Abstract
The present study was undertaken to understand the role of vaccine candidates PhtD and PhtE in pneumococcal nasopharyngeal (NP) colonization, their ability to induce CD4 T cell memory and antibody responses following primary NP colonization, and their contribution to protection against secondary pneumococcal colonization in mice. The study was also aimed at understanding the potential of immunization with PhtD and PhtE in eliciting qualitative CD4 T cell memory responses and protection against pneumococcal NP colonization in mice. PhtD and PhtE isogenic mutants in a TIGR4 background (TIGR4 ΔPhtD and TIGR4 ΔPhtE) were constructed and found to have a significantly reduced colonization density over time in the nasopharynges of mice compared to those of mice colonized with wild-type TIGR4. Mice with primary colonization by wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE were protected against secondary colonization by wild-type TIGR4; nonetheless, the clearance of secondary colonization was slower in mice with primary colonization by either TIGR4 ΔPhtD or TIGR4 ΔPhtE than in mice with primary colonization by wild-type TIGR4. Colonization was found to be an immunizing event for PhtD and PhtE antigens (antibody response); however, we failed to detect any antigen (PhtD or PhtE)-specific CD4 T cell responses in any of the colonized groups of mice. Intranasal immunization with either PhtD or PhtE protein generated robust serum antibody and CD4 Th1-biased immune memory and conferred protection against pneumococcal colonization in mice. We conclude that PhtD and PhtE show promise as components in next-generation pneumococcal vaccine formulations.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial pneumonia, meningitis, and septicemia, causing high morbidity and mortality worldwide, especially among children (1). While the success of pneumococcal conjugate vaccines (PCVs) has been substantial, their high manufacturing complexity and costs limit their use in developing nations, where the health consequences of pneumococcal disease are the highest. Additionally, there are over 90 identified pneumococcal serotypes, and the regional distribution of predominant serotypes varies. Therefore, an affordable vaccine that confers broad, preferably serotype-independent protection from pneumococcal disease remains a major global health priority (2, 3).
Nasopharyngeal (NP) colonization with pneumococcus is common in young children and a crucial first step in the pathogenesis of all pneumococcal diseases (4). Although colonization with pneumococci is mostly asymptomatic, it can progress to respiratory (pneumonia) or even systemic (bacteremia, meningitis) diseases as a result of a temporary defect in mucosal barrier function, e.g., as a result of an upper respiratory viral infection (5, 6). Although capsular serotype-specific antibody responses to PCV formulations have resulted in the widespread reduction of NP carriage and associated invasive pneumococcal diseases (IPDs) in children (3, 7), the duration of pneumococcal carriage is unaffected by PCVs (8). Moreover, without immunization with PCVs, the duration of carriage and the IPD incidence decline several years before naturally acquired serum anticapsular antibody becomes detectable in most children (9, 10). Those studies suggest that additional mechanisms of acquired immunity besides anticapsular antibodies are at play in protection against NP colonization.
Experiments in mouse models have shown that CD4 T cell-mediated immunity has an important role in host immune defense against pneumococcal colonization following immunization with whole-cell vaccine (WCV) (11). Studies of colonization, antibody acquisition, and the relationship with otitis media also suggest that naturally induced antibodies to pneumococcal protein antigens may be protective against disease (12). In fact, in an experimental human pneumococcal carriage model, antibodies to pneumococcal surface protein A (PspA) were inversely correlated with susceptibility to NP carriage (13). A recent experimental human carriage study also explained that mucosal and systemic immunological responses generated as a result of carriage conferred protection against recolonization and invasive pneumococcal disease (14).
A number of pneumococcal surface antigens, i.e., PspA, PsaA, CbpA, Phts, and other proteins, such as pneumolysin and heat shock proteins, have been implicated to be important virulence factors and to play a role in pneumococcal pathogenesis (15–19). Some of these antigens have been shown to be protective against pneumococcal infections in mice (20–22) and to elicit antibody responses against NP colonization in humans (23, 24) and have entered human clinical trials. Though it is well established that several pneumococcal surface antigens confer significant protection in mouse models of pneumococcal infection, the correlate of protection for these antigens remains unresolved. Studies with a pneumococcal WCV have established CD4 Th17-based vaccine-induced immunity to be a correlate of protection against pneumococcal colonization in mice (11). Prior pneumococcal NP colonization in humans is considered to be a protective event for subsequent colonization with the same pneumococcal serotype even before the acquisition of capsular antibodies, suggesting a role for capsular antibody-independent mechanisms of protection against pneumococcal colonization (9). A recent study on experimental human carriage reveals that pneumococcal carriage can increase the proportion of lung interleukin-17A (IL-17A)-secreting CD4+ memory T cells, which may enhance innate cellular immunity against pathogenic challenge (25).
The aim of current study was to comprehensively investigate the role of the histidine triad proteins PhtD and PhtE in pneumococcal NP colonization, colonization-induced immune responses, their role in protection against secondary pneumococcal colonization, and the ability of PhtD and PhtE immunization to elicit CD4 T cell memory responses and protection against pneumococcal colonization. Data reveal that PhtD and PhtE play an important role in pneumococcal colonization and that the PhtD- and PhtE-induced immune response potentially plays some role in primary colonization-induced protection against secondary pneumococcal colonization. Also, PhtD and PhtE intranasal immunization elicits potent CD4 T cell memory and antibody responses in mice and provides protection against pneumococcal colonization.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and reagents.
Pneumococci were grown in manganese-depleted Todd-Hewitt yeast (THY) broth. THY medium was prepared according to the manufacturer's instructions with Chelex-100 (2% [wt/vol]; Sigma-Aldrich, St. Louis, MO), which was added prior to autoclaving. After autoclaving, the THY medium-Chelex mixture was stirred overnight at room temperature and then filter sterilized. Prior to use, the medium was supplemented with 1 mM FeSO4, CaCl2, MgCl2, and ZnCl2 and 0.1 μM MnSO4. Pneumococci were grown at 37°C in the presence of 5% CO2 and harvested at an optical density at 600 nm (OD600) of 0.6. Pneumococcal recombinant proteins (PhtD, N-terminally truncated PhtE, and PcpA) were provided by Sanofi Pasteur. Recombinant choline binding protein A (CbpA) was a gift from Elaine Tuomanen (St. Jude Medical Center). Wild-type TIGR4, TIGR4 ΔPhtD, TIGR4ΔPhtE, and TIGR4ΔPcpA strains were constructed as described earlier (26, 27).
Characterization of mutants.
Mutants (TIGR4 ΔPhtD and TIGR4 ΔPhtE) were characterized by real-time PCR and flow cytometry to study the potential polar effect caused by the introduction of antibiotic cassette. Wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE cultures were grown overnight and reinoculated the next morning as fresh cultures at a dilution of 1:100. Log-phase cultures were harvested; DNA-free RNA was extracted using an RNA minikit (Ambion) and subsequently converted to cDNA using SuperScript III reverse transcriptase (Invitrogen). Real-time PCR was carried out to study the expression of the YfnA, lmb, PhtD, PhtE, PhtF, PspA, PsaA, CbpA, Ply, PcpA, and LytB genes in wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE. For the flow cytometry study, 1 × 107 log-phase bacteria from wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE cultures were incubated with PhtD- and PhtE-specific monoclonal antibody (mouse IgG1) at a dilution of 1:100 and incubated at 37°C for 1 h. Bacteria were washed and incubated with goat anti-mouse secondary antibody conjugated to fluorescein isothiocyanate (FITC) at a dilution of 1:500 at 37°C for 30 min. Bacteria were washed and read on a BD Biosciences LSR II flow cytometer by acquiring 20,000 events. Data were analyzed by FlowJo software (Tree Star).
Animals.
Five-week-old male C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD). The study was carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee (IACUC) for the care and use of animals for scientific purposes (RGHRI protocol approval number 2012-001). All animals were housed in an appropriate biosafety level 2 (BSL-2) containment facility.
Pneumococcal colonization model.
Mice were anesthetized with 5% (vol/vol) isoflurane USP (Isocare) over oxygen (1 liter/min), and 10 μl of phosphate-buffered saline (PBS) containing 107 CFU of wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE pneumococci was distributed equally between both nostrils. On days 7, 14, 21, and 28, groups of 5 mice per time point were euthanized by CO2 inhalation and an upper respiratory wash was done by instilling sterile, nonbacteriostatic saline retrograde through the transected trachea and collecting the first 6 drops (about 200 μl) from the nostrils. The nasal wash from each group was serially diluted, plated on blood agar containing gentamicin, and incubated at 37°C in 5% CO2, and the numbers of CFU were determined the next day.
In order to confirm that the infection dose confined locally in the nasopharynx, blood and lungs were taken out from colonized mice at every time point, homogenized, and plated on blood agar plates containing gentamicin (BD Biosciences). Mice from each group were anesthetized and bled through their lateral tail vein on day 21 postcolonization. All groups of mice (colonized with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE) were recolonized with wild-type TIGR4 on day 35 (1 week after the primary colonization was cleared) to study the primary colonization-induced protection against secondary colonization. The CFU burden in nasal lavage fluid was studied on days 2 (48 h) and 4 after secondary colonization.
Immunizations and pneumococcal challenge.
C57BL/6 mice (age, 6 weeks; n = 5 mice per group) were immunized with PhtD or PhtE at day 0, followed by two boosters on days 14 and 28 by an intranasal route with 4 μg of PhtD or PhtE supplemented with 0.2 μg of Escherichia coli labile toxin (LT) as an adjuvant in the 10-μl volume. Control mice were also injected intranasally with LT (0.2 μg) alone in the same volume. One week after the second booster immunization, mice were bled through the tail vein as described above and challenged intranasally a week later with 107 CFU of the pneumococcal strain TIGR4 in a 10-μl volume as described above. Mice were euthanized on days 2 and 4 after intranasal challenge, and the bacterial load was enumerated by plating serially diluted nasal lavage fluid on blood agar plates containing gentamicin.
Determination of antibody response.
The antigen-specific antibody titers in serum samples from colonized mice (endpoint titers) and immunized mice (quantitative titers) were determined by enzyme-linked immunosorbent assay (ELISA). PhtD, PhtE, PcpA, PspA (family 2), and CbpA recombinant proteins were coated on 96-well plates at a concentration of 1 μg/ml each in coating buffer (1 μg/ml was determined to be the optimal coating concentration in preliminary experiments). After blocking with 3% skim milk, diluted serum samples were added to the wells and the mixture was incubated at room temperature for 1 h. Affinity-purified goat anti-mouse IgG antibody conjugated to horseradish peroxidase was used as the secondary antibody. The reaction products were developed using a tetramethylbenzidine microwell peroxidase substrate system. The reactions were stopped by the addition of 1.0 M phosphoric acid, and the results were read by an ELISA reader at 450 nm. To provide quantitative results of antibody concentrations, the level of the specific antibody present in serum samples was determined by comparison with mouse reference serum (Bethyl Laboratories) included on the same ELISA plates. The IgG titers were expressed as ng/ml. Endpoint titers in sera from colonized mice were calculated from the lowest serum dilution that gave an OD450 of 0.05.
Cell isolations and stimulations.
Mice were euthanized by CO2 inhalation as described above, and spleens were isolated aseptically under BSL-2 containment. Cells were prepared by passage through a 70-μm-pore-size cell strainer (BD Falcon) and placed into RPMI 1640 (Sigma) supplemented with 10% (vol/vol) fetal calf serum (FCS) and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin; Sigma). Following washing by centrifugation at 300 × g for 8 min, cells were resuspended at 5 × 106/ml for assays. One million cells were used for stimulations (both intracellular staining and cytometric bead array) and cultured in the 5% CO2 incubator at 37°C under humidified conditions. One microgram of either PhtD or PhtE was used for stimulation, and phorbol myristate acetate (PMA)-ionomycin (25 ng and 1 μg; Sigma) was used as a positive control in the assay. Pneumococci were grown to mid-log phase, pelleted, washed twice, heat inactivated at 65°C for 10 min, and subsequently used for stimulation at a multiplicity of infection (MOI) of 1:10. No live bacteria were detected after the heat-killed suspension was plated onto blood agar plates.
Intracellular cytokine staining (ICS).
Splenocytes derived from immunized or colonized mice were cultured in 200 μl RPMI 1640 and supplemented with 10% (vol/vol) FCS and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin; Sigma). Splenocytes from colonized mice (colonized with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE) were stimulated with recombinant PhtD or PhtE or heat-killed pneumococci (TIGR4) for 4 h at 37°C in 5% CO2. Splenocytes from PhtD-, PhtE-, or adjuvant (control)-immunized mice were stimulated with either PhtD (splenocytes from PhtD-immunized mice) or PhtE (splenocytes from PhtE-immunized mice) as explained above. Brefeldin A was added at 10 μg/ml, and stimulated cells were further incubated for 16 h (total stimulation time, 20 h). For a positive control, PMA and ionomycin were added together with brefeldin A and the mixture was incubated for 16 h. Cells were washed (300 × g for 5 min) with fluorescence-activated cell sorting (FACS) buffer (PBS plus 5% FBS) and surface stained with CD4 (allophycocyanin [APC]), CD3 (APC-Cy7), CD44 (phycoerythrin [PE]), and CD62L (FITC). Subsequently, the cells were washed, treated with Cytofix/Cytoperm (BD Biosciences) according to the manufacturer's instructions, and stained intracellularly for gamma interferon (IFN-γ; peridinin chlorophyll protein, PE-Cy5.5), IL-2 (Pacific Blue), IL-4 (PE-Texas Red), and IL-17A (Alexa Fluor 700). Both surface and intracellular staining reactions were performed at room temperature for 20 min in the dark, as described earlier (28). All antibody conjugates were purchased from BioLegend, except for CD44 (BD Biosciences). After intracellular staining, cells were further washed 3 times with permeabilization buffer and given a final wash with FACS buffer before they were resuspended in the FACS tubes. A BD Biosciences LSR II flow cytometer (12 colors) was used to collect 200,000 events for each sample, and data were analyzed using FlowJo (Tree Star) software. Gates for cytokine-positive cells were determined on the basis of observations with unstimulated and PMA-ionomycin-stimulated cells. During initial standardization in intracellular cytokine assays, we used an unrelated divergent bacterial protein antigen (protein D of nontypeable Haemophilus influenzae) as a control and found that there was no difference between stimulations by either unrelated antigen or medium (data not shown). Thereafter, in the subsequent assays, medium was added to the unstimulated cells in the same volume as antigen and the cells were termed the unstimulated controls.
Cytometric bead array (CBA).
Splenocytes from colonized mice (colonized with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE) were cultured in 200 μl as described above and stimulated with recombinant PhtD, PhtE, pneumococci, or Staphylococcus enterotoxin B (SEB; positive control) for 36 h at 37°C in 5% CO2. Cell supernatants were collected and stored at −80°C until cytokine testing was performed. IFN-γ and IL-17A were detected simultaneously using a mouse cytometric bead assay kit (BD Biosciences Pharmingen, San Diego, CA) as per the manufacturer's instructions. The data were expressed as pg/ml.
CD4+ T cell proliferation assay.
Mice immunized with PhtD plus adjuvant, PhtE plus adjuvant, or adjuvant only (control) were euthanized, and their spleens were aseptically taken out. Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) dye (eBioscience) according to the manufacturer's instructions, followed by stimulation with either PhtD or PhtE (5 μg/ml) or concanavalin A (ConA; positive control, 5 μg/ml) (1 million cells per stimulation) and incubation at 37°C in a humidified incubator with 5% CO2 for 7 days, as described earlier (29). After the incubation, cells were stained with anti-human CD4-APC antibody (BioLegend) for 20 min at room temperature prior to acquisition on a BD LSR II (20,000 events). Data were subsequently analyzed with FlowJo software (TreeStar), and antigen-induced CD4 T cell proliferation was compared with that for an unstimulated control (treated with medium only).
Statistical analysis.
Data were analyzed using the GraphPad Instant (version 3) statistical package (GraphPad Software). A P value of <0.05 was considered significant in all the comparisons. The mice for experiments whose results are presented in Fig. 2 and 4 were colonized together on day 0 and used further for subsequent studies. NP colonization densities were compared by the use of the Kruskal-Wallis test, and when the results were found to be significant, each colonization group was then compared to the group colonized with the wild type (see Fig. 2) or adjuvant (see Fig. 9) by the use of Dunn's correction for multiple comparisons.
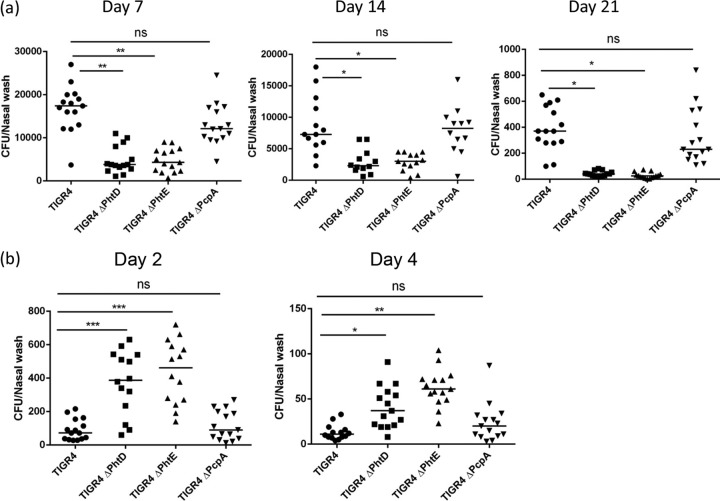
Fig 2.
(a) Nasopharyngeal carriage of wild-type TIGR4, TIGR4 ΔPhtD, TIGR4 ΔPhtE, and TIGR4 ΔPcpA. Twenty mice per group were intranasally infected with wild-type TIGR4, TIGR4 ΔPhtD, TIGR4 ΔPhtE, or TIGR4 ΔPcpA (107 CFU/mouse), and the comparative density and duration of pneumococcal colonization between wild-type TIGR4 and TIGR4 ΔPhtD, TIGR4 ΔPhtE, and TIGR4 ΔPcpA were studied at days 7, 14, and 21. Data were analyzed by the Kruskal-Wallis nonparametric test for differences among groups (P < 0.001), followed by the post hoc Dunn's test for differences between wild-type TIGR4 and a particular mutant group (TIGR4 ΔPhtD, TIGR4 ΔPhtE, or TIGR4 ΔPcpA). Data are representative of 3 separate experiments with 5 mice for each time point on days 7, 14, and 21 and represented as medians. **, P < 0.001; *, P < 0.01; ns, not significant (P > 0.05). (b) Role of prior pneumococcal colonization (with wild-type TIGR4, TIGR4 ΔPhtD, TIGR4 ΔPhtE, and TIGR4 ΔPcpA) in protection against secondary colonization with wild-type TIGR4. Ten mice per group were intranasally infected with wild-type TIGR4, TIGR4 ΔPhtD, TIGR4 ΔPhtE, or TIGR4 ΔPcpA (107 CFU) on day 0 and rechallenged intranasally with the same infection dose of wild-type TIGR4 for secondary colonization on day 35. The density of pneumococcal colonization on day 2 and day 4 is expressed as the number of CFU per nasal wash specimen. Data were analyzed by the Kruskal-Wallis nonparametric test for differences among groups (P < 0.001), followed by the post hoc Dunn's test for differences between wild-type TIGR4 and a particular mutant group (TIGR4 ΔPhtD, TIGR4 ΔPhtE, or TIGR4 ΔPcpA). Data are representative of 3 separate experiments with 5 mice for each time point and represented as medians. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, nonsignificant.
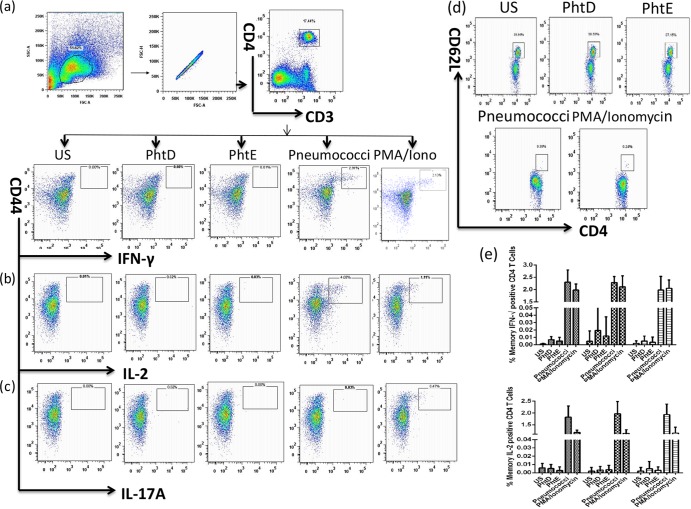
Fig 4.
PhtD- and PhtE-specific CD4 T cell responses following pneumococcal colonization. The gating strategy used to detect CD4+ T cell responses is shown. A flow cytometry 8-color panel was designed to simultaneously analyze IFN-γ-, IL-2-, IL-17A-, and IL-producing CD4+ T cell subsets from splenocytes of mice colonized with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE. Initial gating of total events included selection of lymphocytes on the basis of forward and side scatter properties, followed by gating of single cells and leaving of doublets or aggregates. (a [top]) The CD4 T cell population was gated as CD4+ CD3+ double-positive T cells, followed by gating of CD44hi CD4 T cells positive for cytokines. (a [bottom], b, and c) Dot plots representing CD4+ CD44hi T cells producing IFN-γ (a [bottom]), IL-2 (b), and IL-17A (c). (d) Expression of CD62L on activated CD4 T cells. iono, ionomycin. Dot plots expressed in panels a to d are for splenocytes from mice colonized with wild-type TIGR4. (e) Frequency of IFN-γ- and IL-2-producing antigen-specific CD4 T cells in mice colonized with wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE. Data for stimulations were normalized to total CD4+ T cell counts and further corrected for background with respective unstimulated (US) controls. Bars represent the mean (standard deviation) percent frequency of antigen-specific cytokine-producing CD4 T cells as a percentage of total CD4+ cells. Data represent 3 separate experiments, each with 5 mice per group. One-way analysis of variance was used for comparing significance among groups (P > 0.05).
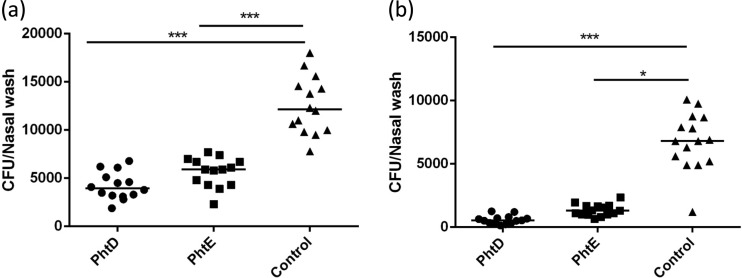
Fig 9.
Role of PhtD and PhtE immunization-induced protection against pneumococcal colonization. The same group of mice used in the experiments whose results are explained in Fig. 5 (10 mice/group) were intranasally challenged 2 weeks after the second booster with 1 × 107 wild-type TIGR4 (the same infection dose used for colonization). Mice were euthanized, and the pneumococcal load in nasal lavage fluid was determined on day 2 (a) and day 4 (b) after intranasal challenge. Data were analyzed by the Kruskal-Wallis test for overall significance among groups (P < 0.001 in panels a and b) and the post hoc Dunn's test for comparison of controls (adjuvant immunized) with either the PhtD-immunized or the PhtE-immunized group of mice. ***, P < 0.001; *, P < 0.01.
RESULTS
Characterization of PhtD and PhtE mutants.
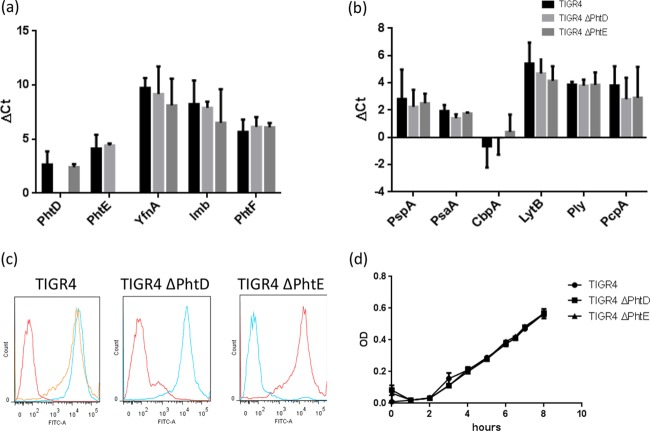
TIGR4 ΔPhtD and TIGR4 ΔPhtE were characterized for any potential polar effect that may have been caused by gene disruption and introduction of an antibiotic resistance cassette in the TIGR4 background. Though the PhtD and PhtE genes are organized as a pair in the TIGR4 genome, they potentially have their own promoters. The genome organization suggested that while the PhtD gene is the last gene of a polycistronic operon consisting of YfnA, Lmb, and PhtD, the PhtE gene is expressed as a bicistronic message with its own promoter (18). Therefore, in order to understand whether the disruption of either the PhtD or the PhtE gene had any effect on the expression of downstream genes, we analyzed the expression of genes downstream of the genes for PhtD and PhtE by real-time PCR. Figure 1a demonstrates that there was no difference in the expression of either the PhtE gene (downstream of the PhtD gene) in TIGR4 ΔPhtD or the PhtF gene (downstream of the PhtE gene) in TIGR4 ΔPhtE compared to their expression in wild-type TIGR4. We also compared the expression of other genes in the close vicinity of PhtD, like YfnA and Lmb, and found that their expression, too, remained unchanged compared to that by the control (Fig. 1a). The comparative expression of the PhtD and PhtE genes in the wild-type and mutant groups was corroborated by flow cytometry and found to be consistent with the findings of real-time PCR (Fig. 1c). In order to further study whether the disruption of one gene was compensated for by the overexpression of other genes in TIGR4 ΔPhtD or TIGR4 ΔPhtE, we checked the comparative expression levels of six other important antigens of pneumococci in wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE by real-time PCR. Figure 1b demonstrates that the expression levels of six important antigens (PspA, CbpA, Ply, PcpA, LytB, and PsaA) in wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE were not significantly different. Finally, we also compared the growth kinetics of wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE and found that there was no difference between the growth rates of wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE (Fig. 1d). Taken together, these data demonstrate the lack of polar effects in TIGR4 ΔPhtD and TIGR4 ΔPhtE and indicate that bacterial physiology and growth remain unaltered in the mutants.
Fig 1.
Characterization of PhtD and PhtE mutants. (a, b) RNA was extracted from log-phase wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE, and cDNA was subsequently synthesized. Real-time PCR was set up and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), along with the genes of interest (the YfnA, Lmb, PhtD, PhtE, PhtF, PspA, PsaA, Ply, CbpA, LytB, and PcpA genes), was used as a housekeeping gene. Results are expressed as the mean and standard deviation of the change in the threshold cycle (ΔCT) from the changes in the threshold cycles for wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE. One-way analysis of variance was used to find the significance in gene expression among TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE (P > 0.05). (c) Log-phase bacteria (1 × 107) from wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE cultures were separately incubated with anti-PhtD and anti-PhtE antibodies for 1 h at 37°C. Bacteria were washed twice and incubated with anti-mouse FITC secondary antibody for 30 min at 37°C. Stained bacteria were read on an LSR II flow cytometer by acquiring 20,000 events. Data were analyzed by FlowJo software (Tree Star). The first histogram of the panel represents wild-type TIGR4 unstained and stained with anti-PhtD and anti-PhtE monoclonal antibodies (geometric mean fluorescence intensities, 85, 3,412, and 3,127); the second histogram (geometric mean fluorescence intensities, 213 and 3,217) and the third histogram (geometric mean fluorescence intensities, 200 and 3,370) represent TIGR4 ΔPhtD and TIGR4 ΔPhtE, respectively, stained with anti-PhtD and anti-PhtE antibodies. (d) Wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE were grown overnight at 37°C in a humidified CO2 incubator. On the next morning, cultures were reinoculated 1:100 in fresh medium and allowed to grow further. The growth kinetics of all three cultures was established by assessing the ODs at periodic intervals. Data represent the means and standard deviations of the ODs of 2 separate experiments. One-way analysis of variance was used to find the significance among TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE (P > 0.05).
PhtD and PhtE play a role in pneumococcal colonization.
Wild-type TIGR4 pneumococci were carried in the nasopharynges of all mice for at least 21 days, but colonization was no longer detectable at 28 days postinoculation (lower limit of detection, 20 CFU/ml). At no point did the mice develop any sign of morbidity, and both lungs and blood were clear of pneumococci at every time point evaluated during the course of NP colonization. To understand the effect of PhtD and PhtE on pneumococcal carriage, TIGR4 ΔPhtD and TIGR4 ΔPhtE were used at the same intranasal challenge dose (10 million cells in a 10-μl volume) as the wild type. The bacterial density in nasal lavage fluid at the first time point tested (day 7) was significantly lower for TIGR4 ΔPhtD (P < 0.001) and TIGR4 ΔPhtE (P < 0.001) (Fig. 2a). Similarly, on days 14 and 21, the bacterial density remained significantly lower for both TIGR4 ΔPhtD (P < 0.01) and TIGR4 ΔPhtE (P < 0.01) (Fig. 2a). It is interesting to note, though, that the colonization density was significantly reduced in mice colonized with TIGR4 ΔPhtD or TIGR4 ΔPhtE than in mice colonized with the wild type but that the duration of their carriage was not largely affected (Fig. 2a). As a control, we studied the colonization dynamics of a TIGR4 strain with a deletion of PcpA, another pneumococcal surface protein of interest. Consistent with the previous finding by Glover et al. (27), there was no significant drop in the rate of carriage of the PcpA mutant (P > 0.05; Fig. 2a) compared to that of the isogenic wild-type strain (6) at any time point.
The experiments presented in Fig. 2a and b were set up together by infecting mice for primary colonization on day 0. The comparative bacterial densities and durations of colonization in nasal lavage fluid of mice colonized with wild-type TIGR4, TIGR4 ΔPhtD, TIGR4 ΔPhtE, and TIGR4 ΔPcpA were studied on days 7, 14, and 21. The remaining mice from each colonized group (10 per group) were rechallenged on day 35 to study the protection against secondary colonization. The bacterial load in nasal lavage fluid was determined on days 2 and 4 after secondary colonization.
PhtD and PhtE play a role in the reduction of bacterial density following secondary NP colonization.
To assess the impact of PhtD and PhtE in protection against secondary pneumococcal colonization, recolonization studies were performed in all groups of mice that cleared primary colonization on day 28. One week following the clearance of primary colonization (day 35), mice colonized with TIGR4 ΔPhtD, TIGR4 ΔPhtE, TIGR4 ΔPcpA, and wild-type TIGR4 were recolonized with wild-type TIGR4 pneumococci and observed for 7 days. In contrast to the colonization of naive mice (Fig. 2a), there was a significant reduction in the density and duration of secondary colonization in the nasopharynges of mice previously colonized with wild-type TIGR4 pneumococci by day 4 (Fig. 2b), demonstrating that primary colonization induced protection against secondary colonization. Mice colonized primarily with TIGR4 ΔPhtD and TIGR4 ΔPhtE also had a huge reduction in bacterial density by day 4 (Fig. 2b), but the colonization density (for secondary colonization) was still higher than that for the wild-type TIGR4-colonized group (day 2, P < 0.001 for TIGR4 ΔPhtD and TIGR4 ΔPhtE; day 4, P < 0.05 for TIGR4 ΔPhD and P < 0.01 for TIGR4 ΔPhtE) (Fig. 2b), suggesting that an immune response to PhtD or PhtE might potentially contribute to some degree to protection against secondary pneumococcal colonization. The colonization density in mice previously colonized with TIGR4 ΔPcpA was not significantly different from that in mice previously colonized with wild-type TIGR4 (P > 0.05).
Pneumococcal colonization is an immunizing event for PhtD and PhtE antigens.
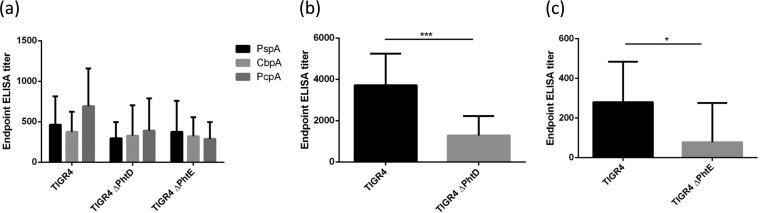
To test whether NP colonization with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE induced antibodies against pneumococcal antigens, blood was collected from colonized mice on day 21 postcolonization (the same colonized mice for which the results are expressed in Fig. 2) and sera were analyzed by ELISA. In the wild-type-colonized group, among the various pneumococcal antigens for which colonization-induced antibodies were tested, PhtD was found to elicit the most robust antibody response (lower 95% confidence interval [CI] of geometric means ± upper 95% CI of geometric means, 2,388.0 ± 4,788). There were low but detectable titers against all the tested antigens: PspA (family 2; 220.3 ± 613.4), TIGR4-derived CbpA (145 ± 535.1), PhtE (139.8 ± 366.4), and PcpA (306.25 ± 982.1) (Fig. 3). It is interesting to note that although PcpA did not play a role in colonization, as described above, antibodies against PcpA were detected, suggesting that the expression of PcpA in the nasopharynges led to the generation of PcpA-specific antibodies. There was also generation of antigen-specific antibodies against PspA, CbpA, and PcpA in the mutant-colonized groups, with no significant difference in the titers between the wild-type TIGR4-, TIGR4 ΔPhtD-, and TIGR4 ΔPhtE-colonized mice being detected (P > 0.05) (Fig. 3a). However, antibodies against PhtD were detected in mice colonized with TIGR4 ΔPhtD, although the titers were significantly reduced compared to those in the wild-type TIGR4-colonized mice (Fig. 3b). The anti-PhtE titers in the group of mice colonized with the TIGR4 ΔPhtE mutant were very low and close to the lower detection limit in the mice (Fig. 3c).
Fig 3.
(a) Endpoint ELISA titers of IgG antibodies against PspA, CbpA, and PcpA on day 21 in mice colonized with WT TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE. (b, c) Anti-PhtD titers in mice colonized with TIGR4 or TIGR4 ΔPhtD (b) and anti-PhtE titers in mice colonized with TIGR4 and TIGR4 ΔPhtE (c). Mice from the same groups used for the experiments whose results are expressed in Fig. 2 were bled on day 21, and ELISA was performed to calculate their endpoint antigen-specific IgG titers. Data are representative of those from 3 separate colonization experiments (5 mice per group in each experiment) and are the geometric mean titer with the 95% CI. Endpoint titers were calculated as the last dilution to give an absorbance at 450 nm of 0.05. Statistical significance in titers among groups (Fig. 3a) was derived from one-way analysis of variance (P > 0.05). The Student t test was used to compare the titers expressed in Fig. 3b and c. ***, P < 0.001; *, P < 0.04.
CD4 T cell memory response against PhtD and PhtE after pneumococcal colonization.
To assess whether pneumococcal colonization induces an antigen-specific CD4 T cell memory response, mice were euthanized and spleens from colonized mice (colonized with wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE) were isolated 1 week after the NP colonization was cleared. Colonization experiments for this part were set up together with the colonization studies expressed in Fig. 2. The splenocytes were stimulated with PhtD, PhtE, heat-killed pneumococci, or PMA-ionomycin (positive control), and subsequently, intracellular cytokine staining was performed for T helper memory cell subsets producing IL-2, IFN-γ, IL-17, and IL-4 by 8-color flow cytometry. CD3, CD4, CD44, and CD62L markers were used for the phenotypic characterization of cytokine-producing CD4 T cell subsets and to discriminate between naive and memory phenotypes. The percentage of cytokine-producing CD4 T cells was calculated by gating CD3+ CD4+ double-positive cells, followed by gating CD44hi and cytokine-producing CD4 T cells (Fig. 4a). Figures 4a and b demonstrate a high frequency of IFN-γ- and IL-2-producing CD4 T cells derived from the CD4+ CD44hi phenotypic subset against whole heat-killed pneumococci in all colonized groups.
Splenocytes stimulated by coculture with heat-killed whole bacteria apparently downregulated the expression of CD62L (Fig. 4d), and T helper cytokine-producing subsets were CD4+ CD62low. Further staining and analysis of these cytokine-producing CD4 T cells by ICS revealed a fraction to be multifunctional for IFN-γ and IL-2 production (0.12%; data not shown). The activation of CD4 T cells by PhtD and PhtE was studied by determination of the expression of CD62L, and Fig. 4d demonstrates that the expression of CD62L remained unchanged in PhtD- or PhtE-stimulated CD4 T cells ex vivo. There was no detectable antigen-specific CD4 T cell response for IL-17A (Fig. 4c) or IL-4 (data not shown) even against whole bacteria. The frequency of cytokine (IFN-γ and IL-2)-producing CD4 T cells among the colonized groups (mice colonized with wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE) remained unchanged (Fig. 4e) (P > 0.05), suggesting that there was no difference in the level of cellular immunity in the colonized groups. As explained above, the antigen-specific responses (against PhtD, PhtE, and heat-killed pneumococci) were similar in the splenocytes of all colonized groups. The dot plots presented in Fig. 4a to d (to depict the pattern) are therefore representative of those for splenocytes from mice colonized with wild-type TIGR4 only.
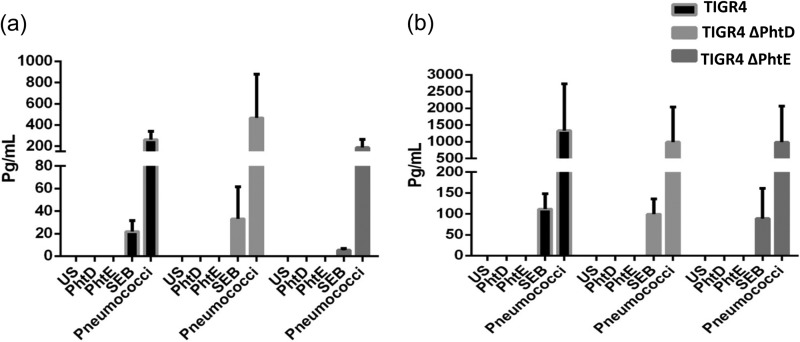
In order to further corroborate the results, a cytometric bead array was performed to analyze the IFN-γ and IL-17A responses. Splenocytes were stimulated with PhtD, PhtE, heat-killed pneumococci, or SEB for 36 h, and IFN-γ and IL-17A responses in stimulated cultured cell supernatant were detected by use of a flow cytometry-based BD multiplex bead array kit. Consistent with the findings presented above, there was no detectable cytokine response in culture supernatants of either PhtD- or PhtE-stimulated cells, but cells stimulated with heat-killed pneumococci elicited similar IL-17A (P = 0.33) and IFN-γ (P = 0.97) levels in all the colonized groups (Fig. 5a and b). A stimulation of 36 h was particularly chosen, as cytokines in culture supernatants derived from longer stimulations could be the result of a mix of both naive and memory responses, and therefore, an attempt was made to capture only antigen-specific memory responses.
Fig 5.
Cytokine bead array for IFN-γ and IL-17A in the culture supernatants of stimulated splenocytes derived from colonized mice; 5 mice per group were intranasally infected with wild-type TIGR4, TIGR4 ΔPhtD, and TIGR4 ΔPhtE on day 0. On day 35, mice were euthanized, their spleens were taken out and processed, and a single-cell suspension was prepared. One million splenocytes were used for stimulation with either PhtD, PhtE, heat-killed pneumococci, or SEB for 36 h. The supernatant was collected, and quantitation of IL-17A (a) and IFN-γ (b) was performed by using a BD multiplex cytokine kit. Data are expressed as means with standard deviations and are representative of one experiment with 5 colonized mice per group. One-way analysis of variance was used for comparing the overall significance among the colonized groups (P > 0.05).
PhtD elicits strong cross-reactivity to PhtB.
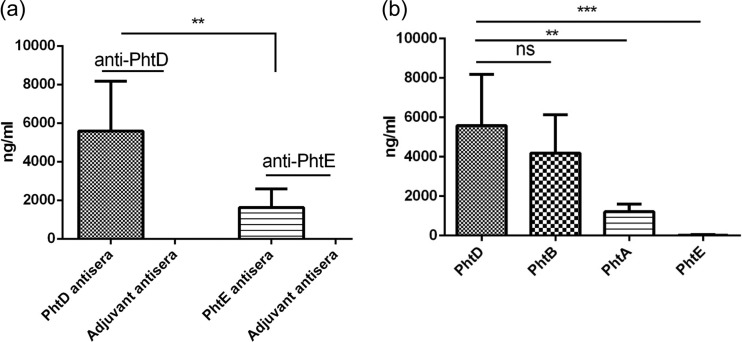
In our intranasal immunization model, PhtD evoked stronger antibody responses than PhtE after immunization (mean, 6528; lower 95% CI of geometric means ± upper 95% CI of geometric means, 3,821 ± 8,178) than PhtE (mean, 1,929; lower 95% CI of geometric means ± upper 95% CI of geometric means, 1,016 ± 2,587) (P = 0.002) (Fig. 6a). It was anticipated that PhtD would elicit antibodies to other Pht proteins because of their sequence homology. We assessed the cross-reactivity of immunization-induced anti-PhtD antibodies with other proteins of the Pht family. Figure 6b demonstrates that anti-PhtD antibodies cross-reacted most strongly with PhtB, followed by PhtA and PhtE. On the basis of this finding, it is plausible that the anti-PhtD antibodies observed in the serum of mice that were colonized with TIGR4 ΔPhtD are a result of cross-reactivity of anti-PhtB and anti-PhtA antibodies to PhtD. PhtD shares maximum sequence homology with PhtB, followed by PhtA, and has the least homology with PhtE, which is phylogenetically more divergent from the rest of the Pht proteins (18); however, it is to be noted that the PhtE used in the study was truncated.
Fig 6.
Anti-PhtD and -PhtE IgG titers in immunized mice. Ten mice per group were immunized with either PhtD plus adjuvant, PhtE plus adjuvant, or adjuvant alone on day 0, followed by two boosters on days 14 and 28. Mice were bled 1 week after the second booster, and analysis of anti-PhtD and anti-PhtE titers was performed by ELISA. Data are expressed as geometric means with 95% CIs and are representative of 3 separate experiments; the mice were later used for challenge studies (1 week after the bleed and 2 weeks after the second booster) (Fig. 9). (a) The first two columns represent the anti-PhtD titers in antisera from mice immunized with PhtD and adjuvant; the last two columns represent the anti-PhtE titers in antisera from mice immunized with PhtE and adjuvant. The Student t test was performed to compare the antibody titers expressed in panel a (**, P = 0.002). (b) Cross-reactivity of PhtD antisera to other Pht proteins. ns, not significant (P = 0.31); **, P = 0.005; ***, P = 0.0001.
Immunization with PhtD and PhtE elicits CD4 T cell memory generation.
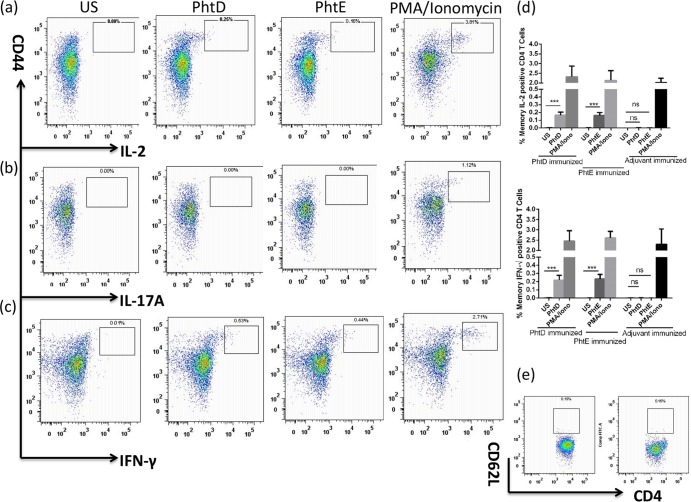
To assess whether immunization with PhtD or PhtE could induce antigen-specific CD4 T cell memory responses, even though natural colonization did not appear to do so, spleens from immunized mice were aseptically removed 1 week after the second booster and stimulated with PhtD, PhtE, or PMA-ionomycin (positive control). We used a multiparameter flow cytometry 8-color panel as described above for detailed immunophenotypic analyses and characterization of cytokine-producing CD4 T cell subsets. CD62Llow and CD44hi CD4 T cells were gated for the evaluation of antigen-specific responses. Cells were analyzed for antigen-specific IFN-γ-, IL-2-, IL-17A-, and IL-4-producing CD4 T cell subsets. Figure 7 demonstrates that immunization with PhtD and PhtE activated CD4 T cell memory for IL-2 (Fig. 7a) and IFN-γ (Fig. 7c). The frequency of IFN-γ- and IL-2-producing CD4 T cell subsets was significantly higher in PhtD- or PhtE-stimulated cells than in their unstimulated controls (P < 0.001) (Fig. 7d). We could not detect IL-17A- (Fig. 7b) or IL-4-producing (data not shown) CD4 T cells in any of the samples from immunized mice, suggesting the predominance of a Th1 response. Dot plots expressed in Fig. 7a to c are representative of those for stimulated splenocytes derived from either PhtD-immunized (PhtD and PMA-ionomycin dot plots) or PhtE-immunized (PhtE dot plots) mice.
Fig 7.
Evaluation of the antigen-specific CD4 T cell response in PhtD- and PhtE-immunized mice. Mice were intranasally immunized with PhtD plus adjuvant, PhtE plus adjuvant, or adjuvant alone (5 mice per group), with two boosters given on days 14 and 28. One week after the second booster, antigen-specific CD4 T cell responses were evaluated in spleens by intracellular cytokine staining by using an 8-color panel. Mice were euthanized, their spleens were taken out and processed, and a single-cell suspension was prepared. One million splenocytes were used for stimulation with either PhtD, PhtE, or PMA-ionomycin (positive control). Data represent the means of 3 separate experiments with standard deviations. (a to c) Dot plot representation of cytokine-producing CD44hi CD4 T cells for IL-2 (a), IL-17A (b), and IFN-γ (c). The PhtD and PhtE dot plots were derived from splenocytes from mice immunized with PhtD and PhtE, respectively. The PMA-ionomycin dot plots were derived from mice immunized with PhtD. (d) Antigen-specific IFN-γ- and IL-2-producing CD4 T cell frequencies in mice immunized with PhtD plus adjuvant, PhtE plus adjuvant, and adjuvant only (control). The bars represent the mean (standard deviation) percent frequency of antigen-specific cytokine-producing cells as a percentage of total CD4+ cells. The Student t test was used for comparing antigen-stimulated cells with their respective unstimulated controls. ***, P < 0.001; ns, not significant (P > 0.2). (e) CD62L expression on ex vivo unstimulated CD4 T cells from PhtD-immunized (PhtD dot plot) and PhtE-immunized (PhtE dot plot) mice (e).
Polyfunctional CD4 T cells that simultaneously produced IFN-γ and IL-2 were also assessed, and a small subset of cells from both PhtD- and PhtE-immunized mice showed polyfunctionality (0.07% ± 0.02%; data not shown). Interestingly, CD4 T cells from the spleens of immunized mice had an apparent downregulation of CD62L expression suggestive of PhtD or PhtE immunization-induced in vivo activation of cells (Fig. 7e). Downregulation of CD62L indicates that cells were activated at some stage of their differentiation, although the cells may not have acquired effector function. Most of the cytokine-producing cells were derived from CD44hi and CD62low subsets of CD4 T cells. The concept of multifunctionality has not been explored in the case of pneumococcal infection or vaccine-induced protection against pneumococcal infections. Nonetheless, the potential of PhtD and PhtE vaccine-induced production of IFN-γ and IL-2 double-positive CD4 T cells is highly encouraging and deserves further study.
PhtD and PhtE antigens induce CD4 T cell proliferative responses.
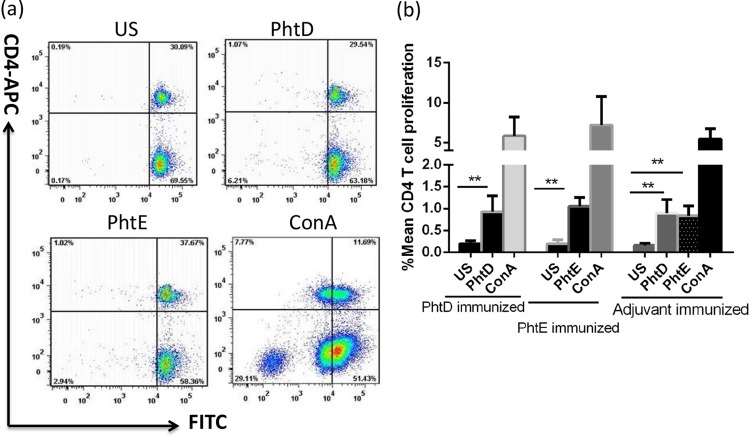
To test the proliferative capacity of CD4 T cells from immunized mice, splenocytes were cultured with recombinant PhtD or PhtE for 7 days. CD4 T cell proliferation was assessed by tracking the dilution of CFSE as a result of cell division after the 3rd and 7th days of stimulations. No early T cell proliferation (on day 3; data not shown) was detected with any of those antigens, and an optimal CD4 T cell proliferation was observed only by day 7. Figure 8a represents the dot plots from PhtD- and PhtE-immunized mice as a demonstration of cell proliferation as a result of CFSE tracking. There was a significantly higher proliferative response from the splenocytes of immunized mice stimulated with both PhtD and PhtE than from the splenocytes of their unstimulated controls (P < 0.02). Adjuvant-immunized mice also had robust proliferative responses when stimulated with PhtD and PhtE (Fig. 8b) compared to the responses of the unstimulated controls (P < 0.02). Ex vivo T cell proliferation after 7 days of stimulation is essentially a naive response, and it indicates that these antigens have the capacity to drive a robust primary immune response.
Fig 8.
PhtD- and PhtE-induced CD4 T cell proliferation. CFSE-stained splenocytes (1 million per stimulation) from mice (the same immunized mice used for the experiments whose results are shown in Fig. 6) immunized with PhtD plus adjuvant, PhtE plus adjuvant, and control (adjuvant only) (5 mice per group) were stimulated with either PhtD, PhtE, or ConA for 7 days (splenocytes from individual mice were stimulated separately). Splenocytes derived from PhtD-immunized mice were stimulated with PhtD and ConA. Similarly, splenocytes from PhtE-immunized mice were stimulated with PhtE and ConA, and splenocytes from adjuvant-immunized mice were stimulated with PhtD, PhtE, and ConA. Stimulated cells were washed with PBS and stained with anti-CD4 antibody (antigen-presenting cells). Cells were acquired on an LSR II flow cytometer. (a) CD4 T cell proliferative dot plots. The PhtD dot plot refers to splenocytes derived from PhtD-immunized mice, the PhtE dot plot refers to splenocytes derived from PhtE-immunized mice, and the ConA dot plot refers to splenocytes derived from PhtD-immunized mice. (b) Mean and standard deviation percent CD4 T cell proliferation from 3 separate experiments. The Student t test was used for comparing antigen-stimulated cells with their respective unstimulated controls. **, P < 0.02.
Immune responses to PhtD and PhtE are protective against pneumococcal colonization.
To assess the protective efficacy of PhtD and PhtE immunization against nasopharyngeal colonization, C57BL/6 mice were immunized intranasally with PhtD and PhtE proteins before they were challenged via the same route with TIGR4 pneumococci. An NP pneumococcal load was observed on days 2 and 4 after intranasal challenge. There was a significant reduction in the nasal bacterial load in mice immunized with either PhtD or PhtE on day 2 postchallenge compared to that in the controls (immunized with adjuvant only) (Fig. 9a) (P < 0.001). By day 4, most of the nasal bacterial load was cleared in both PhtD- (P < 0.001) and PhtE-immunized (P < 0.01) mice (Fig. 9b). Although both PhtD- and PhtE-immunized mice cleared most of their nasal bacterial load by day 4, as shown in Fig. 9b, clearance was faster in PhtD-immunized mice.
DISCUSSION
This study shows that immunization with PhtD and PhtE can produce an antibody and memory CD4 T cell response that affords protection against NP colonization by pneumococcal serotype TIGR4 in mice. We also show that the immune response following natural NP colonization does not predict the results following immunization, since natural colonization did not elicit the same immune response profile observed with PhtD or PhtE immunization. The immune response to PhtD and PhtE sped clearance and reduced the bacterial density in the nasopharynges of mice. PhtD appeared to be a more effective natural and vaccine-inducible immunogen than PhtE, and immunity to PhtD was associated with better protection against pneumococcal NP colonization than PhtE. However, it should also be taken into account that truncated PhtE that retained immunogenicity was used in the present study.
The pneumococcus frequently colonizes the nasopharynges, and the establishment of colonization is a prerequisite for subsequent mucosal infection (acute otitis media, sinusitis) and invasive infection. Protection against pneumococcal NP colonization through vaccination is being sought as an attractive strategy to contain pneumococcal spread (herd immunity) and subsequent invasive pneumococcal diseases (IPDs). The success of PCVs (2, 3) clearly shows that anticapsular antibodies protect against both pneumococcal colonization and invasive disease. However, the shortened duration of pneumococcal carriage and the decline in disease between the first and second year of life for strains expressing serotypes not contained in the current PCV formulations indicate a role for immune responses to antigens other than the pneumococcal capsule in natural development of immunity against pneumococcal colonization and disease. Also, while PCVs reduce the density of NP colonization, once established, the duration of carriage is unaffected (9, 11), suggesting that the immune response to PCVs prevents pneumococcal carriage from taking place rather than clearing carriage.
We have previously demonstrated that PhtD and PhtE play a role in pneumococcal adherence and that humans can raise functional antibodies capable of inhibiting pneumococcal adherence in vitro (30). Pneumococcal colonization is now established to be an immunizing event in both humans (31) and mice (32) that can lead to protection against second colonization events caused by the same serotype (32). However, the role and contribution of protein antigen-specific immunity in the context of prior colonization-induced protection against subsequent pneumococcal colonization(s) have not been described in the past. Here, we found a significant reduction in the rate of carriage of either TIGR4 ΔPhtD or TIGR ΔPhtE over time, supporting their potential involvement in pneumococcal NP colonization.
This is the first study regarding the contribution of PhtD- and PhtE-specific immunity generated as a result of prior pneumococcal colonization in protection against second colonization. PhtD was found to elicit the most robust antibody response, with endpoint titers being 5-fold higher than those of PcpA, 8-fold higher than those of PspA, 10-fold higher than those of CbpA, and 23-fold higher than those of PhtE. We could not correlate the antibody titers with protection from second NP colonization, but protection was observed, since mice without antibody to PhtD or PhtE had a slower NP clearance of pneumococci. Also, the bacterial load was not reduced to the same degree with the second NP colonization challenge if mice lacked exposure to PhtD or PhtE antigen. It is important to note that this difference is observed in a natural colonization setting where the number of bacteria and the expression of genes are important factors for immune activation. Therefore, observation of a small difference in the bacterial load among colonized groups (in secondary colonization) and its correlation with the anti-PhtD or the anti-PhtE immune response could be significant. One important caveat is that we did not include reconstituted mutants in the colonization studies. Thus, despite the real-time PCR and flow cytometry data indicating that TIGR4 ΔPhtD and TIGR4 ΔPhtE have no polar effects, caution is warranted when attributing the attenuated phenotype solely to PhtD and PhtE.
Following NP colonization, we could not detect PhtD- or PhtE-specific cytokine-producing memory CD4 T cells in the spleens of colonized mice. However, there were potent IFN-γ- and IL-2-producing cells against whole pneumococci. IL-17A has been demonstrated in the past to be important in protection against NP colonization in mice (11), but we could not detect IL-17A-producing CD4 T cell subsets in mice colonized with either wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE by intracellular cytokine staining (ICS). To understand whether longer antigenic stimulations could yield an IL-17A response, a cytokine bead array was performed with the culture supernatants of splenocytes derived from the colonized mice. Longer stimulations yielded a detectable IL-17A response, and the response against whole bacteria was equivalent in the wild-type- and mutant-colonized mice. There was no detectable IL-17A response in splenocytes stimulated with either PhtD or PhtE. Therefore, the only immunological difference observed in the mice colonized with pneumococcal wild-type TIGR4, TIGR4 ΔPhtD, or TIGR4 ΔPhtE was the level of the antibodies against PhtD or PhtE, and even the duration of immunological priming did not affect the outcome of the cellular responses.
Protection against secondary pneumococcal colonization in mice is attributed collectively to serotype-specific antibodies that can enhance the opsonic clearance, IL-17A-mediated potentiation of neutrophil activity, and antiadhesin antibodies that can block the bacterial interaction to epithelial cells in the nasopharynx. Since PhtD and PhtE have been demonstrated in the past to have a putative function in pneumococcal adherence, it is plausible that the anti-PhtD and anti-PhtE antibodies generated as a result of pneumococcal primary colonization in mice may potentially have contributed to some extent to protection against secondary pneumococcal colonization.
Godfroid et al. (20) and others (33) have demonstrated in the past that PhtD and PhtE immunization provides a broad range of protection against pneumococcal infections in mice, including colonization. Those prior studies (20, 33) did not compare the PhtD and PhtE immunization-induced antibody responses with the NP colonization-induced responses, as we did here. We also found that anti-PhtD antibodies elicited by immunization include antibodies cross-reactive with other Pht proteins, especially PhtB. The finding of induction of cross-reactive antibodies produced by PhtD immunization of mice is novel and may have great implications in establishing a PhtD-induced correlate of protection against pneumococcal infections in future. However, it remains to be understood whether anti-PhtD antibodies found to be strongly cross-reactive to PhtB by ELISA will be functional and could have the additive effect of neutralizing both PhtD and PhtB antigens in vivo.
This study is the first to evaluate CD4 T cell responses to PhtD and PhtE following NP colonization and immunization. We found that IFN-γ- and IL-2-producing memory CD4 T cells could be detected in the spleens of immunized mice but not following NP colonization. The responses that we observed were consistent with a Th1 profile. The role of CD4 T cells in the protection against pneumococcal colonization has been well documented in mice (34) and attributed to the ability of these cells to produce effector cytokines like IFN-γ (Th1) and IL-17A (Th17). IL-17A has been demonstrated to potentiate the killing mediated by neutrophils and correlated with a reduction in pneumococcal carriage in the past in the context of a pneumococcal whole-cell-based vaccine in mice (11). IFN-γ has a direct effector function and could also significantly enhance activation of the innate immune response, particularly neutrophils, which are vital to bacterial clearance and potent mediators of innate defense (35, 36). The role of IL-2 is critical, as it would help antigen-specific CD4 T cells to survive and persist in the normal homeostatic environment to maintain a stable pool of a memory population. However, more follow-up work is required to further understand the persistence of the antigen-specific memory population. The ability of antigens to initiate an immune response could vary significantly from one antigen to another, and also the way that they shape the immune response and provide protection could vary. This is the first report to investigate CD4 T cell memory responses against pneumococcal purified subunit antigens in mice by multiparametric flow cytometry-based intracellular cytokine staining. By this method, unlike studies using longer stimulations and culture supernatants, we could not detect IL-17A-producing CD4 T cell subsets. Memory CD4 T cells may undergo apoptosis when sustained in culture for longer time periods, and IL-17A responses derived from splenocytes stimulated for longer time intervals might include a mix of both naive and memory responses.
Both PhtD- and PhtE-immunized mice cleared most of their nasal bacterial load by day 4, which was significantly faster than the rate for the controls, and the clearance was faster in PhtD-immunized mice than PhtE-immunized mice. PhtD and PhtE immunization-induced protection against pneumococcal colonization could largely be attributed to their ability to drive strong IFN-γ (Th1) and antibody responses. Further studies are required to elucidate whether PhtD and PhtE immunization in mice could provide broad-range protection against other pneumococcal serotypes. Future studies are also required to understand whether the inability of pneumococcal NP colonization to generate PhtD and PhtE antigen-specific memory CD4 T cell responses is the prime reason behind repeated pneumococcal colonization in children. Overall, our results encourage inclusion of PhtD as an immunogen in a multicomponent protein-based pneumococcal vaccine. Indeed, a multicomponent vaccine that includes PhtD is now in human clinical trials.
ACKNOWLEDGMENTS
This study was supported by NIH NIDCD grant RO1 08671 and Sanofi Pasteur.
Footnotes
Published ahead of print 29 July 2013
REFERENCES
- 1.Paton JC. 2004. New pneumococcal vaccines: basic science developments, p 382–402 In Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG. (ed), The pneumococcus. ASM Press, Washington, DC [Google Scholar]
- 2.Moffitt KL, Malley R. 2011. Next generation pneumococcal vaccines. Curr. Opin. Immunol. 23:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CJ, Moore MR. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 4.Rabquer B, Shriner AK, Smithson SL, Westerink MAJ. 2007. B cell mediated priming following pneumococcal colonization. Vaccine 25:2036–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 6.McCullers JA. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, Lexau CA, Thomas AR, Harrison LH, Reingold AL, Hadler JL, Farley MM, Anderson BJ, Schaffner W. 2006. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 295:1668–1674 [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Lipsitch M. 2004. Changing the ecology of pneumococci with antibiotics and vaccines, p 283–313 In Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG. (ed), The pneumococcus. ASM Press, Washington, DC [Google Scholar]
- 9.Lipsitch M, Whitney CG, Zell E, Kaijalainen T, Dagan R, Malley R. 2005. Age specific incidence of invasive pneumococcal disease by serotype: are anticapsular antibodies the primary mechanism of protection against invasive disease? PLoS Med. 2:e15. 10.1371/journal.pmed.0020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. 2007. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 45:948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgreen A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch MM, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. 10.1371/journal.ppat.1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapola S, Jäntti V, Haikala R, Syrjänen R, Carlone GM, Sampson JS, Briles DE, Paton JC, Takala AK, Kilpi TM, Kaythy H. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146–1152 [DOI] [PubMed] [Google Scholar]
- 13.McCool TL, Cate TR, Gregory M, Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AK, Pennington SH, Moreno LB, Moreno AT, Miyaji EN, Wright AD, Collins AM, Goldblatt D, Kadioglu A, Gordon SB. 2013. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am. J. Respir. Crit. Care Med. 187:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jedrzejas MJ. 2001. Pneumococcal virulence factors: structures and functions. Microbiol. Mol. Biol. Rev. 65:187–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajam G, Anderton JM, Carlone GM, Sampson JS, Ades EW. 2008. Pneumococcal surface adhesin A (PsaA): a review. Crit. Rev. Microbiol. 34:163–173 [DOI] [PubMed] [Google Scholar]
- 17.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819–829 [DOI] [PubMed] [Google Scholar]
- 18.Rioux S, Neyt C, Di EP, Turpin L, Charland N, Labbé S, Mortier MC, Mitchell TJ, Feron C, Martin D, Poolman JT. 2011. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 157:336–348 [DOI] [PubMed] [Google Scholar]
- 19.Rossjohn J, Gilbert RJ, Crane D, Morgan PJ, Mitchell TJ, Rowe AJ, Andrew PW, Paton JC, Tweten RK, Parker MW. 1998. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J. Biol. Chem. 284:449–461 [DOI] [PubMed] [Google Scholar]
- 20.Godfroid F, Hermand P, Verlant V, Denoël P, Poolman JT. 2011. Pre-clinical evaluation of Pht proteins as potential cross protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, Gravenstein S, Braun P, King J, Swift A. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707–1711 [DOI] [PubMed] [Google Scholar]
- 22.Khan MN, Bansal A, Shukla D, Paliwal P, Sarada SK, Mustoori SR, Banerjee PK. 2006. Immunogenicity and protective efficacy of DnaJ (hsp40) of Streptococcus pneumoniae against lethal infection in mice. Vaccine 24:6225–6231 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Bernatoniene J, Bagrade L, Pollard AJ, Mitchell TJ, Paton JC, Finn A. 2006. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur. J. Immunol. 36:46–57 [DOI] [PubMed] [Google Scholar]
- 24.Kaur R, Casey JR, Pichichero ME. 2011. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr. Infect. Dis. J. 30:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, Wright AD, Collins AM, Gordon SB. 2013. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog. 9:e1003274. 10.1371/journal.ppat.1003274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloosterman TG, Bijlsma JJ, Kok J, Kuipers OP. 2006. To have neighbor's fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology 152:351–359 [DOI] [PubMed] [Google Scholar]
- 27.Glover DT, Hollingshead SK, Briles DE. 2008. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 76:2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura L, Maino VC, Maecker HT. 2008. Standardization and optimization of multiparameter intracellular cytokine staining. Cytometry A 73:984–991 [DOI] [PubMed] [Google Scholar]
- 29.Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. 2011. Acquisition of pneumococci specific effector and regulatory Cd4+ T cells localizing within human upper respiratory-tract mucosal lymphoid tissue. PLoS Pathog. 7:e1002396. 10.1371/journal.ppat.1002396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan MN, Pichichero ME. 2012. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmlund E, Quiambao B, Ollgren J, Jaakkola T, Neyt C, Poolman J, Nohynek H, Käyhty H. 2009. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin. Vaccine Immunol. 16:916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards L, Ferreirab DM, Miyajib EN, Andrew PA, Kadioglu A. 2010. The immunizing effect of pneumococcal nasopharyngeal colonization; protection against future colonization and fatal invasive disease. Immunobiology 215:251–263 [DOI] [PubMed] [Google Scholar]
- 33.Adamou JE, Henrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. 2005. CD4T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulendran B, Ahmed R. 2011. Immunological mechanisms of vaccination. Nat. Immunol. 12:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaech SM, Wherry EJ, Ahmed R. 2002. Effector and memory T cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262 [DOI] [PubMed] [Google Scholar]