Abstract
Background
Serum albumin has been suggested to be associated with insulin resistance. We evaluated the association between serum albumin concentration and insulin resistance. We also investigated whether serum albumin level has an independent effect on the development of diabetes.
Methods
In our study, 9,029 subjects without diabetes, who underwent comprehensive health check-ups annually for 5 years, were categorized into tertiles based on their serum albumin levels at baseline. The odds ratio (OR) for the prevalence of insulin resistance, defined as the top quartile of homeostasis model assessment of insulin resistance and the presence of impaired fasting glucose and nonalcoholic fatty liver disease, was evaluated cross-sectionally. Also, the hazard ratio (HR) for incident diabetes was estimated longitudinally, according to the baseline albumin tertiles using Cox proportional hazard analysis respectively.
Results
From the lowest to the highest tertile of albumin, the multivariable-adjusted ORs of insulin resistance increased significantly in both men and women. During the mean follow-up period of nearly 4 years, 556 (6.1%) subjects progressed to diabetes. The multivariable-adjusted HR (95% confidence interval [CI]) of diabetes in men were 1, 1.09 (95% CI, 0.86 to 1.40), and 1.10 (95% CI, 0.86 to 1.41), respectively, from the lowest to the highest tertiles of baseline albumin. Corresponding values for women were 1, 1.21 (95% CI, 0.66 to 2.21), and 1.06 (95% CI, 0.56 to 2.02), respectively.
Conclusion
Our study showed that increased serum albumin level was associated with insulin resistance. However, serum albumin did not have an independent effect on the development of diabetes.
Keywords: Serum albumin, Insulin resistance, Diabetes
INTRODUCTION
Albumin is one of the major proteins synthesized in the liver. Energy supply is a very important determinant for the normal physiology of albumin production [1]. Indeed, reduced serum albumin levels are observed in medical conditions associated with malnutrition [2-4], whereas high serum albumin levels have been reported to be associated with metabolic syndrome, an indicator of obesity and overnutrition [5]. In addition, recently, serum albumin has been suggested to be associated with insulin resistance [6].
In contrast, several studies reported that lower concentrations of serum albumin are associated with an increased risk of coronary heart disease, cardiovascular mortality, and carotid atherosclerosis [6,7]. Both the antioxidant and anti-inflammatory properties of albumin in the atherogenetic process have been suggested as possible mechanisms for this inverse association [6-10]. Considering that oxidative stress and chronic inflammation play crucial roles in the generation of both insulin resistance and type 2 diabetes [11,12], the antioxidant and anti-inflammatory properties of serum albumin may be associated with incident diabetes, as observed in the association with coronary heart disease, cardiovascular mortality, and carotid atherosclerosis.
Patients with nonalcoholic fatty liver disease (NAFLD) or impaired fasting glucose (IFG) are considered to be insulin resistant [13-15]. Homeostasis model assessment of insulin resistance (HOMA-IR) is a useful method for evaluating insulin resistance [16]. Thus, in the present study, we evaluated the association between serum albumin concentration and insulin resistance, as estimated by HOMA-IR and the presence of IFG and NAFLD. We also investigated whether serum albumin level had an independent effect on the development of diabetes.
METHODS
Study population
More than 80,000 people undergo a comprehensive heath check-up each year at Total Healthcare Center at Kangbuk Samsung Hospital. Most of those examinees get a medical check-up on their own initiative or are employees of various companies or their spouses largely paid for the cost by their employer to promote health; considerable proportions of them get a medical check-up annually or biannually. All data containing anthropometric information, laboratory tests, radiology imaging results and coded answers to self-reported questionnaires were stored electronically in medical records.
Our initial data was provided by 10,950 subjects who participated in comprehensive health check-ups annually for 5 years (between January 2005 and December 2009). Based on records from 2005, 1,921 subjects were excluded from the final analysis for the following reasons: 1) positive serologic markers for either hepatitis B (n=558) or C (n=17) virus; 2) liver cirrhosis (n=8); 3) white blood cell count >11,000/mm3 (n=98) or serum creatinine ≥1.5 mg/dL (n=45); 4) self-reported diabetes and undiagnosed diabetes fasting plasma glucose concentration ≥7.0 mmol/L or glycosylated hemoglobin (HbA1c) ≥6.5% (n=437); 5) malignancy (n=250); and 6) absence of data including HbA1c at any visit (n=632). Thus, final analyses were performed on 9,029 subjects (6,654 men and 2,375 women).
The informed consent requirement for this study was exempted by the Institutional Review Board because researchers only accessed the database for analysis purposes, and personal information was not accessed. This study was approved by the Institutional Review Board at Kangbuk Samsung Hospital.
Measurements
Serum albumin was measured by the Bromocresol green dye-binding method, using Bayer reagent packs on an automated chemistry analyzer (Advia 1650 Autoanalyzer, Bayer Diagnostics, Leverkusen, Germany). The intra-assay coefficient of variation was 1.3% and interassay coefficient of variation was 2.1%. Anthropometric variables, blood pressure (BP), and other biochemical markers were measured, as described previously [14]. Lifestyle information was self-reported.
Definitions
As a marker of insulin resistance, HOMA-IR was calculated using the following formula: HOMA-IR=[fasting insulin (µIU/mL)×fasting glycemia (mmol/L)]/22.5. Subjects with HOMA-IR level above the highest quartile (2.51 for men and 2.28 for women) were classified as having insulin resistance. IFG was defined as fasting plasma glucose between 100 and 125 mg/dL. Fatty liver was diagnosed using an abdominal ultrasonogram (Logic Q700 MR, GE, Milwaukee, WI, USA) based on known standard criteria, including hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring. Several experienced radiologists performed the ultrasound exam. The development of diabetes was assessed from the annual records of all subjects and was defined as fasting plasma glucose ≥126 mg/dL or A1c ≥6.5%. In addition, based on the self-reported questionnaire at each visit, subjects who had a history of diabetes or who currently used insulin or other oral antidiabetic drugs were considered to have developed diabetes.
Statistical analysis
Results are expressed as the number of subjects with the percentage (%) and the mean value with standard deviation. One-way analysis of variance and Pearson's chi-squared test were used to analyze any statistical differences in study participant characteristics between tertiles of serum albumin. The odds ratio (OR) for the prevalence of insulin resistance (defined as the top quartile of HOMA-IR and the presence of IFG and NAFLD) was evaluated cross-sectionally according to the baseline albumin tertiles, using binary logistic regression. The hazard ratio (HR) for incident diabetes was estimated longitudinally based on the albumin tertiles at baseline, using binary Cox proportional hazard analysis. When analyzing the OR for the presence of NAFLD, subjects with alcohol intake >20 g/day were excluded.
RESULTS
Table 1 shows the baseline characteristics of the study subjects by tertiles of serum albumin. Fasting glucose, fating insulin, HOMA-IR, systolic BP, uric acid, and low density lipoprotein cholesterol increased with increasing albumin tertiles, in both men and women.
Table 1.
Baseline Characteristics
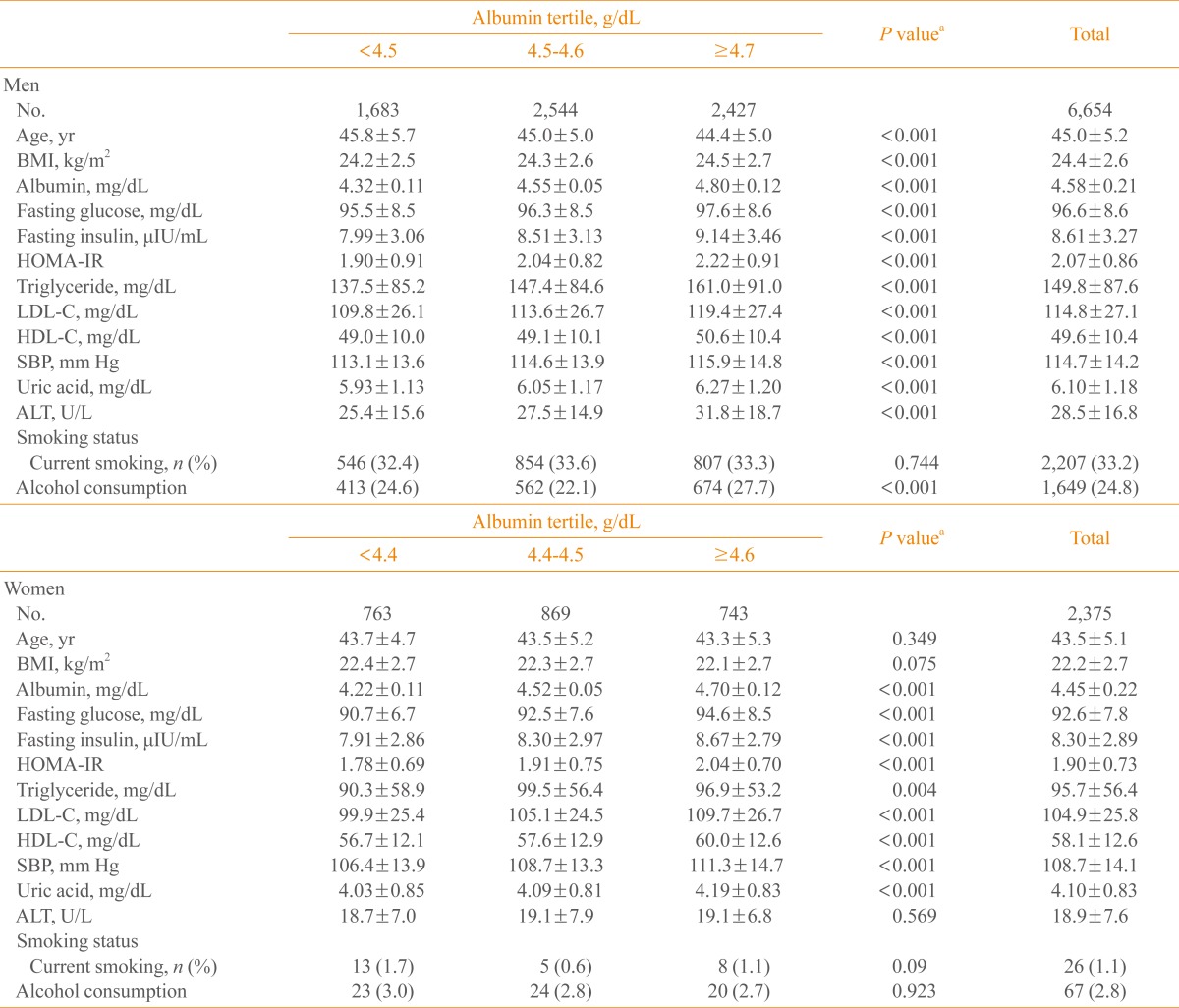
Values are expressed as mean±SD.
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; SBP, systolic blood pressure; ALT, alanine aminotransferase.
aBy one way analysis of variance.
From the lowest to the highest tertile of albumin, the multivariable-adjusted ORs of insulin resistance (defined as the top quartile of HOMA-IR and the presence of IFG and NAFLD) increased significantly in both men and women (Tables 2, 3). During the mean follow-up period of nearly 4 years (47.2 months), 556 (6.1%, 478 men and 78 women) of the 9,029 subjects progressed to diabetes. In the Cox proportional hazard model, after adjusting for age, body mass index, triglyceride, high density lipoprotein cholesterol, systolic BP, presence of IFG and fatty liver, smoking status, and alcohol consumption, the HRs (95% confidence interval [CI]) of diabetes in men were 1, 1.09 (95% CI, 0.86 to 1.40), and 1.10 (95% CI, 0.86 to 1.41), respectively, from the lowest to the highest tertile of albumin. Corresponding values for women were 1, 1.21 (95% CI, 0.66 to 2.21), and 1.06 (95% CI, 0.56 to 2.02), respectively (Table 4).
Table 2.
The Risk for Insulin Resistance Estimated by HOMA-IR and the Presence of IFG and NAFLD According to the Serum Albumin Level in Men
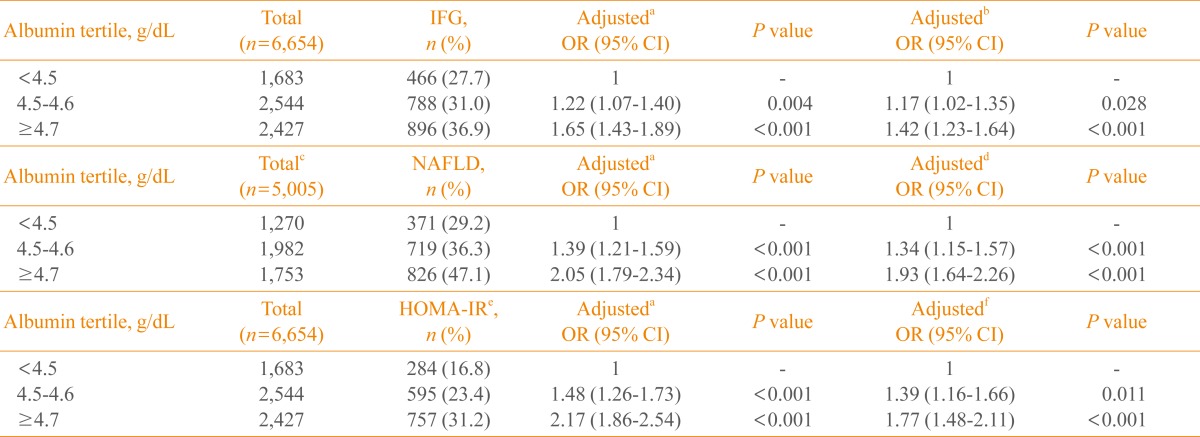
HOMA-IR, homeostasis model assessment of insulin resistance; IFG, impaired fasting glucose; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
aAdjusted for age; bAdjusted for age, triglycerides, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), systolic blood pressure (BP), body mass index (BMI), presence of fatty liver, smoking status, and alcohol consumption; c1,649 were excluded due to alcohol intake >20 g per day; dAdjusted for age, triglycerides, HDL-C, LDL-C, systolic BP, BMI, presence of IFG, and smoking status; eSubjects with HOMA-IR level above the highest quartiles (HOMA-IR >2.51); fAdjusted for age, triglycerides, HDL-C, LDL-C, systolic BP, BMI, presence of fatty liver, presence of IFG, smoking status, and alcohol consumption.
Table 3.
The Risk for Insulin Resistance Estimated by HOMA-IR and the Presence of IFG and NAFLD According to the Serum Albumin Level in Women

HOMA-IR, homeostasis model assessment of insulin resistance; IFG, impaired fasting glucose; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
aAdjusted for age; bAdjusted for age, triglycerides, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), systolic blood pressure (BP), body mass index (BMI), presence of fatty liver, smoking status, and alcohol consumption; c67 were excluded due to alcohol intake >20 g per day; dAdjusted for age, triglycerides, HDL-C, LDL-C, systolic BP, BMI, presence of IFG, and smoking status; eSubjects with HOMA-IR level above the highest quartiles (HOMA-IR >2.28); fAdjusted for age, triglycerides, HDL-C, LDL-C, systolic BP, BMI, presence of fatty liver, presence of IFG, smoking status, and alcohol consumption.
Table 4.
Hazard Ratios for Developing Type 2 Diabetes According to Serum Albumin Tertiles at Baseline
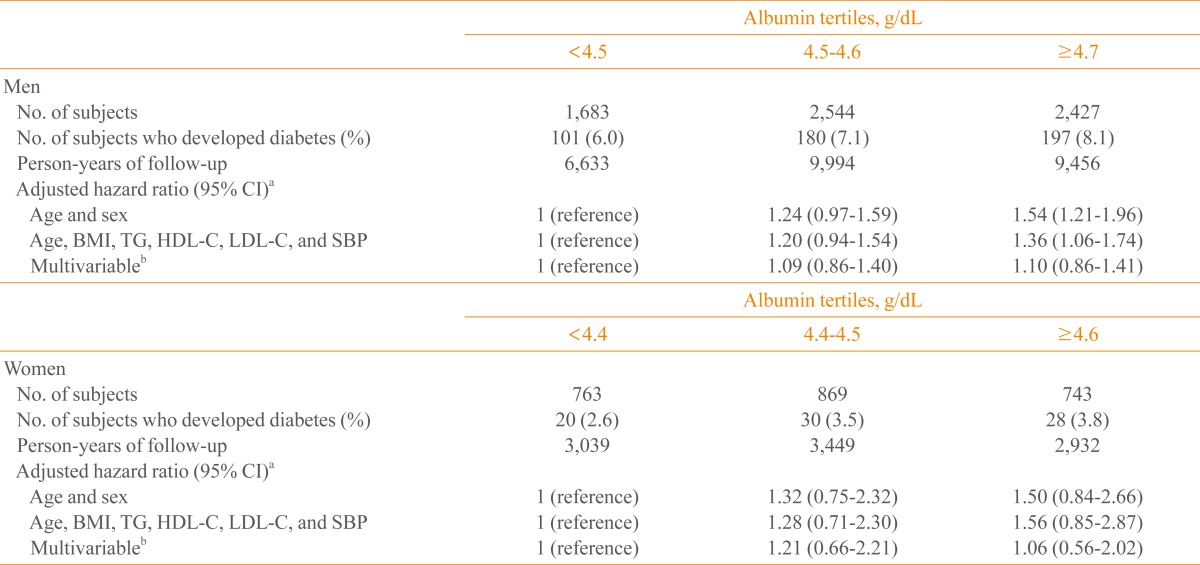
CI, confidence interval; BMI, body mass index; TG, Triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure.
aEstimated by Cox proportional hazard analysis; bAdjusted for age, triglyceride, HDL-C, LDL-C, systolic BP, BMI, presence of IFG and fatty liver, smoking status, and alcohol consumption.
DISCUSSION
In our study, serum albumin concentration was associated with higher levels of HOMA-IR, and the presence of IFG and NAFLD in nondiabetic subjects. Several studies have also reported that serum albumin was positively associated with metabolic syndrome or metabolic risk factors including lipid profile, BP, and body mass index [6,17-19]. These results suggest that serum albumin is associated with insulin resistance.
Insulin resistance is a principal cause of type 2 diabetes [20] and serum albumin has been associated with insulin resistance [6,17-19]. However, in our study, serum albumin did not have independent effect on the development of diabetes. Although it is not clear whether there is causal relationship between insulin resistance and serum albumin levels, our results indicate that insulin resistance may affect serum albumin levels. Insulin resistance is by definition linked to hyperinsulinemia [21]. Nondiabetic patients with insulin resistance have compensatory hyperinsulinemia, a state which predisposes to the development of metabolic impairments, including nonalcoholic fatty liver disease, IFG, and metabolic syndrome [14,22]. This compensatory hyperinsulinemia may contribute to this relationship between insulin resistance and serum albumin levels. Several studies investigating the possibility of a direct relationship between insulin and albumin synthesis have provided insight into that theory. Insulin has effects on the synthesis rates of liver proteins such as albumin and fibrinogen. In vivo in rats and in rat hepatocytes cultures, insulin increased albumin gene transcription and mRNA synthesis in a dose-dependent manner [23,24]. In contrast, insulin deficiency decreased both albumin gene transcription and mRNA concentration with a resultant decrease of albumin synthesis [23-25]. Additionally, in type 1 diabetes patients, insulin withdrawal resulted in a decrease in the albumin synthetic rate, with these changes being reversed by insulin [26]. In diabetic patients, however, plasma albumin concentration has been reported to be inversely related with HbA1c levels, revealing a large proportion of poorly controlled diabetes in patients with lower plasma albumin concentrations [27]. This inverse relationship may also be explained by the fact that poorly controlled type 2 diabetes has been associated with a further decrease in insulin production and secretion by the pancreatic β-cell [28,29].
While our results and those previously reported in other studies show that increased serum albumin is associated with several atherogenic risk factors including lipid profile, BP, body mass index, and insulin resistance [6], several prospective studies have demonstrated the cardioprotective role of serum albumin as lower concentrations of serum albumin were associated with increased risk of coronary heart disease, cardiovascular mortality and carotid atherosclerosis [7,30]. The antioxidant and anti-inflammatory properties of serum albumin in the atherogenetic process have been suggested as possible mechanisms for this inverse association [6-10]. Oxidative stress and chronic inflammation play crucial roles in the generation of both insulin resistance and type 2 diabetes [11,12]. The reported antioxidant and anti-inflammatory properties of serum albumin indicate that serum albumin may have an independent protective effect on incident diabetes, as observed in the association with carotid atherosclerosis and cardiovascular mortality. However, in our study, serum albumin did not have a protective effect on incident diabetes, suggesting that serum albumin does not have anti-inflammatory or antioxidant properties in the development of diabetes.
A limitation in the current study was that we did not use the 2 hours postload glucose test for diagnosing diabetes. This may have included subjects with undiagnosed diabetes at baseline and underestimated the development of diabetes during the follow-up period. Likewise, the self-reporting of diabetes and use of diabetic medications on the questionnaires included in our assessment of diabetes development may have also led to under-reporting of diabetes. Finally, the use of ultrasonography to diagnose NAFLD was another limitation in our study. Although our ultrasonography exams were performed by several experienced radiologists, we did not assess interobserver reliability or consider the degree of fatty liver.
In conclusion, our study showed that increased serum albumin level is associated with insulin resistance. However, analysis of the causal relationship indicated that serum albumin did not have an independent effect on the development of diabetes.
ACKNOWLEDGMENTS
We would like to thank the staff at the Total Healthcare Center at Kangbuk Samsung Hospital, Seoul, Korea. This work was supported by the Samsung Biomedical Research Institute grant, SBRI C-B0-226-1, C-B0-226-2.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology. Part III: albumin and disease states. JPEN J Parenter Enteral Nutr. 1991;15:476–483. doi: 10.1177/0148607191015004476. [DOI] [PubMed] [Google Scholar]
- 2.Kaysen GA. Biological basis of hypoalbuminemia in ESRD. J Am Soc Nephrol. 1998;9:2368–2376. doi: 10.1681/ASN.V9122368. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka H, Nishi M, Kanemaki T, Hosoda N, Hioki K, Yamamoto M. Preoperative nutritional assessment to predict postoperative complication in gastric cancer patients. JPEN J Parenter Enteral Nutr. 1989;13:286–291. doi: 10.1177/0148607189013003286. [DOI] [PubMed] [Google Scholar]
- 4.Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition. 2001;17:496–498. doi: 10.1016/s0899-9007(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 5.Kadono M, Hasegawa G, Shigeta M, Nakazawa A, Ueda M, Yamazaki M, Fukui M, Nakamura N. Serum albumin levels predict vascular dysfunction with paradoxical pathogenesis in healthy individuals. Atherosclerosis. 2010;209:266–270. doi: 10.1016/j.atherosclerosis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Ishizaka N, Ishizaka Y, Nagai R, Toda E, Hashimoto H, Yamakado M. Association between serum albumin, carotid atherosclerosis, and metabolic syndrome in Japanese individuals. Atherosclerosis. 2007;193:373–379. doi: 10.1016/j.atherosclerosis.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Djousse L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation. 2002;106:2919–2924. doi: 10.1161/01.cir.0000042673.07632.76. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Albumin: an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 9.Zoellner H, Hofler M, Beckmann R, Hufnagl P, Vanyek E, Bielek E, Wojta J, Fabry A, Lockie S, Binder BR. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109(Pt 10):2571–2580. doi: 10.1242/jcs.109.10.2571. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55:820–829. doi: 10.1016/s0008-6363(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 12.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 14.Bae JC, Cho YK, Lee WY, Seo HI, Rhee EJ, Park SE, Park CY, Oh KW, Sung KC, Kim BI. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 2010;105:2389–2395. doi: 10.1038/ajg.2010.275. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J. 2001;48:81–86. doi: 10.1507/endocrj.48.81. [DOI] [PubMed] [Google Scholar]
- 17.Hostmark AT, Tomten SE, Berg JE. Serum albumin and blood pressure: a population-based, cross-sectional study. J Hypertens. 2005;23:725–730. doi: 10.1097/01.hjh.0000163139.44094.1d. [DOI] [PubMed] [Google Scholar]
- 18.Danesh J, Muir J, Wong YK, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J. 1999;20:954–959. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- 19.Saito I, Yonemasu K, Inami F. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circ J. 2003;67:323–329. doi: 10.1253/circj.67.323. [DOI] [PubMed] [Google Scholar]
- 20.Kahn CR. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 21.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd CE, Kalinyak JE, Hutson SM, Jefferson LS. Stimulation of albumin gene transcription by insulin in primary cultures of rat hepatocytes. Am J Physiol. 1987;252(2 Pt 1):C205–C214. doi: 10.1152/ajpcell.1987.252.2.C205. [DOI] [PubMed] [Google Scholar]
- 24.Peavy DE, Taylor JM, Jefferson LS. Time course of changes in albumin synthesis and mRNA in diabetic and insulin-treated diabetic rats. Am J Physiol. 1985;248(6 Pt 1):E656–E663. doi: 10.1152/ajpendo.1985.248.6.E656. [DOI] [PubMed] [Google Scholar]
- 25.Kimball SR, Horetsky RL, Jefferson LS. Hormonal regulation of albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1995;268(1 Pt 1):E6–E14. doi: 10.1152/ajpendo.1995.268.1.E6. [DOI] [PubMed] [Google Scholar]
- 26.De Feo P, Gaisano MG, Haymond MW. Differential effects of insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest. 1991;88:833–840. doi: 10.1172/JCI115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Segade S, Rodriguez J, Mayan D, Camina F. Plasma albumin concentration is a predictor of HbA1c among type 2 diabetic patients, independently of fasting plasma glucose and fructosamine. Diabetes Care. 2005;28:437–439. doi: 10.2337/diacare.28.2.437. [DOI] [PubMed] [Google Scholar]
- 28.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 29.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, Cerasi E, Melloul D. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 30.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]


