Abstract
Salmonella spp. are able to form biofilms on abiotic and biotic surfaces. In vivo studies in our laboratory have shown that Salmonella can form biofilms on the surfaces of cholesterol gallstones in the gallbladders of mice and human carriers. Biofilm formation on gallstones has been demonstrated to be a mechanism of persistence. The purpose of this work was to identify and evaluate Salmonella sp. cholesterol-dependent biofilm factors. Differential gene expression analysis between biofilms on glass or cholesterol-coated surfaces and subsequent quantitative real-time PCR (qRT-PCR) revealed that type 1 fimbria structural genes and a gene encoding a putative outer membrane protein (ycfR) were specifically upregulated in Salmonella enterica serovar Typhimurium biofilms grown on cholesterol-coated surfaces. Spatiotemporal expression of ycfR and FimA verified their regulation during biofilm development on cholesterol-coated surfaces. Surprisingly, confocal and scanning electron microscopy demonstrated that a mutant of type 1 fimbria structural genes (ΔfimAICDHF) and a ycfR mutant showed increased biofilm formation on cholesterol-coated surfaces. In vivo experiments using Nramp1+/+ mice harboring gallstones showed that only the ΔycfR mutant formed extensive biofilms on mouse gallstones at 7 and 21 days postinfection; ΔfimAICDHF was not observed on gallstone surfaces after the 7-day-postinfection time point. These data suggest that in Salmonella spp., wild-type type 1 fimbriae are important for attachment to and/or persistence on gallstones at later points of chronic infection, whereas YcfR may represent a specific potential natural inhibitor of initial biofilm formation on gallstones.
INTRODUCTION
Typhoid, or enteric fever, caused primarily by Salmonella enterica serovar Typhi, is a global human-specific disease that is responsible for an estimated 21 million new infections annually, resulting in more than 200,000 deaths worldwide (1). It is an important health problem in developing countries and poses a significant risk to travelers. After ingestion through contaminated water or food, bacteria cross the intestinal epithelial barrier, are phagocytosed by macrophages, and spread systemically, producing acute disease (2–5) with life-threatening complications, including intestinal perforation, septicemia, and meningitis (6). During this systemic infection, S. Typhi can reach the gallbladder from the liver and establish an acute infection with inflammation (cholecystitis) or chronically persist in the organ. It is estimated that between 3 and 5% of typhoid fever patients become chronic carriers, with the gallbladder the primary site of carriage (7, 8). As clinical evidence, inflammation of the gallbladder and sonographic gallbladder abnormalities have been reported in acute and chronic typhoid fever patients (6, 9–12).
Because S. Typhi is a human-specific pathogen, these carriers serve as a critical reservoir for further spread of the disease through bacterial shedding in feces, which is a sporadic and intermittent event (9, 13). Chronic typhoid infections can persist for decades, and although highly contagious, they are typically asymptomatic (14, 15). Approximately 25% of asymptomatic carriers have no history of typhoid fever (16). These factors make the carrier state difficult to confirm and an understudied area of human health and research.
Particularly in areas of high endemicity, the carrier state is linked to the presence of gallstones, as approximately 80 to 90% of chronically infected carriers have this gallbladder abnormality (17–19). Gallstones are primarily composed of cholesterol (up to 70 to 100% cholesterol), although calcium bilirubinate predominates in certain parts of the world (20). Gallstone formation depends on a combination of factors, including the supersaturation of bile with cholesterol, alteration of gallbladder contractility, and hypersecretion of mucin. Patient factors that predispose for gallstone formation include age, obesity, nutrition, female gender, unknown genetic determinants, and chronic bacterial colonization of the gallbladder (21–24).
The increasing incidence of antibiotic-resistant bacteria colonizing chronic typhoid patients exacerbates morbidity and mortality (19, 25–27). In addition, chronic carriers have an approximately 8- to 14-fold-increased risk of developing gallbladder carcinoma and approximately 150-fold-increased risk of developing hepatobiliary carcinoma compared to noncarriers (28–31). Compounding the problem with S. Typhi carriage, nontyphoidal salmonellae have also recently been demonstrated to establish chronic human infections (32). This impact on human health, combined with the high incidence of typhoid fever in many parts of the world and the poor efficacy of current vaccines, highlights the importance of understanding the mechanisms involved in typhoid carriage. To date, removal of the gallbladder (cholecystectomy) is the most common treatment for chronic typhoid carriers and those with gallbladder abnormalities; however, it is both costly and invasive and does not guarantee elimination of the carrier state (33), since additional foci of infection can persist in other locations, including the biliary tree, mesenteric lymph nodes, and liver (34–38). Therefore, alternative treatments are needed to eradicate the gallbladder carriage state.
The known clinical observations of carriers regarding recalcitrance to antibiotics, absence of symptoms, confinement to an organ with shedding, and organ removal as the most successful therapy are consistent with biofilm-related disease (19, 39, 40). Biofilms are communities of microorganisms that adhere to each other and to inert or live substrates. They are typically encased in a self-initiated extracellular matrix composed of exopolysaccharides, proteins, and nucleic acids. Biofilms are associated with many chronic and acute human infections (41–43). The biofilm state can alter the host-pathogen interaction and is often associated with a reduction of the host inflammatory response that has been referred to as “silent chronic inflammation” (44). We have shown that Salmonella can form biofilms on the surfaces of cholesterol gallstones in the gallbladders of mouse and human carriers (45) and on the gallbladder epithelium of mouse carriers (46). This biofilm formation has been demonstrated to be a mechanism of persistence and chronic colonization in the gallbladder (45).
Previous studies have shown that initial interactions between Salmonella and gallstones is likely mediated by OmpC and the flagellar subunit FliC independent of motility (47). The hallmark of a mature biofilm is the development of a self-initiated extracellular matrix. Reports have shown that Salmonella sp. biofilms contain extracellular polymeric substances, including cellulose, colanic acid, curli fimbriae, O-antigen capsule, biofilm-related proteins, and nucleic acids (48–55). Cellulose and colanic acid are important for Salmonella biofilms on abiotic surfaces, HEp-2 cells, and chicken intestinal tissue (49, 56) but are not required for biofilms on human gallstones in vitro (56, 57). These data suggest that the contributions of bacterial factors vary depending on the binding substrates.
In this study, we identified Salmonella genes specifically regulated during biofilm development on cholesterol-coated surfaces using an in vitro flow system that mimics the gallbladder and gallstone environment. The identified genes encode surface (type 1 fimbriae) or outer membrane (YcfR) proteins. We demonstrated in vitro and in vivo that these bacterial surface factors affect the development of biofilms on cholesterol gallstones, unveiling novel mechanisms to modulate biofilms on this specific substrate. By understanding the basis of this biofilm-mediated carriage, it may be possible to identify effective strategies to prevent or eliminate Salmonella carriage and thus the human-to-human spread of typhoid fever.
MATERIALS AND METHODS
Ethics statement.
Mice were housed and used in strict accordance with guidelines established by the Ohio State University (OSU) Institutional Animal Care and Use Committee (IACUC), and all efforts were made to minimize animal suffering. The work performed in this study was approved by the OSU IACUC. The Ohio State University Animal Care and Use Program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The protocol identification number is 2009A0057. All research activities conformed to the statutes of the Animal Welfare Act and the guidelines of the Public Health Service as issued in the Guide for the Care and Use of Laboratory Animals (revised 1996).
Bacterial strains and growth conditions.
Salmonella enterica serovar Typhimurium and S. Typhi behaved similarly in biofilm assays previously conducted in our laboratory. Wild-type S. Typhimurium ATCC 14028 (JSG210) and its derivatives were used in these studies. The strains used are listed in Table 1. All cultures were grown in Luria-Bertani (LB) broth supplemented or not with 3% ox bile (Sigma-Aldrich, St. Louis, MO) at 37°C with aeration. Antibiotics, when needed, were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 45 μg/ml; chloramphenicol, 25 μg/ml.
Table 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Genotype or relevant phenotype | Reference or source |
|---|---|---|
| Strains | ||
| JSG210 | Wild-type S. Typhimurium ATCC 14028 | ATCC |
| JSG3392 | ΔfimAICDHF | 67 |
| JSG3119 | ΔycfR | This study |
| JSG3393 | ΔfimAICDHF ΔycfR | This study |
| JSG3541 | Wild-type S. Typhimurium ATCC 14028 pWSK29 | This study |
| JSG3529 | Wild-type S. Typhimurium ATCC 14028 pWSK129 | This study |
| JSG3542 | ΔfimAICDHF pWSK29 | This study |
| JSG3546 | ΔfimAICDHF pGGE1 | This study |
| JSG3530 | ΔycfR pWSK129 | This study |
| JSG3532 | ΔycfR pGGE2 | This study |
| JSG3526 | Wild-type S. Typhimurium ATCC 14028 pGGE3 | This study |
| Plasmids | ||
| pWSK29 | 91 | |
| pWSK129 | 91 | |
| pFPV25 | 62 | |
| pAZ24 | pET16b (fimAICDHF) | 61 |
| pGGE1 | pWSK29 (fimAICDHF) | This study |
| pGGE2 | pWSK129 (ycfR) | This study |
| pGGE3 | pWSK129 (ycfR/GFP) | This study |
Biofilm growth on microtiter plates.
Glass bottom 12-well plates (14-mm-diameter microwells; glass; no 1.5; MatTek Corp., MA) uncoated or coated by evaporation with 4 mg of cholesterol (diluted in ether; anhydrous; J. T. Baker, NJ) were inoculated with 2 × 108 bacteria in 2 ml of LB bile (3%). The plates were incubated for 24 h or 96 h at 37°C in a GyroMini nutating mixer (LabNet International, Inc., NJ) at 24 rpm. The use of cholesterol-coated surfaces eliminates dependence on human gallstones and replicates the gallstone surface (58).
Flowthrough system assays.
To closely mimic the gallbladder and gallstone environment, a flowthrough system was used for gene expression analysis and biofilm observation by microscopy. Single-channel chambers (24-mm length by 8-mm width by 4-mm height) with glass coverslips (Stovall Life Science Inc., IA) with or without cholesterol coating (8 mg) were inoculated with 2 × 108 exponentially growing S. Typhimurium bacteria. The bacteria were allowed to adhere for 2 h before starting the flow with LB bile (3%) at 270 μl/min for 24 or 72 h.
Crystal violet assays.
Biofilms attached to the microtiter wells or chamber coverslips were washed, heat fixed for 1 h at 60°C, and stained with 0.25% crystal violet for 5 min. After four washes with 1× phosphate-buffered saline (PBS), acetic acid at 33% was used to extract the dye. Determination of the amount of dye retained by the biofilms was performed at an optical density at 570 nm (OD570) in a SpectraMax Spectrophotometer with SoftMaxPro software. Experiments were performed in triplicate. To compensate for background absorbance, values from noninoculated glass and cholesterol-coated coverslips were averaged and subtracted.
Differential gene expression analysis.
A flowthrough system (as described above) was used for gene expression analysis. After 24 h of incubation with flow, planktonic (from the flowthrough) and biofilm (from the flow chamber) cells were collected. After three consecutive washes with 1× PBS, RNA was immediately extracted with an RNeasy Plus Mini Kit (Qiagen), reverse transcribed to cDNA by using random hexamers and SuperScript III following the manufacturer's guidelines (Invitrogen, CA), and submitted to Roche NimbleGen, Inc., for microarray analysis (S. Typhimurium LT2 array). Two biological replicates were used. Differential gene expression analysis was performed using ArrayStar v3.0. To validate the results obtained in the microarray analysis, quantitative real-time PCR (qRT-PCR) was performed using RNA extracted from biofilm cells (grown on glass and cholesterol) after 24 and 72 h of flow. cDNA (20 ng) was added to IQ SYBR green PCR master mix (Bio-Rad) containing 1 μM primers specific for fimC, ycfR, or rpoB (housekeeping gene) (Table 2). Samples were run in triplicate and repeated three times using the Bio-Rad CFX96 iCycler apparatus. Relative copy numbers were calculated according to the 2−ΔΔCT method (59).
Table 2.
Oligonucleotide primers used in the study
| Name | Primer sequence (5′–3′) | Purpose |
|---|---|---|
| JG2081 | ACGGTCGCGTATGTCCTATC | rpoB qRT-PCR |
| JG2082 | GAGTTCGCCTGAGCGATAAC | rpoB qRT-PCR |
| JG1995 | GTTCTGCAACTGGCGATTCT | fimC qRT-PCR |
| JG1996 | GGAATTTGCATGTCGCTTTT | fimC qRT-PCR |
| JG2025 | TAAAAACCCTCATCGCTGCT | ycfR qRT-PCR |
| JG2026 | GGGCCGGTAACAGAGGTAAT | ycfR qRT-PCR |
| JG2055 | ACACCCATTCATCTGCTAAAGGTCATCACTATGAAAAGTGTAGGCTGGAGCTGCTTC | λ-Red deletion of ycfR |
| JG2056 | AATCGCAGCGGCATTAATGAGGGTTAATGCTTACTTATAGATCATATGAATATCCTCCTTAG | λ-Red deletion of ycfR |
| JG2526 | GCTCTAGAAGGAGGAATTCACCATGAAAAACGTAAAAACCCTC | Cloning of ycfR in pWSK129 |
| JG2527 | GGG GTA CCT TAC TTA TAG ATT ACC GCC GTA CCG TG | Cloning of ycfR in pWSK129 |
| JG2512 | GGGGTACCGATGCCGTTGTACCTGTTTAAAGAG | Cloning ycfR promoter in pFPV25 |
| JG2513 | GCTCTAGAGCAGATGAATGGGTGTCGTAAGCAT | Cloning ycfR promoter in pFPV25 |
Generation of mutants and cloning procedures.
Mutation of the ycfR gene was performed by using the λ-red mutagenesis method (60) with primers JG2055 and JG2056 (Table 2). To create a complemented strain of the ycfR mutant, the ycfR gene was cloned in pWSK129 to create pGGE1 by using the XbaI and KpnI restriction sites and the primers JG2526 and JG2527 (Table 2). To obtain a complemented strain of the fimAICDHF mutant, the fimAICDHF cluster of genes was excised from the plasmid pAZ24 (61) and cloned into pWSK29 by using the restriction sites XbaI and BamHI. To create a reporter strain for ycfR expression, the promoter of the gene was cloned in pFPV25 upstream of a promoterless gfp cassette (62) by using the restriction sites KpnI and XbaI and the primers JG2512 and JG2513 (Table 2). The pycfR-gfp fragment was then subcloned in pWSK129 by using SacI and HindIII restriction sites to create pGGE3.
Biofilm treatment with DNase I, proteinase, and cellulase.
Glass bottom 12-well plates (14-mm-diameter microwells; glass; no 1.5; MatTek Corp., MA) with or without 4 mg of cholesterol coating were inoculated with 2 × 108 bacteria and incubated for 24 h and 96 h at 37°C in a GyroMini nutating mixer (LabNet International, Inc.) at 24 rpm. DNase I (56-unit), proteinase (0.53-mg), and cellulase (50-unit) solutions were added to the established biofilms, and the plates were then incubated for 16 h at 37°C in a GyroMini nutating mixer at 24 rpm. Experiments were repeated twice.
FimA and CsgA detection by immunofluorescence.
Glass bottom 12-well plates (14-mm-diameter microwells; glass; no 1.5; MatTek Corp., MA) with or without 4 mg of cholesterol coating were inoculated with 2 × 108 bacteria and incubated for 24 h and 96 h at 37°C in a GyroMini nutating mixer at 24 rpm. The bacteria were stained with FilmTracer calcein red-orange (Invitrogen); fixed with 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4, for 15 min at room temperature; rinsed with water; blocked in 5% bovine serum albumin (BSA) in Tris-buffered NaCl solution with Tween 20, pH 7.6, for 1 h at room temperature; and incubated with preabsorbed rabbit polyclonal anti-FimA (1:500; provided by A. Baumler) or rabbit polyclonal anti-CsgA for 1 h at room temperature (1:500; provided by M. Chapman). Incubation with the secondary antibody Alexa Fluor 488-conjugated goat anti-rabbit (1:1,000; Invitrogen) was also performed for 1 h at room temperature. Mounted slides were observed using an Olympus Fluoview FV10.1. Experiments were repeated two times.
Mouse infections.
Because S. Typhi is a human-restricted pathogen, in vivo studies of S. Typhi pathogenesis typically involve a mouse model of infection using S. Typhimurium. The pathological features and the course of mouse infection with S. Typhimurium are similar to those of human infection with S. Typhi (63). Naturally resistant Nramp1+/+ 129X1/SvJ mice (n = 168) (Jackson Laboratory, ME) were fed with a lithogenic diet (1% cholesterol and 0.5% cholic acid; Sigma) (n = 84) or normal chow (Harlan Laboratory, IN) (n = 84). Nramp1 is a macrophage-associated protein that is a critical factor in controlling the replication of intracellular bacteria (64). It plays this role by stimulating expression of lipocalin 2, which in turn scavenges iron-loaded bacterial siderophores and mediates iron efflux from macrophages (65). After 9 weeks, mice were infected intraperitoneally (n = 120) with 104 S. Typhimurium bacteria or left as uninfected controls (n = 48) and sacrificed at 7, 21, and 60 days postinfection (p.i.). Spleen, liver, pancreas, gallbladder, bile, gallstones, and feces were homogenized and/or diluted for bacterial enumeration using Salmonella-Shigella agar (Difco). Gallstones were washed with 1× PBS and then processed for scanning electron microscopy (SEM) as described below.
Confocal microscopy.
Flowthrough chambers were washed once with 1× PBS and incubated with LIVE/DEAD stain (Invitrogen, CA) for 15 min at room temperature under dark conditions. Biofilms from strains harboring pGGE3 were stained with FilmTracer calcein red-orange (Invitrogen). The chambers were then washed twice with 1× PBS; fixed with 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4, for 15 min at room temperature; rinsed with water; and observed using an Olympus Fluoview FV10.1.
Scanning electron microscopy.
Biofilms on glass, cholesterol, or mouse gallstones were fixed overnight at 4°C with 2.5% glutaraldehyde in 0.1 M phosphate buffer-0.1 M sucrose (pH 7.4), rinsed twice with 0.1 M phosphate buffer, and dehydrated by the addition of solutions of ethanol in a graded series as follows: 35%, 50%, 70%, 80%, 95%, and 100%. Samples were chemically dried with consecutive washes of 25%, 50%, 75%, and 100% hexamethyldisilazane (Ted Pella, CA). Samples were dried overnight in a fume hood, mounted on aluminum stubs, and sputter coated with gold for observation using an FEI Nova NanoSEM at the OSU Campus Microscopy and Imaging Facility (CMIF).
Microarray data accession number.
The microarray data associated with this paper can be found at the GEO repository (GSE48604).
RESULTS
Cholesterol-coated surfaces enhance biofilm formation.
Previous assays using microcentrifuge tubes coated with cholesterol showed that in static cultures this surface mimics gallstones and enhances biofilm formation (58). To validate our model in a flowthrough system, the capacity of S. Typhimurium to form a biofilm was assessed on glass, cholesterol-coated glass surfaces, and glass coated with powdered human gallstones. From the biofilm assays, biofilms formed on cholesterol and powdered gallstones were significantly more robust than those on glass and were similar to one another (Fig. 1A). SEM observations of biofilms on glass and cholesterol-coated surfaces corroborated the results obtained with crystal violet (Fig. 1B). Thus, flow chambers coated with cholesterol represent a good model for mimicking human gallstones. They also allow standardization of the procedures while decreasing the variability that occurs when using intact human gallstones.
Fig 1.
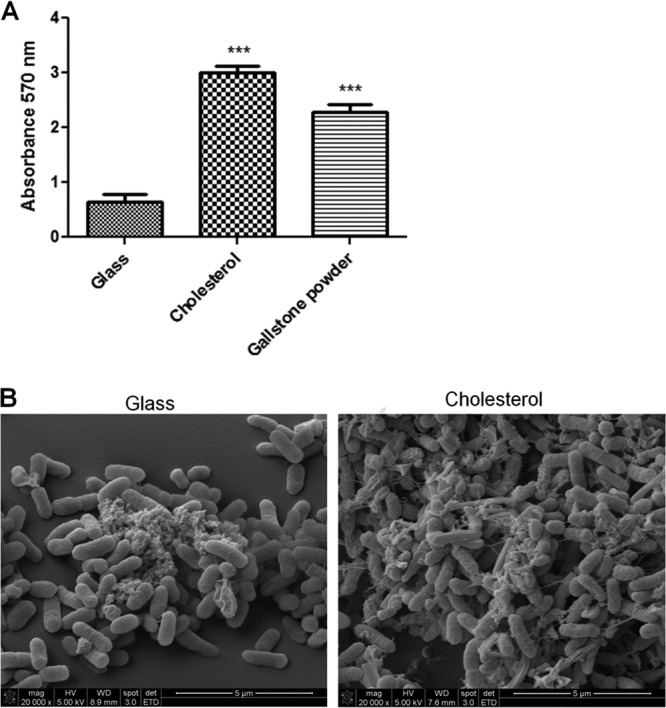
Cholesterol-coated surfaces enhance biofilm formation. (A) Biofilm formation of wild-type S. Typhimurium determined by the crystal violet staining method after 24 h of flowthrough in the presence of bile. Biofilm formation was significantly enhanced on cholesterol-coated surfaces compared with glass surfaces. Similar results were observed using gallstone powder as a coating surface. Experiments were performed in triplicate and repeated four times. Student's t test was used to compare biofilms on glass with those on other surfaces (***, P < 0.001). The error bars indicate standard deviations. (B) Representative SEM images of S. Typhimurium biofilms on glass and cholesterol-coated surfaces that corroborate the crystal violet results.
Differential gene expression between planktonic and biofilm bacterial cells (with and without cholesterol-coated surfaces).
To determine if differential gene regulation occurred during biofilm formation on cholesterol-coated surfaces, we performed microarray analysis on biofilm cells propagated on glass or cholesterol-coated glass or from planktonic cells. Using a 2-fold-change cutoff, comparison of planktonic versus biofilms cells grown on glass showed 1,100 differentially regulated genes, whereas comparison of planktonic versus biofilm cells grown on cholesterol-coated glass showed 935 differentially regulated genes. To summarize the most important findings, csgG (encoding a factor involved in the production of curli fimbriae) was found to be upregulated in biofilms on both surfaces (a 6-fold increase). Additionally, greater than 20-fold upregulation of the followings operons was observed in biofilms regardless of the presence of cholesterol: yciGFE-katN, tdcGECBA (propionate and amino acid metabolism), the eut operon (ethanolamine utilization), the pdu operon (propanediol utilization), yjiHGE, hydrogenase genes, and PTS permease genes. Further analysis of these genes was not the focus of this study, but these data are important for future work regarding Salmonella biofilm development. Notably, a comparison between the two planktonic populations (grown in chambers with a glass surface or cholesterol-coated glass) did not show any genes that were differentially regulated in either replicate.
Type 1 fimbria structural genes (fimAICD) and ycfR were specifically upregulated during biofilm development on cholesterol-coated surfaces.
Because we observed increased biofilm formation on cholesterol-coated surfaces 24 h postinoculation, we hypothesized that there was altered bacterial-gene regulation when biofilms were formed on these gallstone-mimicking surfaces. To examine this, differential gene expression analysis was performed using microarrays. Interestingly, only 7 genes of S. Typhimurium were differentially expressed (all upregulated) when biofilms on glass were compared to biofilms on cholesterol-coated glass (Fig. 2). However, only 5 of these genes were activated in both replicates: fimC (4-fold), fimA (3.9-fold), fimI (3.7-fold), ycfR (3.4-fold), and fimD (2.1-fold). Four of these genes (fimA, fimI, fimC, and fimD) belong to the type 1 fimbria operon. The function of ycfR is unknown in Salmonella, but it is a stress-related gene in Escherichia coli K-12 (66). ycfR was also upregulated when planktonic cells were compared to biofilm cells on cholesterol, but this was not the case when planktonic cells were compared to biofilm cells on glass. These results were validated by qRT-PCR, which showed upregulation of fimC and ycfR in biofilms on cholesterol-coated glass, not only after 24 h of flow (microarray conditions), but also after 72 h of flow (Fig. 3).
Fig 2.
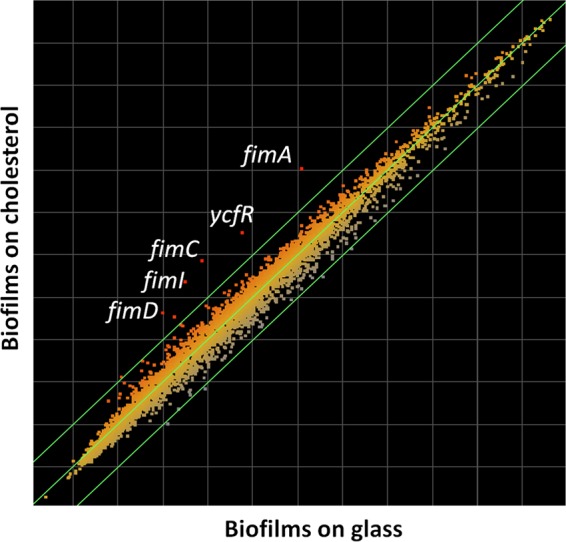
Type 1 fimbria genes (fimAICD) and ycfR were induced during biofilm development on cholesterol-coated surfaces. Shown is a scatter plot of differential gene expression between biofilms on glass (x axis) and biofilms on cholesterol-coated surfaces (y axis). The outermost diagonal green lines represent the cutoff (2-fold change). Four genes of the type I fimbria operon and ycfR were upregulated in both replicates.
Fig 3.
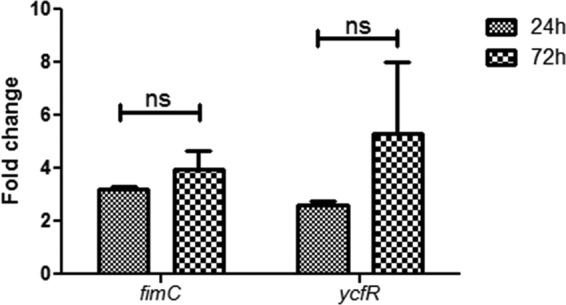
Quantitative real-time PCR validated the increased expression of fimC and ycfR during biofilm formation on cholesterol-coated surfaces. Shown is the fold change of fimC and ycfR expression between biofilms on glass versus biofilms on cholesterol-coated surfaces at 24 and 72 h postinoculation under flowthrough conditions. Experiments were performed in triplicate and repeated four times. Statistical significance was determined using Student's t test (*, P < 0.05; ns, not significant). The error bars indicate standard deviations.
Mutations of type 1 fimbriae and ycfR increased biofilm formation on cholesterol-coated surfaces.
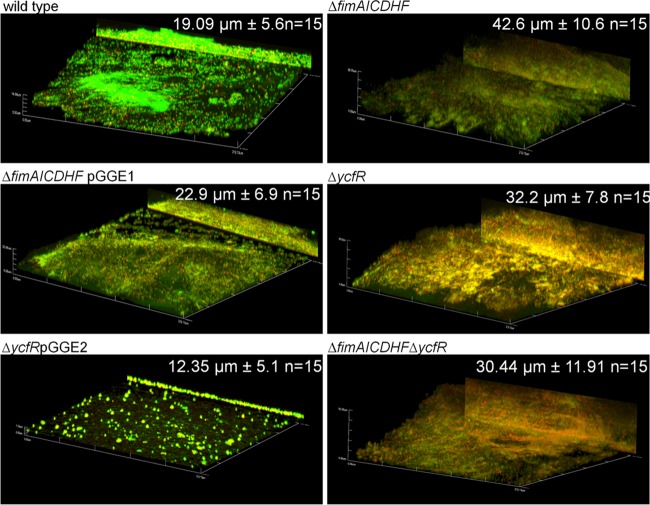
To determine the consequences of the upregulation of type I fimbria-encoding genes and ycfR in biofilms formed on cholesterol-coated surfaces, we utilized a mutant lacking the structural genes of type 1 fimbriae (fimAICDHF) (67) and a ycfR mutant in biofilm assays. Interestingly, biofilm formation on cholesterol-coated surfaces was enhanced compared with that of the wild-type strain at both 24 h and 96 h postinoculation, as determined by crystal violet assays and SEM imaging (Fig. 4 and data not shown). The complemented strains (ΔfimAICDHFpGGE1 and ΔycfRpGGE2) restored the biofilm capacities to wild-type levels (Fig. 4). Confocal imaging demonstrated that these biofilms had more biomass after 24 and 72 h of flow and were thicker (only after 24 h of flow) than wild-type biofilms. The complemented strains restored the biomass to wild-type levels (Fig. 5; see Fig S1 in the supplemental material). Morphologically, the wild-type biofilms looked patchy in comparison with the mutants, which showed more coverage of cholesterol-coated surfaces. In the case of the ycfR mutant, the bacterial membrane integrity was compromised because red-stained (LIVE/DEAD stain) bacterial cells were frequently observed (Fig. 5; see Fig S1 in the supplemental material).
Fig 4.
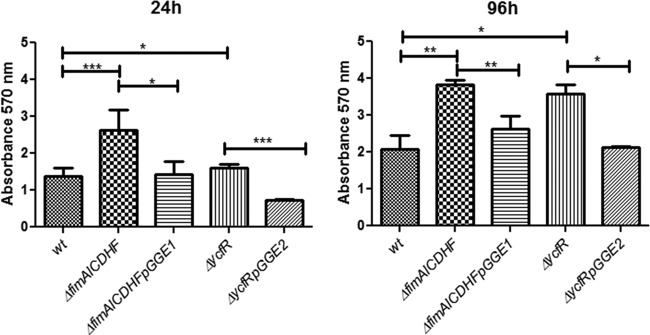
Type 1 fimbria and ycfR mutants showed enhanced biofilm formation on cholesterol-coated surfaces in vitro. Shown is biofilm capacity screening of the wild type (wt), mutants, and complemented strains in glass microtiter plates coated with cholesterol in the presence of bile at 24 and 96 h postinoculation. All strains harbor the empty vector (pWSK29 or pWSK129) or the respective complementation vector (pGGE1 and pGGE2 for fimAICDHF and ycfR, respectively). Biofilm formation was determined by the crystal violet staining method. Experiments were performed in triplicate and repeated three times. Both ΔfimAICDHF and ΔycfR mutants showed increased biofilm formation compared to the wild type at both 24 and 96 h. Student's t test was used to determine significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The error bars indicate standard deviations.
Fig 5.
Biofilms from type 1 fimbria and ycfR mutants are thicker on cholesterol-coated surfaces in vitro. Shown are representative confocal images of biofilms on cholesterol-coated surfaces produced by the wild-type, mutant, and complemented strains after 24 h of flow in the presence of bile. The biofilms were stained with LIVE/DEAD stain (Invitrogen), fixed with 4% paraformaldehyde, and observed by confocal microscopy. All strains harbor the empty vector (pWSK29 or pWSK129) or the respective complementation vector (pGGE1 and pGGE2 for fimAICDHF and ycfR, respectively). All images are at a magnification of ×40, and the y projection of the image is shown on the right. All mutants showed thicker biofilms; the average thickness (n = 15) is shown in the right corner. The ΔycfR strain also showed increased cell damage/death (increased red staining). The complemented strains demonstrated reduction of biofilm thickness and biomass.
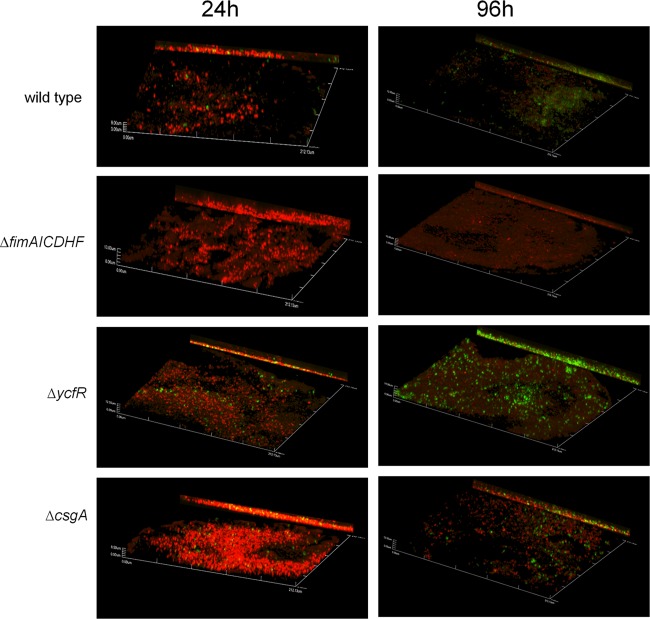
Expression of FimA and ycfR increased during biofilm maturation in vitro.
Type I fimbrial-gene expression was increased in biofilms propagated on cholesterol-coated surfaces in comparison with those on glass surfaces. To elucidate if FimA subunits are actually expressed on the surfaces of bacterial biofilms on cholesterol, we examined FimA by immunofluorescence microscopy. FimA was expressed in biofilms formed by the wild type at both 24 and 96 h. At the latest time point, FimA expression was dramatically increased (Fig. 6). These data corroborate the observed transcriptional upregulation of fimbrial genes in biofilms on cholesterol-coated surfaces. The ycfR mutant showed higher expression of FimA than the wild type in biofilms on cholesterol-coated surfaces at 24 and 96 h (Fig. 6), while no FimA expression was detected in a fimAICDHF mutant. A csgA mutant not only formed robust biofilms but also showed FimA expression similar to that of the wild type.
Fig 6.
FimA expression during biofilm formation on cholesterol-coated surfaces. The representative confocal images of biofilms on cholesterol-coated surfaces show FimA expression by the wild type and mutants. At 24 and 96 h of incubation in the presence of bile, biofilms were stained with FilmTracer calcein red-orange biofilm stain (Invitrogen), fixed, and incubated with primary antibody to FimA. The secondary antibody was Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen; green). All images are at a magnification of ×40, and the y projection of the image is shown on the right.
Because we lack the resource of anti-YcfR antibodies, spatiotemporal expression of ycfR in biofilms was monitored with a wild-type strain harboring a low-copy-number plasmid containing a ycfR::gfp fusion. The expression of ycfR increased over time, with the greatest expression observed at the last time point (72 h) postinoculation (Fig. 7). This shows that ycfR induction is not only a characteristic of early (24-h) biofilms. In addition, ycfR expression in the biofilm was not observed to be restricted to a particular site in the biofilm architecture.
Fig 7.
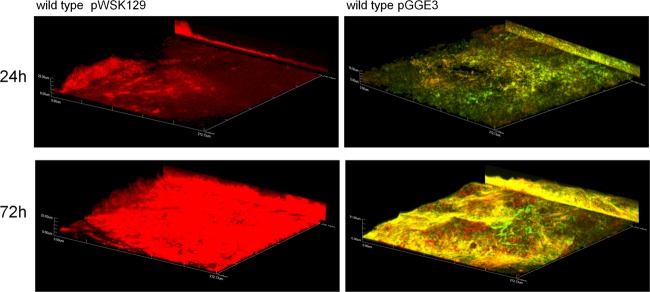
Expression of ycfR increases during biofilm maturation. Shown are representative confocal images of biofilms produced by wild-type S. Typhimurium harboring or not harboring the plasmid pGGE3 (ycfR::gfp). Biofilms were propagated with flow in the presence of bile for 24 and 72 h. ycfR expression increased, along with biofilm maturation. The biofilms were stained with FilmTracer calcein red-orange biofilm stain (Invitrogen) to observe non-green fluorescent protein (GFP)-expressing bacteria. All images are at a magnification of ×40, and the y projection of the image is shown on the right.
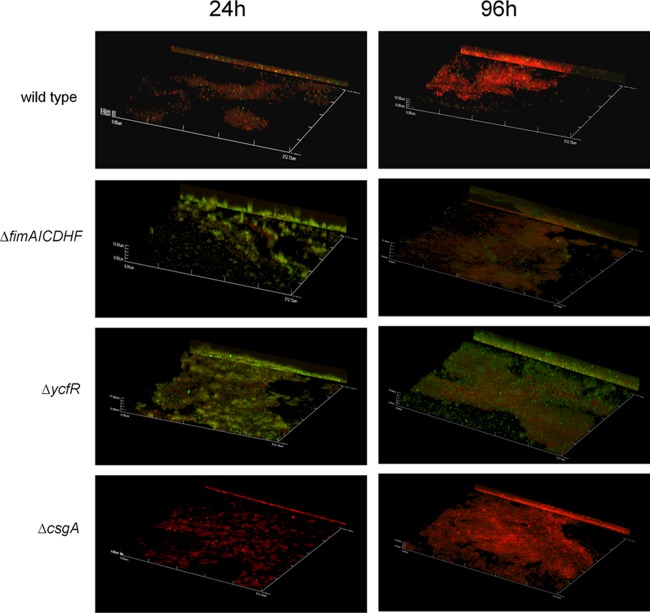
Increased curli expression is observed in biofilms of fimAICDHF and ycfR mutants on cholesterol-coated surfaces.
To investigate the increased biofilm formation of type 1 fimbriae and ycfR mutants on cholesterol-coated surfaces, we tested commonly reported components of biofilms (DNA, proteins, and cellulose) by enzymatic treatment of established biofilms with DNase I (56 units), proteinase (0.53 mg), and cellulase (50 units). Only proteinase treatments significantly affected the biofilm formation of type 1 fimbriae and ycfR mutants (see Fig S2 in the supplemental material), indicating the phenotype could be due to proteins expressed on the surface. Viability assays confirmed that the enzymes did not simply kill the bacteria, indicating that any changes were due solely to effects on the biofilm itself (data not shown). Considering that curli fimbriae are proteins widely reported to be a component of the exopolymeric substance (EPS) of Salmonella biofilms, we examined these surface proteins. Immunofluorescence staining with CsgA antibodies demonstrated that both type 1 fimbriae and ycfR mutants have increased expression of CsgA in vitro (Fig. 8).
Fig 8.
CsgA expression during biofilm formation on cholesterol-coated surfaces. The representative confocal images of biofilms on cholesterol-coated surfaces show CsgA expression by the wild type and mutants. After 24 and 96 h of incubation in the presence of bile, the biofilms were stained with FilmTracer calcein red-orange biofilm stain (Invitrogen), fixed, and incubated with primary antibody to CsgA. The secondary antibody was Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen; green). All images are at a magnification of ×40, and the y projection of the image is shown on the right.
In vivo, type 1 fimbriae and ycfR mutants showed different biofilm capacities, depending on the time of infection.
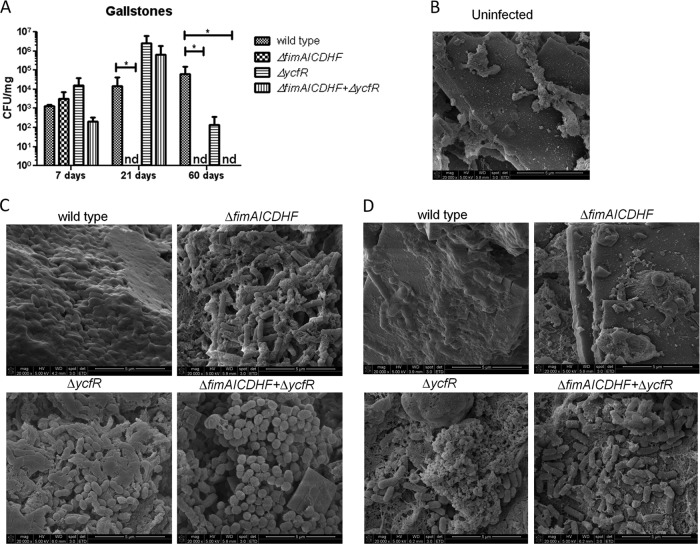
In vitro observations of increased biofilm formation by type 1 fimbriae and ycfR mutants on cholesterol-coated surfaces were examined in the mouse model of chronic typhoid infection (129X1/SvJ). These mice (Nramp1+/+) are resistant to Salmonella infection and have been previously used for modeling chronic Salmonella infection and persistence in a host (35, 45). Although fimAICDHF and ycfR mutants (including a double mutant) colonized the gallstones at early time points (7 days p.i.), no fimAICDHF mutant bacteria were recovered from the gallstones at 21 and 60 days p.i. (Fig. 9A). This phenotype is independent of the ability to colonize the gallbladder and bile, because bacteria were present at these locations at the later time points (see Fig S3 in the supplemental material). Although the ycfR mutant also colonized the gallstones at early time points (7 days p.i.), it was recovered from mouse gallstones in higher numbers than the wild type through 21 days p.i. and was present, though in significantly lower numbers than the wild type, at 60 days p.i. (Fig. 9A). The double mutant, interestingly, mimicked the ycfR mutant at 21 days p.i. (increased numbers of bacteria) but the fimAICDHF mutant at 60 days p.i. (no bacteria were recovered).
Fig 9.
In vivo colonization and biofilm formation on mouse gallstones of S. Typhimurium wild type and mutants. (A) CFU enumeration of wild-type S. Typhimurium and mutants on mouse gallstones 7, 21, and 60 days p.i. The type 1 fimbria mutant was recovered only 7 days p.i., whereas the ycfR mutant was recovered at every time point (increased at 21 days p.i. and decreased at 60 days p.i. relative to the wild type). The double mutant mimicked the ycfR mutant at 21 days p.i. and the fimAICDHF mutant at 60 days p.i. Student's t test was used to determine significant differences (*, P < 0.05). nd, not detected. The error bars indicate standard deviations. (B) SEM micrograph of an uninfected gallstone. (C) SEM micrographs of wild-type and mutant biofilms on gallstones at 7 days p.i. No difference in biofilm-forming ability was observed among the strains, though some morphological differences were noted. (D) SEM micrographs of biofilms on gallstones at 21 days p.i. The ycfR and double mutants clearly showed increased biofilm formation, whereas the type 1 fimbria mutant was not found attached to the surfaces of gallstones. Morphological differences were noted among the strains.
These results were corroborated by SEM imaging of biofilms on mouse gallstones. All the strains were observed to form robust biofilms on the surfaces of cholesterol gallstones at 7 days p.i. (Fig. 9C). However, at 21 days p.i., the type 1 fimbria mutant did not form any microcolonies or biofilms on gallstones (Fig. 9D). Thus, inconsistent with the in vitro phenotype, the type 1 fimbria mutant did not develop robust biofilms in vivo on cholesterol-coated surfaces for extended times postinfection. At 21 days p.i., the ycfR mutant formed more robust biofilms than the wild type (Fig. 9D). Thus, in vivo, the ycfR mutant corroborates its in vitro phenotype of increased biofilm formation on cholesterol-coated surfaces, but only up to 21 days p.i. Morphological differences were noted, depending on the strain and the time of infection. While the wild-type biofilms showed compact bacterium-bacterium association, bacteria in biofilms formed by the mutants were less tightly associated (Fig. 9C and D). In the case of the ycfR mutant and the double mutant of type 1 fimbriae and ycfR, a coccobacillus shape was evident in biofilms formed at 7 days p.i. and defined bacilli in biofilms formed at 21 days p.i. (Fig. 9C and D).
DISCUSSION
The role of gallstones in maintenance of chronic Salmonella carriage has been demonstrated both in the mouse and in humans, as Salmonella readily forms biofilms on human gallstones (45, 56, 57). In this study, we identified specific factors regulated during biofilm formation on cholesterol gallstones and examined the roles of the identified genes in vitro and in vivo. Our flowthrough assays closely mimicked the gallbladder and gallstone environment by using LB bile (3%) and cholesterol, respectively. We demonstrated that cholesterol-coated surfaces enhanced biofilm formation, thus providing a good model for the in vivo interactions of Salmonella with cholesterol gallstones in the gallbladders of chronic carriers.
Microarray data revealed limited genes differentially regulated during biofilm formation on cholesterol in comparison with non-cholesterol-coated glass surfaces. Four of these genes belong to the type 1 fimbria operon, and all are part of the fimAICDHF cluster that encodes the fimbrial shaft. Type 1 fimbriae are proteinaceous filamentous structures 7 nm thick and up to 3 μm in length. They are present on the surfaces of many members of the Enterobacteriaceae (68, 69). These appendages have been associated with attachment and biofilm formation on abiotic surfaces and HeLa, HEp-2, dendritic, and small intestine and bladder epithelial cells (49, 61, 70–74). They have also been associated with colonization of the gut mucosa in vivo (75, 76). Here, we showed that deletion of genes encoding the fimbrial shaft had different phenotypes in vitro and in vivo. In vitro, a fimAICDHF mutant demonstrated increased biofilm formation on both glass and cholesterol-coated surfaces. This increased biofilm formation may be reflective of a compensatory mechanism, as Salmonella possesses several different fimbria types that may be increased in expression when type 1 fimbriae are absent (77, 78). Indeed, we showed that this mutant had increased expression of the curli fimbria main subunit CsgA during biofilm formation on cholesterol-coated surfaces, perhaps partially explaining the phenotype on these surfaces in vitro. These findings could also explain previous in vitro results that showed a negative role of type 1 fimbriae in biofilms formed on cholesterol-coated surfaces. In one of these studies, overexpression of type 1 fimbriae inhibited the initial stages of Salmonella biofilm formation on cholesterol-coated surfaces, but not on glass or plastic (47). In addition, type 1 fimbriae were not required for biofilms in vitro on human gallstones incubated with bile (57). This compensatory mechanism could also explain the increased biofilm formation of the wild type and mutants treated with cellulose (see Fig S2 in the supplemental material). Thus, the absence of a particular EPS component could trigger activation or dysregulation of biofilm-related pathways.
In vivo, however, the role of type 1 fimbriae in biofilm formation on gallstones was different, depending on the time postinfection. We observed type 1 fimbria mutants adhering to the surfaces of mouse gallstones relatively early during colonization (7 days p.i.); however, we did not observe any bacteria attached to gallstones during later times postinfection (21 and 60 days p.i.). Interestingly, bacteria were still observed in the liver and gallbladder, implying that the phenotype is not due to bacterial clearance. Thus, it is likely that during early infection, other fimbriae, such as curli, can replace type 1 fimbriae in initial attachment to gallstones. Later, however, type 1 fimbriae appear to be necessary for permanent adherence/persistence. This is supported by the consistent expression of FimA and CsgA (the main subunits of the type 1 fimbriae and curli fimbriae, respectively) observed during biofilm development in vitro. Together, these data suggest that during initial colonization in the gallbladder, Salmonella finely modulates the expression of different fimbriae, depending on the environment it encounters.
YcfR is an 85-amino-acid putative outer membrane protein that has been reported to be involved in resistance to multiple stresses in E. coli. Its expression in E. coli is induced in the presence of heavy metals, drastic pH changes, heat shock, chlorine, and hydrogen peroxide (79–83). Also in E. coli, ycfR was 12-fold activated in biofilm cells compared to planktonic cells (84). YcfR has been shown to have a negative role in E. coli biofilm formation only in LB with glucose, likely by decreasing bacterial aggregation and cell surface adhesion as a result of decreased hydrophobicity and increased intracellular indole concentrations (66). Others have reported that YcfR lowers the permeability of the outer membrane to copper (85). The ycfR nucleotide sequence is 81% identical between Salmonella and E. coli. We observed that ycfR was specifically induced during S. Typhimurium biofilm formation on cholesterol-coated surfaces, but not on glass. Mutation of ycfR resulted in increased biofilm formation on cholesterol-coated glass and on gallstones in vivo (only up to 21 days p.i.), but not on glass or gallbladder epithelial cells (data not shown), suggesting that ycfR is highly expressed, perhaps to inhibit biofilm formation on this specific surface. We showed that ycfR expression increased as the biofilms matured, further suggesting a role in biofilm regulation. The increased biofilm formation of this mutant may be partially explained by its increased expression of both FimA and CsgA during biofilm formation on cholesterol-coated surfaces. In addition, the absence of ycfR dramatically altered the bacterial-membrane integrity (propidium iodide also stained live cells), likely by increasing cell permeability.
Thus, considering the effect of the ycfR mutation in E. coli (induction of acid, osmotic, oxidative, and heat stress response genes), we believe that during biofilm formation on cholesterol gallstones, ycfR is induced as a response to the stress generated during biofilm formation on cholesterol due to bacterial-membrane damage that has been reported to occur (86, 87). The stress response during biofilm formation is a well-reported phenomenon in E. coli and Salmonella (88–90). The stress and ycfR induction can alter the cell surface hydrophobic properties, resulting in inhibition of biofilm maturation/stability. On glass or epithelial cells, the initiating stress may not be the same, and thus, ycfR may not be needed under these circumstances. Thus, YcfR represents an intrinsic specific inhibitor of the biofilm process on cholesterol gallstones, but not on other surfaces, such as glass, perhaps due to the hydrophobic nature of cholesterol. The induction of ycfR in biofilms on cholesterol-coated surfaces is not a response to bile (bile was always present in our assays), because in biofilm assays using LB only, the ycfR mutant also showed increased biofilm formation on cholesterol (data not shown).
In conclusion, type I fimbria genes and ycfR were specifically upregulated in biofilms formed on cholesterol-coated surfaces. Although mutants of these genes showed increased biofilm formation in vitro, only the ycfR mutant showed this phenotype in vivo, but only up to 21days p.i. In contrast, type 1 fimbria mutants did not persistently attach to the surfaces of gallstones in vivo. Thus, type 1 fimbriae seem to be important for maintaining chronic carriage on gallstones.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andreas Baumler, Torsten Sterzenbach, and Sean Nuccio (University of California, Davis), as well as Matthew Chapman and Dieter M. Schifferli (University of Pennsylvania), for their donation of strains and antibodies. We also thank staff members from the OSU CMIF for their support and contributions to the microscopy.
This work was supported by a grant from the U.S. National Institutes of Health (AI066208).
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00647-13.
REFERENCES
- 1.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346–353 [PMC free article] [PubMed] [Google Scholar]
- 2.Jepson MA, Clark MA. 2001. The role of M cells in Salmonella infection. Microbes Infect. 3:1183–1190 [DOI] [PubMed] [Google Scholar]
- 3.Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808 [DOI] [PubMed] [Google Scholar]
- 4.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, White NJ. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J. Clin. Microbiol. 36:1683–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vladoianu IR, Chang HR, Pechere JC. 1990. Expression of host resistance to Salmonella Typhi and Salmonella Typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8:83–90 [DOI] [PubMed] [Google Scholar]
- 6.Cohen JI, Bartlett JA, Corey GR. 1987. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore) 66:349–388 [DOI] [PubMed] [Google Scholar]
- 7.Levine MM, Black RE, Lanata C. 1982. Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J. Infect. Dis. 146:724–726 [DOI] [PubMed] [Google Scholar]
- 8.Merselis JG, Jr, Kaye D, Connolly CS, Hook EW. 1964. Quantitative bacteriology of the typhoid carrier state. Am. J. Trop. Med. Hyg. 13:425–429 [DOI] [PubMed] [Google Scholar]
- 9.Vogelsang TM, Boe J. 1948. Temporary and chronic carriers of Salmonella Typhi and Salmonella Paratyphi B. J. Hyg. (London) 46:252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaishnavi C, Singh S, Kochhar R, Bhasin D, Singh G, Singh K. 2005. Prevalence of Salmonella enterica serovar Typhi in bile and stool of patients with biliary diseases and those requiring biliary drainage for other purposes. Jpn. J. Infect. Dis. 58:363–365 [PubMed] [Google Scholar]
- 11.Shetty PB, Broome DR. 1998. Sonographic analysis of gallbladder findings in Salmonella enteric fever. J. Ultrasound Med. 17:231–237 [DOI] [PubMed] [Google Scholar]
- 12.Mateen MA, Saleem S, Rao PC, Reddy PS, Reddy DN. 2006. Ultrasound in the diagnosis of typhoid fever. Indian J. Pediatr. 73:681–685 [DOI] [PubMed] [Google Scholar]
- 13.Bhan MK, Bahl R, Bhatnagar S. 2005. Typhoid and paratyphoid fever. Lancet 366:749–762 [DOI] [PubMed] [Google Scholar]
- 14.Sinnott CR, Teall AJ. 1987. Persistent gallbladder carriage of Salmonella Typhi. Lancet i:976. [DOI] [PubMed] [Google Scholar]
- 15.Shpargel JS, Berardi RS, Lenz D. 1985. Salmonella Typhi carrier state 52 years after illness with typhoid fever: a case study. Am. J. Infect. Control 13:122–123 [DOI] [PubMed] [Google Scholar]
- 16.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N. Engl. J. Med. 347:1770–1782 [DOI] [PubMed] [Google Scholar]
- 17.Schioler H, Christiansen ED, Hoybye G, Rasmussen SN, Greibe J. 1983. Biliary calculi in chronic Salmonella carriers and healthy controls: a controlled study. Scand. J. Infect. Dis. 15:17–19 [DOI] [PubMed] [Google Scholar]
- 18.Karaki K, Matsubara Y. 1984. Surgical treatment of chronic biliary typhoid and paratyphoid carriers. Nippon Shokakibyo Gakkai Zasshi 81:2978–2985 (In Japanese.) [PubMed] [Google Scholar]
- 19.Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. 1992. Common bile duct stones: a cause of chronic salmonellosis. Am. J. Gastroenterol. 87:1198–1199 [PubMed] [Google Scholar]
- 20.Kim IS, Myung SJ, Lee SS, Lee SK, Kim MH. 2003. Classification and nomenclature of gallstones revisited. Yonsei Med. J. 44:561–570 [DOI] [PubMed] [Google Scholar]
- 21.Schirmer BD, Winters KL, Edlich RF. 2005. Cholelithiasis and cholecystitis. J. Long Term Eff. Med. Implants 15:329–338 [DOI] [PubMed] [Google Scholar]
- 22.Maurer KJ, Carey MC, Fox JG. 2009. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology 136:425–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paumgartner G. 2010. Biliary physiology and disease: reflections of a physician-scientist. Hepatology 51:1095–1106 [DOI] [PubMed] [Google Scholar]
- 24.Venneman NG, van Erpecum KJ. 2010. Pathogenesis of gallstones. Gastroenterol. Clin. North Am. 39:171–183 [DOI] [PubMed] [Google Scholar]
- 25.Harish BN, Menezes GA. 2011. Antimicrobial resistance in typhoidal salmonellae. Indian J. Med. Microbiol. 29:223–229 [DOI] [PubMed] [Google Scholar]
- 26.Pratap CB, Patel SK, Shukla VK, Tripathi SK, Singh TB, Nath G. 2012. Drug resistance in Salmonella enterica serotype Typhi isolated from chronic typhoid carriers. Int. J. Antimicrob. Agents 40:279–280 [DOI] [PubMed] [Google Scholar]
- 27.Dinbar A, Altmann G, Tulcinsky DB. 1969. The treatment of chronic biliary Salmonella carriers. Am. J. Med. 47:236–242 [DOI] [PubMed] [Google Scholar]
- 28.Shukla VK, Singh H, Pandey M, Upadhyay SK, Nath G. 2000. Carcinoma of the gallbladder—is it a sequel of typhoid? Dig. Dis. Sci. 45:900–903 [DOI] [PubMed] [Google Scholar]
- 29.Caygill CP, Hill MJ, Braddick M, Sharp JC. 1994. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343:83–84 [DOI] [PubMed] [Google Scholar]
- 30.Nath G, Singh H, Shukla VK. 1997. Chronic typhoid carriage and carcinoma of the gallbladder. Eur. J. Cancer Prev. 6:557–559 [DOI] [PubMed] [Google Scholar]
- 31.Welton JC, Marr JS, Friedman SM. 1979. Association between hepatobiliary cancer and typhoid carrier state. Lancet i:791–794 [DOI] [PubMed] [Google Scholar]
- 32.Boisrame-Gastrin S, Tande D, Munck MR, Gouriou S, Nordmann P, Naas T. 2011. Salmonella carriage in adopted children from Mali: 2001–08. J. Antimicrob. Chemother. 66:2271–2276 [DOI] [PubMed] [Google Scholar]
- 33.Ristori C, Rodriguez H, Vicent P, Ferreccio C, Garcia J, Lobos H, D'Ottone K. 1982. Persistence of the Salmonella Typhi-Paratyphi carrier state after gallbladder removal. Bull. Pan Am. Health Organ. 16:361–366 [PubMed] [Google Scholar]
- 34.Nath G, Singh YK, Maurya P, Gulati AK, Srivastava RC, Tripathi SK. 2010. Does Salmonella Typhi primarily reside in the liver of chronic typhoid carriers? J. Infect. Dev. Ctries. 4:259–261 [DOI] [PubMed] [Google Scholar]
- 35.Monack DM, Bouley DM, Falkow S. 2004. Salmonella Typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J. Exp. Med. 199:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaines S, Sprinz H, Tully JG, Tigertt WD. 1968. Studies on infection and immunity in experimental typhoid fever. VII. The distribution of Salmonella Typhi in chimpanzee tissue following oral challenge, and the relationship between the numbers of bacilli and morphologic lesions. J. Infect. Dis. 118:293–306 [DOI] [PubMed] [Google Scholar]
- 37.Rovito V, Bonanno CA. 1982. Salmonella hepatic abscess: an unusual complication of the Salmonella carrier state? Am. J. Gastroenterol. 77:338–339 [PubMed] [Google Scholar]
- 38.Erlik D, Reitler R. 1960. Intrahepatic typhoid infection as cause of the carrier state. Lancet i:1216–1218 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat. Rev. Microbiol. 9:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swidsinski A, Lee SP. 2001. The role of bacteria in gallstone pathogenesis. Front. Biosci. 6:E93–E103 [DOI] [PubMed] [Google Scholar]
- 41.Monds RD, O'Toole GA. 2009. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 17:73–87 [DOI] [PubMed] [Google Scholar]
- 42.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 43.Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40 [DOI] [PubMed] [Google Scholar]
- 44.Cappelli G, Tetta C, Canaud B. 2005. Is biofilm a cause of silent chronic inflammation in haemodialysis patients? A fascinating working hypothesis. Nephrol. Dial. Transplant. 20:266–270 [DOI] [PubMed] [Google Scholar]
- 45.Crawford RW, Rosales-Reyes R, Ramirez-Aguilar MDL, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. U. S. A. 107:4353–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Escobedo G, Gunn JS. 2013. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect. Immun. 81:2920–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford RW, Reeve KE, Gunn JS. 2010. Flagellated but not hyperfimbriated Salmonella enterica serovar Typhimurium attaches to and forms biofilms on cholesterol-coated surfaces. J. Bacteriol. 192:2981–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonas K, Tomenius H, Kader A, Normark S, Romling U, Belova LM, Melefors O. 2007. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ledeboer NA, Jones BD. 2005. Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp-2 cells and chicken intestinal epithelium. J. Bacteriol. 187:3214–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson DL, White AP, Snyder SD, Martin S, Heiss C, Azadi P, Surette M, Kay WW. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J. Bacteriol. 188:7722–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latasa C, Roux A, Toledo-Arana A, Ghigo JM, Gamazo C, Penades JR, Lasa I. 2005. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol. Microbiol. 58:1322–1339 [DOI] [PubMed] [Google Scholar]
- 52.Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U. 2001. The multicellular morphotypes of Salmonella Typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452–1463 [DOI] [PubMed] [Google Scholar]
- 53.Romling U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 62:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain S, Chen J. 2007. Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. J. Food Prot. 70:2473–2479 [DOI] [PubMed] [Google Scholar]
- 55.Johnson L, Horsman SR, Charron-Mazenod L, Turnbull AL, Mulcahy H, Surette MG, Lewenza S. 2013. Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prouty AM, Gunn JS. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect. Immun. 71:7154–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prouty AM, Schwesinger WH, Gunn JS. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70:2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crawford RW, Gibson DL, Kay WW, Gunn JS. 2008. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 60.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo A, Lasaro MA, Sirard JC, Kraehenbuhl JP, Schifferli DM. 2007. Adhesin-dependent binding and uptake of Salmonella enterica serovar Typhimurium by dendritic cells. Microbiology 153:1059–1069 [DOI] [PubMed] [Google Scholar]
- 62.Valdivia RH, Hromockyj AE, Monack D, Ramakrishnan L, Falkow S. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47–52 [DOI] [PubMed] [Google Scholar]
- 63.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3:1335–1344 [DOI] [PubMed] [Google Scholar]
- 64.Forbes JR, Gros P. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397–403 [DOI] [PubMed] [Google Scholar]
- 65.Fritsche G, Nairz M, Libby SJ, Fang FC, Weiss G. 2012. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar Typhimurium in macrophages via stimulation of lipocalin-2 expression. J. Leukoc. Biol. 92:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang XS, Garcia-Contreras R, Wood TK. 2007. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J. Bacteriol. 189:3051–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nuccio SP, Chessa D, Weening EH, Raffatellu M, Clegg S, Baumler AJ. 2007. SIMPLE approach for isolating mutants expressing fimbriae. Appl. Environ. Microbiol. 73:4455–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duguid JP, Anderson ES, Campbell I. 1966. Fimbriae and adhesive properties in salmonellae. J. Pathol. Bacteriol. 92:107–138 [DOI] [PubMed] [Google Scholar]
- 69.Thorns CJ. 1995. Salmonella fimbriae: novel antigens in the detection and control of Salmonella infections. Br. Vet. J. 151:643–658 [DOI] [PubMed] [Google Scholar]
- 70.Pratt LA, Kolter R. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285–293 [DOI] [PubMed] [Google Scholar]
- 71.Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. 2002. Differential binding to and biofilm formation on HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 45:1255–1265 [DOI] [PubMed] [Google Scholar]
- 72.Baumler AJ, Tsolis RM, Heffron F. 1996. Contribution of fimbrial operons to attachment to and invasion of epithelial cell lines by Salmonella Typhimurium. Infect. Immun. 64:1862–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hancox LS, Yeh KS, Clegg S. 1997. Construction and characterization of type 1 non-fimbriate and non-adhesive mutants of Salmonella Typhimurium. FEMS Immunol. Med. Microbiol. 19:289–296 [DOI] [PubMed] [Google Scholar]
- 74.Thankavel K, Shah AH, Cohen MS, Ikeda T, Lorenz RG, Curtiss R, III, Abraham SN. 1999. Molecular basis for the enterocyte tropism exhibited by Salmonella Typhimurium type 1 fimbriae. J. Biol. Chem. 274:5797–5809 [DOI] [PubMed] [Google Scholar]
- 75.Althouse C, Patterson S, Fedorka-Cray P, Isaacson RE. 2003. Type 1 fimbriae of Salmonella enterica serovar Typhimurium bind to enterocytes and contribute to colonization of swine in vivo. Infect. Immun. 71:6446–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Velden AW, Baumler AJ, Tsolis RM, Heffron F. 1998. Multiple fimbrial adhesins are required for full virulence of Salmonella Typhimurium in mice. Infect. Immun. 66:2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ledeboer NA, Frye JG, McClelland M, Jones BD. 2006. Salmonella enterica serovar Typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium. Infect. Immun. 74:3156–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weening EH, Barker JD, Laarakker MC, Humphries AD, Tsolis RM, Baumler AJ. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egler M, Grosse C, Grass G, Nies DH. 2005. Role of the extracytoplasmic function protein family sigma factor RpoE in metal resistance of Escherichia coli. J. Bacteriol. 187:2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. 2005. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 187:304–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richmond CS, Glasner JD, Mau R, Jin H, Blattner FR. 1999. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27:3821–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S, Deng K, Zaremba S, Deng X, Lin C, Wang Q, Tortorello ML, Zhang W. 2009. Transcriptomic response of Escherichia coli O157:H7 to oxidative stress. Appl. Environ. Microbiol. 75:6110–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. 2004. Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 64:515–524 [DOI] [PubMed] [Google Scholar]
- 85.Mermod M, Magnani D, Solioz M, Stoyanov JV. 2012. The copper-inducible ComR (YcfQ) repressor regulates expression of ComC (YcfR), which affects copper permeability of the outer membrane of Escherichia coli. Biometals 25:33–43 [DOI] [PubMed] [Google Scholar]
- 86.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, Kjelleberg S. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Snoussi S, May AE, Coquet L, Chan P, Jouenne T, Landoulsi A, De E. 2012. Adaptation of Salmonella enterica Hadar under static magnetic field: effects on outer membrane protein pattern. Proteome Sci. 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beloin C, Valle J, Latour-Lambert P, Faure P, Kzreminski M, Balestrino D, Haagensen JA, Molin S, Prensier G, Arbeille B, Ghigo JM. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51:659–674 [DOI] [PubMed] [Google Scholar]
- 89.White AP, Weljie AM, Apel D, Zhang P, Shaykhutdinov R, Vogel HJ, Surette MG. 2010. A global metabolic shift is linked to Salmonella multicellular development. PLoS One 5:e11814. 10.1371/journal.pone.0011814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mangalappalli-Illathu AK, Korber DR. 2006. Adaptive resistance and differential protein expression of Salmonella enterica serovar Enteritidis biofilms exposed to benzalkonium chloride. Antimicrob. Agents Chemother. 50:3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang RF, Kushner SR. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195–199 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.