Abstract
Iron acquisition is critical to the growth and virulence of Legionella pneumophila. Previously, we found that L. pneumophila uses both a ferrisiderophore pathway and ferrous iron transport to obtain iron. We now report that two molecules secreted by L. pneumophila, homogentisic acid (HGA) and its polymerized variant (HGA-melanin, a pyomelanin), are able to directly mediate the reduction of various ferric iron salts. Furthermore, HGA, synthetic HGA-melanin, and HGA-melanin derived from bacterial supernatants enhanced the ability of L. pneumophila and other species of Legionella to take up radiolabeled iron. Enhanced iron uptake was not observed with a ferrous iron transport mutant. Thus, HGA and HGA-melanin mediate ferric iron reduction, with the resulting ferrous iron being available to the bacterium for uptake. Upon further testing of L. pneumophila culture supernatants, we found that significant amounts of ferric and ferrous iron were associated with secreted HGA-melanin. Importantly, a pyomelanin-containing fraction obtained from a wild-type culture supernatant was able to stimulate the growth of iron-starved legionellae. That the corresponding supernatant fraction obtained from a nonpigmented mutant culture did not stimulate growth demonstrated that HGA-melanin is able to both promote iron uptake and enhance growth under iron-limiting conditions. Indicative of a complementary role in iron acquisition, HGA-melanin levels were inversely related to the levels of siderophore activity. Compatible with a role in the ecology and pathogenesis of L. pneumophila, HGA and HGA-melanin were effective at reducing and releasing iron from both insoluble ferric hydroxide and the mammalian iron chelates ferritin and transferrin.
INTRODUCTION
The Gram-negative bacterium Legionella pneumophila is the major causative agent of Legionnaires' disease pneumonia (1, 2). One of 58 species of Legionella (3), L. pneumophila lives in natural and human-made freshwater niches mainly in biofilms or as a parasite of protozoa (2, 4–6). When L. pneumophila-containing aerosols are inhaled, the bacterium replicates in alveolar macrophages (2, 7). Iron acquisition is critical for L. pneumophila extracellular growth, intracellular replication, and virulence (2, 8). The first means by which L. pneumophila acquires iron is through the Fe3+-chelating activity of legiobactin (9). LbtA catalyzes the synthesis of legiobactin, LbtB and Cyc4 promote secretion of the siderophore, and LbtU and LbtC mediate uptake of Fe3+-legiobactin complexes (10–14). Ferric reductase enzymes in the periplasm and cytoplasm may help mediate the assimilation of imported ferric iron (15). The second way by which L. pneumophila acquires iron is via the uptake of Fe2+, with the inner membrane protein FeoB translocating ferrous iron into the cell's interior (16). The inability to mutate both feoB and lbtA indicates that having at least one of these two pathways is essential for L. pneumophila viability (10). Legiobactin and FeoB are required for lung infection by L. pneumophila (11, 16).
It has long been known that L. pneumophila secretes a brown pigment (17–24). Early work found that pigment production is enhanced by l-tyrosine in the growth medium and is most obvious when legionellae are experiencing slow growth (17, 18, 25). It was later shown that pigment results from oxidative polymerization of the homogentisic acid (HGA) that is released by the bacteria into the culture supernatant (26). HGA is a product of phenylalanine/tyrosine catabolism; i.e., l-phenylalanine is converted to l-tyrosine by the phenylalanine hydroxylase encoded by phhA, l-tyrosine is then converted to 4-hydroxyphenylpyruvate by an amino acid transferase encoded by hisC2, and 4-hydroxyphenylpyruvate is finally converted to HGA by the 4-hydroxyphenylpyruvate dioxygenase encoded by lly (26–31). The HGA that is made can be either secreted, as noted above, or broken down to 4-maleyl-acetoacetate by the homogentisate 1,2-dioxygenase, encoded by hmgA (31, 32). Thus, the L. pneumophila pigment is HGA-melanin, a type of pyomelanin that is also made by various other environmental bacteria as well as some other pathogens (33–35). For years, the only function ascribed to the HGA-melanin was bacterial resistance to light (36).
While seeking factors involved in the production of legiobactin, we discovered that L. pneumophila culture supernatants can mediate the reduction of ferric iron salts (32). Since the activity was lacking in supernatants from a nonpigmented lly mutant but increased for a hyperpigmented hmgA mutant, we inferred that HGA-melanin, directly or indirectly, facilitates ferric iron reduction. Compatible with the nature of HGA-melanin, the wild-type activity was positively influenced by the amount of tyrosine in the medium, resistant to protease treatment, acid precipitable, and variable in size (32). In light of these results, we posited that the secreted pyomelanin represents another facet of L. pneumophila iron acquisition; i.e., the reduction of extracellular Fe3+ by HGA-melanin generates Fe2+ that can be internalized via FeoB transport. We now report confirmation of this hypothesis as well as demonstrate the ability of HGA-melanin to stimulate L. pneumophila growth and act upon iron chelates that are prominent within the mammalian host and natural environmental habitats.
MATERIALS AND METHODS
Bacterial strains.
L. pneumophila strain 130b, also known as strain AA100 or Wadsworth, served as our principal wild-type strain. Table 1 lists additional wild-type legionellae, as well as mutants of strain 130b that were also examined in this study. In order to construct the lbtA lly double mutant NU424, plasmid pUClly::GNT (31) was introduced into the lbtA mutant NU302 by transformation. Transformants were selected on antibiotic-containing agar medium, and insertion of the gentamicin resistance cassette into the lly gene was confirmed by PCR using primers llyLpgFBcl1 and llyLpgXho1, as previously described (31). Escherichia coli strain DH5α (Invitrogen, Carlsbad, CA) was used as the host for recombinant plasmids.
Table 1.
Wild-type and mutant Legionella strains used in this study
| Species and straina | Description | Reference or source |
|---|---|---|
| Wild-type strains | ||
| L. pneumophila | ||
| 130b (ATCC BAA-74) | Clinical isolate, serogroup 1 | 58 |
| Philadelphia 1 (ATCC 33152) | Clinical isolate, serogroup 1 | 59 |
| Los Angeles 1 (ATCC 33156) | Clinical isolate, serogroup 4 | 60 |
| L. anisa (ATCC 35292) | Environmental isolate | 61 |
| L. jamestowniensis (ATCC 35298) | Environmental isolate | |
| L. micdadei (ATCC 33218) | Clinical isolate | 62 |
| Mutants of strain 130b | ||
| NU269 | feoB mutant | 16 |
| NU275 | lspF mutant | 63 |
| NU287 | tatB mutant | 64 |
| NU292 | ccmB mutant | 65 |
| NU302 | lbtA mutant | 10 |
| NU302(plbtA) | lbtA-complemented NU302 | 10 |
| NU326 | hmgA mutant | 32 |
| NU326(phmgA) | hmgA-complemented NU326 | 32 |
| NU408 | lly mutant | 31 |
| NU424 | lbtA lly double mutant | This study |
| GG105 | dotA mutant | 66 |
L. pneumophila wild-type strains are listed with their original designations, followed, in parentheses, by the corresponding American Type Culture Collection (ATCC) strain number.
Bacteriological media and growth assays.
L. pneumophila was routinely cultured at 37°C on buffered charcoal yeast extract (BCYE) agar or in buffered yeast extract (BYE) broth (37). These media contain an iron supplement consisting of 0.25 g of ferric pyrophosphate per liter. E. coli strains were grown in LB medium. When appropriate, the following antibiotics were added to the medium at the indicated final concentrations (in μg/ml): ampicillin, 100; gentamicin, 2.5 for L. pneumophila and 5 for E. coli; chloramphenicol, 3 for L. pneumophila and 30 for E. coli; and kanamycin, 25 for L. pneumophila and 50 for E. coli. All chemicals, unless otherwise noted, were from Sigma-Aldrich (St. Louis, MO). In order to prepare L. pneumophila for iron uptake assays or to obtain supernatants containing siderophore activity, bacteria were grown at 37°C in deferrated chemically defined medium (CDM) (10, 38). Briefly, CDM ordinarily consists of the 20 amino acids and nine trace metals, in addition to iron, pyruvate, glutathione, α-ketogluturate, MOPS [3-(N-morpholino)propanesulfonic acid] buffer, KH2PO4, and NaCl. Deferrated CDM is CDM that lacks the iron component and is made with deferrated water. In order to obtain bacterial HGA-melanin, legionellae were grown at 37°C in CDMP, a variant of CDM that contained extra l-tyrosine (32). To monitor the growth of L. pneumophila strains, bacteria were grown in BYE, CDM, or CDMP broth, and the optical density at 660 nm (OD660) of the cultures was determined using a DU720 spectrophotometer (Beckman, Fullerton, CA) or a Synergy H1 hybrid reader (Biotek, Winooski, VT) (10, 32).
Preparation of HGA, synthetic HGA-melanin, and bacterial HGA-melanin.
Unpolymerized HGA was prepared as a fresh, 200-μg/ml (1.19 mM) solution in phosphate-buffered saline (PBS). Synthetic HGA-melanin (i.e., oxidized and polymerized HGA) was made by incubating the HGA solution for 3 days at 37°C with agitation at 225 rpm. To obtain L. pneumophila HGA-melanin, wild-type strain 130b that had been grown in non-iron-supplemented BYE broth until mid-log phase (i.e., OD660, ∼1.0) was inoculated into deferrated CDMP and incubated for 3 days with shaking. The cell-free supernatant was then acidified to pH 1.5 with 11 M hydrochloric acid (HCl). After precipitation at 4°C for 16 h, the HGA-melanin was collected by centrifugation at 5,000 × g for 1 h and washed once in 10 ml of 10 mM HCl. The resulting pellet was air dried and reconstituted in PBS to the same OD400 (as read by the Synergy H1 hybrid reader) as the synthetic HGA-melanin. In order to obtain an appropriate negative control for the bacterially derived HGA-melanin, the nonpigmented lly mutant was grown in an identical fashion, and its culture supernatant was acidified and processed as described above.
To obtain iron-loaded HGA-melanin, strain 130b was cultured in CDMP containing 30 μM ferric chloride for 3 days. The resulting supernatant was consecutively filtered through 50-kDa-, 30-kDa-, 10-kDa-, and 3-kDa-cutoff cellulose filters (Millipore, Billerica, MA). The melanin-containing fractions collected on the filters were washed once with 15 ml of deferrated CDM and then reconstituted in 20 ml of deferrated CDM. The OD400 of each fraction was measured in order to judge the amount of melanin, and the ferrozine assay (32) was used to determine the concentration of iron in the sample. For the ferrozine assay, 100 μl of the bacterial fraction was mixed with 100 μl of 1 mM ferrozine (Acros Organics, Geel, Belgium), and the reaction mixture was incubated overnight at room temperature (RT). The color change associated with ferrozine binding to ferrous iron was measured at 562 nm using the Synergy H1 hybrid reader. In order to judge the total amount of iron in the sample, the reducing agent vitamin C was added to a final concentration of 1 mM. In order to ascertain the levels of ferrous iron in the samples, treatment with vitamin C was omitted. To quantify the amount of product in our reaction mixtures, a standard curve was prepared using known concentrations of ferrous sulfate (FeSO4) covering the range of 0 to 180 μM. In order to have the appropriate negative control for the iron-loaded bacterial melanin, the nonpigmented lly mutant was grown and its supernatant was processed and tested in parallel.
Ferric iron reduction assays.
Ferric iron reduction was done as described before (32), with the following details and modifications. Reactions were carried out in 25 mM Tris buffer (pH 7.5) in 96-well microtiter plates in a final volume of 250 μl containing 400 μM ferrozine. The initial substrates added included ferric nitrate, ferric chloride, or ferric ammonium citrate, all at 120 μM, or ferric pyrophosphate at 30 μM. To assay for potential reducing reagents, we added either 10 μl of synthetic HGA-melanin, 10 μl of unpolymerized HGA (200 μg/ml), 100 μl of bacterial HGA-melanin prepared from the acid-precipitated supernatants of strain 130b, or 100 μl of acid-precipitated supernatant material obtained from the nonpigmented lly mutant. PBS served as the negative control, and the known reducing agent vitamin C, at a concentration of 1 mM, was the positive control. The reaction was allowed to proceed at RT for 1 h when testing HGA and the synthetic HGA or overnight when assessing bacterial supernatant samples. As described above, the color change associated with ferrozine binding to ferrous iron was measured at OD562. For later reduction reactions, we employed as the substrates 100 μl of (1:500) diluted equine holoferritin (catalog no. F4503; Sigma-Aldrich) and 100 μl of 50 μM human holotransferrin (in 25 mM Tris buffer, pH 7.5; catalog no. T0665; Sigma-Aldrich), and the reaction proceeded for 24 h at 37°C in a 5% CO2 incubator. Because it was not possible to assay for the reduction of insoluble ferric hydroxide under the above-mentioned reaction conditions, we inoculated the bacterial strains in 100 ml of deferrated CDMP containing as a supplement 1 g of ferric hydroxide, and after 3 days of incubation at 37°C with shaking (225 rpm), we used the ferrozine assay to determine the amount of total iron and ferrous iron released into culture supernatants.
Iron uptake assays.
Bacterial strains were first grown in non-iron-supplemented BYE broth until mid-log phase (OD660, 1.0). Next, the bacteria were centrifuged at 5,000 × g, washed, and resuspended in deferrated CDM to an OD660 of 0.3. After further incubation for 13 h at 37°C with shaking (i.e., 225 rpm), the bacterial culture was centrifuged and then washed three times in a base buffer consisting of CDM containing 50 mM MOPS, 2 mM monobasic potassium phosphate (KH2PO4), and 50 mM sodium chloride (NaCl). The final bacterial pellets were resuspended in base buffer to an OD660 of 1.0, and 55FeCl3 (PerkinElmer, Boston, MA) in 10 mM HCl was added to a final concentration of 1 μCi/ml (37 kBq/ml). To each milliliter of this reaction mixture was added 16.7 μl of unpolymerized HGA, synthetic HGA-melanin, acid-precipitated HGA-melanin from strain 130b supernatants, acid-precipitated supernatant material from the nonpigmented lly mutant, or PBS. In some cases, vitamin C was added to a final concentration of 1 mM. After 0, 60, and 120 min incubation at RT, 1 ml of the suspension (n = 3) was filtered through a 0.45-μm-pore-size nitrocellulose membrane (Millipore, Billerica, MA) and washed with 5 ml of a 0.5% solution of thioglycolic acid to remove the extracellular iron. The number of counts per minute of radioactivity associated with the bacteria was measured with a Beckman LS6500 scintillation counter, and the mean of the counts per minute over a 5-min period was recorded (13). To confirm that the method is detecting only cell-associated iron, a control experiment was carried out in the absence of bacteria, and in this case, very little radioactivity remained on the filters after washing with thioglycolate.
Determining the effect of iron-loaded melanin on bacterial growth.
Wild-type strain 130b was precultured in non-iron-supplemented BYE until mid-log phase, washed, and then resuspended in deferrated CDM to an OD660 of 0.3. Two hundred microliters of the bacterial suspension was aliquoted into the wells of a 96-well microtiter plate. Into the bacterial culture was then added 10 μl of either standard CDM (which contains iron), deferrated CDM, or the >50-kDa, 30- to 50-kDa, 10- to 30-kDa, 3- to 10-kDa, or <3-kDa supernatant fraction obtained from CDMP cultures of either strain 130b or the nonpigmented lly mutant (see above). The inoculated plates were then incubated in the Synergy H1 hybrid reader at 37°C with a 1-min period of shaking every 10 min. The OD660 of each well was recorded every 10 min for a period of 40 h. To prevent condensation, the lids of the microtiter plates were precoated with 10 ml of 0.05% Triton X-100 in 20% ethanol for 30 min and air dried (39).
Siderophore assay.
Chrome azurol S (CAS) assays were done as described before (9, 10, 13). Briefly, 100 μl of deferrated CDM culture supernatant was mixed with 100 μl of CAS solution in the wells of a 96-well plate, and after 30 min of incubation at RT, the OD630 of the mixture was measured using the Synergy H1 hybrid reader. Desferrioxamine was used to generate a standard curve for quantifying siderophore activity.
Statistical analysis.
The unpaired t test was used in experiments with two-group comparisons. The one-way analysis of variance (ANOVA) was used in experiments with >2 experimental groups. P values of <0.05 were considered different. With the exception that the control experiment whose results are presented in Fig. S3 in the supplemental material was done twice, all experiments were done on at least three independent occasions. In all cases, the multiple trials yielded similar results, and therefore, the results of a representative experiment are presented in each of the figures. Although the absolute levels of iron reductase and CAS activity in a given type of supernatant/HGA sample varied from 1 to 9% from day to day and the absolute levels of iron uptake for a particular bacterial strain typically varied from 10 to 30% from day to day, all of the statistically significant differences that we observed between different supernatant or bacterial samples were observed in each of the corresponding repeat experiments.
RESULTS
HGA-melanin and HGA can directly mediate ferric iron reduction.
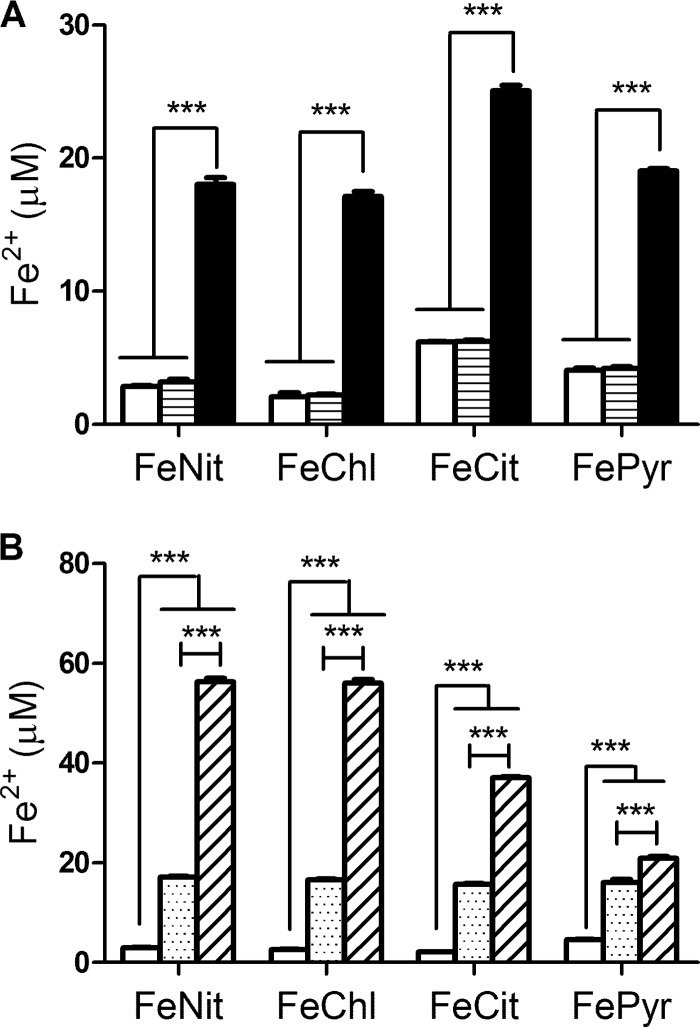
Previously, we determined that acid-precipitated material obtained from culture supernatants of L. pneumophila strain 130b can mediate reduction of ferric nitrate and ferric chloride (32). To begin this study, we sought to verify that this activity was dependent upon partially purified HGA-melanin versus another supernatant component that might have been in the precipitated samples. Thus, we compared acid-precipitated material obtained from wild-type 130b versus lly mutant supernatants for their ability to reduce Fe3+. Whereas 130b samples reduced ferric nitrate, ferric chloride, ferric ammonium citrate, and ferric pyrophosphate, the nonpigmented mutant samples had a level of activity that was no greater than that of the PBS control (Fig. 1A). Next, in order to determine if pyomelanin itself reduces ferric iron or is merely required for the activity of a coprecipitating substance, we used pure HGA to generate HGA-melanin in the test tube and then tested the ability of the synthetic HGA-melanin to mediate ferric iron reduction. Synthetic HGA-melanin had activity against all of the iron substrates (Fig. 1B). Ferric iron reduction was also seen when we tested pure, unpolymerized HGA (Fig. 1B). In fact, the activity of HGA was greater than that of HGA-melanin, suggesting that some reducing capacity is lost when HGA is oxidized and polymerized. Also, HGA, unlike HGA-melanin, showed greater activity against ferric nitrate and ferric chloride than against ferric ammonium citrate and ferric pyrophosphate. Taken together, these data indicate that both the HGA-melanin and unpolymerized HGA present in L. pneumophila supernatants can directly mediate the reduction of ferric iron.
Fig 1.
Reduction of ferric iron by HGA and HGA-melanin. Acid-precipitated HGA-melanin obtained from wild-type strain 130b culture supernatants (black bars) or acid-precipitated material obtained from lly mutant NU408 culture supernatants (bars with horizontal lines) (A), synthetic HGA-melanin (stippled bars) or unpolymerized HGA (bars with diagonal lines) (B), or PBS (white bars) (A, B) was mixed with ferric nitrate (FeNit), ferric chloride (FeChl), ferric ammonium citrate (FeCit), and ferric pyrophosphate (FePyr). After 1 h of incubation for HGA and synthetic HGA-melanin samples or overnight incubation for supernatant samples, the amount of ferrous iron generated was determined. Data are the means and standard deviations obtained from triplicate samples. In panel A, the levels of Fe2+ generated by the wild-type sample were significantly greater than those produced by the mutant sample and the PBS control (***, P < 0.001). In panel B, the levels of Fe2+ generated by HGA were significantly greater than those produced by synthetic melanin (***, P < 0.001), with the results for both HGA and HGA-melanin being greater than those for the PBS control (***, P < 0.001).
HGA-melanin and HGA promote iron uptake by Legionella.
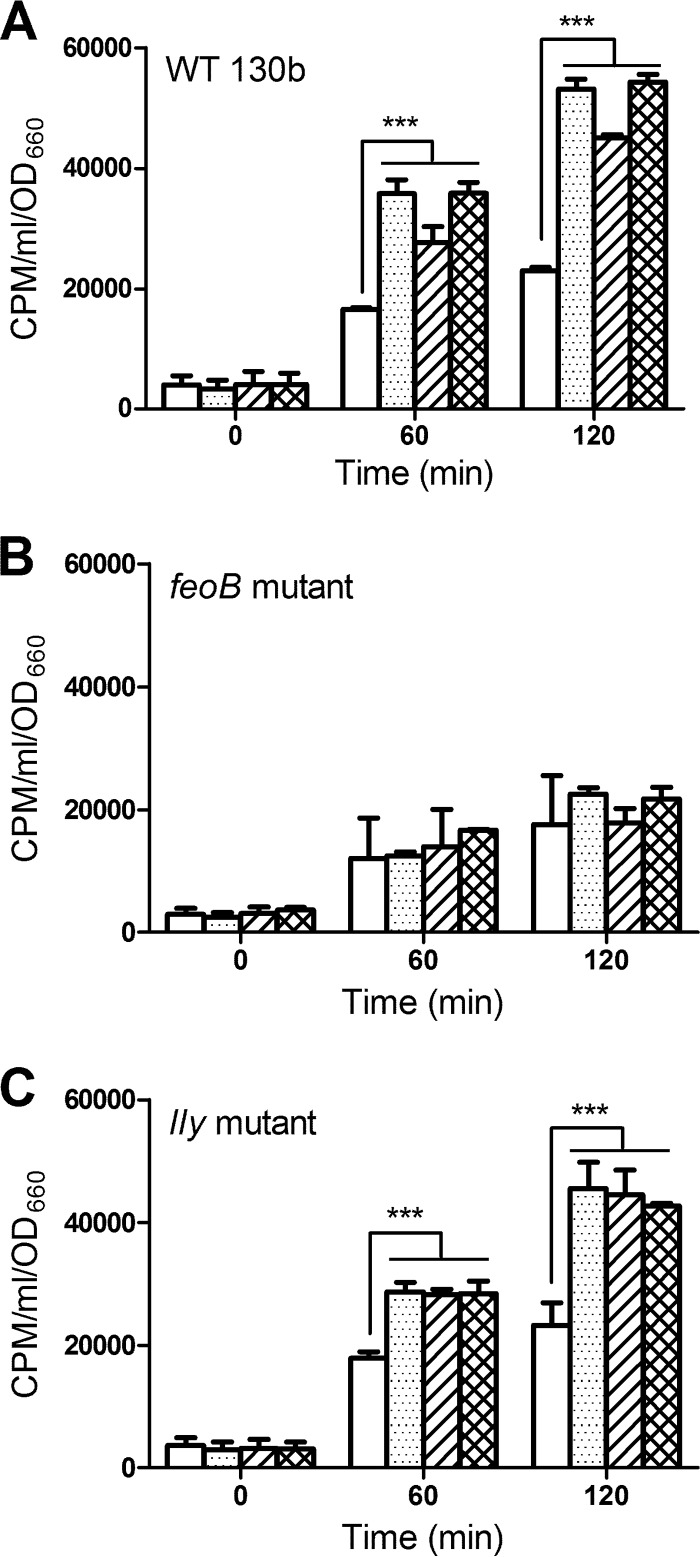
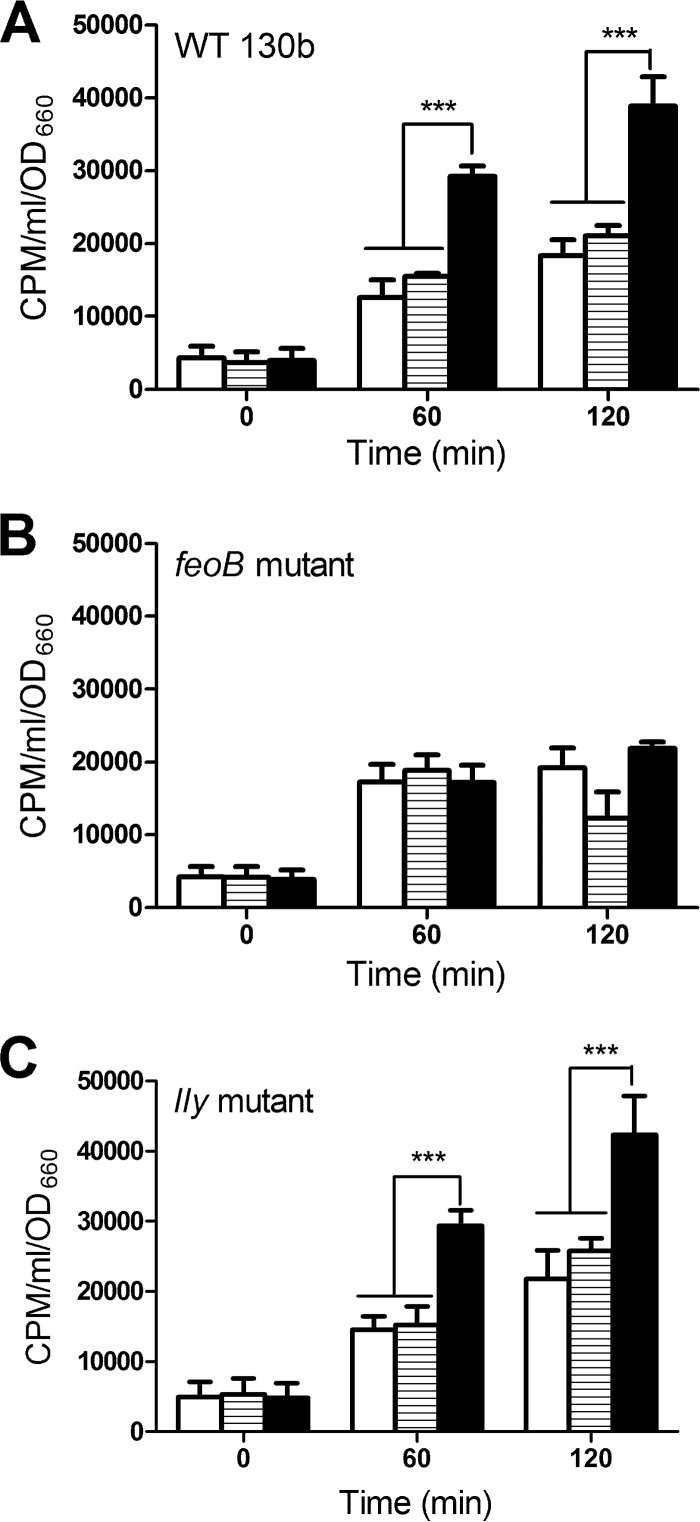
We hypothesized that the ability of HGA-melanin and HGA to reduce extracellular ferric iron sources would generate ferrous iron that can be internalized by L. pneumophila via its FeoB transport system. To test this hypothesis, bacteria that had been grown in deferrated CDM and washed to remove conditioned medium (which contains legiobactin and potential reducing agents, e.g., glutathione) were suspended in buffer containing radiolabeled ferric chloride and then incubated in the presence or absence of HGA-melanin, unpolymerized HGA, or the known reducing agent vitamin C. At various times, the level of cell-associated radiolabel was determined. After 60 and 120 min of incubation, HGA, synthetic HGA-melanin, and HGA-melanin from L. pneumophila culture supernatants all significantly increased the amount of radiolabeled iron taken up by wild-type strain 130b (Fig. 2A and 3A). Although enhanced iron uptake was seen for a variety of mutants lacking membrane proteins (see Fig. S1 in the supplemental material), HGA and HGA-melanin did not increase iron uptake by a feoB ferrous iron transport mutant (Fig. 2B and 3B). Thus, the enhanced uptake exhibited by wild type upon exposure to HGA and HGA-melanin was due to increased levels of available ferrous iron. Compatible with this conclusion, there was no enhancement of uptake by 130b if the radiolabel presented to the bacterium was already in the ferrous form (see Fig. S2 in the supplemental material). In terms of its iron uptake, the nonpigmented lly mutant behaved similarly to parental strain 130b (Fig. 2C and 3C), indicating that the ability to take up the Fe2+ that is generated by HGA or HGA-melanin is independent from the bacterium's ability to produce HGA.
Fig 2.
Effect of HGA and synthetic HGA-melanin on iron uptake by L. pneumophila 130b. Wild-type (WT) strain 130b (A), feoB mutant strain NU269 (B), and lly mutant strain NU408 (C) that had been grown in deferrated CDM and then resuspended in buffer were mixed with 55FeCl3 in the presence of PBS (white bars), synthetic HGA-melanin (stippled bars), unpolymerized HGA (bars with diagonal lines), or vitamin C (hatched bars), and after 0, 60, and 120 min of incubation, the levels of intracellular radiolabeled iron were determined. The data represent the means and standard deviations obtained from triplicate samples. In panels A and C, HGA, synthetic HGA, and vitamin C resulted in significantly greater levels of iron uptake relative to the level for the PBS control (***, P < 0.001).
Fig 3.
Effect of bacterial HGA-melanin on iron uptake by L. pneumophila 130b. Wild-type strain 130b (A), feoB mutant strain NU269 (B), and lly mutant strain NU408 (C) that had been grown in deferrated CDM and then resuspended in buffer were mixed with 55FeCl3 in the presence of PBS (white bars), acid-precipitated HGA-melanin obtained from 130b culture supernatants (black bars), or acid-precipitated material from lly mutant strain NU408 culture supernatants (bars with horizontal lines), and after 0, 60, and 120 min of incubation, the levels of intracellular radiolabeled iron were determined. The data are the means and standard deviations obtained from triplicate samples. In panels A and C, 130b melanin resulted in greater levels of uptake relative to those for the mutant sample and the PBS control (***, P < 0.001).
HGA and HGA-melanin most likely promote iron uptake by strain 130b by acting upon extracellular Fe3+, with the Fe2+ made moving toward the bacterial surface and then crossing the cell envelope. However, it is formally possible that exposure to HGA and HGA-melanin induces a change in the bacterial surface triggering an alternative iron reduction and/or uptake pathway. To distinguish between these possibilities, 130b bacteria were preincubated with HGA, synthetic HGA-melanin, or the vitamin C control for 2 h (to allow for a possible association with or modification of the bacterial cell), then washed to remove all unbound reducing agents, and finally, incubated with radiolabeled FeCl3. In contrast to results obtained when bacteria were exposed to HGA or HGA-melanin at the same time as radiolabeled iron (Fig. 2), preincubation with HGA or the pyomelanin (or vitamin C) did not enhance iron uptake over that observed with the PBS control (see Fig. S3 in the supplemental material). Thus, HGA-melanin and HGA enhance iron assimilation and do so by generating Fe2+ in a form that is compatible with uptake.
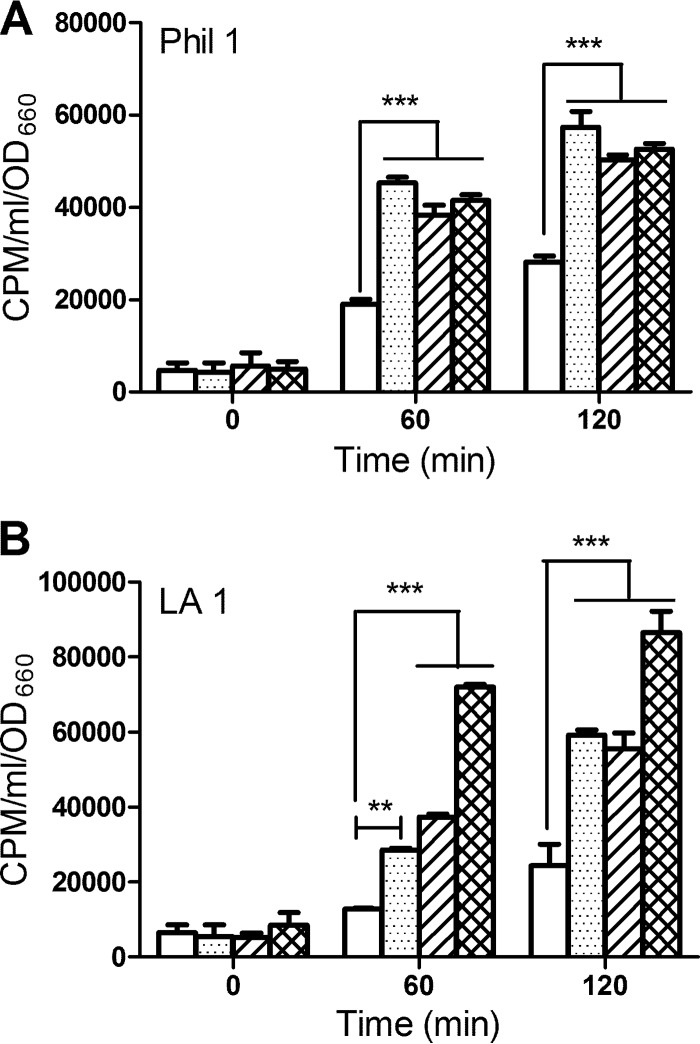
HGA and HGA-melanin also promoted iron uptake by L. pneumophila Philadelphia 1 and Los Angeles 1 (Fig. 4), and a similar result was obtained when we examined several other Legionella species, i.e., L. anisa, L. jamestowniensis, and L. micdadei (see Fig. S4 in the supplemental material). That L. micdadei does not produce pigment (40, 41) indicates, once again, that the ability to take up Fe2+ generated by HGA or HGA-melanin is independent from a bacterium's ability to produce HGA and pyomelanin. In sum, the ability of HGA and HGA-melanin to promote iron uptake is not peculiar to L. pneumophila strain 130b and may, in fact, be conserved across the Legionella genus.
Fig 4.
Iron uptake by other L. pneumophila strains upon exposure to HGA and HGA-melanin. Strains Philadelphia 1 (Phil 1) (A) and Los Angeles 1 (LA 1) (B) that had grown in deferrated CDM were mixed with 55FeCl3 in the presence of either PBS (white bars), synthetic HGA-melanin (stippled bars), unpolymerized HGA (bars with diagonal lines), or vitamin C (hatched bars), and after 0, 60, and 120 min of incubation, the levels of intracellular radiolabeled iron were determined. The data are the means and standard deviations from triplicate samples. In panels A and B, HGA, synthetic HGA, and vitamin C resulted in levels of iron uptake greater than the level for the PBS control (**, P < 0.01; ***, P < 0.001).
HGA-melanin can stimulate L. pneumophila growth under low-iron conditions.
When strain 130b was grown in CDMP supplemented with ferric chloride, HGA-melanin was detected in supernatant fractions that were >50 kDa, 10 to 30 kDa, 3 to 10 kDa, and <3 kDa (Fig. 5A). These findings were compatible with the variable size that has been observed for HGA-melanin and other melanins (32). No melanin was detected in the 30- to 50-kDa fraction (Fig. 5A), suggesting that the polymers of HGA-melanin that are ≤30 kDa may self-aggregate in a specific manner or form large complexes with other components of the supernatant, such that the HGA-melanin shows a discontinuous distribution of sizes. Interestingly, all of the HGA-melanin-containing fractions, but not the 30- to 50-kDa fraction, contained significant amounts of iron (Fig. 5B), with approximately one-half of that iron being in the ferrous form (Fig. 5C compared to B). Eighty-three percent of the total iron was in the >50-kDa, 10- to 30-kDa, and 3- to 10-kDa fractions, and the remaining 27% was in the <3-kDa fraction, indicating that the majority of the iron in the culture supernatants was not free ferric chloride or in low-molecular-mass chelates. In contrast, the >50-kDa, 10- to 30-kDa, and 3- to 10-kDa fractions obtained from cultures of the nonpigmented lly mutant had significantly less iron, and in the case of the 10- to 30-kDa fraction, no iron was detected (Fig. 5B and C). Unlike the wild-type situation, most of the iron in the lly mutant supernatants was in the <3-kDa fraction. Taken together, these data suggested that when L. pneumophila grows in culture, a substantial portion of the available iron associates, directly or indirectly, with HGA-melanin.
Fig 5.
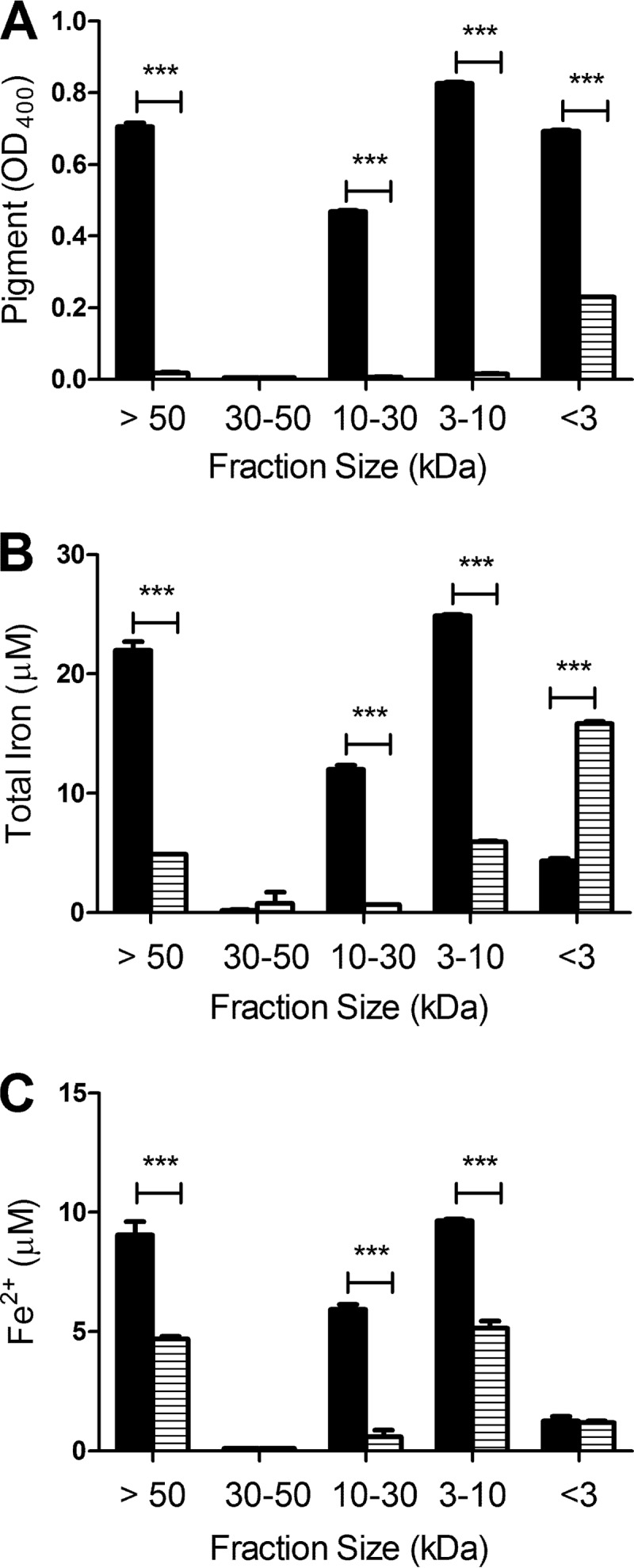
Association of iron with bacterial HGA-melanin. Wild-type strain 130b (black bars) and lly mutant strain NU408 (bars with horizontal lines) were grown in CDMP supplemented with FeCl3 for 3 days at 37°C, and then cell-free supernatants were collected and consecutively filtered through 50-, 30-, 10-, and 3-kDa cellulose filters. For each fraction, the amount of HGA-melanin was determined by measuring the OD400 of the sample (A), the level of total associated iron was determined by the ferrozine assay in the presence of the reducing agent vitamin C (B), and the amount of associated ferrous iron was ascertained by the ferrozine assay in the absence of vitamin C (C). The data are the means and standard deviations obtained from triplicate samples. In panel A, all wild-type fractions contained more pigment than did the corresponding mutant samples (***, P < 0.001), with the exception of the 30- to 50-kDa fraction, which was devoid of melanin in both cases. In panels B and C, the >50-kDa, 10- to 30-kDa, and 3- to 10-kDa fractions of the wild type contained more iron than did the corresponding fractions of the mutant (***, P < 0.001), whereas the <3-kDa fraction of the mutant had more total iron than that of the wild type (***, P < 0.001).
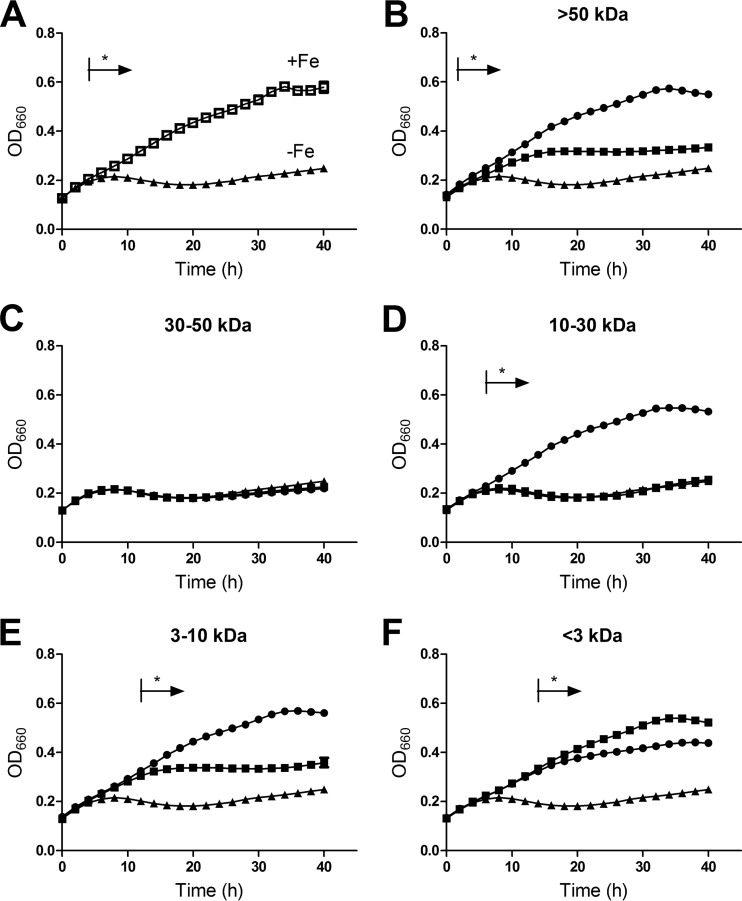
When L. pneumophila is grown in low-iron BYE medium and then inoculated into deferrated CDM, the bacteria become iron starved and exhibit very minimal growth, unless the CDM is supplemented with an iron source (Fig. 6A). To determine if the iron that associates with secreted HGA-melanin is capable of stimulating bacterial growth, we examined the various supernatant fractions described above for their ability to stimulate the growth of strain 130b in the deferrated CDM. The >50-kDa (Fig. 6B), 10- to 30-kDa (Fig. 6D), and 3- to 10-kDa (Fig. 6E) fractions obtained from wild-type supernatants, which contained both iron and HGA-melanin, significantly improved the growth of the iron-starved legionellae above that observed with the deferrated CDM control. The wild type's 30- to 50-kDa supernatant fraction, which contained neither iron nor HGA-melanin, gave no growth stimulation (Fig. 6C). In marked contrast to the results obtained with wild type, the >50-kDa (Fig. 6B) and 3- to 10-kDa (Fig. 6E) fractions obtained from cultures of the nonpigmented lly mutant stimulated growth to a significantly lesser degree, and in the case of the mutant's 10- to 30-kDa fraction, there was no growth enhancement at all (Fig. 6D). When the <3-kDa fractions were tested, the mutant sample promoted growth to a slightly greater degree than did the wild-type sample (Fig. 6F), in line with the larger amount of iron in that mutant sample. Together, these data, especially those obtained when examining the 10- to 30-kDa fractions, indicate that HGA-melanin can stimulate the growth of L. pneumophila under iron-limiting conditions. Given the ability of HGA-melanin to associate with iron, mediate ferric iron reduction, and then promote ferrous iron uptake, we infer that the growth stimulation caused by HGA-melanin is due to increased iron assimilation.
Fig 6.
Effect of HGA-melanin on L. pneumophila growth under iron-limited conditions. Wild-type strain 130b that had been grown in non-iron-supplemented BYE was inoculated into deferrated CDM that was supplemented with an additional aliquot of deferrated CDM (black triangles), standard CDM which contains iron (open squares, in panel A only), or a CDMP culture supernatant fraction that was obtained from 130b (black circles) or the lly mutant NU408 (black squares) and that was either >50 kDa (B), 30 to 50 kDa (C), 10 to 30 kDa (D), 3 to 10 kDa (E), or <3 kDa (F) in size. Samples were incubated at 37°C for up to 40 h, and bacterial growth was monitored by measuring the OD660 of the cultures. Data are the means and standard deviations from 3 to 4 replicates, although all the error bars are too small to be seen above the data point symbols. In panels B, D, and E, the cultures treated with the melanin-containing supernatant fractions from the wild type grew better than did those cultures supplemented with either supernatant material from the lly mutant or deferrated CDM (*, P < 0.05). In panel F, the cultures treated with the fraction from the mutant grew better than did those cultures supplemented with either supernatant material from the wild type or deferrated CDM (*, P < 0.05).
The production of HGA-melanin and the elaboration of siderophore activity are inversely related.
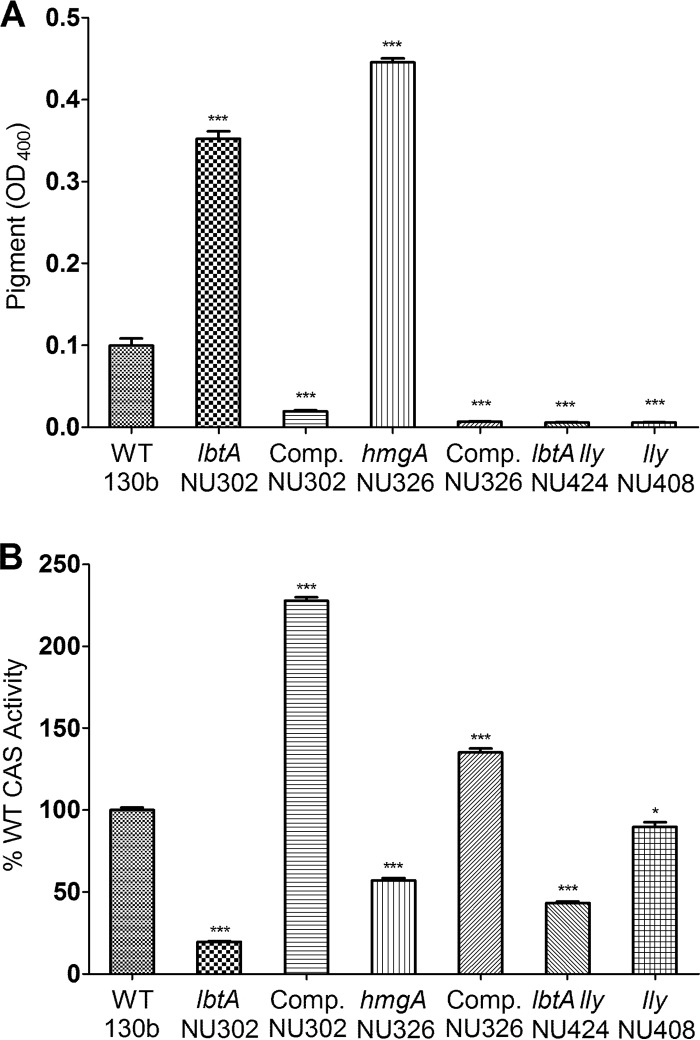
Given the ability of HGA-melanin to promote iron uptake and bacterial growth under iron-limited conditions, we hypothesized that the production of the pyomelanin might be complementary to that of the L. pneumophila siderophore; i.e., HGA-melanin levels would be increased in strains that do not elaborate legiobactin. Indeed, an lbtA mutant produced 3- to 4-fold more HGA-melanin than did wild-type strain 130b and nearly as much as did a hyperpigmented hmgA mutant (Fig. 7A). When an intact copy of lbtA was introduced into the legiobactin mutant on a multicopy plasmid, there was a large drop in pyomelanin production, compatible with hyperexpression of siderophore activity in the complemented mutant (10) (Fig. 7A). As expected, the increase in HGA-melanin production associated with the loss of lbtA was abolished when the lly gene was also mutated in an lbtA lly double mutant (Fig. 7A). In light of these results, we examined whether or not legiobactin levels were altered in the lly and hmgA mutants. Although the nonpigmented lly mutant did not exhibit increased levels of siderophore activity, the hyperpigmented hmgA mutant (but not its hmgA-complemented derivative) did display significantly lower levels of siderophore (Fig. 7B). Interestingly, the lbtA lly double mutant showed an increased level of CAS reactivity compared to that of the lbtA mutant (P < 0.05), suggesting that the loss of HGA-melanin might trigger the production of an additional, as-yet-unknown siderophore (Fig. 7B). The apparent inverse relationship between the production of HGA-melanin and the elaboration of siderophore activity further indicates that the pyomelanin can be involved in iron assimilation by L. pneumophila.
Fig 7.
Relationship between secreted HGA-melanin and siderophore activity. As indicated, wild-type strain 130b, lbtA mutant strain NU302, complemented (comp.) lbtA mutant strain NU302, hmgA mutant strain NU326, complemented hmgA mutant strain NU326, lbtA lly double mutant strain NU424, and lly mutant strain NU408 were grown in deferrated CDMP for 24 h, and then the levels of HGA-melanin (A) and relative siderophore activity (B) in cell-free supernatants were determined by measuring the OD400 and CAS reactivity, respectively. The data are the means and standard deviations from duplicate samples. All mutant and complemented mutant strains had levels of pigment and siderophore activity that were different from those of the wild type (***, P < 0.001; *, P < 0.05).
HGA and HGA-melanin release iron from naturally occurring iron chelates.
Within the mammalian host, the vast majority of iron is tightly bound by protein iron chelators, such as (holo-)transferrin and ferritin (42). Furthermore, ferritin levels are elevated in the serum of some Legionnaires' disease patients (43). Therefore, we next determined whether HGA and HGA-melanin are capable of releasing and reducing iron from purified ferritin and transferrin. HGA, synthetic HGA-melanin, and HGA-melanin precipitated from wild-type 130b culture supernatants all were capable of releasing ferrous iron when mixed with purified ferritin (Fig. 8A). In a similar vein, HGA and the HGA-melanins were able to release and reduce iron from purified transferrin (Fig. 8B). In contrast, acid-precipitated materials obtained from lly mutant supernatants did not show any activity against ferritin or transferrin (Fig. 8).
Fig 8.
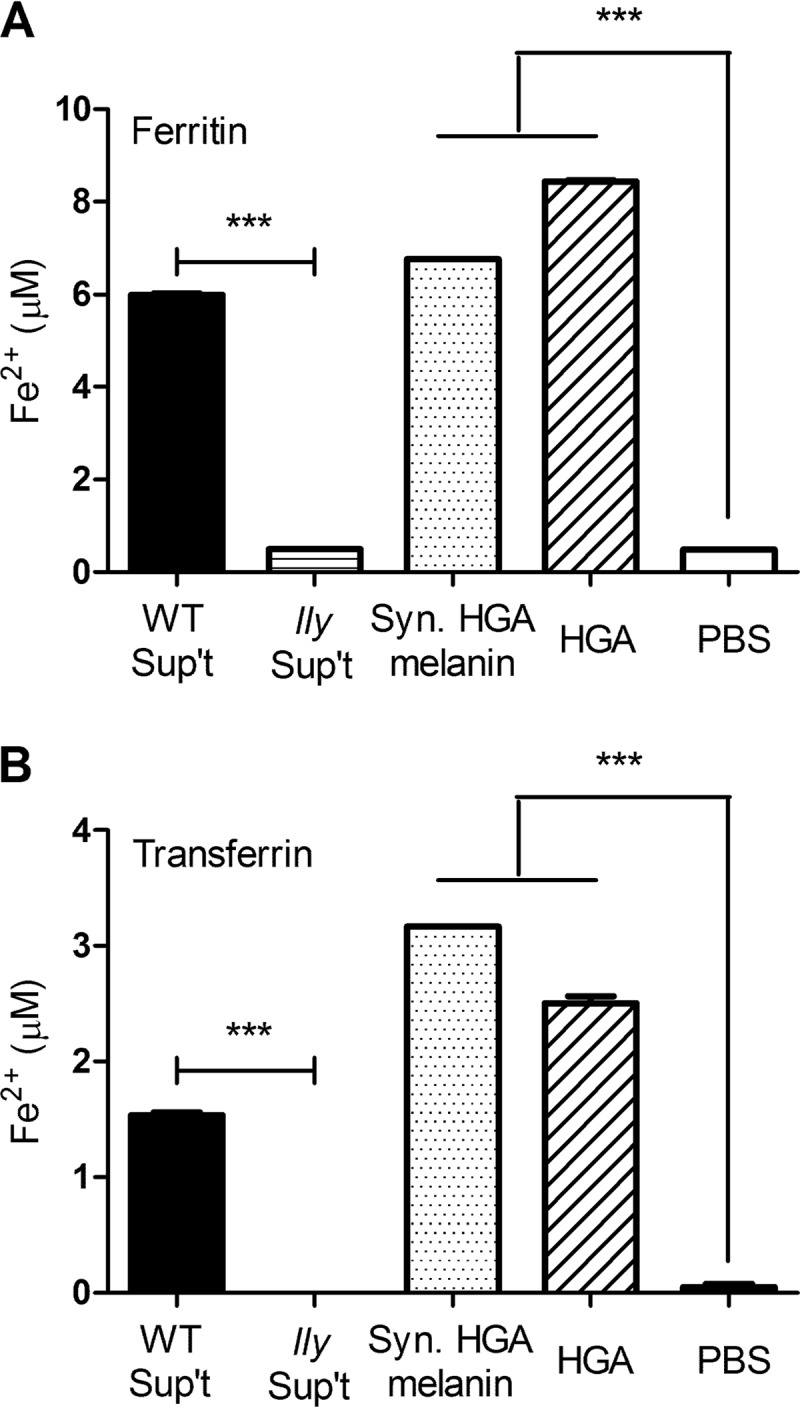
Effect of HGA and HGA-melanin on host iron chelates. As indicated, HGA-melanin obtained from wild-type strain 130b supernatants (WT Sup't), acid-precipitated material obtained from lly mutant strain NU408 supernatants (lly Sup't), synthetic HGA-melanin (Syn. HGA-melanin), unpolymerized HGA (HGA), or the PBS control (PBS) was mixed with ferritin (A) or transferrin (B), and then after 24 h at 37°C, the amount of ferrous iron generated and released into solution was determined by the ferrozine assay. The data are the means and standard deviations obtained from triplicate samples. The levels of ferrous iron generated by HGA, synthetic HGA-melanin, and bacterial HGA-melanin were greater than those produced by the mutant sample and the PBS control (***, P < 0.001).
Within many aquatic habitats, iron is in its ferric form as insoluble minerals, such as ferric hydroxide (44). Thus, we next sought to determine if HGA and HGA-melanin were capable of releasing ferrous iron from ferric hydroxide. However, when the purified reagents were mixed together in the test tube, the ferric hydroxide appeared to remain completely insoluble and no soluble iron was detected (data not shown). Therefore, we cultured L. pneumophila in CDMP with ferric hydroxide added and examined culture supernatants for the presence of solubilized iron. The pigmented supernatants obtained from wild-type strain 130b cultures contained approximately 6-fold more total iron than did the nonpigmented supernatants obtained from lly mutant cultures (Fig. 9A). Essentially, the same result was obtained when we specifically examined the supernatants for levels of ferrous iron (Fig. 9B). Taken together, these data indicate that HGA and HGA-melanin produced by L. pneumophila are capable of reducing and releasing the iron contained within iron chelates that the bacterium likely encounters within both its natural aquatic habitats and its mammalian hosts.
Fig 9.
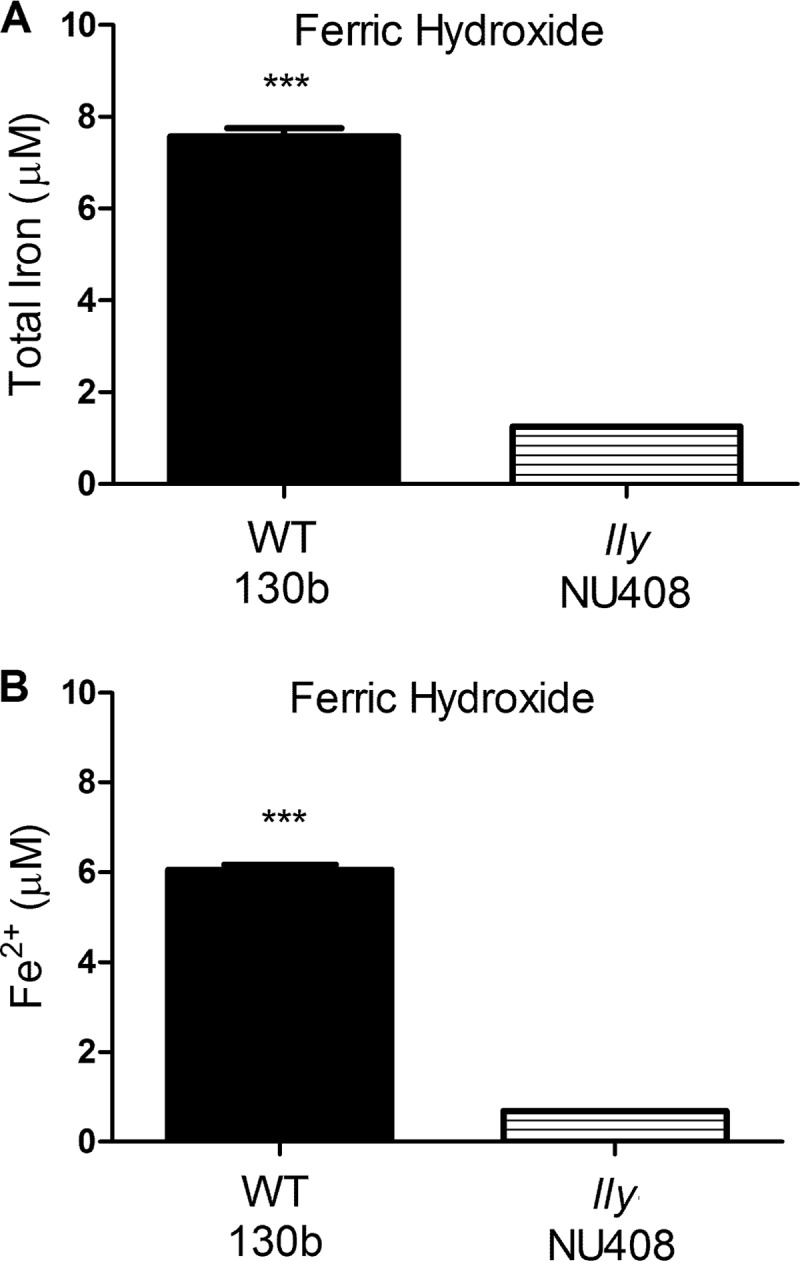
Effect of bacterial HGA-melanin on ferric hydroxide. As indicated, wild-type strain 130b and lly mutant strain NU408 were grown in CDMP containing added ferric hydroxide (1%, wt/vol), and then after 72 h of incubation at 37°C, the levels of total iron in cell-free culture supernatants were determined by the ferrozine assay in the presence of the reducing agent vitamin C (A) and the level of ferrous iron in cell-free supernatants was ascertained by the ferrozine assay in the absence of vitamin C (B). Data are the means and standard deviations obtained from triplicate samples. The levels of iron released into the supernatant were greater for the wild type than the mutant (***, P < 0.001).
DISCUSSION
Summarizing the results of this study, we have demonstrated that secreted HGA and HGA-melanin (i) directly mediate the reduction of ferric iron substrates, including ones that L. pneumophila likely encounters in natural aquatic and mammalian host environments, (ii) enhance iron uptake by Legionella strains, because of the ferrous iron generated by the reduction reactions, (iii) stimulate L. pneumophila growth under iron-limiting conditions, and (iv) are made in inverse fashion to secreted siderophore activity. In light of these data, we conclude that HGA and HGA-melanin are key components of L. pneumophila iron acquisition. Thus, upon encountering ferric iron in its extracellular milieu, L. pneumophila appears to have at least two options for iron acquisition. The first pathway, as we previously described, entails chelation of the Fe3+ by legiobactin and then the subsequent binding of the ferrisiderophore to the LbtU receptor on the bacterial surface (9–13). The second pathway, identified here, involves reduction of the ferric iron source by HGA or HGA-melanin and then subsequent uptake of the generated ferrous iron via the FeoB pathway. Although we know that FeoB is the inner membrane transporter for the ferrous iron (16), the identity of the outer membrane porin is unknown for L. pneumophila, as it is for the many other FeoB-containing bacteria. That all three of the other Legionella species tested were able to utilize HGA-melanin for enhanced iron uptake suggests that this second pathway for iron assimilation is present in many, if not all, other legionellae. Given the critical role of iron acquisition in L. pneumophila growth, we suspect that the function of HGA and HGA-melanin uncovered here is relevant to the natural ecology and pathogenesis of the bacterium. Supporting this viewpoint are the findings that genes involved in HGA production are expressed in L. pneumophila biofilms and in Legionella-infected macrophages and the pigment is seen in L. pneumophila-infected lungs and sputum (6, 31, 45–47). That nonpigmented mutants are still able to grow in amoebae and macrophage host cells (28, 30, 36) is compatible with the fact that the bacterium has multiple means of iron uptake. Indeed, siderophore mutants are also not impaired for intracellular infection (10).
To our knowledge, the data reported here represent the first documentation of a role for HGA and a pyomelanin in bacterial iron acquisition and assimilation. The fact that many other bacteria, including a variety of environmental bacteria as well as several key (plant and/or animal) pathogens, produce HGA-melanin (32) suggests that our observations have broad implications and other bacteria should be tested for the role of their secreted HGA in iron acquisition. Aside from L. pneumophila, the one bacterium whose secreted HGA-melanin has been studied at a functional level is the Gram-negative, environmental bacterium Shewanella algae; in that case, the pyomelanin provides a soluble electron shuttle or a membrane-associated electron conduit to reduce ferric iron to get energy (48, 49). Among the other bacteria known to produce HGA-melanin, key human pathogens include Burkholderia cenocepacia, Pseudomonas aeruginosa, and Vibrio cholerae (32, 50–53).
In considering the mechanism by which HGA and HGA-melanin promote iron acquisition, it would appear that they, by virtue of their ferric iron reduction reactions, generate ferrous iron that diffuses to the bacterial surface and is internalized through the yet-to-be-defined outer membrane porin/channel. However, since Fe2+ is generally not stable at neutral pH under aerobic conditions, this mechanism may not be able to fully explain our results. Given the finding that iron associates with the HGA-melanin in the bacterial culture supernatants, it is also possible that the pyomelanin provides a shuttle or trap protecting and bringing the ferrous iron closer to the surface of L. pneumophila. In support of this type of scenario, we observed that HGA-melanin promoted iron uptake to the same degree as the stronger reducing agents vitamin C and unpolymerized HGA (compare Fig. 1 and 2). Thus, the amount of iron assimilated by the bacterium may not be solely dependent on the concentration of the Fe2+ generated but may be influenced by the polymerized status of the reducing agent or the nature of a HGA-melanin complex. Other pyomelanins present in the soil have been linked to the sequestration of other metals, including uranium, molybdenum, lead, bismuth, and arsenic (54). In thinking about the ferric iron reduction reaction itself, it is possible that HGA and HGA-melanin do not always act alone; e.g., a secreted protease of L. pneumophila that degrades transferrin (55) may improve the access of HGA to the ferric iron in that host iron chelate.
Over the years, a wide variety of activities have been linked to microbial melanins and pigments overall (33, 56, 57). In addition to the link between L. pneumophila HGA-melanin and resistance to light that was mentioned in the introduction (36), microbial melanins have been linked to antioxidant and antiphagocytic activities and the ability to blunt antimicrobials. Other virulence-related functions ascribed to different types of microbial pigments include cytotoxicity, disabling of ciliary action, induction of apoptosis, induction of inflammation, and immunosuppression (57). Thus, finding a role for HGA-melanin in L. pneumophila iron assimilation significantly increases our appreciation for what pigments do for microorganisms and pathogens.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the N. P. Cianciotto lab for their help and support.
This study was funded by NIH grants AI089712 and AI034937 awarded to N.P.C.
Footnotes
Published ahead of print 26 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00858-13.
REFERENCES
- 1.Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cianciotto N, Hilbi H, Buchrieser C. Legionnaires' disease. In Rosenberg E. (ed), The prokaryotes, 4th ed. Springer-Verlag, Berlin, Germany [Google Scholar]
- 3.Pearce MM, Theodoropoulos N, Mandel MJ, Brown E, Reed KD, Cianciotto NP. 2012. Legionella cardiaca sp. nov., isolated from a case of native valve endocarditis in a human heart. Int. J. Syst. Evol. Microbiol. 62:2946–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyson JY, Pearce MM, Vargas P, Bagchi S, Mulhern BJ, Cianciotto NP. 2013. Multiple Legionella pneumophila type II secretion substrates, including a novel protein, contribute to differential infection of amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis. Infect. Immun. 81:1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Declerck P. 2010. Biofilms: the environmental playground of Legionella pneumophila. Environ. Microbiol. 12:557–566 [DOI] [PubMed] [Google Scholar]
- 6.Hindre T, Bruggemann H, Buchrieser C, Hechard Y. 2008. Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology 154:30–41 [DOI] [PubMed] [Google Scholar]
- 7.Newton HJ, Ang DK, van Driel IR, Hartland EL. 2010. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23:274–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cianciotto NP. 2008. Iron assimilation and type II protein secretion, p 33–48 In Hoffman PS, Friedman H, Bendinelli M. (ed), Legionella pneumophila: pathogenesis and immunity. Springer, New York, NY [Google Scholar]
- 9.Liles MR, Aber Scheel T, Cianciotto NP. 2000. Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila. J. Bacteriol. 182:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard KA, Viswanathan VK, Cianciotto NP. 2006. lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin. J. Bacteriol. 188:1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allard KA, Dao J, Sanjeevaiah P, McCoy-Simandle K, Chatfield CH, Crumrine DS, Castignetti D, Cianciotto NP. 2009. Purification of legiobactin and the importance of this siderophore in lung infection by Legionella pneumophila. Infect. Immun. 77:2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatfield CH, Mulhern BJ, Burnside DM, Cianciotto NP. 2011. Legionella pneumophila LbtU acts as a novel, TonB-independent receptor for the legiobactin siderophore. J. Bacteriol. 193:1563–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatfield CH, Mulhern BJ, Viswanathan VK, Cianciotto NP. 2012. The major facilitator superfamily-type protein LbtC promotes the utilization of the legiobactin siderophore by Legionella pneumophila. Microbiology 158:721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip ES, Burnside DM, Cianciotto NP. 2011. Cytochrome c4 is required for siderophore expression by Legionella pneumophila, whereas cytochromes c1 and c5 promote intracellular infection. Microbiology 157:868–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poch MT, Johnson W. 1993. Ferric reductases of Legionella pneumophila. Biometals 6:107–114 [DOI] [PubMed] [Google Scholar]
- 16.Robey M, Cianciotto NP. 2002. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 70:5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baine WB, Rasheed JK. 1979. Aromatic substrate specificity of browning by cultures of the Legionnaires' disease bacterium. Ann. Intern. Med. 90:619–620 [DOI] [PubMed] [Google Scholar]
- 18.Baine WB, Rasheed JK, Feeley JC, Gorman GW, Casida LE. 1978. Effect of supplemental l-tyrosine on pigment production in cultures of the Legionnaires' disease bacterium. Curr. Microbiol. 1:93–94 [Google Scholar]
- 19.Ristroph JD, Hedlund KW, Gowda S. 1981. Chemically defined medium for Legionella pneumophila growth. J. Clin. Microbiol. 13:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeley JC, Gorman GW, Weaver RE, Mackel DC, Smith HW. 1978. Primary isolation media for Legionnaires disease bacterium. J. Clin. Microbiol. 8:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren WJ, Miller RD. 1979. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J. Clin. Microbiol. 10:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pine L, George JR, Reeves MW, Harrell WK. 1979. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 9:615–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vickers RM, Yu VL. 1984. Clinical laboratory differentiation of Legionellaceae family members with pigment production and fluorescence on media supplemented with aromatic substrates. J. Clin. Microbiol. 19:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orrison LH, Cherry WB, Fliermans CB, Dees SB, McDougal LK, Dodd DJ. 1981. Characteristics of environmental isolates of Legionella pneumophila. Appl. Environ. Microbiol. 42:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg JD, Hoff JC, Roberts PV, Matin A. 1985. Growth of Legionella pneumophila in continuous culture. Appl. Environ. Microbiol. 49:1534–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinert M, Flugel M, Schuppler M, Helbig JH, Supriyono A, Proksch P, Luck PC. 2001. The Lly protein is essential for p-hydroxyphenylpyruvate dioxygenase activity in Legionella pneumophila. FEMS Microbiol. Lett. 203:41–47 [DOI] [PubMed] [Google Scholar]
- 27.Wintermeyer E, Rdest U, Ludwig B, Debes A, Hacker J. 1991. Characterization of legiolysin (lly), responsible for haemolytic activity, colour production and fluorescence of Legionella pneumophila. Mol. Microbiol. 5:1135–1143 [DOI] [PubMed] [Google Scholar]
- 28.Wintermeyer E, Flugel M, Ott M, Steinert M, Rdest U, Mann KH, Hacker J. 1994. Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect. Immun. 62:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie DE, Brady SF, Bettermann AD, Cianciotto NP, Liles MR, Rondon MR, Clardy J, Goodman RM, Handelsman J. 2002. Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl. Environ. Microbiol. 68:4301–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiater LA, Sadosky AB, Shuman HA. 1994. Mutagenesis of Legionella pneumophila using Tn903 dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641–653 [DOI] [PubMed] [Google Scholar]
- 31.Flydal MI, Chatfield CH, Zheng H, Gunderson FF, Aubi O, Cianciotto NP, Martinez A. 2012. Phenylalanine hydroxylase from Legionella pneumophila is a thermostable enzyme with a major functional role in pyomelanin synthesis. PLoS One 7:e46209. 10.1371/journal.pone.0046209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatfield CH, Cianciotto NP. 2007. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 75:4062–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plonka PM, Grabacka M. 2006. Melanin synthesis in microorganisms—biotechnological and medical aspects. Acta Biochim. Pol. 53:429–443 [PubMed] [Google Scholar]
- 34.Weiner RM. 1997. Biopolymers from marine prokaryotes. Trends Biotechnol. 15:390–394 [DOI] [PubMed] [Google Scholar]
- 35.Turick CE, Tisa LS, Caccavo F., Jr 2002. Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl. Environ. Microbiol. 68:2436–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert M, Engelhard H, Flugel M, Wintermeyer E, Hacker J. 1995. The Lly protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmannella vermiformis. Appl. Environ. Microbiol. 61:2428–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edelstein PH. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeves MW, Pine L, Hutner SH, George JR, Harrell WK. 1981. Metal requirements of Legionella pneumophila. J. Clin. Microbiol. 13:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewster JD. 2003. A simple micro-growth assay for enumerating bacteria. J. Microbiol. Methods 53:77–86 [DOI] [PubMed] [Google Scholar]
- 40.Edelstein PH, Cianciotto NP. 2010. Legionella, p 2969–2984 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed, vol 2 Elsevier Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 41.Bender L, Ott M, Debes A, Rdest U, Heesemann J, Hacker J. 1991. Distribution, expression, and long-range mapping of legiolysin gene (lly)-specific DNA sequences in legionellae. Infect. Immun. 59:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hentze MW, Muckenthaler MU, Andrews NC. 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297 [DOI] [PubMed] [Google Scholar]
- 43.Cunha BA. 2008. Highly elevated serum ferritin levels as a diagnostic marker for Legionella pneumonia. Clin. Infect. Dis. 46:1789–1791 [DOI] [PubMed] [Google Scholar]
- 44.Challis GL. 2005. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. Chembiochem 6:601–611 [DOI] [PubMed] [Google Scholar]
- 45.Suffin SC, Kaufmann AF, Whitaker B, Muck KB, Prince GA, Porter DD. 1980. Legionella pneumophila: identification in tissue sections by a new immunoenzymatic procedure. Arch. Pathol. Lab. Med. 104:283–286 [PubMed] [Google Scholar]
- 46.Fujita J, Touyama M, Chibana K, Koide M, Haranaga S, Higa F, Tateyama M. 2007. Mechanism of formation of the orange-colored sputum in pneumonia caused by Legionella pneumophila. Intern. Med. 46:1931–1934 [DOI] [PubMed] [Google Scholar]
- 47.Faucher SP, Mueller CA, Shuman HA. 2011. Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turick CE, Caccavo F, Jr, Tisa LS. 2008. Pyomelanin is produced by Shewanella algae BrY and affected by exogenous iron. Can. J. Microbiol. 54:334–339 [DOI] [PubMed] [Google Scholar]
- 49.Turick CE, Beliaev AS, Zakrajsek BA, Reardon CL, Lowy DA, Poppy TE, Maloney A, Ekechukwu AA. 2009. The role of 4-hydroxyphenylpyruvate dioxygenase in enhancement of solid-phase electron transfer by Shewanella oneidensis MR-1. FEMS Microbiol. Ecol. 68:223–235 [DOI] [PubMed] [Google Scholar]
- 50.Keith KE, Killip L, He P, Moran GR, Valvano MA. 2007. Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J. Bacteriol. 189:9057–9065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter RC, Newman DK. 2010. A putative ABC transporter, HatABCDE, is among molecular determinants of pyomelanin production in Pseudomonas aeruginosa. J. Bacteriol. 192:5962–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Rojas A, Mena A, Martin S, Borrell N, Oliver A, Blazquez J. 2009. Inactivation of the hmgA gene of Pseudomonas aeruginosa leads to pyomelanin hyperproduction, stress resistance and increased persistence in chronic lung infection. Microbiology 155:1050–1057 [DOI] [PubMed] [Google Scholar]
- 53.Valeru SP, Rompikuntal PK, Ishikawa T, Vaitkevicius K, Sjoling A, Dolganov N, Zhu J, Schoolnik G, Wai SN. 2009. A role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect. Immun. 77:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turick CE, Knox AS, Leverette CL, Kritzas YG. 2008. In situ uranium stabilization by microbial metabolites. J. Environ. Radioact. 99:890–899 [DOI] [PubMed] [Google Scholar]
- 55.James BW, Mauchline WS, Dennis PJ, Keevil CW. 1997. A study of iron acquisition mechanisms of Legionella pneumophila grown in chemostat culture. Curr. Microbiol. 34:238–243 [DOI] [PubMed] [Google Scholar]
- 56.Nosanchuk JD, Casadevall A. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 50:3519–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu GY, Nizet V. 2009. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 17:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart CR, Rossier O, Cianciotto NP. 2009. Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion. J. Bacteriol. 191:1537–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenner DJ, Steigerwalt AG, McDade JE. 1979. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann. Intern. Med. 90:656–658 [DOI] [PubMed] [Google Scholar]
- 60.McKinney RM, Thacker L, Harris PP, Lewallen KR, Hebert GA, Edelstein PH, Thomason BM. 1979. Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann. Intern. Med. 90:621–624 [DOI] [PubMed] [Google Scholar]
- 61.Gorman GW, Feeley JC, Steigerwalt A, Edelstein PH, Moss CW, Brenner DJ. 1985. Legionella anisa: a new species of Legionella isolated from potable waters and a cooling tower. Appl. Environ. Microbiol. 49:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hebert GA, Steigerwalt AG, Brenner DJ. 1980. Legionella micdadei species nova: classification of a third species of Legionella associated with human pneumonia. Curr. Microbiol. 3:255–257 [Google Scholar]
- 63.Rossier O, Starkenburg S, Cianciotto NP. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72:310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossier O, Cianciotto NP. 2005. The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect. Immun. 73:2020–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naylor J, Cianciotto NP. 2004. Cytochrome c maturation proteins are critical for in vivo growth of Legionella pneumophila. FEMS Microbiol. Lett. 241:249–256 [DOI] [PubMed] [Google Scholar]
- 66.Zink SD, Pedersen L, Cianciotto NP, Abu-Kwaik Y. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.