated with BaPGN alone and BaPGN plus AT were harvested after 24 h, and images were taken using an IX51 Olympus microscope with a DP70 camera. Representative images from at least three independent experiments are shown at ×600 magnification. BaPGN-treated WBC supernatants markedly reduced the vegetative bacilli after 24 h of growth, which was restored by a combination of anthrax toxin and BaPGN, suggesting a role of AT in the suppression of antimicrobial effects of factors released by BaPGN-treated WBCs.
Experiments were next performed to determine if ET and LT were both necessary for suppressing the production of antimicrobial factors. WBCs were treated with BaPGN, along with either ET or LT, and the supernatants were tested for inhibitory effects against B. anthracis. BaPGN treatments that included ET did not affect the ability of BaPGN to induce production of factors that altered the growth profile of B. anthracis (Fig. 6A). In contrast, LT completely suppressed the production of growth-inhibitory factors by WBCs during exposure to BaPGN (Fig. 6B).
Fig 6.
WBC-mediated antimicrobial effects are FtsX dependent and are suppressed by lethal toxin. Experiments were performed to determine the individual effects of ET and LT on the production of antimicrobial factors by human WBCs. The requirement for B. anthracis FtsX was also determined. (A) Growth of B. anthracis Sterne in the presence of supernatants from BaPGN-treated WBCs or BaPGN-treated WBCs cotreated with ET. (B) Growth of B. anthracis Sterne in the presence of supernatants from BaPGN-treated WBCs or BaPGN-treated WBCs cotreated with LT. (C) B. anthracis ΔftsX mutant growth in the presence of supernatants from BaPGN-treated WBCs. At least three independent experiments were performed with three individual donors, and the error bars represent the standard deviations from the mean of three samples. ●, WBC supernatant alone (untreated control); ■, supernatants from BaPGN-treated WBCs; ▽, supernatants from WBCs treated with BaPGN and ET; △, supernatants from WBCs treated with BaPGN and LT.
Previous work has shown that FtsX, a protein associated with an ABC transporter complex in the cell membrane of B. anthracis, is necessary for CXCL10 to exert an antimicrobial effect against vegetative cells (24). FtsX mutants demonstrate resistance to the observed CXCL10 antimicrobial effect (24). Accordingly, experiments were next performed to determine if FtsX is also involved in susceptibility to supernatants from BaPGN-treated WBCs. An ftsX deletion mutant of B. anthracis (24) designated B. anthracis ΔftsX was incubated with supernatants from BaPGN-treated WBCs, and growth was assessed. As shown in Fig. 6C, B. anthracis ΔftsX growth was not suppressed by supernatants from BaPGN-treated WBCs. These data indicate that, similar to the effects of CXCL10, FtsX is required for the antimicrobial effects of supernatants collected from BaPGN-treated WBCs.
Based on these results, we reasoned that the factor(s) mediating antimicrobial effects should be present in BaPGN-treated samples only at the later time points and should be suppressed by AT. Thus, we examined cytokine and chemokine profiles at the 15-h time point and compared them with the data shown in Fig. 1, where the cytokine and chemokine profiles in BaPGN-treated cells were measured at 6 h. Analysis of the profile of BaPGN-induced cytokines and chemokines at 15 h indicated that the inflammatory response had significantly waned by this time point, despite the increase in antimicrobial activity against B. anthracis. Only MCP-1, C5a, and GRO-α levels were increased at 15 h (Fig. 7), while those of all the other cytokines and chemokines upregulated at 6 h had declined. MCP-1, C5a, and GRO-α were also suppressed by AT at 15 h, and as such, these three factors fulfilled the criteria for possible candidates for mediating antimicrobial activity based on what was found on the array. Previous studies have found that MCP-1 (CCL2) and GRO-α exhibit antimicrobial effects (30), and in line with this, both chemokines exhibited antimicrobial effects against B. anthracis. As shown in Fig. 7, similar to the previously described effects of CXCL10 (recapitulated in Fig. 7C as a positive control), addition of purified MCP-1 or GRO-α altered the overall growth profile of B. anthracis.
Fig 7.
AT modulation of cytokine/chemokine production in BaPGN-treated WBCs at 15 h and impacts of candidate antimicrobial chemokines on B. anthracis growth. Purified human WBCs were treated with BaPGN and BaPGN plus AT for 15 h, and cytokine/chemokine profiles were assessed using an antibody array. (A) Representative antibody array highlighting BaPGN-induced factors modulated by AT. BaPGN-induced GRO-α (circled 1) and MCP1 (circled 3) were greatly suppressed by the AT treatment; in contrast, CXCL10 (circled 2) expression was slightly reduced. (B to D) Growth profile of B. anthracis in the presence of increasing concentrations of recombinant human rhGRO-α (CXCL1), CXCL10 (IP-10), and MCP1 (CCL2). The growth curves were curve fitted (nonlinear regression) using the statistics module of GraphPad Prism. The numbers in parentheses refer to the circled numbers in panel A. The array and growth curves are representative of three independent experiments.
DISCUSSION
The findings of this study support a model in which BaPGN triggers a host response that is inflammatory at early time points (6 h postexposure) and antimicrobial at later time points (15 h postexposure). We predicted that BaPGN would stimulate a strong inflammatory response in human blood, correlating with earlier studies (21) and supported by the fact that monomers of the molecule are detected by nucleotide oligomerization domain (NOD) sensors 1 and 2 in phagocytic cells (22). It also seemed likely that AT could suppress the response to BaPGN, given the well-established immunosuppressive effects of the toxin. The bimodal nature of the response and the different impacts of LT and ET on this response came as unexpected findings, and the results of these experiments reveal a previously undescribed aspect of anthrax disease as it might occur in mid- to late-stage illness in humans.
An important objective of these studies was to mimic events that occur in later stages of anthrax disease in humans. In this regard, we felt there were three critical parameters to consider. First, we used human WBCs prepared from freshly isolated human blood as the system for testing the effects of BaPGN and AT. Second, we selected BaPGN as a B. anthracis-derived factor that would be sensed by human immune cells during the bacteremic stage of infection. Although the exact level of BaPGN present in humans with late-stage anthrax is not known, we selected the 10-μg/ml dose because this is a high end approximation of what has been associated with 107 to 109 CFU of Gram-positive bacteria (31). By selecting this higher dose, we believe we have captured effects that might be due to cell surface PGN, as well as to PGN shed from the organism. Most importantly, this dose primed a robust inflammatory response in human WBCs. Third, we selected a dose of AT based on the levels of PA detected in vivo in animal models of anthrax. The circulating level of AT in humans with systemic anthrax is not known. PA levels are generally around 1 μg/ml (32) in guinea pigs, but levels are much higher in rabbits, with PA detected at 100 μg/ml. Thus, we started with a dose of 1 μg/ml with the intent of adjusting to larger amounts if the BaPGN effects were not modulated. Collectively, the experimental system attempts to model the events likely to occur during middle to late stages of human anthrax when the host is detecting B. anthracis PAMPs and the pathogen is countering these responses through the activity of anthrax toxin.
The data suggest that BaPGN induces an antimicrobial response in human WBCs and becomes more prominent as the proinflammatory responses subside. We first became interested in the possibility of a BaPGN-induced antimicrobial response when we noted the increased production of CXCL10 in response to BaPGN. A series of recent studies found that interferon-inducible Glu-Leu-Arg (ELR)-negative CXC chemokines (CXCL9, CXCL10, and CXCL11) had antimicrobial effects against B. anthracis (6, 29). This led us to test the supernatants for antimicrobial activity, and we were interested to find that the early response, with CXCL10, was not antimicrobial but the later response, with lower levels of CXCL10, was antimicrobial. Upon further examination of the early response, we found that the concentration of CXCL10, determined by enzyme-linked immunosorbent assay (ELISA) (data not shown), does not reach the amounts known to repress growth of B. anthracis (6, 24, 29). Moreover, when candidate antimicrobials, such as MCP-1 and GRO-α, were tested for antimicrobial effects against B. anthracis, they, too, were found to suppress growth of the organism (Fig. 7). Identification of the specific BaPGN-inducible antimicrobial factors is the focus of ongoing studies, but we predict that no single factor will be sufficient to block the growth B. anthracis and that multiple factors are likely to be involved in the process. Finally, it will be important to determine the role of FtsX in conferring susceptibility to BaPGN-induced antimicrobial effects. Previous work showed that B. anthracis FtsX is required for ELR-negative CXCL chemokine antimicrobial activity (24); however, the current work suggests that FtsX may have an even broader role in this event and that it confers sensitivity beyond just these chemokines.
Although LT and ET have been studied extensively in recent years and both are known to suppress immune responses (9, 14–19), little is known about the immunomodulatory effects unique to LT or ET. Where differences have been found, LT seems to be the dominant toxin for immunosuppression. For example, both LT and ET can suppress monocyte-derived dendritic cell production of proinflammatory chemokines; among these factors, LT completely abrogated IL-8 production, but ET produced only a relative reduction in IL-8 production (33). In a like manner, we observed that ET was unable to block the BaPGN-induced expression of IL-1β, IL-6, GRO-α, IL-8, and CXCL10, while LT reduced these cytokines and chemokines to below the levels of detection. The findings of this study indicate LT, but not ET, is capable of blocking the production of antimicrobial factors produced by WBCs, but the underlying reasons for this differential effect are not clear. The ability of LT to disrupt mitogen-activated protein kinase signaling and modulate activation of the inflammasome may allow LT to impact many different signaling events, some of which extend beyond those influenced by ET's capacity to increase intracellular levels of cAMP. While the mechanism of LT-mediated suppression of antimicrobial production by WBCs has not yet been defined, the findings of this study reveal a dynamic host-pathogen interaction that may occur during bacteremia. The accumulation of B. anthracis in the bloodstream is almost certainly a signal for innate responses by circulating immune cells, and it appears that monocytes and neutrophils secrete antimicrobial factors in response to BaPGN. Our current working model suggests that this host-favorable event is dampened by LT, which further supports the growth of B. anthracis in the bloodstream.
Unlike in mice, expression levels of TEM-8 (ANTXR1) appeared to be higher than those of CMG-2 (ANTXR2) in human monocytes and neutrophils. In addition, PA preferentially bound to human monocytes, while in mice, neutrophils appear to be the primary target of AT. These findings suggest that ANTXR1 may be a more relevant receptor for anthrax toxin-induced immunosuppression in humans than in mice. However, it is important to note that ANTXR1 exhibits a much lower affinity for PA than ANTXR2, and a direct comparison of the two receptors may not accurately or totally reflect the reason for higher PA binding to monocytes (34). Second, macrophages from certain inbred strains of mice are susceptible to LT-induced apoptosis or rapid necrotic cell death, but the toxin did not appear to cause cell death in human WBCs under our experimental conditions (data not shown). It is not known whether this reflects the differences between rodent and human WBCs or the differences between circulating and tissue-resident innate immune cells, but in either case, these data support the idea that AT is not cytotoxic to human WBCs and that the effects of AT on mouse cells may not accurately reflect what happens in humans.
Collectively, the current studies reveal new aspects of B. anthracis interactions with human immune cells. By all indications, systemic anthrax is a complex event influenced by host responses that are modulated by AT in order to provide an advantage to the pathogen. Studies are beginning to integrate single elements, such as BaPGN, ET, AT, CXCL chemokines, antimicrobial factors, and FtsX, into a detailed model of the pathogenesis of B. anthracis. Moreover, studies using freshly isolated primary human cells enhance the relevance of the findings and raise confidence that the information gained will be directly applicable to anthrax disease in humans.
ACKNOWLEDGMENTS
This work was supported by NIH grant U19 AI 062629 (K.M.C. and J.D.B.).
We have no conflicting or competing financial interests.
Footnotes
Published ahead of print 22 July 2013
REFERENCES
- 1.Guarner J, Jernigan JA, Shieh WJ, Tatti K, Flannagan LM, Stephens DS, Popovic T, Ashford DA, Perkins BA, Zaki SR. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mina B, Dym JP, Kuepper F, Tso R, Arrastia C, Kaplounova I, Faraj H, Kwapniewski A, Krol CM, Grosser M, Glick J, Fochios S, Remolina A, Vasovic L, Moses J, Robin T, DeVita M, Tapper ML. 2002. Fatal inhalational anthrax with unknown source of exposure in a 61-year-old woman in New York City. JAMA 287:858–862 [DOI] [PubMed] [Google Scholar]
- 3.Smith H, Keppie J. 1954. Observations on experimental anthrax; demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. Nature 173:869–870 [DOI] [PubMed] [Google Scholar]
- 4.Smith H, Keppie J, Stanley JL. 1954. Observations on the cause of death in experimental anthrax. Lancet 267:474–476 [DOI] [PubMed] [Google Scholar]
- 5.Rooijakkers SH, Rasmussen SL, McGillivray SM, Bartnikas TB, Mason AB, Friedlander AM, Nizet V. 2010. Human transferrin confers serum resistance against Bacillus anthracis. J. Biol. Chem. 285:27609–27613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford MA, Zhu Y, Green CS, Burdick MD, Sanz P, Alem F, O'Brien AD, Mehrad B, Strieter RM, Hughes MA. 2009. Antimicrobial effects of interferon-inducible CXC chemokines against Bacillus anthracis spores and bacilli. Infect. Immun. 77:1664–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer-Scholl A, Hurwitz R, Brinkmann V, Schmid M, Jungblut P, Weinrauch Y, Zychlinsky A. 2005. Human neutrophils kill Bacillus anthracis. PLoS Pathog. 1:e23. 10.1371/journal.ppat.0010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang TJ, Fenton MJ, Weiner MA, Hibbs S, Basu S, Baillie L, Cross AS. 2005. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect. Immun. 73:7495–7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi Paccani S, Tonello F, Patrussi L, Capitani N, Simonato M, Montecucco C, Baldari CT. 2007. Anthrax toxins inhibit immune cell chemotaxis by perturbing chemokine receptor signalling. Cell. Microbiol. 9:924–929 [DOI] [PubMed] [Google Scholar]
- 10.Paccani SR, Tonello F, Ghittoni R, Natale M, Muraro L, D'Elios MM, Tang WJ, Montecucco C, Baldari CT. 2005. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 201:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. 2012. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8:e1002638. 10.1371/journal.ppat.1002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leppla SH. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guichard A, Park JM, Cruz-Moreno B, Karin M, Bier E. 2006. Anthrax lethal factor and edema factor act on conserved targets in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 103:3244–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi SK, Lang GA, Larabee JL, Devera TS, Aye LM, Shah HB, Ballard JD, Lang ML. 2009. Bacillus anthracis lethal toxin disrupts TCR signaling in CD1d-restricted NKT cells leading to functional anergy. PLoS Pathog. 5:e1000588. 10.1371/journal.ppat.1000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barson HV, Mollenkopf H, Kaufmann SH, Rijpkema S. 2008. Anthrax lethal toxin suppresses chemokine production in human neutrophil NB-4 cells. Biochem. Biophys. Res. Commun. 374:288–293 [DOI] [PubMed] [Google Scholar]
- 16.Fang H, Cordoba-Rodriguez R, Lankford CS, Frucht DM. 2005. Anthrax lethal toxin blocks MAPK kinase-dependent IL-2 production in CD4+ T cells. J. Immunol. 174:4966–4971 [DOI] [PubMed] [Google Scholar]
- 17.Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C, Pulendran B. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329–334 [DOI] [PubMed] [Google Scholar]
- 18.Erwin JL, DaSilva LM, Bavari S, Little SF, Friedlander AM, Chanh TC. 2001. Macrophage-derived cell lines do not express proinflammatory cytokines after exposure to Bacillus anthracis lethal toxin. Infect. Immun. 69:1175–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.During RL, Li W, Hao B, Koenig JM, Stephens DS, Quinn CP, Southwick FS. 2005. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 192:837–845 [DOI] [PubMed] [Google Scholar]
- 20.Iyer JK, Khurana T, Langer M, West CM, Ballard JD, Metcalf JP, Merkel TJ, Coggeshall KM. 2010. Inflammatory cytokine response to Bacillus anthracis peptidoglycan requires phagocytosis and lysosomal trafficking. Infect. Immun. 78:2418–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer M, Malykhin A, Maeda K, Chakrabarty K, Williamson KS, Feasley CL, West CM, Metcalf JP, Coggeshall KM. 2008. Bacillus anthracis peptidoglycan stimulates an inflammatory response in monocytes through the p38 mitogen-activated protein kinase pathway. PLoS One 3:e3706. 10.1371/journal.pone.0003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer JK, Coggeshall KM. 2011. Cutting edge: primary innate immune cells respond efficiently to polymeric peptidoglycan, but not to peptidoglycan monomers. J. Immunol. 186:3841–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cataldi A, Labruyere E, Mock M. 1990. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol. Microbiol. 4:1111–1117 [DOI] [PubMed] [Google Scholar]
- 24.Crawford MA, Lowe DE, Fisher DJ, Stibitz S, Plaut RD, Beaber JW, Zemansky J, Mehrad B, Glomski IJ, Strieter RM, Hughes MA. 2011. Identification of the bacterial protein FtsX as a unique target of chemokine-mediated antimicrobial activity against Bacillus anthracis. Proc. Natl. Acad. Sci. U. S. A. 108:17159–17164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batty S, Chow EM, Kassam A, Der SD, Mogridge J. 2006. Inhibition of mitogen-activated protein kinase signalling by Bacillus anthracis lethal toxin causes destabilization of interleukin-8 mRNA. Cell. Microbiol. 8:130–138 [DOI] [PubMed] [Google Scholar]
- 26.Raymond B, Batsche E, Boutillon F, Wu YZ, Leduc D, Balloy V, Raoust E, Muchardt C, Goossens PL, Touqui L. 2009. Anthrax lethal toxin impairs IL-8 expression in epithelial cells through inhibition of histone H3 modification. PLoS Pathog. 5:e1000359. 10.1371/journal.ppat.1000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225–229 [DOI] [PubMed] [Google Scholar]
- 28.Scobie HM, Rainey GJ, Bradley KA, Young JA. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. U. S. A. 100:5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford MA, Burdick MD, Glomski IJ, Boyer AE, Barr JR, Mehrad B, Strieter RM, Hughes MA. 2010. Interferon-inducible CXC chemokines directly contribute to host defense against inhalational anthrax in a murine model of infection. PLoS Pathog. 6:e1001199. 10.1371/journal.ppat.1001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf M, Moser B. 2012. Antimicrobial activities of chemokines: not just a side-effect? Front. Immunol. 3:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mabry R, Brasky K, Geiger R, Carrion R, Jr, Hubbard GB, Leppla S, Patterson JL, Georgiou G, Iverson BL. 2006. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleret-Buhot A, Mathieu J, Tournier JN, Quesnel-Hellmann A. 2012. Both lethal and edema toxins of Bacillus anthracis disrupt the human dendritic cell chemokine network. PLoS One 7:e43266. 10.1371/journal.pone.0043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Goot G, Young JA. 2009. Receptors of anthrax toxin and cell entry. Mol. Aspects Med. 30:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]