Abstract
Helicobacter pylori infection is the leading cause for peptic ulcer disease and gastric adenocarcinoma. Mucosal T cell responses play an important role in mediating H. pylori-related gastric immunopathology. While induced regulatory T (iTreg) cells are required for chronic colonization without disease, T helper 1 (Th1) effector responses are associated with lower bacterial loads at the expense of gastric pathology. Pigs were inoculated with either H. pylori strain SS1 or J99. Phenotypic and functional changes in peripheral blood mononuclear cell (PBMC) populations were monitored weekly, and mucosal immune responses and bacterial loads were assessed up to 2 months postinfection. Both H. pylori strains elicited a Th1 response characterized by increased percentages of CD4+Tbet+ cells and elevated gamma interferon (IFN-γ) mRNA in PBMCs. A subset of CD8+ T cells expressing Tbet and CD16 increased following infection. Moreover, a significant increase in perforin and granzyme mRNA expression was observed in PBMCs of infected pigs, indicating a predominant cytotoxic immune response. Infiltration of B cells, myeloid cells, T cells expressing Treg- and Th17-associated transcription factors, and cytotoxic T cells was found in the gastric lamina propria of both infected groups. Interestingly, based on bacterial reisolation data, strain SS1 showed greater capacity to colonize and/or persist in the gastric mucosa than did strain J99. This novel pig model of infection closely mimics human gastric pathology and presents a suitable avenue for studying effector and regulatory responses toward H. pylori described in humans.
INTRODUCTION
Helicobacter pylori is the dominant member of the gastric microbiota and colonizes the stomach of more than 50% of the human population worldwide (1). H. pylori colonization usually does not cause illness, since 85% of infected people remain asymptomatic throughout life, but infection with strains bearing the cag (cytotoxin-associated gene) pathogenicity island can result in peptic ulcer disease, gastric lymphoma, and gastric adenocarcinoma, the second leading cause of cancer-related deaths, in 15% of infected individuals (2, 3). Conversely, there also is increasing evidence of H. pylori providing protection against esophageal and cardial pathologies (4–7), childhood asthma (8–10), childhood allergies (9, 11), and diabetes and obesity (12). This Gram-negative microaerophilic bacterium of the Epsilonproteobacteria has coevolved with humans for at least 50,000 years, indicating high adaptation capacity to the environmental niche of the human gastric mucosa and suggesting the ability to evade the immune system (13) through mechanisms that are incompletely understood. Infection with H. pylori in humans is associated mainly with a mucosal Th1 response, which is unsuccessful in clearing the bacteria from the stomach and can lead to more severe immunopathology (14). The pathogenicity of H. pylori is determined by various host- and pathogen-related factors, including the host's genetic background, age, and immune status and the bacterium's ability for antigenic variation, molecular mimicry, intracellular persistence, and expression of pathogenicity factors (15).
Regulatory T (Treg) cells play a crucial role in H. pylori's ability to evade the immune system and persist in the gastric mucosa. Specifically, H. pylori can trigger a reprogramming of dendritic cells (DC) by downregulating major histocompatibility complex II (MHC-II) and inducing interleukin-10 (IL-10) and inhibiting IL-12 secretion, thereby inducing H. pylori-specific Treg cells (16–18). B cells also play a regulatory role by promoting IL-10 production in cocultured CD4+ cells and subsequent conversion into a T regulatory 1 (Tr1)-like phenotype (19). In children, H. pylori infection favors the induction of mucosal Treg responses, which are associated with reduced gastric inflammatory lesions compared to those of adults (20). A study in neonatal mice infected with H. pylori demonstrated the induction of immunological tolerance and the subsequent protection from T cell-driven immunopathology and gastric cancer precursor lesions, suggesting that the age at the time of H. pylori infection may delineate health outcomes (21). Thus, patients with fewer or less functional Treg cells are more likely to develop peptic ulcers and are afflicted by more intense gastritis (13). Cytotoxic T lymphocytes (CTL) have also been recently implicated in immune responses toward H. pylori in clinically relevant settings. Specifically, an increased number of CD8+ T lymphocytes were found in the gastric epithelium and lamina propria (LP) of H. pylori-infected children with grade I to III gastritis (22, 23). Furthermore, the CD8+HLA-DR+ chronically activated memory T cell subset was expanded in peripheral blood of H. pylori-colonized children with duodenal ulcers (24), suggesting a role for CD8+ T cells in H. pylori-mediated pathology.
The majority of in vivo studies on the host responses to H. pylori are based on mouse models; however, in contrast to human H. pylori infections, CD8+ T cell responses to the bacterium have been detected only in immunodeficient mice lacking CD4+ T cells (25, 26). Results from these studies indicate that CD8+ T cells also contribute to the development of gastric lesions, which traditionally has been attributed to effector CD4+ T cells (27, 28). Even though H. pylori infection has been studied in gnotobiotic piglets, the main focus of previous pig challenge studies was on humoral immune responses, vaccine-induced protection, or gastric pathology (29–31). To overcome the limitations in the study of CD8+ T cell responses to H. pylori, we have developed the first pig model to study mucosal and systemic Th1 and CD8+ cytotoxic immune responses to H. pylori infection. Specifically, we use this newly developed model to characterize the mechanisms of immunoregulation underlying immune responses to H. pylori strains SS1 and J99 systemically and in the gastric mucosa. Thus, in addition to developing a novel pig model that closely resembles human gastric pathology, we report the predominance of CTL responses during H. pylori infection.
MATERIALS AND METHODS
Animal procedures.
Two independent pig challenge studies were performed. The second study was designed to validate results from the first one. Pigs used in this study were weaned at 3 weeks of age and transferred to an animal biosafety level 2 (ABSL2) facility at Virginia Tech. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Tech and met or exceeded requirements of the Public Health Service/National Institutes of Health and the Animal Welfare Act.
Pigs (study 1, n = 8; study 2, n = 26) were divided into 3 groups: noninfected (n1 = 2, n2 = 9), infected with H. pylori strain J99 (n1 = 3, n2 = 9), and infected with H. pylori strain SS1 (n1 = 3, n2 = 8). In the case of study 2, 9 blocks of 2 or 3 pigs were designed on the basis of litter of origin and initial body weight and randomly assigned to the 3 treatment groups. Following a 12-hour fasting period, bacterial challenge was performed by orogastric gavage with 1 × 108 CFU/ml H. pylori live organisms (strain J99 or SS1) resuspended in 100 ml sterile brucella broth administered on days 0 and 2 of the study. As a control, the noninfected group received sterile brucella broth without any bacteria. Due to constraints associated with the use of a large-animal model, the infection dose was optimized by conducting a series of dose-response experiments in mice as previously described (32). We chose doses that provided detectable host responses and long-term colonization, which were then adapted to the pig model.
A delay in gastric emptying was ensured by oral administration of agar in 1% brucella broth supplemented with 10% fetal bovine serum (FBS) prior to the bacterial or mock challenges (33). To suppress gastric acidity and to increase the efficiency of H. pylori colonization, all pigs received famotidine (1 mg/kg of body weight) intramuscularly 90 min prior to each bacterial and control inoculation (34), and 5% urea was added to the drinking water for 7 days postinfection to provide a sufficient substrate for H. pylori urease and to increase gastric pH (35). Pigs were scored for clinical signs of disease daily, and peripheral blood was collected from the vena cava weekly to study systemic immune responses. Pigs were euthanized between day 51 and day 59 postinfection to assess gastric colonization with H. pylori and to study local immune responses in the gastric mucosa. The stomach was scored based on macroscopic lesions, excised, and processed for further analysis. Tissue was collected from three major regions, i.e., fundus gland (F), pyloric gland (P), and cardiac gland (C), and these were further subdivided in two sections (F-A, F-B, P-A, P-B, C-A, and C-B; see Fig. S1 in the supplemental material), which were analyzed separately for bacterial reisolation and histopathology.
Culture of H. pylori, preparation of bacterial antigens, and bacterial reisolation.
H. pylori strains J99 (ATCC 700824) and SS1 (kindly provided by Richard Peek, Vanderbilt University) were used in these studies. J99 is an African strain that was isolated from a patient in the United States in 1994 (36), whereas SS1 is a European mouse-adapted strain that is widely used in animal models of H. pylori infection (37). We chose an African strain and a European strain for this study based on differences observed in a previously conducted comparative genomics study of H. pylori (38). Both strains are CagA positive but differ in VacA, with SS1 expressing the s2m2 and J99 the s1m1 isoforms (36, 39). The latter has been associated with higher cytotoxin activity and increased risk for gastroduodenal disease (40). H. pylori was grown on plates prepared with Difco Columbia blood agar base (BD Biosciences) supplemented with 7% horse blood (Lampire) and Helicobacter pylori selective supplement (containing 10 mg/liter vancomycin, 5 mg/liter trimethoprim, 5 mg/liter amphotericin, and 5 mg/liter polymyxin from Oxoid) at 37°C under microaerophilic conditions. The challenge inoculum was prepared by harvesting bacteria into brucella broth (BD Biosciences) and adjusting to an optical density at 600 nm (OD600) of 1.0, which was estimated as a concentration of 1 × 108 CFU/ml as previously determined by a growth curve correlating OD measurements with colony counts on blood agar plates. For reisolation of H. pylori from pigs, tissues from six different stomach regions (see Fig. S1 in the supplemental material) were weighed and homogenized using a grinder. The homogenate was plated onto Columbia blood agar plates and incubated for 4 to 5 days under the conditions described above. Serial dilutions (1:100, 1:1,000, 1:10,000) of the tissue homogenate were plated for samples derived from infected pigs.
To prepare whole-cell sonicated (WCS) bacterial antigens, H. pylori strains J99 and SS1 were inactivated with 4% formaldehyde for 26 h followed by two washing steps with 1× phosphate-buffered saline (PBS). Inactivated whole-cell H. pylori preparations were resuspended in 1× PBS and sonicated five times on ice for 20 s at 1-min intervals. Protein concentration of WCS antigen preparations were quantified and stored at −20°C until further use. Bacterial inactivation was confirmed by culturing formaldehyde-treated H. pylori for at least 4 days as described above.
Histopathology.
Sections of all six stomach regions (see Fig. S1 in the supplemental material) were fixed in 10% buffered neutral formalin, later embedded in paraffin, and then sectioned and stained with hematoxylin and eosin (H&E) for histological examination.
Isolation of splenocytes and cells from GLN.
Spleen and gastric lymph nodes (GLN) were excised and crushed in 1× PBS–5% FBS using the frosted ends of two sterile microscope slides. Single cell suspensions were centrifuged at 300 × g for 10 min and washed once with 1× PBS. Leukocytes were isolated using a discontinuous 44/67% Percoll gradient (GE Healthcare). After density gradient centrifugation for 20 min at 770 × g, the interphase containing viable leukocytes was harvested, washed once in 1× PBS–5% FBS, and resuspended in fluorescence-activated cell sorter (FACS) buffer (1× PBS supplemented with 5% FBS and 0.09% sodium azide) or complete RPMI (cRPMI) (41) for subsequent analysis.
Isolation of gastric leukocytes.
Different parts of the stomach (see Fig. S1 in the supplemental material) were excised, and lamina propria leukocytes (LPL) were isolated. Tissue pieces were washed in CMF (1× Hanks balanced salt solution [HBSS]–10% FBS–25 mM HEPES–100 μg/ml gentamicin), excess mucus and fat were removed, and the tissue was sectioned into 5- to 6-mm pieces. Tissue from the 3 main regions (pyloric, cardiac, and fundus) was vortexed in medium for 2 min to remove particulates and mucus. After washing with 1× PBS, tissue was further digested in RPMI/FBS (RPMI 1640–10% FBS–25 mM HEPES–100 μg/ml gentamicin–3 mM CaCl2) supplemented with 300 U/ml type VIII collagenase and 50 U/ml DNase I (both from Sigma-Aldrich) for 1.5 h at 37°C with stirring. Cell suspensions were filtered through a 100-μm strainer and pelleted. Cells were subjected to purification by discontinuous Percoll density gradient as described for splenocyte isolation above and resuspended in cRPMI for further analyses.
PBMC isolation.
Porcine peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation. Briefly, leukocyte separation medium (specific density, 1.077 to 1.080 g/ml; Mediatech) was overlayered with diluted whole blood (1:4 in 1× PBS) and centrifuged for 20 min at 770 × g without break. The PBMC-containing interphase was collected, red blood cells were removed by osmotic lysis, and cells were washed with 1× PBS and resuspended in cRPMI.
ELISpot.
For the enzyme-linked immunosorbent spot assay (ELISpot), 96-well multiscreen plates with a polyvinylidene difluoride (PVDF) membrane (Millipore) were coated with 50 μl of 4-μg/ml anti-pig IL-17 (Bethyl Laboratories) or 100 μl anti-pig IFN-γ (ELISpot Development Module; RnD Systems) in 1× PBS overnight at 4°C in a humidified chamber. Wells were washed 4 times with 1× PBS and blocked with 1× PBS–5% sucrose–1% bovine serum albumin (BSA) for 2 h. After a washing step with cRPMI, a total of 2 × 105 splenocytes or cells from GLN were stimulated with 5 μg/ml of H. pylori SS1 or J99 WCS prepared as described above or maintained in cRPMI only. Cells were incubated at 37°C, 5% CO2, and 95% humidity. After 36 h, cells were removed from plates and bound cytokines were incubated with either 100 μl of 0.5-μg/ml biotinylated anti-pig IL-17 (Bethyl Laboratories) or biotinylated anti-pig IFN-γ in 1× PBS–1% BSA overnight at 4°C in a humidified chamber. Spots of cytokine-secreting cells were detected by incubating wells with 100 μl streptavidin-horseradish peroxidase (HRP) (Blue Color Development Module; RnD Systems) in 1× PBS–1% BSA for 2 h, followed by developing with enzyme substrate for 30 min in the dark. Between incubations, wells were washed 3 or 4 times with 1× PBS–0.05% Tween 20 (Bio-Rad). After formation of colored spots, wells were washed with distilled water and left for drying overnight. Plates were analyzed on an ELISpot reader (AID) using AID ELISpot reader software. Results were expressed as spot-forming units (SFU)/1 × 106 cells and displayed as fold of induction (FOI) compared to the nontreated cRPMI control.
Immunophenotyping and cytokine analysis by flow cytometry.
To assess the distribution of immune cell subsets, 4 × 105 to 6 × 105 PBMCs, splenocytes, cells from GLN, or LPL or 10 μl of whole blood was incubated for 20 min with fluorochrome-conjugated or unconjugated primary pig-specific antibodies: anti-CD3-PerCP-Cy5.5/phycoerythrin (PE)-Cy7 clone BB23-8E6-8C8, anti-CD4 fluorescein isothiocyanate (FITC)/PerCP-Cy5.5/PE-Cy7/AF-647 clone 74-12-4, anti-CD8α PE clone 295/33-25, anti-TCRγδ APC clone MAC320, anti-CD16 Biotin clone FcG7 (all from BD Biosciences), anti-SWC3a clone 74-22-15A, anti-CD8β clone PG164A, and anti-CD45 clone 74-9-3A1(VMRD), as well as anti-human CD21 PE-Cy7/PE-Cy5 clone B-Ly4 (BD Biosciences). To detect binding of unlabeled antibodies (SWC3a, CD8β, CD45), cells were incubated for another 20 min with isotype-specific, fluorochrome-conjugated secondary antibodies: goat anti-mouse IgG2b APC-Cy7, IgG2a PE, IgG1 APC, IgM FITC, or APC-Cy7 (Southern Biotech). For intracellular staining of transcription factors, cells were fixed and permeabilized using a commercial kit according to the manufacturer's instructions (eBioscience). Briefly, cells were fixed and permeabilized for 20 min, Fc receptors were blocked with mouse anti-CD16/CD32 FcBlock (BD Biosciences), and cells were incubated with fluorochrome-conjugated antibodies toward anti-mouse/human Tbet PerCP-Cy5.5 clone 4-B10, anti-mouse RORγt PE clone AFKJS-9, and FOXP3 FITC/APC clone FJK-16S (eBioscience) (BD Biosciences). Due to the lack of pig-specific antibodies to transcription factors, mouse-specific antibodies were used for experiments after testing for cross-reactivity. All samples were stored fixed at 4°C in the dark until acquisition on an LSR II flow cytometer (BD Biosciences). A live cell gate (FSC-A, SSC-A) was applied to all samples followed by single cell gating (FSC-H, FSC-W) before cells were analyzed for the expression of specific markers. Data analysis was performed with Flow Jo (Tree Star Inc.).
qRT-PCR.
RNA was isolated from PBMCs using the RNeasy Minikit (Qiagen). mRNA concentrations were quantified by optical density at 260 nm with a Nanodrop spectrophotometer (Invitrogen). Up to 1 microgram of RNA per sample was used to synthesize cDNA using the iSCRIPT cDNA Synthesis kit (Bio-Rad) and stored at −20°C. Reverse transcription (RT)-PCR was performed to assess the absolute expression of IFN-γ, granzyme A, granzyme B, and perforin using the primers listed in Table 1. Standard curves were created using diluted cDNA at known concentrations ranging from 5 to 5 × 10−6 pg per reaction volume. RT-PCR was performed using a CFX96 Real Time system (Bio-Rad). Target gene expression was normalized to the housekeeping gene RPL-19.
Table 1.
Primer sequences and properties
| Target | Sensea | Primer sequence | Annealing temp (°C) | Amplicon length (bp) | Accession no. |
|---|---|---|---|---|---|
| RPL-19 | S | AACTCCCGTCAGCAGATC | 57 | 147 | AF435591 |
| AS | AGTACCCTTCCGCTTACCG | ||||
| IFN-γ | S | GCTCTGGGAAACTGAATGAC | 60 | 167 | NM_213948 |
| AS | TCTCTGGCCTTGGAACATAG | ||||
| Granzyme A | S | GGAGATGTCAAGCAAAGC | 57.3 | 91 | NM_001143709 |
| AS | AGTCAGGCTATCGTTTGG | ||||
| Granzyme B | S | CCCACAACATCAAGAAAC | 57 | 85 | NM_001143710 |
| AS | CGCTTCTCGTTATAGTCT | ||||
| Perforin 1 | S | GTTTTGTTTTCAATGAGGTGGC | 56.5 | 84 | XM_003483492 |
| AS | TGCCAGGTTGCTTCTGTT |
S, sense; AS, antisense.
Statistics.
Data were analyzed using analysis of variance (ANOVA) followed by Scheffe's multiple-comparison method. For repeated analyses on the same animal (i.e., flow cytometry data over time), we used repeated-measures ANOVA. ANOVA was performed by using the general linear model procedure of SAS, release 9.2 (SAS Institute). Statistical significance was assessed at P values of ≤0.05.
RESULTS
H. pylori infection induces a predominant systemic Th1 response in vivo.
To assess whether H. pylori infection affects the expansion of specific T cell subsets, we evaluated the expression of the main transcription factors involved in the regulation of CD4+ T cell phenotype: FOXP3 (iTreg and nTreg), Tbet (Th1), and RORγt (Th17). Overall numbers of circulating CD4+ T cell populations did not change over time due to infection.
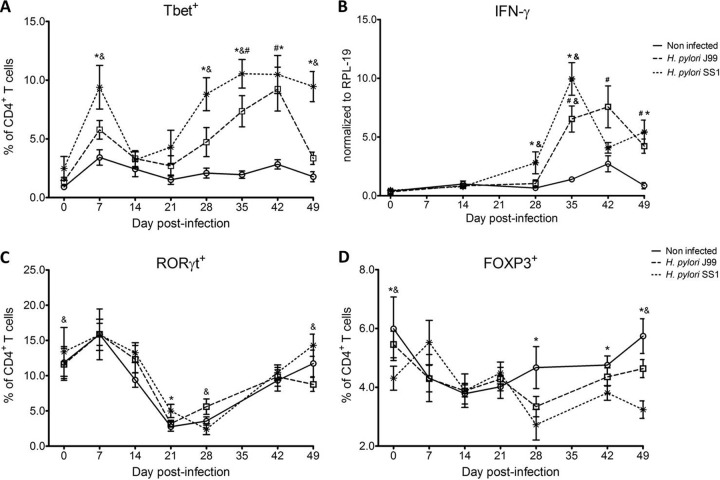
Further analysis revealed an elevated proportion of CD4+ T cells expressing the Th1-associated transcription factor Tbet in response to H. pylori (Fig. 1A). We observed a transient single peak at day 7 postinfection followed by a sustained increase in CD4+Tbet+ cells from day 28 to day 49 postchallenge in SS1-infected pigs. The same pattern was found in J99-infected pigs, except that the response declined by day 49 postchallenge. The percentage of RORγt+CD4+ T cells was significantly different on day 21 postinfection in SS1-infected pigs and on days 28 and 49 postinfection in J99-infected pigs (Fig. 1C). There was a more notable decline in the expression of the Treg-associated transcription factor FOXP3 in CD4+ T cells from pigs infected with strain SS1 on day 28 postinfection (Fig. 1D), which mirrored the increase in Tbet and coincided with increased transcripts of IFN-γ mRNA in PBMCs for both SS1- and J99-infected pigs (Fig. 1B). These results provided the first indication of a predominant Th1 response induced in our experimental model by H. pylori.
Fig 1.
Increase in circulating Th1 cells and IFN-γ expression upon H. pylori infection. CD4+ T cells expressing the T cell lineage-specific transcription factors Tbet (Th1) (A), RORγt (TH17) (C), and FOXP3 (iTreg, nTreg) (D) were detected by flow cytometry. IFN-γ mRNA levels in PBMC were measured by qRT-PCR (B). Data were derived from study 2. Symbols indicate statistical differences between either the H. pylori J99 (#)- or the SS1 (*)-infected group and the control group and between the two infected groups (&); n = 8 or 9; data are means ± standard errors of the means (SEM); P ≤ 0.05.
Infection with H. pylori upregulates expression of Tbet in various immune cell subsets.
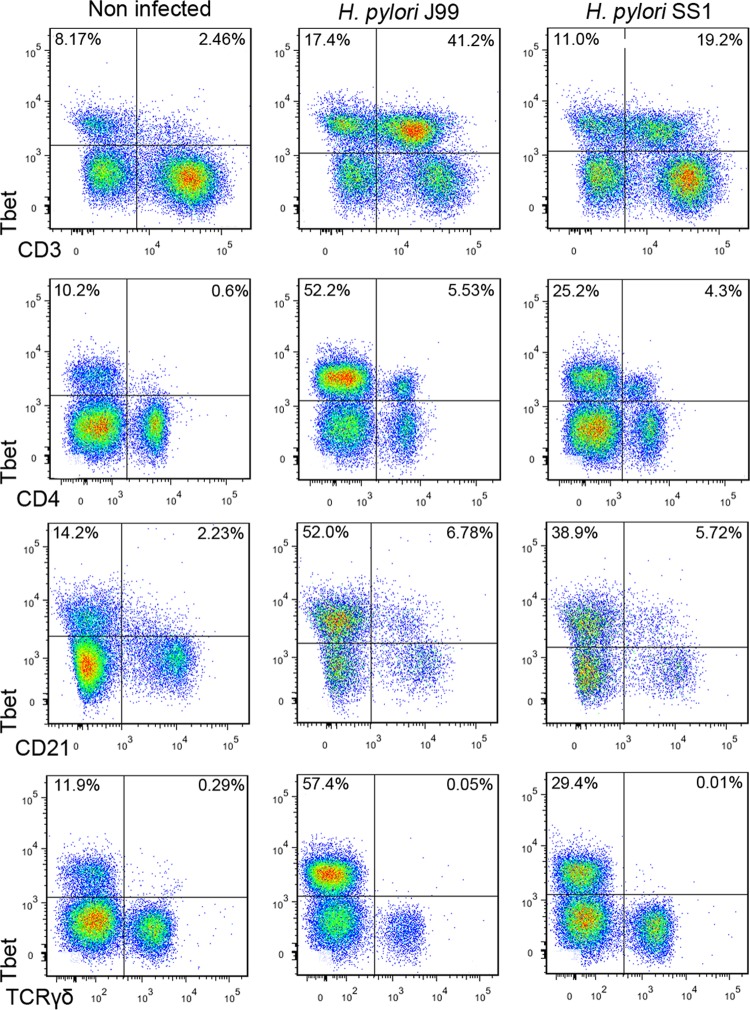
Besides the clear increase of Tbet expression in CD4+ T cells upon infection, flow cytometry data from study 1 also showed that the majority of Tbet+ cells in H. pylori-infected pigs were CD4− and that a fraction of those were also CD3− (Fig. 2). We performed an in-depth immunophenotypic analysis, which led to the identification of a subset of CD21lo B cells expressing Tbet that was increased due to infection (Fig. 2). This overall increase of Tbet in several cell subsets was not due to unspecific binding of the anti-Tbet antibody since TCRγδ+ T cells did not express any (Fig. 2).
Fig 2.
T cells and B cells upregulate Tbet expression upon infection with H. pylori. Expression of Tbet was determined in peripheral T cells (CD3+), T helper cells (CD4+), B cells (CD21+), and γδ T cells (TCRγδ+). Representative flow cytometry dot blots for uninfected and H. pylori J99- and SS1-infected pigs at day 42 postinfection of study 1 are presented. Numbers indicate the percentages of positive cells within the single cell population.
Infection with H. pylori results in expansion of NK and cytotoxic T cells.
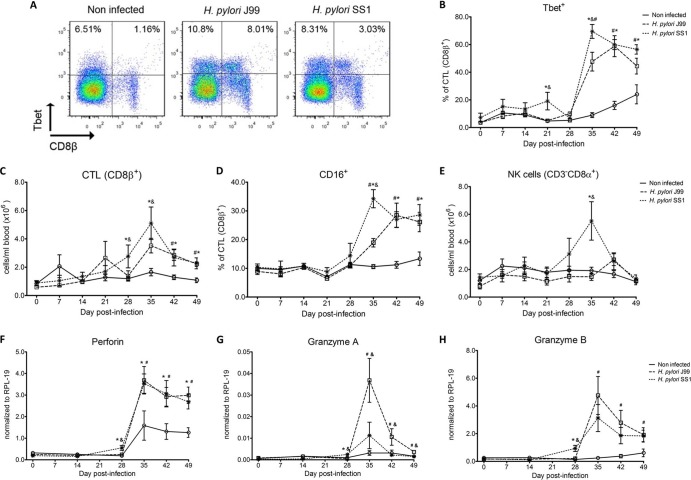
Study 1 demonstrated that a significant fraction of CD3+CD4− T cells that were not γδ T cells upregulated Tbet. Figure 3A shows flow cytometry analysis demonstrating the induction of Tbet in CD8+ T cells on day 42 of study 1. In study 2, we performed a systematic assessment of Tbet expression in CD8ab+ T cells. Of note, CD8β is the most specific marker for CTL in pigs, since the CD8αα homodimer is also expressed by other cell subsets (42). These cells specifically expressed low levels of the CD8β coreceptor, which is characteristic of CTL that have been previously exposed to their cognate antigen (43).
Fig 3.
Increased circulating and functional cytotoxic cells upon H. pylori infection. Expression of Tbet was assessed in peripheral CTL (CD8β+) on day 42 postinfection (A) and over time (B). Representative flow cytometry dot blots for noninfected and H. pylori J99- and SS1-infected pigs are presented. Numbers indicate percentages of positive cells within the single cell population (A). CTL (CD8β+) (C) and NK cells (CD3−CD8α+) (E) were enumerated in PBMC over time. Numbers of cells per ml of blood were calculated by applying the percentage of immune cells obtained by flow cytometry to the concentration of cells in whole blood. The percentage of CD16-expressing CTL was assessed throughout the study (D). Gene expression levels of perforin (F), granzyme A (G), and granzyme B (H) in PBMC were analyzed over time. Data were derived from study 2. Symbols indicate statistical differences between either the H. pylori J99 (#)- or the SS1 (*)-infected group and the control group and between the two infected groups (&); n = 8 or 9; data are means ± SEM; P ≤ 0.05.
Our analysis revealed a sharp increase of CD8β+Tbet+ cells in H. pylori-infected pigs (Fig. 3B), which was slightly higher in SS1-carrying pigs. The shift in Tbet expression was first detected on day 35 postinfection, when on average 62% of cells had detectable amounts of this transcription factor. The percentage of CD8β+Tbet+ cells declined thereafter, although toward the end of the study, on day 49 postinfection, there were still significant differences between infected and noninfected pigs. A closer analysis revealed the expansion of circulating CD8β+ T cells (Fig. 3C) and an increase of CD16 expression on those circulating CTL (Fig. 3D) upon infection. Furthermore, CD3−CD8a+ NK cells were significantly increased in blood of SS1-infected pigs on day 35 postinfection (Fig. 3E).
In concordance with the observed expansion of circulating cytotoxic T cells, we detected a significant upregulation in the expression of genes involved in the cytotoxic activity of CTL and NK cells, perforin, granzyme A, and granzyme B (Fig. 3F to H). Overall, our data suggest the initial induction of an IFN-γ-producing Th1 response orchestrated by the transcription factor Tbet and executed by cytotoxic T cells.
Local gastric responses to H. pylori.
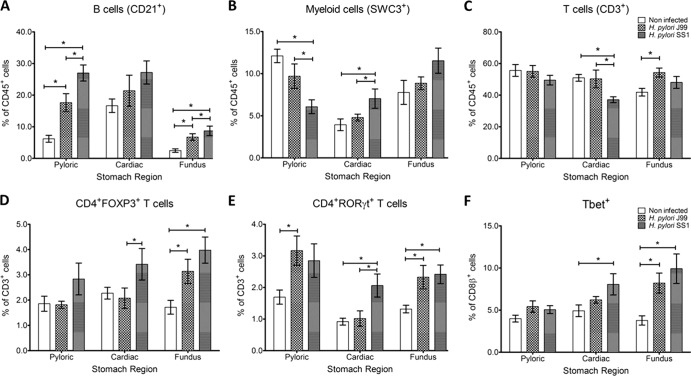
H pylori colonizes mainly the stomach mucosa. To evaluate local immune responses, at the end of the 60-day infection study, we performed a phenotypic analysis of the leukocyte subsets present in different stomach regions. Our data show significant accumulation of B cells in the fundic and pyloric regions (Fig. 4A). There was an increased presence of SWC3+ myeloid cells in the cardiac region and decreased numbers in the pyloric region of SS1-infected pigs (Fig. 4B). We also found increased numbers of CD4+FOXP3+ T cells in the cardiac region of SS1-challenged pigs and in the pyloric region of SS1- and J99-infected pigs (Fig. 4D). The percentage of CD4+RORγt+ cells, which would suggest the presence of IL-17-producing cells, was increased in the pyloric and fundic regions of infected pigs, irrespective of the strain (Fig. 4E). Finally, Tbet expression in CD8+ T cells significantly increased in the cardia of SS1-infected pigs and in the fundus of SS1- and J99-infected pigs (Fig. 4F).
Fig 4.
Infiltration of immune cells into the gastric lamina propria upon H. pylori infection. The phenotypes of lamina propria leukocytes within the pyloric, cardiac, and fundus regions of the pig stomach were determined by flow cytometry. The percentages of B cells (CD21+) (A), myeloid cells (SWC3+) (B), T cells (CD3+) (C), CD4+ T cells expressing FOXP3 (D) or RORγt (E), and CD8β+Tbet+ T cells (F) were assessed in noninfected and H. pylori J99- and SS1-infected pigs. Data were derived from study 2, and statistically significant differences are represented by an asterisk (*); n = 8 or 9; data are means ± SEM; P ≤ 0.05.
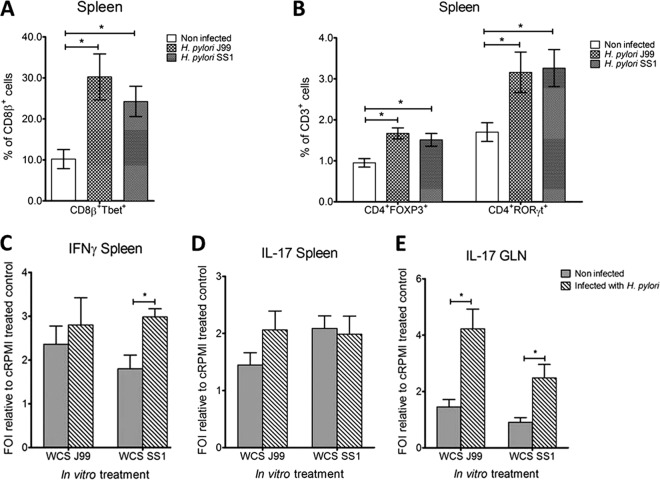
Similar to the findings in PBMCs and gastric lamina propria, we also found elevated Tbet levels in CD8β+ splenocytes upon infection (Fig. 5A). Furthermore, the overall number of CD4+ T cells significantly increased in the spleen of both infected groups (data not shown). More specifically, we detected a significant increase in FOXP3- and RORγt-expressing CD4+ T cells (Fig. 5B). A significantly elevated number of cultured splenocytes from SS1-infected pigs secreted IFN-γ upon reexposure to bacterial antigen ex vivo compared to cells from noninfected controls (Fig. 5C). In addition, we observed a trend for an increase in the number of splenocytes secreting IL-17 only in the J99-infected group (Fig. 5D). The number of IL-17-secreting cells significantly increased upon reexposure of cells isolated from GLN to H. pylori crude antigen (Fig. 5E).
Fig 5.
Increased Tbet expression in spleen and induction of cytokine secretion in cells from GLN and spleen upon reexposure to antigen. The percentages of CD8β+ Tbet+ (A) and CD4+ FOXP3+ and CD4+ RORγt+ T cells (B) were assessed in spleen of noninfected and H. pylori J99- and SS1-infected pigs. The number of splenocytes secreting IFN-γ (C) and IL-17 (D) and the number of cells from GLN secreting IL-17 (E) were analyzed by ELISpot. Cells from noninfected and infected pigs were stimulated with whole-cell sonicated (WCS) bacterial antigen in vitro for 36 h. Cells treated with cRPMI alone served as negative control. Data were expressed as fold of induction (FOI) to the negative control. Data were derived from study 2, and statistically significant differences are represented by an asterisk (*); n = 8 or 9; data are means ± SEM; P ≤ 0.05.
Persistence of H. pylori regardless of increased cytotoxic cell populations.
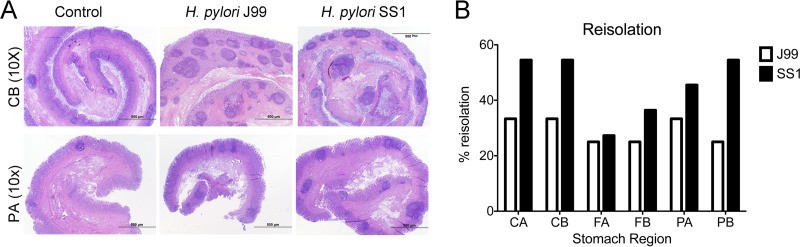
Reisolation of H. pylori from infected pigs was performed at the end of the study. Overall, H. pylori SS1 was recovered from the stomach of all pigs in that group, while H. pylori J99 could be reisolated from only 8 of 12 pigs. With regard to the level of colonization for each of the 3 regions and 2 subregions (see Fig. S1 in the supplemental material), we found that the percentage of reisolation was consistently higher in the SS1-infected group than in the J99 group, with the exception of the FA subregion, which showed similar frequencies for the two strains (Fig. 6B). Microscopic changes were present in the stomach of both infected groups and were characterized by significant expansion and development of organized lymphoid aggregates and diffuse leukocytic infiltration. Both strains of H. pylori induced organized lymphoid tissue in the stomach mucosa, which was more predominant in the cardiac region (Fig. 6A).
Fig 6.
Lesion development upon H. pylori infection and lack of bacterial clearance upon long-term infection with strain SS1. Representative images were taken from hematoxylin- and eosin-stained specimens collected from the stomach regions cardiac B (CB) and pyloric A (PA) of noninfected and infected pigs at a magnification of ×10 (A). H. pylori J99 and SS1 were reisolated from 6 stomach locations (see Fig. S1 in the supplemental material) at 2 months postinfection. Reisolation data from studies 1 and 2 were combined and expressed as percentages (B).
DISCUSSION
Although initially considered exclusively an extracellular bacterium, H. pylori also exploits intracellular niches in its host (44). Several studies have demonstrated that H. pylori can persist in hepatocytes (45) and replicate in macrophages (46) and bone marrow-derived dendritic cells (17) as well as gastric epithelial cells in vitro, thus providing evidence for its role as a facultative intracellular organism with the ability to reside, replicate, and successfully evade antibiotic therapy within host cells (47). Recent in vivo studies have further strengthened the role of H. pylori as an intracellular pathogen in mice and humans. More specifically, H. pylori not only was localized to murine gastric epithelial progenitor cells (48) but also was identified in human tissue specifically residing within gastric epithelial cells, parietal cells, and lamina propria macrophages (49, 50). In macrophages, some H. pylori strains have the ability to prevent phagosome maturation, allowing the bacterium to survive and replicate by escaping phagocytic killing (51, 52). Furthermore, H. pylori has been found in gastric lymph nodes, suggesting lymphatic dissemination (49). One report described the systemic presence of H. pylori in peripheral blood of an H. pylori-seropositive breast cancer patient with bacteremia (53), providing further in vivo evidence that H. pylori can spread beyond the gastric mucosa invading other organs and tissues.
Our findings that H. pylori elicits Th1 and CTL responses in a novel pig model correlate well with its role as a facultative intracellular pathogen. Greater numbers of circulating NK cells and CTL characterized by increased CD16 surface expression have been found in H. pylori-infected pigs. In concordance with this, we found increased gene expression of CTL-associated factors including granzyme A, granzyme B, and perforin upon H. pylori infection.
Clinical and in vitro studies with human cells provide increasing evidence that cytotoxic immune responses play a crucial role in H. pylori pathogenesis. Sugita et al. (54) described a case report on an H. pylori-infected woman with gastric peripheral T cell lymphoma characterized by elevated markers of cytotoxic cells such as CD3, CD8, granzyme B, and perforin. It has also been demonstrated that H. pylori-reactive CD8+ T cells can be activated by B cells and DC that have been pulsed with H. pylori antigens in vitro. Furthermore, memory CD8+ T cells sorted from peripheral blood of H. pylori-infected individuals were highly responsive to H. pylori urease (55). Additionally, clinical studies in children have revealed higher numbers of circulating CD8+CD45RO+ and CD4+CD45RO+ memory as well as NK cells upon infection with H. pylori (56).
Our data demonstrate for the first time an increase in circulating CTL and NK cells peaking on day 35 postinfection, which coincides with increased IFN-γ gene expression. NK cells have been shown to secrete IFN-γ upon stimulation with H. pylori lysate and IL-12 in vitro (57), which is attributed to recognition of the membrane-bound H. pylori lipoprotein HpaA by Toll-like receptor 2 (TLR2) (58). Lindgren et al. (59) recently identified a subset of human CD8− NK cells residing in the gastrointestinal tract, which exerted cytotoxic activity and, in contrast to the CD8+ NK cell subset, secreted IFN-γ upon stimulation with H. pylori lysates, thus providing strong evidence for the importance of innate immune responses against H. pylori. We provide novel evidence in support of the infiltration of cytotoxic cells in the gastric mucosa of H. pylori-infected pigs, thus indicating a specific role for these cell subsets in the host's local immune response toward the bacterium. Interestingly, while signatures of CTL have been described in humans, mouse models of H. pylori have focused mainly on the balance between CD4+ T cell phenotypes. In addition, while it is well recognized that H. pylori induces a predominant Th1 response, the potential induction of cytotoxic responses has been only marginally addressed in the mouse model. In support of the role of cytotoxic responses in the pathogenesis of H. pylori-induced gastric disease, it was reported that mice deficient in CD4+ T cells developed severe gastritis following infection characterized by infiltration of CD8+ T cells and B cells. Moreover, the authors suggest that the regulatory role of CD4+ T cells may be important for suppressing excessive or tissue-damaging CD8+ T cell responses (26). Thus, the mouse model does not seem appropriate to study CD8+ T cell responses to H. pylori.
The transcription factor Tbet was found to play a crucial role in driving this cytotoxic response. We show a pronounced increase in circulating and splenic Tbet-positive CD4+ T cells, CTL, and B cells, which further demonstrates the predominance of a Th1 immune response upon H. pylori infection in pigs. Our CD4-specific Tbet flow cytometry data indicate a dual wave of expression showing a first peak at day 7 postinfection followed by a significant upregulation starting at day 28 postinfection. The latter corresponded to increased IFN-γ gene expression starting between days 21 and 28 postinfection. This was followed by increased expression of Tbet by CD8β+ T cells on day 35 postinfection, suggesting that CD8+ T cell responses might be triggered by the upregulation of Tbet in CD4+ T cells, corresponding to a Th1 response. Although Tbet has a unique role in orchestrating the differentiation of naive CD4+ T cells into Th1 cells, it is also expressed in other cell types including NK cells, NKT cells, CTL, dendritic cells, and B cells, thus affecting immunoregulation at various levels of the immune response (60, 61). In mice, Tbet expression in B cells has been associated with class switching from the Th2-related isotypes IgG1 and IgE to the Th1-related isotype IgG2a (61). In human B cells, an upregulation of Tbet expression was caused by IL-27 (62), which has also been found responsible for increased granzyme B expression by murine naive CD8+ T cells (63). Similar to Th1 cells, Tbet expression in mature B cells is induced by IFN-γ, thereby promoting a type 1-like cell fate (64). B effector 1 cells can further promote Th1 differentiation by secreting IFN-γ and thereby also sustain their own development (65). Thus, our findings that B cells upregulate Tbet expression upon infection are in line with the observed increase in Th1 and CTL responses.
Overall, the immune response to H. pylori and the mechanisms that allow chronic colonization of the human gastric mucosa continue to be an enigma. The innate and adaptive responses do not resolve the infection, and so far, antibiotic therapy has been the only avenue to eradicate H. pylori. Several H. pylori-related factors have been characterized as contributors to immune evasion. Specifically, H. pylori-derived γ-glutamyl transpeptidase has been shown to arrest antigen-activated T cells in the G1 phase of the cell cycle (19). Furthermore, vacuolating cytotoxin (VacA) can be endocytosed into activated human primary T cells (66), where it inhibits cell proliferation and thus the clonal expansion of H. pylori antigen-specific T cells (67). For this study, we used two different H. pylori strains: SS1 and J99. Both of them carry the pathogenicity island cagPAI and vacA. However, strains SS1 and J99 carry different genetic variants of vacA, s2m2 and s1m1, respectively. Recently, we described the whole genome of the cag-positive strain V225d, cultured from a Venezuelan Piaroa Amerindian subject. Phylogenetic analysis of the host-interactive genes vacA and cagA of strain V225d shows substantial divergence of Amerindian from Old World forms and indicates a new genotype (e.g., vacA m3) with potentially decreased cytotoxin activity (38). Variation in the vacuolating activity of H. pylori strains is associated mainly with differences in the signal (s) and middle (m) regions of the vacA gene. Strains bearing the s1m1 variants have been shown to possess highest cytotoxin activity in vitro and have been associated with increased risk for gastroduodenal disease compared to the s2 or m2 strains (40). s1 and s2 VacA isoforms also differ in their ability to induce autophagosome formation by epithelial cells. More specifically, s1 VacA binds to the low-density lipoprotein receptor-related protein-1 triggering reactive oxygen species-induced autophagy (68). Compromised autophagy has been associated with increased capacity of H. pylori to survive in the intracellular environment (69). Interestingly, while both strains induced systemic Th1 responses and caused an expansion in the cytotoxic cell compartments, we found differences in their ability to persist in the stomach mucosa. The increase in cytotoxic cell populations did not correlate with reduced bacterial loads in SS1-infected pigs, suggesting a possible tolerogenic mechanism counteracting the cytotoxic response. Our reisolation results indicate strain-specific differences in long-term colonization, whereby H. pylori J99 shows diminished capacity to persist in the stomach compared to H. pylori SS1. This might be in part related to more-pronounced infiltration of CD4+FOXP3+ T cells into the gastric LP in SS1-infected pigs than in J99-infected pigs. In parallel to bacterial reisolation data, the main histopathological finding of this study is the development of large lymphoid aggregates in H. pylori-infected pigs. Interestingly, the same types of lesions were described in the stomachs of humans that were experimentally infected with H. pylori, and these lesions were still detectable, although of smaller size, after antibiotic therapy to eliminate the bacteria (70).
The exact role of CD8+ T cells and whether they contribute to the depletion of H. pylori from the gastric mucosa deserve further investigation. Findings from reisolation and histopathology suggest that CD8+ T cell responses elicited upon infection might be ineffective in the elimination of bacteria but rather contribute to tissue damage. Furthermore, the infiltration of regulatory cells found in the stomach at least partially counteracts proinflammatory responses and contributes to bacterial persistence. The elucidation of the mechanisms of persistence is warranted in future studies using pig models. The two strains had in common their ability to induced cytotoxic responses. Of notable interest is the identification of the antigenic determinants from H. pylori that are recognized by CD8+ T cells and the pathways involved in processing and presentation of H. pylori antigens through the MHC-I pathway. Here, we present the first pig model of H. pylori infection that corroborates in an experimental setting that the predominant Th1 response induced by the bacterium leads to the expansion of cytotoxic cells, including CTL and NK cells. The hallmark of the immune response to H. pylori in humans is the infiltration of Treg cells, neutrophils, and Th1 cells (35). We have been able to reproduce these findings in our pig model, showing infiltration of myeloid cells and FOXP3+ T cells, which suggests the presence of Tregs in the gastric mucosa. Furthermore, our model shows a strong systemic Th1 response followed by cytotoxic T cell responses. Similar to what occurs with H. pylori-mediated chronic gastritis in humans, bacteria are able to persist in the pig stomach but at the expense of lesion development. Recent studies have attributed a crucial role to cytotoxic cells in H. pylori-mediated pathology (22–24). While the role of CD8+ T cells in mouse models of H. pylori has been studied only with immunodeficient mice lacking CD4+ T cells (25, 26), our pig model provides a more suitable in vivo system to study cytotoxic immune responses toward H. pylori observed in humans and an ideal setting for testing new therapeutic approaches.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by NIAID Contract No. HHSN272201000056C to J.B.-R. and funds from the Nutritional Immunology and Molecular Medicine Laboratory.
We thank Richard Peek from Vanderbilt University for providing H. pylori strain SS1.
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00660-13.
REFERENCES
- 1.Cave DR. 1997. How is Helicobacter pylori transmitted? Gastroenterology 113:S9–S14 [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Blaser MJ. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28–37 [DOI] [PubMed] [Google Scholar]
- 3.Polk DB, Peek RM., Jr 2010. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer 10:403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ. 2008. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev. Res. (Phila.) 1:308–311 [DOI] [PubMed] [Google Scholar]
- 5.Vieth M, Masoud B, Meining A, Stolte M. 2000. Helicobacter pylori infection: protection against Barrett's mucosa and neoplasia? Digestion 62:225–231 [DOI] [PubMed] [Google Scholar]
- 6.Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. 2000. CagA-positive strains of Helicobacter pylori may protect against Barrett's esophagus. Am. J. Gastroenterol. 95:2206–2211 [DOI] [PubMed] [Google Scholar]
- 7.Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H, West AB, Fraumeni JF. 1998. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 58:588–590 [PubMed] [Google Scholar]
- 8.Blaser MJ, Chen Y, Reibman J. 2008. Does Helicobacter pylori protect against asthma and allergy? Gut 57:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Blaser MJ. 2007. Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med. 167:821–827 [DOI] [PubMed] [Google Scholar]
- 10.Lang L. 2007. Childhood acquisition of Helicobacter pylori linked to reduced asthma and allergy risk. Gastroenterology 133:6. 10.1053/j.gastro.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 11.McCune A, Lane A, Murray L, Harvey I, Nair P, Donovan J, Harvey R. 2003. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 15:637–640 [DOI] [PubMed] [Google Scholar]
- 12.Bassaganya-Riera J, Dominguez-Bello MG, Kronsteiner B, Carbo A, Lu P, Viladomiu M, Pedragosa M, Zhang X, Sobral BW, Mane SP, Mohapatra SK, Horne WT, Guri AJ, Groeschl M, Lopez-Velasco G, Hontecillas R. 2012. Helicobacter pylori colonization ameliorates glucose homeostasis in mice through a PPAR gamma-dependent mechanism. PLoS One 7:e50069. 10.1371/journal.pone.0050069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atherton JC, Blaser MJ. 2009. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J. Clin. Invest. 119:2475–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962–967 [PubMed] [Google Scholar]
- 15.Ricci V, Romano M, Boquet P. 2011. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J. Gastroenterol. 17:1383–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell P, Germain C, Fiori PL, Khamri W, Foster GR, Ghosh S, Lechler RI, Bamford KB, Lombardi G. 2007. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect. Immun. 75:810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Gorvel JP, Chu YT, Wu JJ, Lei HY. 2010. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One 5:e10844. 10.1371/journal.pone.0010844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Liu M, Luther J, Kao JY. 2010. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes 1:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller A, Oertli M, Arnold IC. 2011. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun. Signal. 9:25. 10.1186/1478-811X-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD. 2008. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134:491–499 [DOI] [PubMed] [Google Scholar]
- 21.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Muller A. 2011. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krauss-Etschmann S, Gruber R, Plikat K, Antoni I, Demmelmair H, Reinhardt D, Koletzko S. 2005. Increase of antigen-presenting cells in the gastric mucosa of Helicobacter pylori-infected children. Helicobacter 10:214–222 [DOI] [PubMed] [Google Scholar]
- 23.Lopes AI, Victorino RM, Palha AM, Ruivo J, Fernandes A. 2006. Mucosal lymphocyte subsets and HLA-DR antigen expression in paediatric Helicobacter pylori-associated gastritis. Clin. Exp. Immunol. 145:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueiredo Soares T, Aguiar Rocha G, Camargos Rocha AM, Correa-Oliveira R, Martins-Filho OA, Teles Carvalho AS, Souto Bittencourt PF, Afonso Oliveira C, Ferreira Nogueira AM, Alvares Cabral MM, Caetano Faria AM, Queiroz DM. 2007. Differences in peripheral blood lymphocyte phenotypes between Helicobacter pylori-positive children and adults with duodenal ulcer. Clin. Microbiol. Infect. 13:1083–1088 [DOI] [PubMed] [Google Scholar]
- 25.Fukui T, Nishio A, Okazaki K, Kasahara K, Saga K, Tanaka J, Uza N, Ueno S, Kido M, Ohashi S, Asada M, Nakase H, Watanabe N, Chiba T. 2007. Cross-primed CD8+ cytotoxic T cells induce severe Helicobacter-associated gastritis in the absence of CD4+ T cells. Helicobacter 12:486–497 [DOI] [PubMed] [Google Scholar]
- 26.Tan MP, Pedersen J, Zhan Y, Lew AM, Pearse MJ, Wijburg OL, Strugnell RA. 2008. CD8+ T cells are associated with severe gastritis in Helicobacter pylori-infected mice in the absence of CD4+ T cells. Infect. Immun. 76:1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton KA, Mefford M, Thevenot T. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456–7461 [DOI] [PubMed] [Google Scholar]
- 28.Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Muller A. 2009. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J. Immunol. 182:7085–7101 [DOI] [PubMed] [Google Scholar]
- 29.Eaton KA, Ringler SS, Krakowka S. 1998. Vaccination of gnotobiotic piglets against Helicobacter pylori. J. Infect. Dis. 178:1399–1405 [DOI] [PubMed] [Google Scholar]
- 30.Krakowka S, Eaton KA. 2002. Helicobacter pylori-specific immunoglobulin synthesis in gnotobiotic piglets: evidence for the induction of mucosal immunity in the stomach. Vet. Immunol. Immunopathol. 88:173–182 [DOI] [PubMed] [Google Scholar]
- 31.Krakowka S, Eaton KA, Rings DM. 1995. Occurrence of gastric ulcers in gnotobiotic piglets colonized by Helicobacter pylori. Infect. Immun. 63:2352–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbo A, Bassaganya-Riera J, Pedragosa M, Viladomiu M, Marathe M, Eubank S, Wendelsdorf K, Bisset K, Hoops S, Deng X, Alam M, Kronsteiner B, Mei Y, Hontecillas R. Predictive computational modeling of the mucosal immune responses during Helicobacter pylori infection. PLoS One, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga T, Shimada Y, Sato K, Takahashi K, Kikuchi Miura T, Takenouchi T, Narita T, Iwata M. 2002. Experimental Helicobacter pylori gastric infection in miniature pigs. J. Med. Microbiol. 51:238–246 [DOI] [PubMed] [Google Scholar]
- 34.Treem WR, Davis PM, Hyams JS. 1991. Suppression of gastric acid secretion by intravenous administration of famotidine in children. J. Pediatr. 118:812–816 [DOI] [PubMed] [Google Scholar]
- 35.Kusters JG, van Vliet AH, Kuipers EJ. 2006. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19:449–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176–180 [DOI] [PubMed] [Google Scholar]
- 37.Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386–1397 [DOI] [PubMed] [Google Scholar]
- 38.Mane SP, Dominguez-Bello MG, Blaser MJ, Sobral BW, Hontecillas R, Skoneczka J, Mohapatra SK, Crasta OR, Evans C, Modise T, Shallom S, Shukla M, Varon C, Megraud F, Maldonado-Contreras AL, Williams KP, Bassaganya-Riera J. 2010. Host-interactive genes in Amerindian Helicobacter pylori diverge from their Old World homologs and mediate inflammatory responses. J. Bacteriol. 192:3078–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Doorn NE, Namavar F, Sparrius M, Stoof J, van Rees EP, van Doorn LJ, Vandenbroucke-Grauls CM. 1999. Helicobacter pylori-associated gastritis in mice is host and strain specific. Infect. Immun. 67:3040–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771–17777 [DOI] [PubMed] [Google Scholar]
- 41.Hontecillas R, Bassaganya-Riera J. 2007. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J. Immunol. 178:2940–2949 [DOI] [PubMed] [Google Scholar]
- 42.Piriou-Guzylack L, Salmon H. 2008. Membrane markers of the immune cells in swine: an update. Vet. Res. 39:54. 10.1051/vetres:2008030 [DOI] [PubMed] [Google Scholar]
- 43.Werwitzke S, Tiede A, Drescher BE, Schmidt RE, Witte T. 2003. CD8beta/CD28 expression defines functionally distinct populations of peripheral blood T lymphocytes. Clin. Exp. Immunol. 133:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen LA. 1999. Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J. Leukoc. Biol. 66:753–756 [DOI] [PubMed] [Google Scholar]
- 45.Ito K, Yamaoka Y, Ota H, El-Zimaity H, Graham DY. 2008. Adherence, internalization, and persistence of Helicobacter pylori in hepatocytes. Dig. Dis. Sci. 53:2541–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YH, Wu JJ, Lei HY. 2009. The autophagic induction in Helicobacter pylori-infected macrophage. Exp. Biol. Med. (Maywood) 234:171–180 [DOI] [PubMed] [Google Scholar]
- 47.Chu YT, Wang YH, Wu JJ, Lei HY. 2010. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect. Immun. 78:4157–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh JD, Karam SM, Gordon JI. 2005. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. U. S. A. 102:5186–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N, Furukawa A, Muraoka H, Ikeda S, Sekine M, Ando N, Suzuki Y, Yamada T, Suzuki T, Eishi Y. 2008. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab. Invest. 88:664–681 [DOI] [PubMed] [Google Scholar]
- 50.Ozbek A, Ozbek E, Dursun H, Kalkan Y, Demirci T. 2010. Can Helicobacter pylori invade human gastric mucosa?: an in vivo study using electron microscopy, immunohistochemical methods, and real-time polymerase chain reaction. J. Clin. Gastroenterol. 44:416–422 [DOI] [PubMed] [Google Scholar]
- 51.Borlace GN, Keep SJ, Prodoehl MJ, Jones HF, Butler RN, Brooks DA. 2012. A role for altered phagosome maturation in the long term persistence of Helicobacter pylori infection. Am. J. Physiol. Gastrointest. Liver Physiol. 503:G169–G179 [DOI] [PubMed] [Google Scholar]
- 52.Borlace GN, Jones HF, Keep SJ, Butler RN, Brooks DA. 2011. Helicobacter pylori phagosome maturation in primary human macrophages. Gut Pathog. 3:3. 10.1186/1757-4749-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han XY, Tarrand JJ, Dickey BF, Esteva FJ. 2010. Helicobacter pylori bacteremia with sepsis syndrome. J. Clin. Microbiol. 48:4661–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugita S, Iijima T, Furuya S, Kano J, Yanaka A, Ohta K, Kojima H, Noguchi M. 2007. Gastric T-cell lymphoma with cytotoxic phenotype. Pathol. Int. 57:108–114 [DOI] [PubMed] [Google Scholar]
- 55.Azem J, Svennerholm AM, Lundin BS. 2006. B cells pulsed with Helicobacter pylori antigen efficiently activate memory CD8+ T cells from H. pylori-infected individuals. Clin. Immunol. 118:284–291 [DOI] [PubMed] [Google Scholar]
- 56.Helmin-Basa A, Michalkiewicz J, Gackowska L, Kubiszewska I, Eljaszewicz A, Mierzwa G, Bala G, Czerwionka-Szaflarska M, Prokurat A, Marszalek A. 2011. Pediatric Helicobacter pylori infection and circulating T-lymphocyte activation and differentiation. Helicobacter 16:27–35 [DOI] [PubMed] [Google Scholar]
- 57.Yun CH, Lundgren A, Azem J, Sjoling A, Holmgren J, Svennerholm AM, Lundin BS. 2005. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infect. Immun. 73:1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindgren A, Pavlovic V, Flach CF, Sjoling A, Lundin S. 2011. Interferon-gamma secretion is induced in IL-12 stimulated human NK cells by recognition of Helicobacter pylori or TLR2 ligands. Innate Immun. 17:191–203 [DOI] [PubMed] [Google Scholar]
- 59.Lindgren A, Yun CH, Lundgren A, Sjoling A, Ohman L, Svennerholm AM, Holmgren J, Lundin SB. 2010. CD8- natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J. Innate Immun. 2:294–302 [DOI] [PubMed] [Google Scholar]
- 60.Lazarevic V, Glimcher LH. 2011. T-bet in disease. Nat. Immunol. 12:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng SL, Szabo SJ, Glimcher LH. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. U. S. A. 99:5545–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. 2006. Differential effects of IL-27 on human B cell subsets. J. Immunol. 176:5890–5897 [DOI] [PubMed] [Google Scholar]
- 63.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. 2005. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 175:1686–1693 [DOI] [PubMed] [Google Scholar]
- 64.Peng SL. 2006. The T-box transcription factor T-bet in immunity and autoimmunity. Cell. Mol. Immunol. 3:87–95 [PubMed] [Google Scholar]
- 65.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. 2005. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J. Immunol. 174:6781–6790 [DOI] [PubMed] [Google Scholar]
- 66.Sewald X, Jimenez-Soto L, Haas R. PKC-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary T lymphocytes. Cell. Microbiol. 13:482–496 [DOI] [PubMed] [Google Scholar]
- 67.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. U. S. A. 101:7727–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. 2012. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12:764–777 [DOI] [PubMed] [Google Scholar]
- 69.Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang HG, Fang Y, Yu B, Zhang JY, Xie QH, Chen L, Jiang XJ, Xiao B, Zou QM, Mao XH. 2012. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy 8:1045–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham DY, Opekun AR, Osato MS, El-Zimaity HM, Lee CK, Yamaoka Y, Qureshi WA, Cadoz M, Monath TP. 2004. Challenge model for Helicobacter pylori infection in human volunteers. Gut 53:1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.