Abstract
Mammalian hosts often develop distinct immune response against the diverse parasitic helminths that have evolved for immune evasion. Interleukin-25 (IL-25), an IL-17 cytokine family member, plays a key role in initiating the protective immunity against several parasitic helminths; however, the involvement and underlying mechanisms by which IL-25 mediates immune response against Trichinella spiralis infection have not been investigated. Here we showed that IL-25 functions in promoting protective immunity against T. spiralis infection. Mice treated with IL-25 exhibited a lower worm burden and fewer muscle larvae in the later stage of T. spiralis infection. In contrast, mice treated with neutralizing antibody against IL-25 failed to expel T. spiralis effectively. During T. spiralis infection, intestinal IL-25 expression was rapidly elevated before the onset of IL-4 and IL-9 induction. While antigen-specific Th2 and Th9 immune responses were both developed during T. spiralis infection, an antigen-specific Th9 response appeared to be transiently induced in the early stage of infection. Mice into which antigen-specific T cells deficient in IL-9 were transferred were less effective in worm clearance than those given wild-type T cells. The strength of the antigen-specific Th9 immune response against T. spiralis could be enhanced or attenuated after treatment with IL-25 or neutralizing antibody against IL-25, respectively, correlating positively with the levels of intestinal mastocytosis and the expression of IL-9-regulated genes, including mast cell- and Paneth cell-specific genes. Thus, our study demonstrates that intestinal IL-25 promotes protective immunity against T. spiralis infection by inducing antigen-specific Th9 immune response.
INTRODUCTION
Gastrointestinal roundworm parasites such as Trichuris muris, Trichinella spiralis, and Strongyloides stercolaris affect people worldwide, especially in developing countries (1). Each of these parasites resides in a distinct anatomical compartment of the host, which launches a protective immune response against the invading parasite (2). Trichinella spiralis is known to be a food-borne, zoonotic parasite that infects the small intestine. Following parasite infection, encysted first-stage larvae mature into adults in the small intestine, where they reside and reproduce within the intestinal epithelial cells (1). Female adult worms will produce larvae which then migrate to muscle. The host protective mechanism in gastrointestinal helminth infection is known to be mediated by Th2 immune responses (3). Although most components of the Th2 immune response exhibit stereotypical activation against these intestinal helminth parasites, certain effector molecules are capable of mediating specific protective effects against a particular parasite (1).
Interleukin-25 (IL-25) (IL-17E), a cytokine of the IL-17 family, is involved in the initiation of type 2 immune responses (4–6). Several lines of experimental evidence indicate that IL-25 is derived from epithelial cells and plays important roles in mucosal immunity (5, 7). IL-25 is known to mediate host protective immunity to several intestinal helminthes. During Trichuris muris infection, IL-25 could promote a Th2 cytokine-dependent immune response and goblet cell hyperplasia, while proinflammatory cytokine production and chronic intestinal inflammation were limited (7). Other studies demonstrated that IL-25-deficient mice had impaired Th2 protective immunity and diminished intestinal smooth muscle and epithelial responses to Nippostrongylus brasiliensis infection, thereby resulting in the failure to expel N. brasiliensis efficiently (8), (9). Whether IL-25 also plays a critical role in the rapid expulsion of T. spiralis has not been addressed.
Cytokines secreted by Th2 cells, such as IL-4 and IL-13 but not IL-5, are known to be effective against tissue-dwelling intestinal nematode parasites, including T. spiralis. IL-9, a known Th2-associated cytokine, enhances the biological function of IL-4 in accelerating worm expulsion (10). In the intestinal mucosa, IL-9 modulates epithelial cell function by upregulating the expression of the genes for several innate immunity mediators, including the Paneth cell marker angiogenin 4 (Ang4), cryptdins, and phospholipase A2 (11). IL-9-mediated worm expulsion is correlated with mast cell expansion and secretion of specific proteases such as mouse mast cell protease 1 (mMCPT-1) (1, 12, 13). Accumulating studies suggest that IL-9 may be a pivotal cytokine to mediate effective expulsion of T. spiralis, possibly via triggering the epithelial cell response and amplification of intestinal mastocytosis and mMCPT-1 release (2, 12, 14). Indeed, IL-9 transgenic mice exhibited elevated intestinal mastocytosis and had increased levels of serum mMCPT-1, which were associated with the rapid expulsion of T. spiralis from the gut (14). Mice that lack mast cells failed to expel worms during T. spiralis infection (12, 15). These lines of evidence support a role for IL-9 as a specific effector molecule against T. spiralis infection.
Recent studies demonstrate that IL-9 can be produced by a specialized population of T cells, termed Th9 cells (16, 17). It was suggested that Th9 cells are distinct from the Th2 cell lineage and function mainly in mucosal immunity (16). Transforming growth factor β (TGF-β) and IL-4 potentiated the differentiation of Th9 cells from naive CD4+ T cells by enhancing IL-9 production from activated T cells in vitro (16, 17). Inhibition of Th9 cell development by blocking TGF-β signaling resulted in a diminished immune response to T. muris, indicating the importance of Th9 cells in the protective immunity to helminth parasites (16). Other factors, such as IL-1 (18), IL-33 (19), and IL-25 (20), were also shown to enhance IL-9 production by Th9 cells. In the absence of IL-25, allergic asthma was alleviated in association with reduced Th2 cytokines and IL-9 (20). Notably, IL-25 potentiated the effect of TGF-β and IL-4 in promoting Th9 differentiation in vitro. Whether IL-25 plays a critical role in regulating the kinetics and function of the antigen-specific IL-9-producing T cell response that has the potential to mediate protective immunity against T. spiralis infection in vivo remains unclear.
In this study, we demonstrated that IL-25 mediates the protective immune response to T. spiralis by enhancing antigen-specific Th9 cell function. Following T. spiralis infection, IL-25 mRNA and protein were induced before the expression of IL-9 in the intestine. Indeed, the antigen-specific Th9 response occurred transiently in the early stage and appeared to be important for mediating an effective worm clearance. We also showed that exogenous IL-25 treatment enhanced antigen-specific IL-9 production, which was associated with the increased worm expulsion in the intestine and muscle, while IL-25 blockade reduced the antigen-specific IL-9 response and worm expulsion. These changes in the antigen-specific Th9 response mediated by IL-25 treatment or blockade correlated with the alteration of mast cell number and the expression levels of IL-9-regulated genes, including those for mast cell protease 1 and Paneth cell markers cryptdin and Ang4 in the intestine. In contrast, IL-25 treatment failed to modulate the expression of these IL-9-regulated genes in IL-9-deficient mice during T. spiralis infection. These results suggest that IL-25 mediates an effective immune response to expel T. spiralis infection through the induction of an antigen-specific Th9 immune response.
MATERIALS AND METHODS
Animals.
C57BL/6 mice and BALB/c mice were obtained from The National Laboratory Animal Center, Mahidol University. Female 6- to 8-week-old mice were used for experiments. IL-9-deficient mice in the BALB/c background were kindly provided by Andrew McKenzie (Medical Research Council, Laboratory of Molecular Biology, Cambridge, United Kingdom). All animal studies were approved by the Thammasat University Animal Care and Use Committee.
MAbs and flow cytometry.
Recombinant mouse IL-25–Ig protein was prepared as we previously reported (5). Anti-IL-25 monoclonal antibodies (MAbs) were generated as previously described (5). Peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 (GK1.5), phycoerythrin (PE)-conjugated anti-IL-4 (11B11), and PE-conjugated anti-IL-17 (TC11-18H10) antibodies were from BD Pharmingen. Allophycocyanin (APC)-conjugated anti-IL-9 (RM9A4) antibody was from BioLegend. Cells were analyzed using a FACSCalibur cytometer (BD Biosciences).
Parasite infection and worm expulsion.
T. spiralis (ISS62) (21) originated from the outbreak in the Mae Hong Son Province in 1986 and was obtained from the Department of Parasitology, Faculty of Medicine, Khon Kaen University, and maintained through infection in ICR mice (22). The larvae were obtained from infected mice after 30 days postinfection by homogenizing muscle by pepsin-HCl digestion. C57BL/6, BALB/c, or IL-9-deficient mice were infected orally with 400 T. spiralis larvae and sacrificed at various time points postinfection. Anti-IL-25 antibody (100 μg/mouse) (5), control rat IgG (100 μg/mouse), or IL-25–Ig (5 μg/mouse) (5) was given intraperitoneally at days 0, 1, and 3 after infection. For worm burden analysis, small intestines of infected mice at day 7 and day 14 postinfection were removed, opened longitudinally, and incubated in Hanks' balanced salt solution (HBSS) at 37°C for 3 h. Following incubation, intestines were agitated, and worms were then counted using inverted microscope. Muscle larval burdens were assessed at 30 days postinfection in whole carcasses as described previously (23).
Histology.
Intestinal tissue samples (jejunum) were taken 10 cm from the pylorus, fixed in 10% buffered formalin, and subsequently dehydrated in ethanol and embedded in paraffin wax. Sections were stained with Leder stain for mast cells. Numbers of mast cells were expressed per villus crypt unit (VCU).
Evaluation of cytokine production.
Mesenteric lymph nodes or spleens were harvested from mice infected with T. spiralis at various time points after infection or from naive mice (23). Single-cell suspensions were prepared and further subjected to intracellular cytokine analysis and enzyme-linked immunosorbent assay (ELISA) (5, 20). For intracellular cytokine analysis, cells were restimulated with 500 ng/ml ionomycin and 50 ng/ml phorbol myristate acetate (PMA) in the presence of GolgiStop (BD Biosciences) for 5 h (5, 20). Cells were permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) and analyzed for the expression of IL-9, IL-4, and IL-17. For ELISA, single-cell suspensions were stimulated with or without T. spiralis extract antigens (50 μg/ml). Following a 3-day incubation at 37°C with 5% CO2, culture supernatants were collected and analyzed for the production of cytokines. For restimulation experiments, single-cell suspensions were cultured with antigen for 7 days, followed by purifying for CD4+ cells using anti-CD4-conjugated microbeads and separating out the CD4+ cell population by magnetically activated cell sorting (MACS) according to the manufacturer's instruction (Miltenyi-Biotec). CD4+ cells were restimulated with anti-CD3 overnight, and culture supernatants were collected and analyzed for cytokine production by ELISA. The antibody pairs for IL-4, IL-9, and IL-17 were obtained from BD Pharmingen, and assays were performed according to the manufacturer's instructions. For quantitative measurement of intestinal IL-25, the intestines (jejunum) were excised and homogenized in cold phosphate-buffered saline (PBS) (24), and the resulting supernatants were measured for IL-25 using the IL-25 ELISA kit from R&D Systems.
Real-time reverse transcription-PCR (RT-PCR) analysis.
The small intestines (jejunum) were removed from naive or T. spiralis-infected mice and homogenized in TRIzol reagent (Invitrogen). Total RNA extracted using TRIzol reagent was used to generate cDNA using oligo(dT), random hexamers, and Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) (5, 20). For quantitation of cytokines, cDNA samples were amplified in IQ SYRB green Supermix (Bio-Rad Laboratories). The data were normalized to actin expression (Actb). The primer pairs for analysis of cytokines (20) and for Mcpt1, Mcpt2, Ang4, and Cryptdins were as previously described (11).
Cell transfer experiment.
Mesenteric lymph node cells were obtained on days 7 and 14 after infection with 400 T. spiralis larvae. Single-cell suspensions were prepared and cultured with T. spiralis antigen (10 μg/ml). After 7 days, cells were washed and resuspended in PBS. Culture cells were then pooled and enriched for CD4+ cells by using anti-CD4 microbeads (L3T4), followed by positive magnetic bead separation (Miltenyi Biotec). We also tested for the induction of IL-9-producing T cells by staining the restimulated cultured cells before performing transfer experiments. CD4+ cell-enriched cells from mice infected for 7 days (Th2+ Th9 cells) or for 14 days (Th2 cells) were intravenously injected into syngeneic recipient mice with 2 × 107 cells 24 h before oral infection with 400 T. spiralis larvae. Infected mice were killed on day 6 after infection with T. spiralis, and the intestines were harvested for the analysis of cytokine gene expression, worm burdens, and mast cell numbers. For some adoptive transfer experiments, IL-9-deficient mice were transferred with antigen-specific T cells prepared from IL-9-deficient mice or wild-type (BALB/c) mice after 7 days postinfection as described above. Mice were then orally infected with 400 T. spiralis larvae on the next day and analyzed for antigen-specific cytokine production in mesenteric lymph nodes. Worm burdens were counted on day 6 after T. spiralis infection.
Statistical analysis.
Each experiment was conducted two or three times. Data are presented as mean values and standard deviations (SD). Data were analyzed using the Student t test or one-way analysis of variance (ANOVA) with Turkey's post hoc analysis. Statistical analysis was performed with GraphPad Prism 5 software. A P value of <0.05 was considered significant.
RESULTS
IL-25 is involved in the host protective immune responses against T. spiralis infection.
To test whether IL-25 is involved in the host protective immune responses to T. spiralis infection, we assessed worm burdens in the intestines of T. spiralis-infected mice following IL-25 cytokine treatment. Mice were treated with IL-25 fusion protein at days 0, 1, and 3 following T. spiralis infection, and the numbers of worms present in the intestines of infected mice were determined at 7 and 14 days postinfection. Compared to untreated mice, mice treated with IL-25 had significantly reduced worm burdens in the intestine at 7 days postinfection (Fig. 1A). At 14 days postinfection, a few worms remained in untreated mice; however, IL-25 treatment resulted in complete worm expulsion in infected mice (Fig. 1A). To investigate the biological significance of the rapid expulsion observed in the IL-25-treated mice, muscle larval burdens were assessed at 30 days postinfection. As expected, mice treated with IL-25 fusion proteins had a 3-fold reduction of muscle larva number compared to that in mice without treatment (Fig. 1B).
Fig 1.
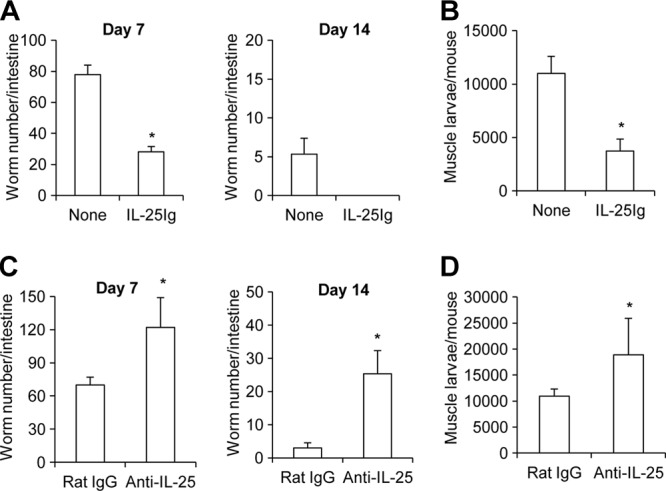
IL-25 mediates protective immunity to T. spiralis infection. (A and B) C57BL/6 mice were untreated or administered IL-25–Ig intraperitoneally at days 0, 1, and 3 following T. spiralis infection. (A) At day 7 and day 14 postinfection, whole intestines were harvested and analyzed for adult worms in the intestine. (B) At day 30 postinfection, whole carcasses of infected mice from different groups were analyzed for muscle larval burden. (C and D) C57BL/6 mice were administered control rat IgG or anti-IL-25 neutralizing antibody intraperitoneally at days 0, 1, and 3 following T. spiralis infection. (C) Adult worms in small intestine were counted at day 7 and day 14 postinfection. (D) Muscle larvae were analyzed in mice sacrificed at day 30 postinfection. Graphs depict mean ± SD and are representative of three independent experiments with three or four mice per group. Significance was determined by Student's t test. *, P < 0.05 (compared with data for the control-treated group).
To further examine the protective role of IL-25 in mediating immune responses against T. spiralis infection, mice were treated with IL-25 neutralizing antibody or control rat IgG antibody at days 0, 1, and 3 following T. spiralis infection, and their numbers of intestinal adult worms and muscle larvae were counted and compared. We found that the administration of IL-25 neutralizing antibody in T. spiralis-infected mice resulted in a 2-fold increase in the worm burden (Fig. 1C) (P < 0.05, compared with those in the rat IgG antibody-treated group). Compared to the control antibody-treated group at 14 days postinfection, mice treated with IL-25 neutralizing antibody were less competent to expel worms efficiently (Fig. 1C). Moreover, muscle larval depositions in mice treated with IL-25 neutralizing antibody were significantly increased compared to those in rat IgG-treated mice at 30 days postinfection (Fig. 1D), supporting the finding of the delayed worm expulsion rate in the intestines. These results demonstrate that IL-25 plays important roles in mediating the host protective immunity against T. spiralis infection.
Temporal IL-25 expression precedes the intestinal IL-9 induction during T. spiralis infection.
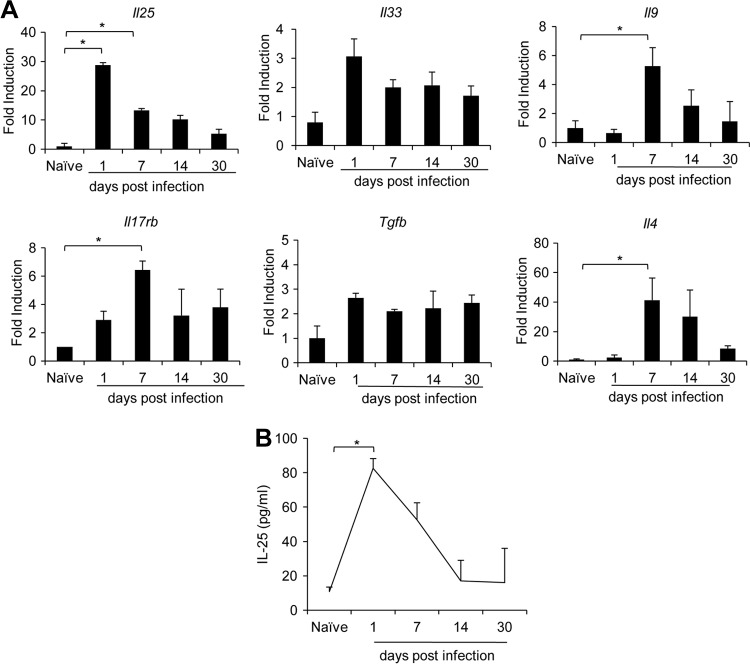
To begin to address the mechanisms underlying the IL-25-mediated protective immune response against T. spiralis, we set out to examine intestinal IL-25 expression during helminth infection. RNA samples isolated from the small intestines of mice sacrificed on days 1, 7, 14, and 30 postinfection with T. spiralis were reversed transcribed and served as templates for assessments of targeted gene expression using quantitative real-time PCR analyses. Notably, intestinal Il25 expression was significantly elevated (>25-fold) in mice infected with T. spiralis on day 1 postinfection compared to that in naive mice (Fig. 2A). The helminth-induced intestinal Il25 expression declined gradually and was still detectable at day 30 postinfection (Fig. 2A). Consistent with these data, the kinetics of intestinal IL-25 protein secretion measured by ELISA peaked at day 1 postinfection but were normalized at day 14 postinfection (Fig. 2B). In addition to cytokine IL-25, we also observed significantly elevated expression of IL-25's cognate receptor Il17rb (>6-fold) at day 7 postinfection. At 1 day postinfection, we observed a trend of an increase of intestinal Tgfb (>3-fold) and Il33 (>2-fold) transcript expression (though this was not statistically significant), and the expression of these genes remained elevated at days 7, 14, and 30 postinfection (Fig. 2A). Since the TGF-β, IL-25, and IL-33 cytokines were shown to promote the induction of IL-9-producing T cells (16, 19, 20), we examined the expression of Il9 as well as Il4 transcripts during T. spiralis infection. In contrast to the early induction of Il25, Il33, and Tgfb, the expression of intestinal Il9 and Il4 transcripts was not induced at day 1 but increased significantly at day 7 postinfection (Fig. 2A). These results reveal that T. spiralis infection triggers a rapid intestinal IL-25 production that precedes the induction of Il4 and Il9 gene expression in mice.
Fig 2.
Il25 expression is upregulated transiently and precedes intestinal Il9 induction in T. spiralis-infected mice. C57BL/6 mice were infected with T. spiralis for 1, 7, 14, or 30 days. Small intestines (jejunum) were harvested from naive mice or mice infected at the indicated time points. (A) Total RNA was isolated and subjected to cDNA synthesis and subsequent real-time PCR analysis of cytokine gene expression. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the naive group set as 1. (B) The small intestines (jejunum) of naive and infected mice were homogenized in cold PBS, and supernatant was analyzed for IL-25 content by ELISA. Graphs depict mean ± SD and are representative of at least two independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis (*, P < 0.05).
T. spiralis infection induces a transient antigen-specific IL-9-producing T cell response.
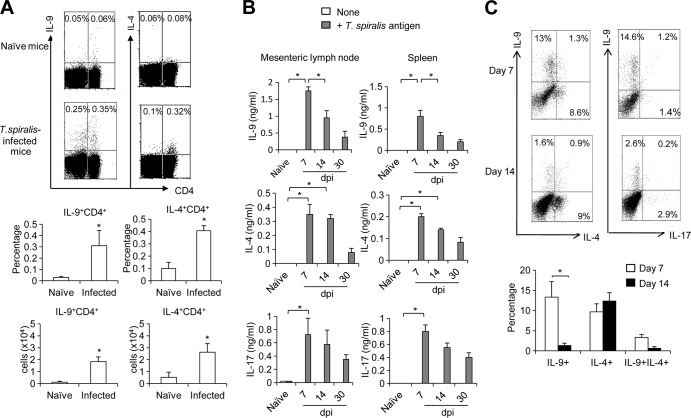
Previous studies suggest that the induction of an IL-9-producing T cell response is essential for the host to mount protective immunity against helminth parasite infection (16). Having observed elevated intestinal IL-9 expression, we next addressed whether IL-9-producing T cell response are involved in the protective immunity against T. spiralis infection. Indeed, a significant induction of both IL-4- and IL-9-producing CD4+ T cells in the mesenteric lymph nodes was detected 7 days after T. spiralis infection (Fig. 3A). Notably, we also observed an increase in a population of non-CD4 cells producing IL-9 following T. spiralis infection (Fig. 3A). To examine the duration of the induced IL-4- and IL-9-producing T cell response against parasitic antigens, immune cells isolated from splenocytes or mesenteric lymph nodes of mice infected with T. spiralis for 7 days, 14 days, and 30 days were stimulated ex vivo with T. spiralis extract for 3 days before collecting culture supernatants for measurement of their cytokine production by ELISA. Compared to that in naive mice, we detected a greater induction of antigen-specific IL-9 and IL-4 production in mice after 7 days of infection (Fig. 3B). While antigen-specific IL-4 production in mesenteric lymph nodes was sustained on day 14 postinfection, antigen-specific IL-9 production declined after 7 days of infection (Fig. 3B). Notably, the appearance of IL-4- or IL-9-producing CD4+ T cell response in mesenteric lymph nodes corresponded to the temporal expression pattern of intestinal Il9 and Il4 transcripts shown in Fig. 2. Since Th17 cells were recently found to be associated with muscle contraction during T. spiralis infection (25), we investigated the appearance of these cells during infection. Compared to naive mice, mice infected with T. spiralis also displayed enhanced antigen-specific IL-17 production on day 7 postinfection (Fig. 3B). To investigate whether IL-9- and IL-4-producing CD4+ T cells can be expanded ex vivo in the presence of T. spiralis extract antigen, mesenteric lymph node cells collected from mice at days 7 and 14 postinfection were cultured with T. spiralis extract antigens for 7 days before restimulation for intracellular cytokine analysis. Notably, the frequencies of IL-9-producing (10% to 15%) and IL-4-producing (8% to 11%) CD4+ T cells from mesenteric lymph node of mice at 7 days after infection were significantly increased ex vivo in the presence of T. spiralis extract antigens (Fig. 3C). While the frequency of IL-4-producing CD4+ T cells (7% to 12%) that could be activated by T. spiralis extract antigens and expanded ex vivo remained constant in mesenteric lymph node of mice at 14 days postinfection, the frequency of antigen-specific IL-9-producing CD4+ T cells (1% to 3%) in these infected mice declined (Fig. 3C). Notably, these IL-9-producing CD4+T cells did not produce IL-4, IL-5, and IL-17 concomitantly (Fig. 3C; see Fig. S1 in the supplemental material). Our data suggest that both antigen-specific Th2 (IL-4-producing CD4+ T cells) and Th9 (IL-9-producing CD4+ T cells) responses occur concurrently during T. spiralis infection; however, an antigen-specific Th9 immune response may be induced transiently at the early stage of T. spiralis infection.
Fig 3.
Kinetics of antigen-specific IL-9- and IL-4-producing T cell responses during infection with T. spiralis. (A) C57BL/6 mice were infected with T. spiralis for 7 days. Mesenteric lymph nodes from naive mice and infected mice were harvested and analyzed for surface CD4 staining and intracellular cytokine staining of IL-4 and IL-9. The results are presented as the percentage of the cells and the total cell number (P < 0.05 compared with the number in naive mice). (B) C57BL/6 mice were infected with T. spiralis. At the indicated time points (days postinfection [dpi]), mesenteric lymph nodes or spleens from naive and infected mice were harvested and restimulated with or without T. spiralis extract antigen (concentration of 50 μg/ml) for 3 days. The cytokine levels in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). (C) Mesenteric lymph node cells from naive mice or mice infected with T. spiralis for 7 or 14 days were cultured with T. spiralis extract antigen (50 μg/ml) for 7 days and then were enriched for CD4+ T cells by MACS. Enriched CD4+ cells were then restimulated and analyzed for intracellular cytokine staining (plots are gated on CD4+ cells). The results are also presented as the percentage of the cells. Graphs depict mean ± SD and are representative of three experiments with three or four mice per group. Significance was determined by Student's t test (A and C) or one-way ANOVA with Tukey's post hoc analysis (B). *, P < 0.05.
Antigen-specific Th9 immune responses facilitate the expulsion of T. spiralis.
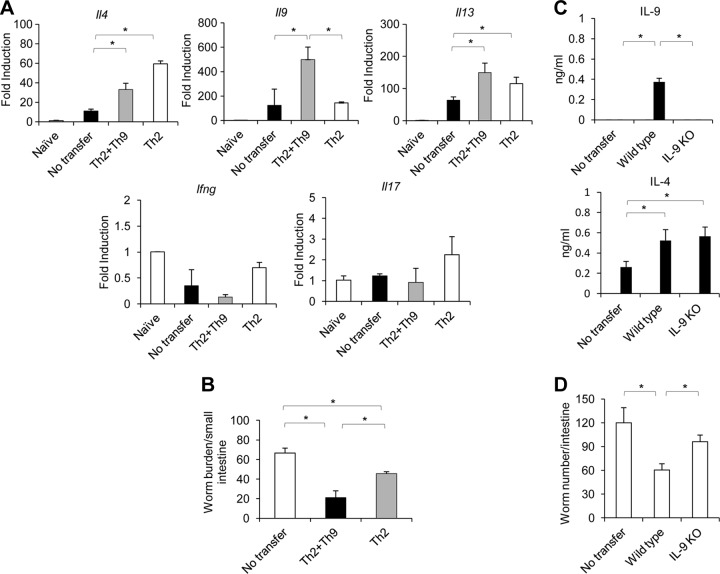
To examine whether antigen-specific Th9 immune response can facilitate the expulsion of T. spiralis, we compared worm burdens in mice that were adoptively transferred with both antigen-specific Th9 and Th2 cells or with antigen-specific Th2 cells at 24 h before infection with T. spiralis larvae. As shown in Fig. 4A, mice that received enriched antigen-specific Th9 and Th2 cells that were generated from mesenteric lymph nodes of mice infected for 7 days (see Fig. S2 in the supplemental material) exhibited high levels of intestinal Il9 expression compared to those in mice receiving enriched antigen-specific Th2 cells (Fig. 4A). We did not see the induction of gamma interferon (IFN-γ) and IL-17 expression in mice transferred with cells from mice infected for 7 or 14 days. Compared to mice without transfer, both groups of infected mice into which antigen-specific Th9 plus Th2 cells or only antigen-specific Th2 cells were adoptively transferred, had reduced worm burdens. However, the infected mice that were received antigen-specific Th9 plus Th2 cells were more competent to expel worms than those received only antigen-specific Th2 cells (Fig. 4B).
Fig 4.
Antigen-specific Th9 cells enhance effective worm expulsion. (A and B) C57BL/6 mice were infected with T. spiralis. At day 7 or day 14 postinfection, mice were sacrificed, and their mesenteric lymph nodes were harvested and cultured with T. spiralis extract antigen. After 7 days of culture, cells of 7-day- or 14-day-infected mice were then collected and enriched for CD4+ cells. Both antigen-specific Th2 and Th9 cells (2 × 107 cells) obtained from 7-day-infected mice or antigen-specific Th2 cells obtained from 14-day-infected mice were transferred into C57BL/6 mice. After 24 h, the recipient mice were then infected with T. spiralis. At 6 days postinfection, mice were sacrificed and analyzed for cytokine gene expression (A) and worm burden (B). (C and D) Antigen-specific CD4+ T cells prepared as described above from wild-type or IL-9-deficient mice were intravenously transferred into IL-9-deficient mice, and the mice were then infected with T. spiralis. At 6 days postinfection, mice were then analyzed for antigen-specific cytokine by ELISA (C) and worm burden (D). Graphs depict mean ± SD and are representative of at least two independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05.
To provide direct evidence that IL-9 derived from antigen-specific CD4+ T cells is involved in T. spiralis worm clearance, we reconstituted IL-9-deficient mice with antigen-specific CD4+ T cells from wild-type mice or mice deficient in IL-9 and then assessed and compared their antigen-specific cytokine production and worm burdens after 6 days after T. spiralis infection. Indeed, we could detect antigen-specific IL-9 and IL-4 production by mesenteric lymph node cells from IL-9-deficient mice after the reconstitution with wild-type antigen-specific CD4+ T cells but could detect only antigen-specific IL-4 production after reconstitution with IL-9-deficient antigen-specific CD4+ T cells (Fig. 4C). Notably, these IL-9-deficient mice reconstituted with wild-type antigen-specific CD4+ T cells had less of a worm burden than those reconstituted with IL-9-deficient antigen-specific CD4+ T cells (Fig. 4D). Collectively, our results demonstrate that the infected mice may be more competent to expel T. spiralis after the acquisition of antigen-specific Th9 cells.
The antigen-specific Th9 immune response to T. spiralis is regulated by IL-25.
We previously showed that IL-25 promotes the induction of IL-9 in allergic asthma (20). The finding that IL-25 expression preceded the induction of IL-9 in the intestines of T. spiralis-infected mice prompted us to test whether the modulation of IL-25 activity can alter the T. spiralis-induced Th9 immune response. At day 7 postinfection, mesenteric lymph node cells isolated from mice treated with IL-25 fusion protein or IL-25 neutralizing antibody or from untreated mice were activated with T. spiralis antigens for 3 days before collection of supernatants for the analyses of antigen-specific cytokine production by ELISA. We found that antigen-specific T cells in T. spiralis-infected mice treated with IL-25 secreted significantly larger amounts of Th2 cytokines IL-4, IL-13, and IL-9 than those in infected mice without IL-25 treatment, while no significant change in IFN-γ and IL-17 production was observed after IL-25 treatment (Fig. 5A). In contrast, anti-IL-25 neutralizing antibody treatment significantly attenuated the induction of antigen-specific IL-9 and other Th2 cytokine production in the mesenteric lymph nodes of infected mice (Fig. 5B) (P < 0.05 for IL-4, IL-5, IL-9, and IL-13, compared with those in the rat IgG antibody-treated group). Notably, IL-25 blockade resulted in the increase of antigen-specific IFN-γ production but not antigen-specific IL-17 production (Fig. 5B). Our data thus suggest that IL-25 promotes a protective immune response against T. spiralis helminth infection through the induction of antigen-specific Th2 and Th9 immune responses.
Fig 5.
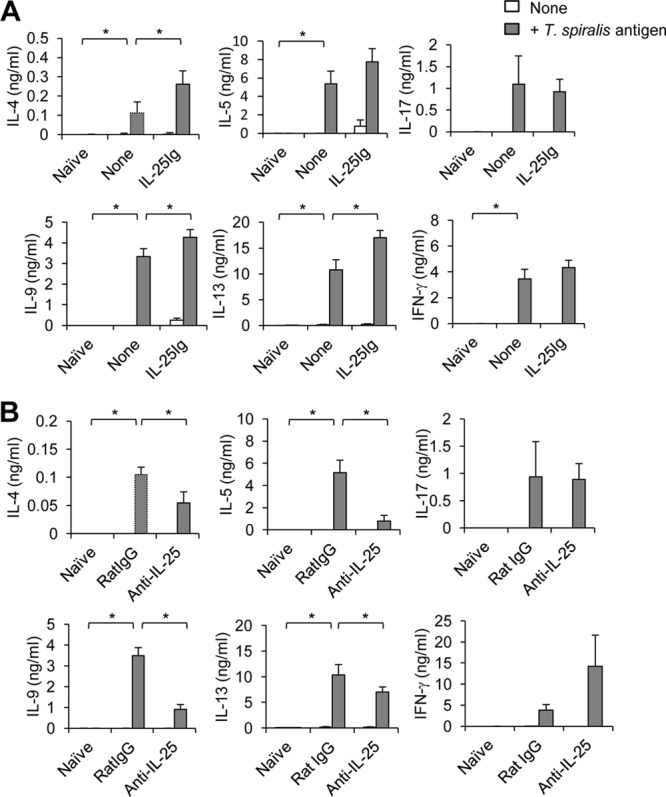
The antigen-specific IL-9 response to T. spiralis is regulated by IL-25. C57BL/6 mice were untreated or administered IL-25–Ig (A) or administered rat IgG antibody or IL-25-neutralizing antibody (B) intraperitoneally at days 0, 1, and 3 following T. spiralis infection. At day 7 postinfection, mesenteric lymph node cells were harvested, and single-cell suspensions were then cultured with or without T. spiralis extract antigen (50 μg/ml). After 3 days, supernatant was collected and analyzed for T. spiralis-specific cytokine production by ELISA. Graphs depict mean ± SD and are representative of at least three independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05.
IL-25 regulates IL-9-mediated effector function during T. spiralis infection.
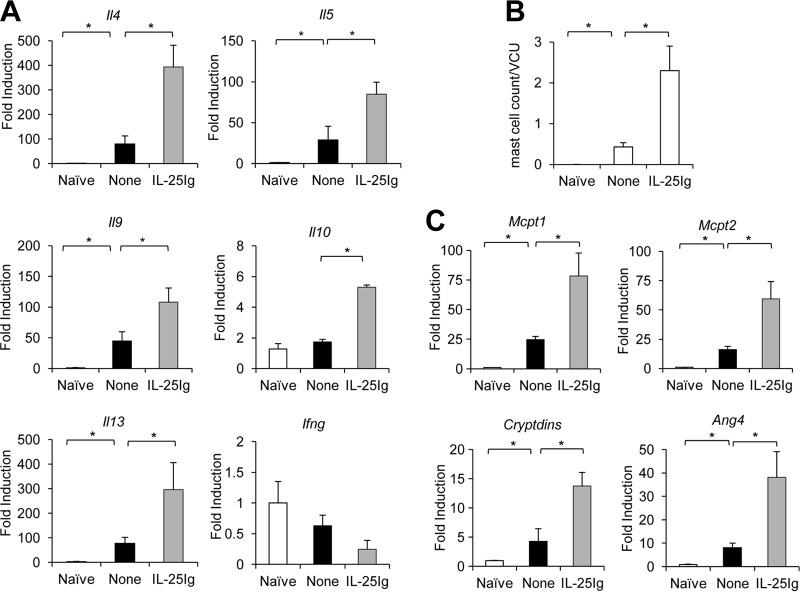
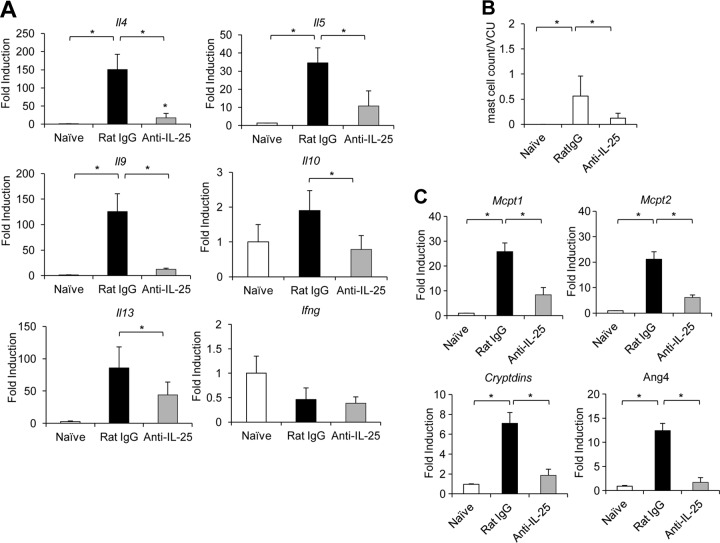
Previous studies indicate that IL-9 can regulate several innate immune cells in the intestinal mucosa, including epithelial cells (goblet and Paneth cells) and mast cells (11). IL-9-promoted T. spiralis expulsion was found to be associated with the presence of mast cells and the expression of mouse mast cell proteases (12, 14, 15). These studies led us to hypothesize that the IL-25-induced Th9 immune response can trigger an IL-9-mediated effector function that leads to the efficient expulsion of intestinal T. spiralis. To begin to test this hypothesis, we first examined the effect of IL-25 on modulating intestinal Il9 expression in mice infected with T. spiralis. At day 7 postinfection, the expression of Il9 and other Th2 cytokine genes (Il4, Il5, Il3, and Il10) in the intestines of mice after IL-25 treatment at days 0, 1, and 3 following T. spiralis infection was significantly elevated (Fig. 6A). In contrast, IL-25 blockade using IL-25 neutralizing antibody resulted in a significant reduction of T. spiralis-induced expression of intestinal Il9 and other Th2 cytokine genes (Il4, Il5, Il3, and Il10) (Fig. 7A) (P < 0.05, compared with those in the rat IgG antibody-treated group). We did not detect a significant induction of IFN-γ gene expression in the intestines of T. spiralis-infected mice after either IL-25 treatment or IL-25 blockade (Fig. 6A and 7A). Notably, the elevation of intestinal Il9 gene expression after IL-25 treatment was positively correlated with the numbers of intestinal mast cells (Fig. 6B). Moreover, IL-25 treatment induced significant increases in the expression of IL-9-regulated genes, such as mouse mast cell protease genes Mcpt1 and Mcpt2, as well as Cryptdins and Ang4, which participate in mast cell and Paneth cell responses, respectively, in the intestine (Fig. 6C). In contrast, treatment with IL-25 neutralizing antibody attenuated T. spiralis-induced intestinal mast cell recruitment and expression of Mcpt1, Mcpt2, Cryptdins, and Ang4 transcripts compared to those in infected mice received control rat IgG antibody (Fig. 7B and C). Thus, our data suggest that IL-25 may promote the induction of an IL-9-mediated immune response that triggers intestinal mastocytosis and Paneth cells, resulting in effective T. spiralis expulsion.
Fig 6.
Exogenous IL-25 treatment during T. spiralis infection enhances intestinal IL-9-, mast cell-, and Paneth cell-specific gene expression. C57BL/6 mice were untreated or administered IL-25–Ig intraperitoneally at days 0, 1, and 3 following T. spiralis infection. At day 7 postinfection, small intestines (jejunum) were harvested. (A) Small intestines were subjected to RNA extraction, followed by cDNA synthesis and analysis of cytokine gene expression by real-time PCR. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the naive group set as 1. (B) Small intestines (jejunum) were fixed with 10% formalin buffer and subjected to histological analysis of mast cells by Leder staining. Numbers of mast cells are expressed per villus crypt unit (VCU). (C) cDNA was analyzed for the expression of mouse mast cell protease 1 (Mcpt1), mouse mast cell protease 2 (Mcpt2), and Paneth cell markers Cryptdins and Ang4 by real-time PCR. Graphs depict mean ± SD and are representative of at least two independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05.
Fig 7.
IL-25 blockade during T. spiralis infection reduces intestinal IL-9-, mast cell-, and Paneth cell-specific gene expression. C57BL/6 mice were administered rat IgG (control) or anti-IL-25 neutralizing antibody intraperitoneally at days 0, 1, and 3 after T. spiralis infection. At day 7 postinfection, the small intestines (jejunum) were removed. (A) Cytokine gene expression analysis by real-time RT-PCR. Data are expressed as fold induction over actin (Actb) expression, with the mRNA levels in the naive group set as 1. (B) Jejunum tissue was fixed in 10% formalin buffer and subjected to Leder staining. Numbers of mast cells are expressed per villus crypt unit (VCU). (C) cDNA was analyzed for mast cell- and Paneth cell-specific gene expression by real-time PCR. Graphs depict mean ± SD and are representative of at least three independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05.
Next, we examine whether IL-25 can enhance IL-9-mediated effector functions that promote effective protective immunity against T. spiralis infection in vivo. While wild-type mice treated with IL-25 were more competent in expelling worms than those without treatment, IL-9-deficient mice failed to respond to IL-25 treatment and remained ineffective in worm expulsion (Fig. 8A). As expected, the enhanced immune response in expelling worm infection in wild-type mice after IL-25 treatment was correlated with increased expression of IL-9-regulated genes Mcpt1, Mcpt2, and Cryptdins (Fig. 8B). Furthermore, the failure to mount an enhanced immune response against T. spiralis infection in IL-9-deficient mice after IL-25 treatment coincided with the findings that the expression of IL-9-regulated genes, including Mcpt1, Mcpt2, and Cryptdins, in these mice remained unchanged (Fig. 8B). Interestingly, while the significantly increased intestinal Il5 expression induced by IL-25 treatment was comparable in both wild-type and IL-9-deficient mice (>200-fold), the increased intestinal Il13 expression was less pronounced in mice deficient in IL-9 than in wild-type mice after IL-25 treatment (Fig. 8B). These data indicate that IL-25-regulated IL-9 effector function plays important roles in immunity to T. spiralis infection.
Fig 8.
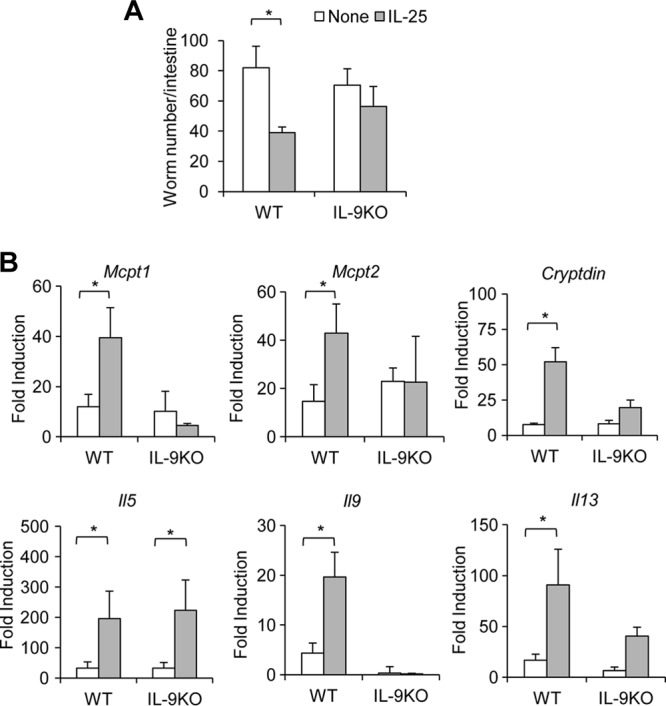
IL-9 is required for IL-25-enhanced T. spiralis worm clearance. IL-9-deficient or wild-type mice were untreated or administered IL-25–Ig intraperitoneally at days 0, 1, and 3 following T. spiralis infection. At day 7 postinfection, small intestines were harvested and analyzed for worm burden (A) or for gene expression by real-time PCR (B). Graphs depict mean ± SD and are representative of at least two independent experiments with three or four mice per group. Significance was determined by one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05.
DISCUSSION
IL-25 is an important cytokine in the initiation of type 2 immune responses (4). There is strong evidence supporting a crucial role of IL-25 in mediating the protective immunity to gastrointestinal helminthes, such as Nippostrongylus braziliensis (8, 9) and Trichuris muris (7); however, the involvement of this cytokine in driving the immune response against Trichinella spiralis infection has not been addressed. In this study, we showed that IL-25 enhanced effective protective immunity to T. spiralis infection. Following T. spiralis infection, the expression of intestinal IL-25 was upregulated and preceded IL-9 expression. IL-25-mediated host protective immune responses to T. spiralis were associated with the induction of antigen-specific Th9 and Th2 immune responses. Treatment with exogenous IL-25 induced the increased antigen-specific IL-9 production, expression of Mcpt1, Mcpt2, Cryptdins, and Ang4 transcripts, and intestinal mastocytosis, which resulted in enhanced worm expulsion. In contrast, IL-25 blockade resulted in an inefficacy in worm expulsion, correlating with the reduced intestinal IL-9 expression, mast cell number, and mast cell- and Paneth cell-specific gene expression. These findings substantiate the function of IL-25 in evoking protective immunity against T. spiralis infection by regulating IL-9 effector function.
We showed that during T. spiralis infection, IL-25 expression was increased in the intestine on day 1 after infection, suggesting that IL-25 may function in the early stage of T. spiralis infection. The kinetics of intestinal IL-25 expression during T. spiralis infection appeared to be different from that in mice infected with N. braziliensis, which peaked at day 9 postinfection (9). The difference in the kinetics of IL-25 expression may be due to the distinct life cycles of these parasites. During T. spiralis infection, parasitic larvae initiate the process by penetrating the columnar epithelium of the small intestine, and the larvae rapidly develop into adults. Previous studies indicate that epithelial cells of the lung and intestine are the major IL-25 producers (5, 7, 9). T. spiralis might induce IL-25 expression while penetrating into the epithelial layer. Thus, early induction of intestinal IL-25 may be a critical step in initiating the protective immunity against T. spiralis infection.
In addition to IL-25, intestinal Il4 and Il9 expression was also induced at 7 days postinfection. Unlike intestinal Il4 expression, which was sustained from day 7 to day 14 postinfection, intestinal Il9 expression was transiently induced, peaked at day 7 postinfection, and then declined at day 14 postinfection. Our finding of a transient Il9 induction in vivo is coincided with a recent study showing that IL-9 was produced but declined rapidly during in vitro differentiation of naive T cells with TGF-β and IL-4 (26). It is possible that the strong signals from the antigens or environmental stimuli induced by the parasite in the early stage of infection are required for the induction and maintenance of Il9 expression and that the absence of these signals in subsequent stages of infection results in the decline in its expression. Correlating with the IL-9 expression pattern in the intestine, we showed that the antigen-specific Th9 response in mesenteric lymph nodes declined rapidly after day 7 postinfection. Mice into which antigen-specific T cells deficient in IL-9 were transferred were less effective in T. spiralis worm clearance than those receiving wild-type antigen-specific T cells, suggesting that the combination of antigen-specific Th9 and Th2 responses may be required for effective clearance of T. spiralis in the intestine. Interestingly, we also observed increased frequencies of IL-9 production by CD4− cells in some experiments. Whether these non-T/non-B IL-9 producers during T. spiralis infection are the recently described type 2 innate lymphoid cells (ILC2) remains to be investigated (27).
IL-9 production can be regulated by several cytokines (16–20); however, the regulation of Th9 cell differentiation during helminth infection is less clear. In vitro, TGF-β and IL-4 were the major stimulators of Th9 cell induction (16, 17). The absence of TGF-β signaling resulted in an impaired IL-9 production that correlated with an increased worm burden (16). Furthermore, our previous findings demonstrated that IL-25 can promote Th9 cell differentiation (20). IL-25 blockade resulted in alleviated allergic asthma that coincided with reduced Il9 expression in the lung (20). In this study, we showed that T. spiralis infection triggers early induction of Il25 as well as Tgfb and Il4 expression, which may be essential for the optimal generation of Th9 cells in vivo. Indeed, when IL-25 production was abrogated during T. spiralis infection, we detected an increased worm burden that was associated with a reduction of the frequency of antigen-specific Th9 and Th2 cells. Thus, early induction of IL-25 after T. spiralis infection may evoke protective immunity against the parasite through promoting the induction of Th9 and Th2 immune responses. Indeed, we demonstrated that the enhanced worm clearance driven by the IL-25-induced Th9 immune response occurred only in wild-type mice, not IL-9-deficient mice, suggesting that IL-9 function participates in IL-25-enhanced protective immunity to T. spiralis. Notably, we observed that IL-9-deficient mice were competent in T. spiralis worm clearance. Consistent with our findings, neutralization of IL-9 using anti-IL-9 antibody had no significant effect on worm expulsion, while overexpression of IL-9 or exogenous IL-9 treatment in mice resulted in accelerating worm expulsion in T. spiralis infection (10, 14, 28). It is likely that the upregulation of IL-9 expression induced by IL-25 may be important for the optimal induction of IL-4 and IL-13. Our results thus could not rule out the possibility that other Th2 cytokines may participate in the regulation of IL-25-induced protective immunity to T. spiralis. Previous studies showed that IL-33 can initiate IL-9 protein secretion in vitro in human CD4+ T cells (19). However, we observed that IL-33 expression was not significantly induced by T. spiralis infection.
Helminth genera and species possess distinct features that stimulate immune responses; therefore, a host may deploy different sets of defense mechanisms against these separate parasites. The principal function of cytokine IL-9 in the intestine is to regulate innate immune cells, including mast cells and epithelial cells. In the small intestine, IL-9 administration not only induced mast cell-specific genes but also upregulated innate immunity genes, including genes for Paneth cell markers such as angiogenin 4, cryptdins, and phospholipase A2 (11). Mast cells seem to be important for T. spiralis worm expulsion, while they have little involvement in N. braziliensis expulsion (1, 15, 29). The number of Paneth cells was found to be increased in the epithelial monolayers of T. spiralis-infected mice. Enhanced secretion of Paneth cell products such as cryptdins and other antimicrobial proteins shown to be regulated by mucosal T cells is expected to contribute to immunity against T. spiralis infection (30). Our finding that IL-9 is important for IL-25-enhancing protective immunity against T. spiralis infection prompted us to investigate the numbers of mast cells and the expression of mast cell protease- and Paneth cell-specific genes in the intestines following IL-25 cytokine or antibody treatment. The modulation of IL-25 production during T. spiralis infection could alter intestinal expression of mast cell protease genes (Mcpt1 and Mcpt2) and Paneth cell-specific genes (Cryptdins and Ang4), which was positively correlated with the changes in the antigen-specific Th9 response, thus linking the role of IL-25 in regulating IL-9-mediated effector function. Indeed, our finding that IL-25 treatment failed to induce the increased expression of those IL-9-targeted genes in mice deficient in IL-9 further substantiates the role of the IL-25/IL-9 axis in promoting the function of mast cells and Paneth cells, which leads to protective immunity against T. spiralis infection. In contrast to our findings, the number of mast cells and level of mouse mast cell protease 1 were not changed in the guts of IL-25-deficient mice infected with T. muris (7). It has been reported that a mucosal mast cell response was not required for protection against infection with T. muris (31). Indeed, the specific intestinal habitats of parasites may influence types of effector immune responses. T. muris eggs are hatched in the ileum of the small intestine, and the larvae then migrate to the cecum, where they invade the mucosal epithelial cells at the crest of the crypt, while T. spiralis larvae migrate to small intestinal sites at the base of villi, where they reside in a syncytium of epithelial cells (2). Together, our findings and other studies highlight the view that cytokines such as IL-25 may play a crucial role in mediating effective protective immune responses against distinct type of helminth infection.
In conclusion, we provide in vivo evidence that IL-25 promotes protective immunity against T. spiralis infection with a distinct pathway by inducing a Th9 cell response that drive intestinal mastocytosis and Paneth cell function. Future investigations on understanding the roles of type 2 innate lymphoid cells (ILC2), as well as Th9 and Th2 cells, in contributing to effective immune responses against parasite infection may provide novel insights into designing better approaches to prevent parasitic infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrew McKenzie (Medical Research Council, Laboratory of Molecular Biology, Cambridge, United Kingdom) for IL-9-deficient mice, Wanchai Maleewong and Pewpan Maleewong (Department of Parasitology, Faculty of Medicine, Khon Kaen University) for T. spiralis strain information. the Faculty of Allied Health Sciences, Thammasat University, for support, and Pattra Moonjit and the Faculty of Veterinary Medicine, Kasetsart University (Kamphaeng Saen Campus), for help with histology.
This work was supported by the Research Grant for New Scholar (cofunded by the Thailand Research Fund [TRF] and Commission on Higher Education, MRG5380229), the Coordinating Center for Thai Government Science and Technology Scholarship Students of the National Science and Technology Development Agency (CSTS, NSTDA), and the National Research University Project of Thailand, Office of the Higher Education Commission.
We declare no conflicting financial interests.
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00646-13.
REFERENCES
- 1.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505–533 [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Kreider T, Urban JF, Jr, Gause WC. 2009. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int. J. Parasitol. 39:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Else KJ, Finkelman FD. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145–1158 [DOI] [PubMed] [Google Scholar]
- 4.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985–995 [DOI] [PubMed] [Google Scholar]
- 5.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. 2007. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. 2007. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 204:1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203:843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. 2006. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203:1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao A, Urban JF, Jr, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. 2010. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J. Immunol. 185:6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner H, Renauld JC, Van Snick J, Grencis RK. 1998. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun. 66:3832–3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC. 2009. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 182:4737–4743 [DOI] [PubMed] [Google Scholar]
- 12.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. 2003. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. U. S. A. 100:7761–7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. 2000. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192:1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. 1997. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur. J. Immunol. 27:2536–2540 [DOI] [PubMed] [Google Scholar]
- 15.Ha TY, Reed ND, Crowle PK. 1983. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect. Immun. 41:445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. 2008. Transforming growth factor-beta ‘reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9:1341–1346 [DOI] [PubMed] [Google Scholar]
- 17.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. 2008. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 9:1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uyttenhove C, Brombacher F, Van Snick J. 2010. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur. J. Immunol. 40:2230–2235 [DOI] [PubMed] [Google Scholar]
- 19.Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. 2011. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One 6:e21695. 10.1371/journal.pone.0021695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. 2010. Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 11:250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozio E, Khamboonruang C. 1989. Trichinellosis in Thailand: epidemiology and biochemical identification of the aethiological agent. Trop. Med. Parasitol. 40:73–74 [PubMed] [Google Scholar]
- 22.Srimanote P, Ittiprasert W, Sermsart B, Chaisri U, Mahannop P, Sakolvaree Y, Tapchaisri P, Maleewong W, Kurazono H, Hayashi H, Chaicumpa W. 2000. Trichinella spiralis-specific monoclonal antibodies and affinity-purified antigen-based diagnosis. Asian Pac. J. Allergy Immunol. 18:37–45 [PubMed] [Google Scholar]
- 23.Helmby H, Grencis RK. 2002. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. J. Immunol. 169:2553–2560 [DOI] [PubMed] [Google Scholar]
- 24.Ganeshan K, Bryce PJ. 2012. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J. Immunol. 188:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y, Wang W, Tong J, Pan Q, Long Y, Qian W, Hou X. 2009. Th17 cells influence intestinal muscle contraction during Trichinella spiralis infection. J. Huazhong Univ. Sci. Technol. Med. Sci. 29:481–485 [DOI] [PubMed] [Google Scholar]
- 26.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, Gery I. 2010. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J. Immunol. 185:6795–6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. 2011. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12:1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. 2003. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect. Immun. 71:2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh H, Ide H, Ishikawa N, Nawa Y. 1994. Mast cell protease inhibitor, trypstatin, is a fragment of inter-alpha-trypsin inhibitor light chain. J. Biol. Chem. 269:3818–3822 [PubMed] [Google Scholar]
- 30.Kamal M, Wakelin D, Ouellette AJ, Smith A, Podolsky DK, Mahida YR. 2001. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin. Exp. Immunol. 126:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama K, Ito Y. 2000. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunol. 22:13–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.