Abstract
VirF is an AraC family transcriptional activator that is required for the expression of virulence genes associated with invasion and cell-to-cell spread by Shigella flexneri, including multiple components of the type three secretion system (T3SS) machinery and effectors. We tested a small-molecule compound, SE-1 (formerly designated OSSL_051168), which we had identified as an effective inhibitor of the AraC family proteins RhaS and RhaR, for its ability to inhibit VirF. Cell-based reporter gene assays with Escherichia coli and Shigella, as well as in vitro DNA binding assays with purified VirF, demonstrated that SE-1 inhibited DNA binding and transcription activation (likely by blocking DNA binding) by VirF. Analysis of mRNA levels using real-time quantitative reverse transcription-PCR (qRT-PCR) further demonstrated that SE-1 reduced the expression of the VirF-dependent virulence genes icsA, virB, icsB, and ipaB in Shigella. We also performed eukaryotic cell invasion assays and found that SE-1 reduced invasion by Shigella. The effect of SE-1 on invasion required preincubation of Shigella with SE-1, in agreement with the hypothesis that SE-1 inhibited the expression of VirF-activated genes required for the formation of the T3SS apparatus and invasion. We found that the same concentrations of SE-1 had no detectable effects on the growth or metabolism of the bacterial cells or the eukaryotic host cells, respectively, indicating that the inhibition of invasion was not due to general toxicity. Overall, SE-1 appears to inhibit transcription activation by VirF, exhibits selectivity toward AraC family proteins, and has the potential to be developed into a novel antibacterial agent.
INTRODUCTION
Shigella is a major cause of bacillary dysentery (shigellosis) in humans (1), a disease characterized by a short period of watery diarrhea with intestinal cramps, followed by bloody mucoid stools. Shigella is responsible for 165 million cases of illness and more than 1.1 million deaths worldwide each year, and 70% of those dying from illnesses due to Shigella are children under the age of 5 (2, 3). Among Shigella species, Shigella flexneri causes more mortality than any other; as few as 100 cells are sufficient to cause disease (4). The first step in Shigella pathogenesis is invasion of colonic and rectal epithelial cells. After invasion, Shigella replicates and spreads from cell to cell within the colonic and rectal epithelia. In addition to direct damage by Shigella, host inflammatory responses also contribute to the epithelial cell damage leading to shigellosis (5–12).
Given the enormous clinical relevance of Shigella infections, it is important to find an effective treatment strategy to combat them. Despite many efforts, no vaccine has been released for public use, although a few vaccine candidates are currently in clinical trials (13–16). A major obstacle to vaccine development is the substantial heterogeneity of surface antigens among different strains of this pathogen (1). Further, Shigella is rapidly developing resistance to currently available antibiotics (17). In recent years, the development of new antibiotics has generally been limited to modifications of existing antibiotics (18–21), which primarily target essential bacterial proteins and put extensive selective pressure on bacteria to develop resistance (22). In addition, broad-spectrum antibiotics adversely affect the resident gut microbiota (23). Thus, new and innovative approaches are needed to circumvent the problem of Shigella infections.
Anti-infective strategies that target several individual AraC family bacterial virulence gene activator proteins have been reported and have the potential to be developed into alternatives to traditional antibiotics (24–28). AraC family proteins share sequence similarity in a region of approximately 100 amino acids that functions as a DNA-binding domain (DBD) (29) and are present in 70% of sequenced bacterial genomes (30). The DBDs of AraC family proteins have two conserved helix-turn-helix motifs, through which they bind to DNA and activate, or sometimes repress, transcription (29). AraC family proteins activate virulence gene expression in many pathogenic bacteria, including Shigella flexneri (VirF), Vibrio cholerae (ToxT), enterotoxigenic Escherichia coli (ETEC) (Rns/CfaD), and Pseudomonas aeruginosa (ExsA) (29). Importantly, mutations that disrupt the function of AraC family virulence activators reduce bacterial virulence without affecting the growth of the bacteria (26, 31). Thus, inhibition of AraC family virulence activators is expected to exert less selective pressure on bacteria to develop resistance than currently available antibiotics (22, 32–34). Multiple lines of experimental evidence indicate that targeting AraC family virulence activators can dramatically reduce the severity of infections in animal models, suggesting that AraC family proteins may be excellent targets for the development of novel antimicrobials (24–28).
The AraC family activator VirF is required for Shigella virulence gene expression and is encoded on a 220-kb virulence plasmid (35). VirF expression has been shown to be temperature dependent, with 3- to 4-fold-lower expression at 30°C than at 37°C (36, 37). The nucleoid-associated protein H-NS represses VirF expression at 30°C by binding to sites within an intrinsically curved region of the virF promoter (38, 39). At 37°C, a change in DNA structure results in the release of H-NS from DNA, thereby facilitating VirF expression (36, 38, 40, 41).
VirF activates the expression of a cascade of genes responsible for the formation of the type three secretion system (T3SS) machinery, the invasion of host epithelial cells by Shigella, and cell-to-cell spread (5–8, 42). VirF directly activates the expression of the icsA and virB virulence genes (43, 44). The icsA gene encodes the IcsA (VirG) protein, which aids the intracellular movement of the pathogen by mediating actin-based motility (45–48). The virB gene encodes a transcriptional activator, VirB, which in turn activates the expression of many virulence-associated genes (including the mxi, spa, and ipa operons) (37). virB expression is also regulated by H-NS and thus has VirF-dependent and VirF-independent mechanisms that increase its expression at 37°C over that at 30°C (11, 49). Genes in the mxi and spa operons encode the T3SS machinery, through which effectors are released into host cells (50). Genes in the ipa operon encode effector proteins (IcsB, IpaA, IpaB, IpaC, and IpaD) that translocate directly into host cells. Among the effectors, IpaB plays major roles in the formation of pores in the host cell membrane, the lysis of phagosomes, and macrophage apoptosis (7, 51–54). Another effector, IcsB, prevents the triggering of host autophagy, a process that can be used by host cells to export invading bacteria to lysosomes for degradation (55, 56). Given that VirF is required for VirB expression and that VirB activates the expression of multiple virulence-associated genes that are required for the earliest steps in the successful invasion of host cells by Shigella, VirF is considered the master regulator of Shigella virulence (5–8, 42). Shigella mutants that do not express VirF are avirulent but grow at the same rate as the wild-type bacteria (57, 58). We therefore reasoned, as have others (59), that VirF has potential as a target for novel antibacterial agents and that its nonessential nature has the potential to reduce the development of resistance.
We recently identified an AraC family inhibitor, SE-1 (formerly called OSSL_051168), that selectively inhibited two AraC family activators, RhaS and RhaR, despite their limited sequence similarity with each other (60). We further found that SE-1 inhibited RhaS and the RhaS DNA-binding domain (RhaS-DBD) to the same extent and that it blocked DNA binding by RhaS and RhaR (60). Furthermore, SE-1 had little or no impact on DNA binding by the non-AraC family proteins LacI and cyclic AMP receptor protein (CRP), indicating selectivity for at least the AraC family proteins tested (60). Taken together, our results support the hypothesis that SE-1 acts on the RhaS and RhaR DBDs—the structurally conserved domain in AraC family proteins (29, 60). These observations lead us to propose the hypothesis that this compound might selectively inhibit additional AraC family activators.
Here we have tested the ability of SE-1 to inhibit another AraC family activator, the master Shigella virulence activator VirF. Our results showed that the compound effectively inhibited the binding of VirF to DNA and therefore its ability to activate transcription in vivo. Analysis of mRNA levels for direct and indirect targets of VirF showed a significant reduction in the level of expression of these genes in the presence of SE-1. SE-1 also demonstrated significant inhibition of invasion of epithelial cells in tissue culture by Shigella without showing any detrimental effects on the growth of the bacterial cells or the metabolism of the host cells. We hypothesize that the reductions in the levels of gene expression and invasion are due to inhibition of VirF activity by SE-1. Overall, we have demonstrated that SE-1 inhibits transcription activation by the Shigella master virulence regulator, VirF.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. Escherichia coli strains were grown in tryptone-yeast extract (TY) broth (0.8% Difco tryptone, 0.5% Difco yeast extract, and 0.5% NaCl [pH 7.0]). Cultures for phage P1vir infection (generalized transduction) were grown in TY broth supplemented with 5 mM CaCl2. Difco nutrient agar (1.5% agar) (Becton Dickinson and Company [BD], Cockeysville, MD) was used routinely to grow E. coli strains on solid medium. S. flexneri was cultured in tryptic soy broth (TSB; pH 7.0) (BD) or Luria-Bertani broth (1% tryptone, 0.5% yeast extract, and 1% NaCl) or on tryptic soy agar (TSA; 1.5% agar) (BD) containing Congo red dye (0.025%). All Shigella strains were picked as red colonies from TSA plates containing Congo red. Cultures were grown at 37°C unless otherwise specified. Appropriate antibiotics (ampicillin, 100 μg/ml; tetracycline, 20 μg/ml; chloramphenicol, 30 μg/ml; gentamicin, 10 μg/ml) were used as indicated. The entire open reading frames of all cloned genes were sequenced on both strands. L-929 mouse fibroblasts (ATCC, Manassas, VA) were routinely cultured in RPMI 1640 tissue culture medium (Mediatech, Manassas, VA) supplemented with 10 μg/ml gentamicin (MP Biomedicals, Santa Ana, CA) and 5% fetal bovine serum (FBS; Thermo Fisher, Waltham, MA) in a humidified 5% CO2 incubator at 37°C.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristicsb | Source or reference |
|---|---|---|
| Strains | ||
| E. coli strains | ||
| AB97 | MC4100 λMAD102 [Φ(virB-lacZ)]a | A. Maurelli |
| SME4021 | ECL116 λMAD102 [Φ(virB-lacZ)]a | This study |
| SME4240 | SME4021 malP::lacIq zhc-511:: Tn10 | This study |
| SME4259 | SME4240 recA::cat | This study |
| SME4382 | SME4259 + pHG165virF | This study |
| ECL116 | F− ΔlacU169 endA hsdR thi | 62 |
| SME1048 | ECL116 recA::cat | Laboratory collection |
| MC4100 | F− araD139 Δ(argF-lac)U169 rpsL150 relA1 flhD5301 deoC1 ptsF25 rbsR | 97 |
| SG22166 | MC4100 malP::lacIq ftsH1(Ts) zgj::Tn10 | 66 |
| SME3358 | ECL116 ΔrhaS λ Φ(Phts-lacZ) recA::cat | This study |
| SME3359 | SME3358 + pHG165lacI | This study |
| SME4319 | KS1000 + pMAL virF | This study |
| KS1000 | F′ lacIq lac+ pro+ ara Δ(lac-pro) Δ(tsp) Δ(prc)::Kanr eda51::Tn10 Tetr gyrA (Nalr) rpoB thi-1 argE(Am) | 98 |
| Shigella strains | ||
| SME4331 | S. flexneri ipgD | W. D. Picking |
| SB116 | S. flexneri mxiH | 6 |
| BS536 | S. flexneri virB-lacZ | A. Maurelli |
| Plasmids | ||
| pHG165 | lacZα+ rop+ Ampr; essentially pUC8 with pBR322 copy number | 61 |
| pHG165virF | This study | |
| pHG165lacI | This study | |
| pMAL-c2 | lacIq malE-lacZα; Ampr | New England BioLabs |
| pMAL virF | This study |
The virB promoter portion of the fusion comprised nucleotides −259 to +308 relative to the transcription start site (+1).
Kanr, kanamycin resistant; Nalr, nalidixic acid resistant; Ampr, ampicillin resistant.
Construction of strains for in vivo dose-response studies.
For dose-response studies, a virB-lacZ reporter strain that allowed isopropyl-β-d-thiogalactopyranoside (IPTG)-induced expression of VirF from plasmid pHG165 was constructed (61). To construct this virB-lacZ reporter strain, λMAD102 was isolated from strain AB97 and was used to infect strain ECL116 (62), and single-copy lysogens were identified (63–65). P1vir-mediated generalized transductions were then used to add malP::lacIq and recA::cat to this strain, as described previously (60, 66). The virF gene was amplified (forward oligonucleotide, 5′-GATGAATTCTAAATATAGTTTGGTTATTCTGTTGAATTTATG-3′; reverse oligonucleotide, 5′-GTGCTGCAGTTAAAATTTTTTATGATATAAGTAAAATTTCTTTGGAG-3′), digested with EcoRI and PstI, and then ligated into the same sites of plasmid pHG165. The resulting plasmid, pHG165virF, was transformed into SME4259 to make SME4382 (see Fig. S1 [top] in the supplemental material). A control strain, SME3359, which carries a single-copy hts-lacZ reporter fusion in the chromosome and plasmid pHG165lacI, expressing LacI protein (see Fig. S1, bottom), was constructed as described previously (60). Briefly, the hts-lacZ reporter fusion consists of a synthetic promoter that is repressed by LacI. The control strain is isogenic with SME4382, except that it does not carry malP::lacIq, and therefore, LacI is expressed from pHG165lacI regardless of the presence of IPTG.
In vivo dose-response experiments with E. coli.
In vivo dose-response assays were performed using a procedure described previously (60). In brief, cultures of SME4382 and SME3359 grown overnight were diluted to an optical density at 600 nm (OD600) of ∼0.1. SE-1 (1-butyl-4-nitromethyl-3-quinolin-2-yl-4H-quinoline; formerly designated OSSL_051168) was obtained from eMolecules, Inc., Solana Beach, CA (catalog no. 3761-0013), or Princeton BioMolecular Research, Princeton, NJ (catalog no. OSSL_051168) (Fig. 1A), and was dissolved in 100% dimethyl sulfoxide (DMSO). Bacterial cultures were mixed with varying concentrations of SE-1 (final concentration of DMSO, 2.2%), induced with 6.5 mM IPTG for 3 h at 37°C, and then lysed, and β-galactosidase activity was measured (60). Uninduced (no IPTG and no SE-1) and uninhibited (6.5 mM IPTG and no SE-1) controls were included for each strain and were used to normalize β-galactosidase activity, as described previously (60). Fifty percent inhibitory concentrations (IC50s) were calculated, and graphs were drawn, using GraphPad Prism (GraphPad, La Jolla, CA). Error bars in figures represent the standard errors of the means for three independent experiments with two replicates each.
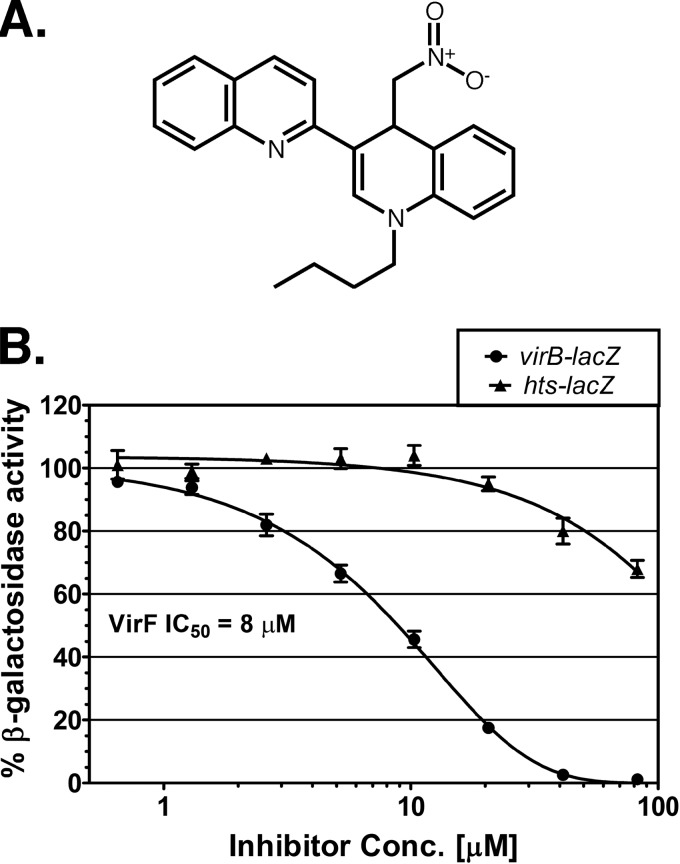
Fig 1.
Chemical structure of SE-1 and inhibition of in vivo VirF activation in E. coli. (A) Chemical structure of SE-1 (1-butyl-4-nitromethyl-3-quinolin-2-yl-4H-quinoline). (B) β-Galactosidase (LacZ) activities from two reporter fusions in E. coli, VirF-activated virB-lacZ (SME4382) (circles) and LacI-repressed hts-lacZ (SME3359) (triangles), were assayed at the indicated concentrations of the inhibitor SE-1. VirF and LacI were each expressed from plasmid pHG165. Activity in the absence of SE-1 was set to 100% in each case and corresponded to approximately 950 Miller units for virB-lacZ and 350 Miller units for hts-lacZ. Results are averages for three independent experiments with two replicates each.
E. coli growth curves.
To test the impact of SE-1 on the growth of the bacterial strains used for in vivo dose-response assays (SME4382 and SME3359), we compared growth rates in the presence and absence of SE-1 by using a procedure described previously (60), but without the addition of rhamnose. Briefly, the cells were grown at 37°C with shaking in morpholinepropanesulfonic acid (MOPS)-buffered minimal medium with glycerol plus 6.5 mM IPTG, in 24-well plates, either with SE-1 (44 μM SE-1, 0.3% DMSO) or with DMSO (0.3%) alone, in a PowerWave XS plate reader equipped with KC4 data analysis software (BioTek Instruments).
Heterologous expression and purification of VirF.
The IPTG-inducible MBP-VirF expression plasmid pMALvirF was constructed by cloning virF downstream and in frame with malE (encoding maltose binding protein [MBP]) in the pMAL-c2X vector (New England BioLabs, Beverly, MA). MBP-VirF protein (referred to below as VirF for simplicity) was purified using amylose affinity chromatography at 4°C. Briefly, pMALvirF was transformed into E. coli strain KS1000 (New England BioLabs). The cells were grown to an OD600 of 0.5 and were transferred to 15°C; 0.1 mM IPTG was added; and the cells were then incubated overnight with shaking. Cells were harvested and resuspended in 10 ml of binding buffer (20 mM Tris, 500 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol [DTT] [pH 7.4]). Cells were lysed by three freeze-thaw cycles with lysozyme (0.4 mg/ml), tris(2-carboxyethyl)phosphine (TCEP; 1 mM), and phenylmethylsulfonyl fluoride (PMSF; 1 mM) at −80°C, followed by sonication, and were then centrifuged to remove cell debris. The supernatant was applied to an amylose resin column using a BioLogic low-pressure (LP) chromatography system (both from Bio-Rad Laboratories). The column was preequilibrated with 80 ml binding buffer and was then washed with 120 ml binding buffer. VirF was eluted with 40 ml of elution buffer (binding buffer plus 15% [wt/vol] glycerol and 10 mM maltose) and was used directly for subsequent assays. The purified protein was more than 90% pure (see Fig. S2 in the supplemental material), although some older preparations showed partial breakdown to MBP and nonfused VirF. Electrophoretic mobility shift assays (EMSAs) showed no evidence of nonfused VirF binding to DNA (data not shown), suggesting that the nonfused VirF protein was inactive (likely due to aggregation).
EMSAs.
The binding of purified VirF to a DNA fragment containing the VirF binding site at virB was analyzed by EMSAs (67) in the absence or presence of SE-1. DNA probes were generated by hybridizing (68) the following oligonucleotides (Eurofins MWG Operon, Huntsville, AL): a short IR700-labeled LUEGO (labeled universal electrophoretic gel shift oligonucleotide) (5′-GTGCCCTGGTCTGG-3′) (68), a top oligonucleotide containing the virB binding sites (underlined in sequences) (5′-AGAATATTATTCTTTTATCCAATAAAGATAAATTGCATCAATCCAGCTATTAAAATAGTA-3′), and a bottom oligonucleotide that was complementary to both of the preceding oligonucleotides (5′-TACTATTTTAATAGCTGGATTGATGCAATTTATCTTTATTGGATAAAAGAATAATATTCT CCAGACCAGGGCAC-3′). Reactions and electrophoresis were performed as described previously (60) but without any of the additives described; however, all reaction mixtures contained 10% DMSO (used to dilute SE-1). Gels were scanned using an Odyssey infrared imager (Li-Cor, Lincoln, NE) and were quantified using Odyssey software, version 3.0.30. Graphs were drawn using GraphPad Prism (GraphPad).
Fluorescence-based thermal shift assay.
Binding of SE-1 to purified VirF was tested by comparing the VirF melting temperature (Tm) in the absence of SE-1 to that in its presence using a thermal shift assay with the fluorescent dye Sypro Orange (69, 70) (Molecular Probes). This method is also known as differential scanning fluorimetry (DSF) (71). We used the protocol of Ericsson et al. (70) with the following minor modifications. As a control, we tested the binding of SE-1 to the unrelated purified protein MBP-NS1-NTD (more than 95% pure). Reaction mixtures contained the following (final concentrations): 1 μM protein, 20× Sypro Orange (a 250-fold dilution of the 5,000× stock solution purchased), 0.76× EMSA buffer (7.6 mM Tris-acetate [pH 7.4], 0.76 mM K-EDTA, 38 mM KCl, and 0.76 mM DTT), 4% DMSO, and either no SE-1 or 80 μM SE-1. The reactions were carried out in low-profile 0.1-ml PCR 8-tube strips with optically clear flat caps (USA Scientific) in a StepOnePlus real-time PCR system (Applied Biosystems), with heating from 25 to 99°C in increments of 0.8°C. The data were analyzed using the Boltzmann sigmoidal model in GraphPad Prism software (GraphPad). Data points before and after the fluorescence intensity minimum and maximum, respectively, were excluded from fitting. The values for ΔTm (Tm changes) are averages for three independent experiments with two replicates in each experiment. Results of a single representative experiment are shown.
Shigella virB-lacZ reporter gene assays.
Colonies of S. flexneri strain BS536 that were red on TSA plates containing Congo red were grown overnight in Luria-Bertani broth at 30°C to maintain low basal expression from the virB promoter. The overnight-grown cultures were diluted 1:100 into 10 ml of the same medium with either 0.3% DMSO or different concentrations of SE-1 dissolved in DMSO (final concentration, 0.3% DMSO). Cultures were grown at 37°C in a shaking incubator to an OD600 of ∼0.4, centrifuged to pellet the cells, and then resuspended in Z buffer for a 2-fold concentration of the cells (63). Control cultures were grown at 30°C, a temperature at which virF expression is not induced (36), to illustrate the basal level of expression from the virB promoter region. β-Galactosidase assays were performed as described previously using the method of Miller (63), except that incubation with the substrate o-nitrophenyl-β-d-galactopyranoside (ONPG) was carried out at room temperature. Activities are presented as percentages of the activity of the uninhibited, DMSO-only control sample. Three independent assays were performed, with two replicates in each assay. Error bars in figures represent the standard errors of the means.
Shigella gene expression analysis.
Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed to test the effects of SE-1 on the expression of the VirF-regulated genes virB, icsA, icsB, and ipaB. The gapA and rrsA transcripts were used as internal controls. These are constitutively expressed Shigella genes commonly used to normalize mRNA levels (72–74). S. flexneri strain SME4331, which carries a null mutation in ipgD, was grown at 30°C overnight in TSB with ampicillin and was then diluted into the same medium to an OD600 of ∼0.1, and 1-ml aliquots were further grown at 30°C (with DMSO only) or at 37°C (with SE-1 at 20 or 40 μM, or with DMSO only; in all cases, the concentration of DMSO was 0.3%). Cells were grown to an OD600 of 1.0 (∼2.5 h), and RNA was isolated using an RNeasy MinElute cleanup kit (Qiagen, Germantown, MD); DNA contamination was eliminated by using a Turbo DNase kit (Ambion, Austin, TX). cDNA was synthesized by random priming using a cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Primers (Table 2) were tested for PCR specificity using a genomic DNA template to ensure a single amplicon of the expected size for each primer pair and were validated using 5-fold dilutions of cDNA (2,000 to 0.0256 ng) to ensure a linear plot of the cycle threshold (CT) versus cDNA concentration. Validated primer sets were used to test mRNA profiles with reaction mixtures that contained 10 ng of cDNA, 10 μl of SYBR green master mix (Applied Biosystems), specific primers (0.5 μM each), and water to a final volume of 20 μl, using a StepOnePlus real-time PCR system (Applied Biosystems). The data were analyzed using the 2−ΔΔCT method, as described previously (75), and also by Applied Biosystems real-time analysis software. The data analyzed by the 2−ΔΔCT method are reported; however, the two analyses produced comparable results. In brief, ΔΔCT values [calculated as (CT for the target in the presence of SE-1 − CT for the internal control in the presence of SE-1) − (CT for the target in the absence of SE-1 − CT for the internal control in the absence of SE-1)] were obtained separately for each sample relative to two internal controls, gapA and rrsA. The ΔΔCT values for each sample relative to the two internal controls were averaged and were then used to calculate the relative expression levels (2−ΔΔCT). As a control, we also normalized each internal control to the other internal control. Examples of data and calculated values are shown in Table S1 in the supplemental material. Control reactions used templates generated without reverse transcriptase to ensure low genomic DNA contamination. To ensure that products were not likely to be due to primer dimer artifacts, additional control reactions were performed in which the reaction mixtures contained primers alone (no template).
Table 2.
Primers for qRT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Amplicon size (bp) |
|---|---|---|---|
| virB | GGAAGGGAGATTGATGGTAG | GAACTTCAAGATCTGCTCCTGC | 84 |
| icsA | CTTTCGGGTACTCAAGAAC | GAGAAAGTCCATCAACAGG | 76 |
| icsB | CTCAATTCAACACTCTTTCACAG | GGCTGTACCGATGCCATGAAAACG | 82 |
| ipaB | CTGCATTTTCAAACACAGC | GAGTAACACTGGCAAGTC | 78 |
| gapA | GTTCTGACATCGAGATCGTTGC | GTGAGTGGAGTCATATTTCAGCATG | 83 |
| rrsA | AACGTCAATGAGCAAAGGTATTAA | TACGGGAGGCAGCAGTGG | 140 |
Shigella growth curves.
The S. flexneri ipgD-null strain (SME4331) was grown overnight at 30°C and was then diluted to an OD600 of ∼0.1 in TSB supplemented with ampicillin. SE-1 was diluted in DMSO and either was not added to the SME4331 cultures (0.3% DMSO only) or was added at a final concentration of 20 or 40 μM with 0.3% DMSO. These cultures were grown at 37°C in 24-well plates containing 1.2 ml of each sample in the temperature-controlled chamber of a PowerWave XS plate reader equipped with KC4 data analysis software (BioTek Instruments). Bacterial growth was continued at 37°C for 8 h with shaking, and the OD600 was monitored every 5 min for growth curves (20-min time intervals were plotted).
Shigella invasion assays.
The S. flexneri ipgD-null (SME4331) and mxiH-null (SB116) (6) mutant strains were grown overnight at 30°C and were then diluted to an OD600 of ∼0.1 into TSB plus ampicillin. SE-1 (in DMSO) either was not added to the cultures (0.3% DMSO only) or was added at a final concentration of 20 or 40 μM SE-1 with 0.3% DMSO. These cultures and SB116 (no SE-1) were grown at 37°C in 24-well plates (each with 1.2 ml) in the temperature-controlled chamber of a PowerWave XS plate reader (BioTek Instruments). Bacterial growth was continued at 37°C with shaking, and the OD600 was monitored every 15 min. A separate sample of SME4331 was grown at 30°C. L-929 cells (mouse fibroblasts, used previously for Shigella invasion assays [76–79]) that had been grown overnight to ∼90% confluence in 24-well plates were washed three times with 1 ml of RPMI medium each (without FBS or antibiotics), and the final wash was removed by aspiration. (All L-929 incubation steps were carried out at 37°C under a 5% CO2 humidified atmosphere.) When the OD600 of the bacterial cultures reached 1.0 (∼2.5 h), samples were diluted 100-fold in prewarmed RPMI medium (without FBS or antibiotics), and then 300 μl was added to each monolayer of L-929 cells, for a multiplicity of infection of roughly 20. In a control sample, 40 μM SE-1 and bacteria grown at 37°C without SE-1 were added at the same time to L-929 cells. The 24-well plate was centrifuged (2,000 × g) for 5 min at room temperature to initiate bacterium-host cell contact, incubated for 45 min at 37°C, washed three times with 1 ml RPMI medium containing 50 μg/ml gentamicin (or without gentamicin for the control), and incubated at 37°C for 2 h. Cells were again washed three times with 1 ml RPMI medium and were then lysed in 100 μl of phosphate-buffered saline (PBS) (Mediatech) containing 0.1% Triton X-100 (Promega, Madison, WI) for 10 min at room temperature. The 100-μl lysates from the gentamicin-treated and untreated L-929 cells were mixed with 400 μl TSB, and 100 μl of further dilutions (1:50 and 1:250) was plated onto TSB agar containing ampicillin. Plates were incubated overnight at 37°C, and colonies were counted. The number of colonies obtained from gentamicin-treated samples was divided by the number of colonies from control samples without gentamicin, and the quotient was multiplied by 100 to calculate the invasion index, as described previously (80).
alamarBlue assays were performed as a control to test whether SE-1 had any impact on the metabolic activity of the host cells, as follows. L-929 cells were seeded into 96-well plates and were grown overnight to ∼90% confluence. SE-1 was serially diluted in RPMI medium supplemented with 10 μg/ml gentamicin and 5% FBS, added to the wells, and incubated at 37°C for 3 h. Cells were washed with 200 μl Hanks' balanced salt solution (Mediatech) and were incubated with 200 μl RPMI medium (without phenol red) containing 10% alamarBlue (also known as resazurin; Invitrogen, Grand Island, NY). Following 8 h of incubation at 37°C, the absorbance was measured at 570 nm and 600 nm using a PowerWave XS plate reader, and the metabolic activity of the cells (as a percentage of that of untreated controls) was calculated using the following formula provided by the manufacturer: [(117,216 × A570) − (80,586 × A600)]/[(117,216 × A570′) − (80,586 × A600′)] × 100, where A is the absorbance of test wells and A′ is the absorbance of positive-control wells (mock infection) (81).
RESULTS
SE-1 inhibited VirF activation of a virB-lacZ fusion in E. coli.
The small molecule SE-1 (Fig. 1A) was previously found to inhibit DNA binding and transcription activation by the E. coli AraC family proteins RhaS and RhaR (60). Given that our findings indicated that SE-1 interacted with the relatively conserved DNA-binding domains of these AraC family proteins, we tested whether it might also inhibit VirF, an AraC family activator required for virulence in Shigella. SE-1 inhibition of transcription activation by VirF was first tested using cell-based assays in E. coli. We performed β-galactosidase assays in the absence or presence of the inhibitor with a nonpathogenic strain of E. coli that carried a single copy of the lacZ reporter gene under the control of the virB promoter (virB-lacZ) in the chromosome (see Fig. S1 [top] in the supplemental material) and a plasmid with IPTG-inducible expression of VirF. A control strain carried plasmid-encoded LacI and the lacZ reporter gene under the control of the synthetic, LacI-repressible hts promoter (hts-lacZ) (60) (see Fig. S1, bottom). This control strain served to distinguish the inhibition of overall transcription or β-galactosidase activity, for example, from selective inhibition of transcription activation by VirF. IPTG was added to the cells (to induce VirF expression or to release LacI from repressing hts-lacZ) at the same time as the inhibitor. Thus, β-galactosidase was maintained at an uninduced level until the inhibitor was added, and it was not necessary to wait for the decay of preformed β-galactosidase to detect inhibition. The expression of lacZ from uninduced and induced controls was set to 0% and 100%, respectively, thus normalizing the effect of the inhibitor on the VirF-activated fusion and the LacI-repressed fusion.
SE-1 inhibition of transcription activation by VirF was predicted to decrease lacZ expression from virB-lacZ with little or no effect on hts-lacZ expression. In contrast, a decrease in lacZ expression from both virB-lacZ and hts-lacZ would indicate that inhibition was not specific for VirF. We found that SE-1 showed substantially greater, dose-dependent inhibition of the VirF-activated lacZ fusion than of the LacI-repressed fusion (Fig. 1B). The IC50 of SE-1 for expression from the VirF-activated fusion was 8 μM. This is a typical potency for a hit from a high-throughput screen (low μM to high nM potency range) that has not yet been chemically optimized (82) and a higher potency than the IC50 of 30 μM found in similar assays with RhaS (60). As in the previous report in which we used hts-lacZ as a control (60), there was also some nonspecific inhibition of LacZ expression from the LacI-repressed fusion at higher doses of SE-1. Overall, these cell-based assays suggest that SE-1 inhibits transcription activation by VirF, at least in E. coli, with reasonable selectivity.
SE-1 did not impact the growth of E. coli.
Bacterial growth assays were performed to ensure that the observed inhibition of VirF activation could not be attributed to toxicity for the strains of E. coli used in the cell-based reporter assays. Strains carrying the VirF-activated and LacI-repressed fusions were grown for 8 h at 37°C in the same minimal medium used for the in vivo dose-response assays. We detected no effect of SE-1 on the growth of either strain, indicating that the inhibition observed in the whole-cell assays could not be attributed to effects on cell growth (see Fig. S3 in the supplemental material). As a control, we tested whether 0.3% DMSO (the solvent for SE-1) had any impact on the growth rates of these strains of E. coli and found that it did not (see Fig. S3). The finding that the concentrations of SE-1 tested had no detectable effects on bacterial growth rates further supports the conclusion that SE-1 exhibits selectivity for VirF inhibition.
SE-1 inhibited in vitro DNA binding by VirF.
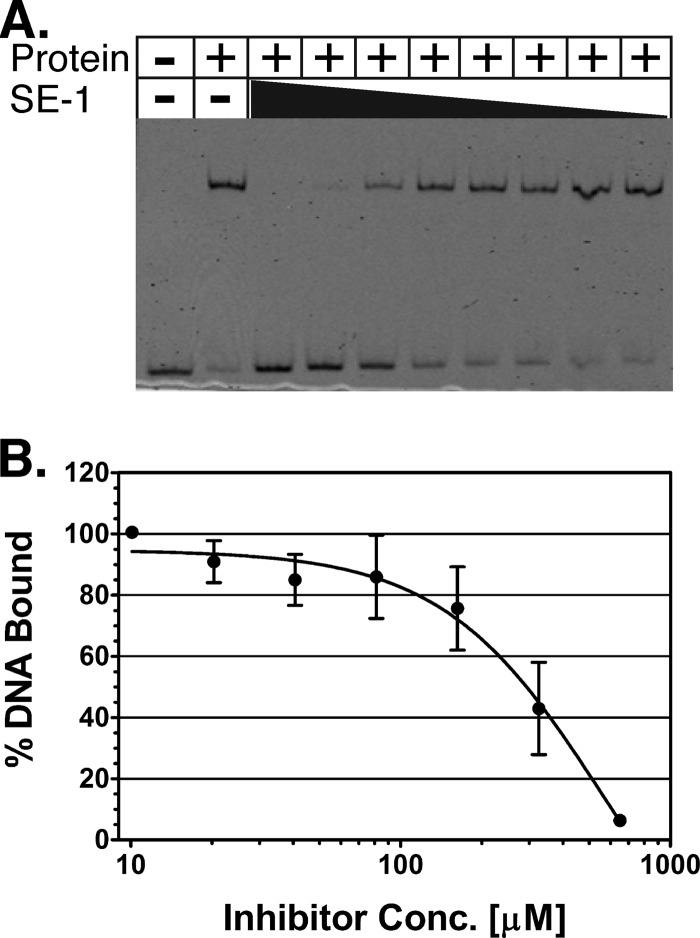
Electrophoretic mobility shift assays (EMSAs) were performed to investigate whether, as with RhaS and RhaR (60), SE-1 inhibits DNA binding by purified VirF protein (MBP-VirF). The VirF protein was purified using amylose affinity chromatography, and the DNA tested included the VirF binding site sequence from the virB promoter region. Our results indicate that SE-1 was able to fully inhibit DNA binding by VirF in a dose-dependent manner (Fig. 2). The concentration of SE-1 required for half-maximal inhibition of DNA binding was higher than the IC50 for the cell-based assays. However, it was not possible to calculate an accurate IC50 from the DNA binding assays due to limitations of the sensitivity of the detection method, the solubility of SE-1, and residual aggregation of the purified VirF protein. We showed previously that SE-1 did not inhibit DNA binding by the LacI protein or CRP (60). LacI and CRP are not members of the AraC family; each is a founding member of its own protein family (83–85). Overall, our EMSA results indicate that SE-1 can block the ability of VirF to bind to its specific DNA site.
Fig 2.
SE-1 inhibition of in vitro DNA binding by VirF. (A) A representative EMSA gel image. The black triangle represents decreasing concentrations of SE-1, from 1.3 mM to 10 μM, with serial 2-fold dilutions. (B) The binding of purified VirF to a DNA fragment containing the VirF binding site from the virB promoter was assayed using EMSAs with the inhibitor SE-1 at concentrations from 10 to 650 μM (serial 2-fold dilutions). DNA and protein were used at final concentrations of 2 and 300 nM, respectively. The DNA shifted (bound by VirF) was quantified, and the value at the lowest concentration of SE-1 was set to 100%. Results are averages for three independent replicates.
SE-1 binds directly to VirF.
The finding that SE-1 blocked DNA binding by VirF but not by the non-AraC family proteins LacI and CRP suggested that SE-1 might bind directly to VirF. (At least in the simplest case, SE-1 binding to DNA would be expected to inhibit DNA binding by any protein tested.) To test the hypothesis that SE-1 binds to VirF, we performed thermal shift assays (69, 70) using the dye Sypro Orange. In this assay, there is an increase in fluorescence intensity upon the binding of Sypro Orange to the exposed hydrophobic residues of a thermally unfolding protein. The unfolding transition allows calculation of the protein's Tm and any melting temperature changes (ΔTm) due to the increased protein stability imparted by ligand binding.
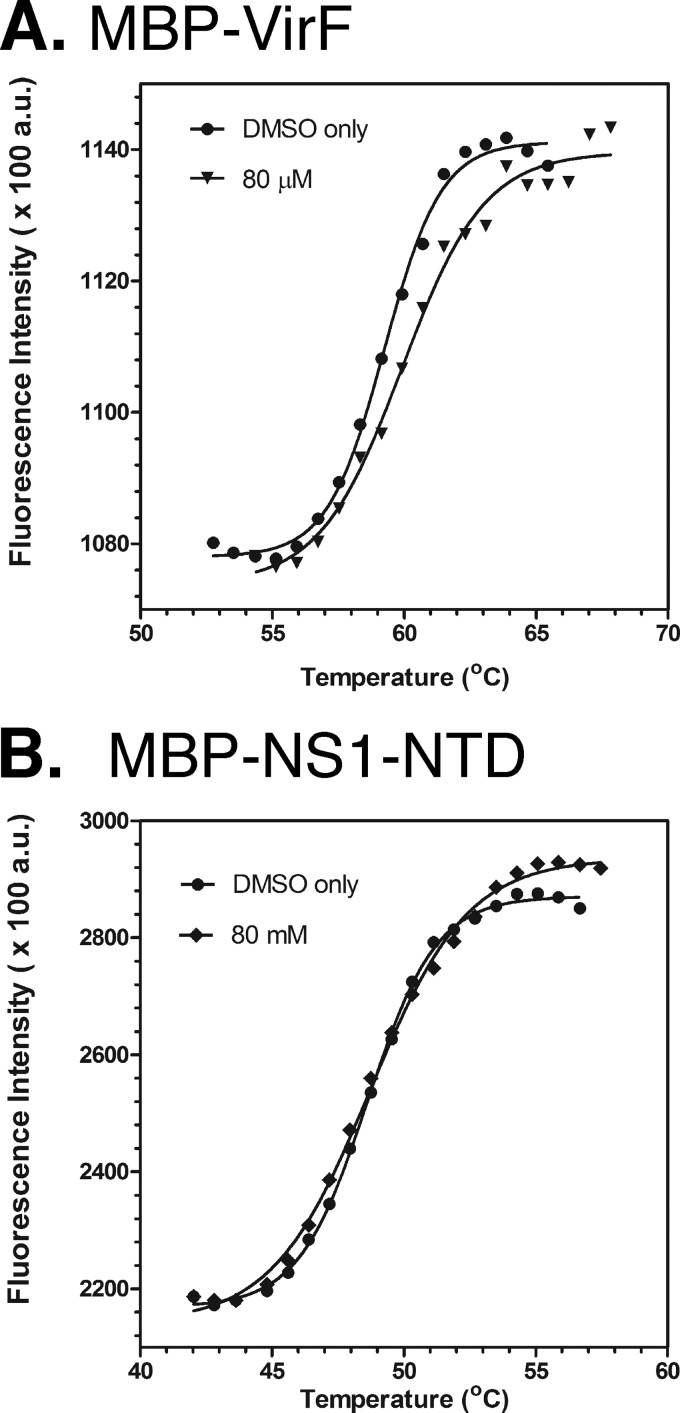
Using the thermal shift assay, we found that the addition of 80 μM SE-1 increased the Tm of VirF (1 μM) by 0.61°C (±0.09) (Fig. 3A). While this appears to be a relatively small change in melting temperature, the following evidence supports the conclusion that this likely indicates binding of SE-1 to VirF. Pantoliano et al. (69) used a melting temperature change cutoff of 0.5°C or greater to indicate ligand binding to the target protein after testing more than 100 different proteins. Further, the ΔTm for VirF with 80 μM SE-1 is much greater than that found with 40 μM SE-1 (0.15°C ± 0.04 [data not shown]), suggesting that VirF is likely not saturated with SE-1 at 80 μM and that a higher ΔTm would likely be found if we could test higher concentrations of SE-1 (86). Finally, multiple reports of ΔTm values similar to ours have been validated as indicating true binding to a protein (69, 87–89).
Fig 3.
Thermal shift assay showing that SE-1 binds to VirF protein. The thermal denaturation of MBP-VirF (A) and MBP-NS1-NTD (B) was measured using the dye Sypro Orange. Assays were carried out in the absence (DMSO only) or presence of 80 mM SE-1. Readings were taken from 25 to 99°C. Only the relevant portions of the curves are shown. Results of a single experiment (representative of three independent assays with two replicates each) are shown.
Since our VirF protein is a fusion with MBP, we tested an unrelated MBP fusion protein (MBP-NS1-NTD) to determine whether SE-1 bound to MBP. NS1 is a poxvirus protein that is not a member of the AraC family. MBP-NS1-NTD showed only a 0.15°C (±0.06) change in melting temperature, and the melting curve with SE-1 was not well separated from that with the DMSO-only control (Fig. 3B), suggesting that SE-1 did not bind to MBP. We conclude that SE-1 binds directly to VirF and hypothesize that this binding is responsible for the inhibition of DNA binding, and thus of transcription activation, by VirF.
SE-1 inhibited activation of a virB-lacZ fusion by VirF in Shigella.
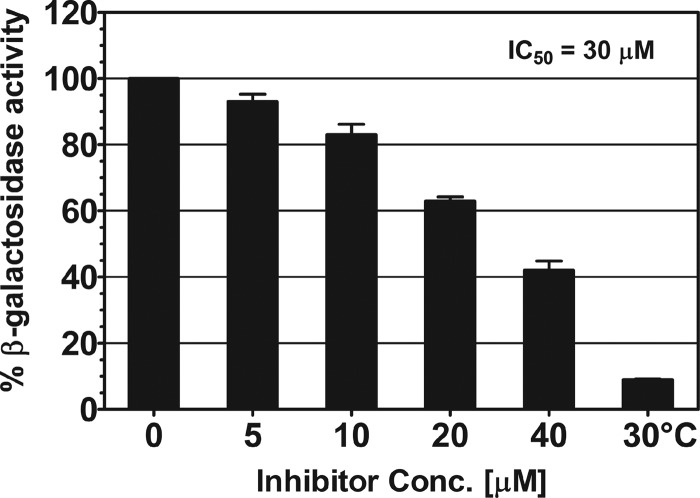
Our cell-based assays in E. coli indicated that SE-1 was able to effectively inhibit activation by VirF at the virB promoter region. To test whether similar inhibition occurred in Shigella, we assayed the effect of SE-1 on VirF activity in an S. flexneri strain carrying a virB-lacZ fusion. β-Galactosidase assays were performed on bacterial cultures grown at 37°C in the absence or presence of varying concentrations of the inhibitor. Our results show a dose-dependent inhibition of virB-lacZ expression, with a maximum inhibition of >2-fold at 40 μM SE-1 (Fig. 4). This inhibition was most likely due to inhibition of VirF activity. We calculated an IC50 of approximately 30 μM, somewhat higher than the IC50 of 8 μM achieved in E. coli. S. flexneri samples grown in 80 μM SE-1 were not analyzed, because they exhibited growth defects. Shigella cultures grown at 30°C in the absence of SE-1 were used to identify the basal level of virB-lacZ expression (36, 38, 40, 41). These assays, like the cell-based assays in E. coli, indicate that SE-1 inhibits transcription activation by VirF at the virB-lacZ promoter region and, further, provide evidence that SE-1 is effective in Shigella.
Fig 4.
Inhibition by SE-1 of in vivo VirF activation in Shigella. β-Galactosidase (LacZ) activity from a virB-lacZ transcriptional fusion in Shigella, with native VirF expression, was assayed at the indicated concentrations of the inhibitor SE-1. Activity in the absence of SE-1 was set to 100% and corresponded to approximately 3,000 Miller units. The result for Shigella grown at 30°C illustrates the basal expression of virB-lacZ. Results are averages for three independent experiments with two replicates each.
SE-1 reduced VirF-regulated virulence gene expression in Shigella.
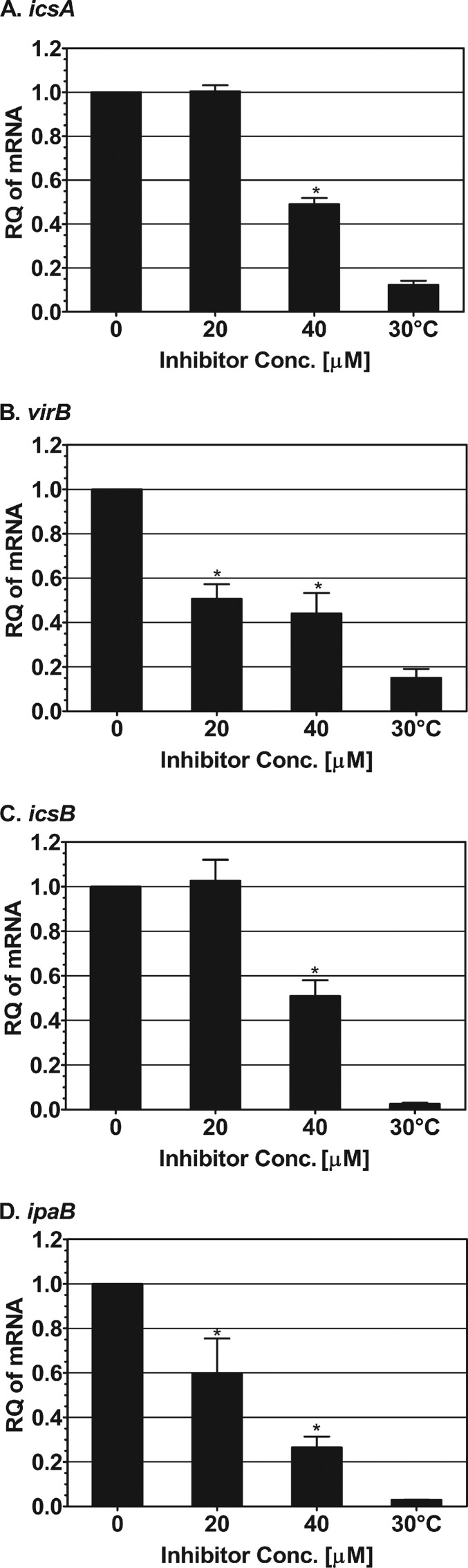
Our next goal was to test the impact of SE-1 on the expression of other VirF-regulated genes in Shigella. Previous reports have shown that the icsA and virB genes are direct targets of VirF activation at 37°C (36). VirB, in turn, activates the expression of many operons that play a crucial role in Shigella pathogenesis (37). Thus, any compound that inhibits transcription activation by VirF should also affect the expression levels of genes that either directly or indirectly require VirF for their expression. To test this hypothesis, we used qRT-PCR to quantify the mRNA levels of two genes that are direct targets of VirF, icsA and virB, and two that are indirect targets of VirF, icsB and ipaB (both directly activated by VirB) (37). S. flexneri samples were grown to an OD600 of ∼1.0 in the absence or presence of two different concentrations of SE-1 (20 μM and 40 μM), and the mRNA levels for each of these genes were quantified. S. flexneri samples grown at 30°C in the absence of SE-1 were included to illustrate the basal expression levels of these genes. The results were normalized to those for the gapA (encoding glyceraldehyde-3-phosphate dehydrogenase) and rrsA (encoding 16S rRNA) genes, two constitutively expressed genes commonly used as qRT-PCR controls in Shigella (72–74).
Our qRT-PCR results showed that the icsA expression level remained essentially unchanged at 20 μM inhibitor but was significantly reduced (P < 0.05) at 40 μM inhibitor (Fig. 5A). Expression of virB, another direct target of VirF, decreased significantly with increasing concentrations of the inhibitor, with a maximal inhibition of >2-fold at 40 μM SE-1 (P < 0.05) (Fig. 5B). Expression of icsB, an indirect target of VirF (55, 56), showed a significant 2-fold reduction at 40 μM SE-1 (P < 0.05) (Fig. 5C). The greatest inhibition was detected for the expression of ipaB, another indirect target of VirF, which was reduced as much as 4-fold at 40 μM inhibitor (P < 0.05) (Fig. 5D). As a control, we normalized each of the internal control genes relative to the other internal control gene. We found that there was no decrease in the expression of either control gene with 40 μM SE-1 (see Fig. S4 in the supplemental material), arguing that SE-1 did not result in a global decrease in gene expression. Together, our data demonstrate that SE-1 is capable of reducing the expression of at least four VirF-regulated virulence-associated genes in Shigella, presumably through its inhibition of transcription activation by VirF.
Fig 5.
Inhibition by SE-1 of the expression of VirF-regulated virulence genes in Shigella. qRT-PCR was used to determine the relative quantities (RQ) of icsA (A), virB (B), icsB (C), and ipaB (D) expressed in Shigella grown at 30°C (without SE-1) and at 37°C (without or with 20 or 40 μM SE-1). RNA levels were normalized to those of two constitutively expressed genes, gapA and rrsA. RNA levels were set to 1 in the absence of SE-1. The results for Shigella grown at 30°C illustrate the basal expression of each gene. Error bars represent the standard errors of the means calculated from three independent replicates. The significance of values for inhibitor-treated bacteria was calculated using a nonparametric Mann-Whitney test; P values less than 0.05 were considered significant (*). Results are averages for three independent experiments with one replicate each.
SE-1 inhibited the invasion of host cells by Shigella.
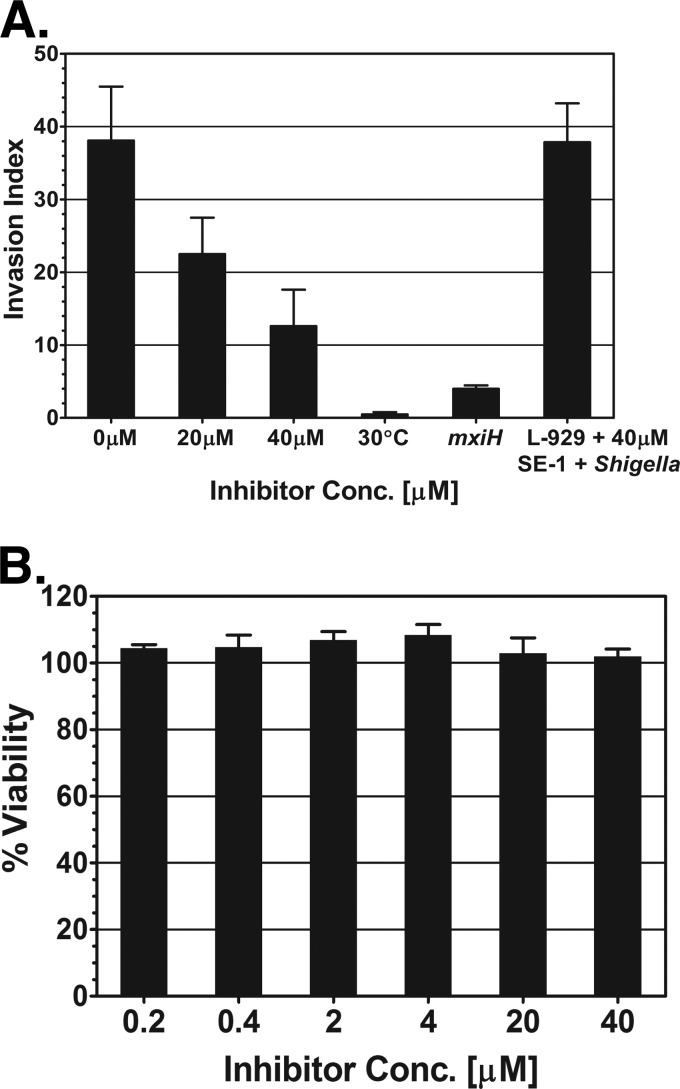
Our qRT-PCR experiments with SE-1 showed a decrease in the expression of virulence genes in Shigella. In order to test the impact of this decrease in virulence gene expression on the ability of Shigella to invade host cells, invasion assays (80) were performed with the mouse fibroblast line L-929. L-929 cells have been used previously to study the invasion of host cells by Shigella (76–79). The S. flexneri strain used in the assays was an ipgD-null strain that has hemolysis and invasion properties similar to those of the wild type but is safer to work with due to its reduced ability to cause human infection (90–92). The cells were grown at 30°C overnight and were then diluted and grown at 37°C (to induce the expression of VirF-regulated genes) in the presence or absence of various concentrations of SE-1. In addition, S. flexneri strain SB116 (a mxiH-null mutant) was used as a negative control for invasion, since it does not form a T3SS needle and thus is unable to invade host cells (6). S. flexneri grown at 30°C was used as a second negative control for invasion, since these bacteria have reduced expression of VirF and VirB and thus are defective for invasion of host cells (37). Our results showed a dose-dependent decrease in the invasion of L-929 cells by Shigella upon addition of SE-1 (Fig. 6A). SE-1 resulted in invasion decreases of 1.7-fold at 20 μM and 3-fold at 40 μM from levels with no inhibitor. As expected, both of the negative controls (wild-type S. flexneri grown at 30°C and the mxiH-null strain) showed substantially decreased invasion relative to that of the wild-type strain grown at 37°C. As in our growth assays with E. coli, we found that 0.3% DMSO did not detectably slow the growth of Shigella (data not shown), and SE-1 resulted in no detectable decrease in the Shigella growth rate at concentrations as high as 40 μM (see Fig. S5 in the supplemental material).
Fig 6.
Inhibition by SE-1 of invasion of host cells by Shigella but not of host cell metabolism. (A) The index of invasion of L-929 (mouse fibroblast) cells by Shigella was determined by a gentamicin protection assay. Cultures were grown in the absence or presence of SE-1 (20 μM or 40 μM). An mxiH-null Shigella strain (SB116) and an ipgD-null strain (SME4331) grown at 30°C were used as negative controls for invasion. As an additional control, 40 μM SE-1 and Shigella grown at 37°C without the inhibitor were added to L-929 cells at the same time to test the effect of the inhibitor on invasion by a Shigella strain with a preformed T3SS and on host cells. (B) alamarBlue assays to test the effect of SE-1 (at the indicated concentrations) on the metabolic activity (viability) of L-929 cells. The absorbance level in the absence of the inhibitor and the presence of DMSO was set to 100%. Assays for both panels were performed in triplicate, and error bars represent the standard errors of the means.
Overall, our results suggest the hypothesis that SE-1 inhibits Shigella invasion by decreasing the expression of the genes in the VirF regulon, including the genes that encode the T3SS. Thus, Shigella grown under inducing conditions (37°C) without SE-1 would not be inhibited by the addition of SE-1 unless growth with SE-1 was continued for a period sufficient for the loss of preformed VirF-regulated gene products, such as the T3SS. To test this hypothesis, Shigella bacteria were grown at 37°C in the absence of the inhibitor, and then Shigella and SE-1 were added to L-929 cells at the same time. This control also tested whether SE-1 had any impact on the L-929 cells that affected invasion by Shigella. Our result suggests that the inhibition of invasion by SE-1 was likely not due to posttranscriptional effects on Shigella or to effects on the eukaryotic cells, since this control sample invaded to the same extent as Shigella grown in the absence of the inhibitor (Fig. 6A). Overall, our results support the hypothesis that SE-1 decreases the expression of VirF-regulated virulence genes and that this decrease, in turn, results in a reduction in the ability of Shigella to invade host cells.
In principle, it is possible that the decrease in the level of invasion of L-929 cells by Shigella was the result of SE-1 compromising host cellular processes and thereby decreasing the viability of the host cells. To test this possibility, the effect of SE-1 on the viability of L-929 cells was assayed using an alamarBlue cell viability assay (Invitrogen, Grand Island, NY). The alamarBlue assay is a fluorescence-based assay of the viability of host cells based on their metabolic activity (93). During our invasion assays, the L-929 cells were exposed only to SE-1 concentrations of 0.2 and 0.4 μM, due to dilution of the SE-1-containing Shigella culture. Our alamarBlue assays indicated that these concentrations of SE-1 did not detectably decrease the viability of L-929 cells (Fig. 6B). In fact, a 100-fold-higher concentration (40 μM) had no effect on host cell viability. These results support the hypothesis that the effect of SE-1 on invasion by Shigella was not an indirect effect on L-929 cell viability. Further, the results provide evidence that SE-1 did not have detectable toxicity for the L-929 cells at concentrations as high as 40 μM.
DISCUSSION
Many bacterial pathogens require AraC family transcriptional regulators to cause disease (24–29). VirF, one such AraC family regulator, activates the expression of a cascade of virulence genes required for successful infection by Shigella (43, 44). Thus, inhibitors that block transcription activation by VirF (preferably without detrimental effects on the growth of the bacteria) have potential to be developed into novel antimicrobial agents. The nonessential nature of VirF may reduce the probability that Shigella will develop resistance to VirF inhibitors (22, 32–34). A high-throughput screen performed by a different group identified several inhibitors of VirF activation of virB-lacZ reporter expression; however, the mechanisms of inhibition and further effects on Shigella infections were not tested (59). We recently identified an inhibitor, SE-1, that inhibited transcription activation by the AraC family proteins RhaS and RhaR by blocking their ability to bind to DNA (60). In the present study, we tested SE-1 for inhibition of VirF and of VirF-dependent Shigella virulence.
Using a cell-based reporter gene assay to test the ability of SE-1 to inhibit transcription activation by the VirF protein, we found that SE-1 decreased virB-lacZ reporter fusion expression in an E. coli strain with plasmid-expressed VirF. The inhibition was dose dependent, with an IC50 of approximately 8 μM, suggesting that SE-1 inhibited transcription activation by VirF. An initial indication that this inhibition was selective for VirF activity was the finding that SE-1 inhibited lacZ expression from a control fusion (hts-lacZ) substantially less than it inhibited VirF-dependent lacZ expression. The strains carrying the VirF-dependent and control lacZ fusions showed no detectable growth defects in the presence of SE-1, indicating that SE-1 did not have general toxicity for these E. coli strains at the concentrations tested.
We showed previously that the inhibition of the AraC family activators RhaS and RhaR by SE-1 involved blocking their binding to DNA (60). Thus, we tested SE-1 for inhibition of the binding of purified VirF protein to DNA carrying a VirF binding site, and we found that SE-1 was able to fully inhibit DNA binding by VirF. We also showed previously that SE-1 did not inhibit DNA binding by two proteins that are not related to the AraC family, LacI and CRP (60), suggesting that SE-1 is selective for AraC family proteins. Our thermal shift assay results suggest that SE-1 can bind directly to the VirF protein. Overall, our results support the hypothesis that the mechanism of action of SE-1 inhibition involves selectively blocking DNA binding by VirF by directly binding to the protein.
We next tested inhibition by SE-1 in Shigella. Initially, we tested SE-1 for the ability to inhibit endogenously expressed VirF in a Shigella strain carrying a virB-lacZ reporter construct. As in E. coli, we observed dose-dependent inhibition of VirF activation of virB-lacZ in Shigella. In addition, Shigella grown in the presence of SE-1 showed decreases both in the transcription (assayed by qRT-PCR) of genes that are directly activated by VirF (virB, icsA) and in the transcription of genes that are directly activated by VirB (indirect VirF targets; icsB, ipaB). In these experiments, we observed decreases of as much as 2- to 4-fold in the expression of these VirF-dependent genes; the values were consistent with those obtained in the virB-lacZ assays in Shigella. However, the same concentration of SE-1 resulted in lower levels of inhibition in Shigella than in E. coli, suggesting differences in the uptake, efflux, and/or stability of SE-1 between these two bacteria. Alternatively, as with many commonly used antibiotics (94), there may be differences in sensitivity to SE-1 between organisms grown in minimal medium (used for E. coli) and those grown in rich medium (used for Shigella).
Several findings argue that the decreases in virB-lacZ, virB, icsA, icsB, and ipaB expression in Shigella were due to inhibition of VirF activity and not to a global decrease in gene expression in the presence of SE-1. First, there was no detectable decrease in the growth rate of Shigella in the presence of as much as 40 μM SE-1, whereas global decreases in gene expression of a comparable magnitude would be expected to affect the growth rate. Second, the qRT-PCR results were normalized to results for two different internal-control genes (which would be expected to exhibit decreased expression if there was a global decrease in gene expression). Normalization of each of the internal-control genes relative to the other showed that there was no significant change in the expression of the control genes in the presence of 40 μM SE-1. Finally, the magnitudes of inhibition of virB expression by SE-1 detected in the virB-lacZ assays and the qRT-PCR assays were essentially the same, indicating that the normalization of the qRT-PCR results to those for the internal controls did not alter the extent of the inhibition (as would have been expected if expression of the internal control genes was also inhibited).
Finally, we investigated the effect of SE-1 on the ability of Shigella to invade mouse fibroblasts (L-929 cells) in tissue culture. A dose-dependent decrease in invasion was observed, with a maximum inhibition of 70% at 40 μM inhibitor. This inhibition required preincubation of Shigella with SE-1, in agreement with the hypothesis that SE-1 did not destabilize preassembled T3SS or other Shigella virulence components, inhibit posttranscriptional processes in Shigella, or cause cytotoxicity to L-929 cells at the concentrations tested. The latter notion is further supported by the finding that at the same concentrations, SE-1 did not result in a detectable decrease in the metabolism of L-929 cells in alamarBlue assays.
Overall, our results suggest a model in which SE-1 inhibits VirF-dependent activation of the transcription of Shigella virulence genes, including at least icsA, virB, icsB, and ipaB. The decrease in invasion we detected is consistent with, and can be attributed to, the effect of decreased expression of these virulence genes in the VirF regulon. The 2- to 4-fold decreases in the expression of these genes appear to be sufficient to decrease the level of Shigella invasion by 70% from that without the inhibitor. The ipaB gene exhibited the largest decrease in expression, 4-fold, suggesting that a reduction in IpaB expression may be associated with reduced invasion in the presence of SE-1. This is a reasonable hypothesis given the central role IpaB plays in Shigella invasion. IpaB is a component of the translocon pores in the host cell membrane (51), which enable the translocation of Shigella effectors into host cells (54), ultimately modulating host cell cytoskeletal dynamics and leading to bacterial invasion (52). In addition, only a subset of the VirF-regulated virulence genes that are required for invasion were assayed (43, 44); thus, decreased expression of other genes likely also contributed to the invasion defects.
We have now shown that SE-1 selectively inhibits DNA binding by three different AraC family proteins (60). The VirF protein shares only about 15% amino acid sequence identity and 40% similarity with RhaS and RhaR (the pairwise RhaS-VirF and RhaR-VirF comparisons are nearly the same) (see Fig. S6 in the supplemental material). Given our prior finding that SE-1 blocks transcription activation by the RhaS DBD to at least the same extent as that by the full-length RhaS protein (60), we hypothesize that the VirF DNA-binding domain is the likely site of action of SE-1. The sequence similarities in this domain are somewhat higher than those of the full-length proteins but are still relatively low: the VirF DBD shares about 22% identity and 52% similarity with the RhaS and RhaR DBDs. This finding supports the hypothesis that SE-1 may bind to a relatively conserved region of AraC family DNA-binding domains. We cannot rule out the possibility that SE-1 inhibits other AraC family activators in addition to VirF in Shigella; however, it is likely that inhibition of VirF would exert the greatest impact on genes in the VirF regulon and on host cell invasion. In addition to the inhibitory effects of SE-1 on VirF from the S. flexneri strains used in our work, SE-1 may also be effective against other pathogenic Shigella species (Shigella dysenteriae, S. sonnei, and S. boydii), since VirF is the master virulence regulator in all these species and shares ∼100% identity (1).
In conclusion, we have identified SE-1 as an inhibitor of transcription activation by VirF by using cell-based reporter gene assays, DNA binding assays, and invasion assays. SE-1 is noncytotoxic toward the eukaryotic cells tested (at concentrations as high as 40 μM), is nonbactericidal toward E. coli and Shigella (at concentrations as high as 40 μM), and has potential to be developed into a novel antibacterial agent. However, SE-1 does show evidence of nonspecific inhibition at higher concentrations (partial inhibition of hts-lacZ at higher concentrations and inhibition of Shigella growth at 80 μM SE-1), and its current potency is not sufficient for therapeutic applications. Thus, chemical optimization of SE-1 will be needed to improve both its potency and its specificity before it can be considered a lead compound for further development. In addition, once Shigella cells invade host epithelial cells, they are able to spread directly from cell to cell and are no longer exposed to the extracellular environment (95). We do not know whether SE-1 can penetrate host cells; however, it is known that intracellular Shigella requires VirF for growth, viability, and cell-to-cell spread (96). Therefore, if SE-1 (or a chemically optimized analog) were able to penetrate host epithelial cells, it might reduce growth and cell-to-cell spread and increase host-mediated killing of Shigella bacteria that have already invaded host cells. It is also not yet known whether SE-1 inhibits the activity of MxiE, an AraC family activator that regulates the expression of T3SS effectors after invasion and is required for Shigella virulence in an animal model (50).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to A. T. Maurelli (Uniformed Services University) for the generous gift of Shigella strain BS536, to G. P. Munson (University of Miami) for E. coli strain AB97, to L. Swint-Kruse (University of Kansas Medical Center) for pHG165lacI, to W. D. Picking (Oklahoma State University) for Shigella strains SB116 and SME4331, and to L. Tang (University of Kansas) for purified MBP-NS1-NTD.
The research reported in this publication was supported by the National Institute of General Medical Sciences, National Institutes of Health, under award R01GM55099 and by the University of Kansas. J.M.S. was partially supported by award T32GM008359. I.O. was supported by NIH AI082697. P.S.H. was supported by NIH AI079083.
Footnotes
Published ahead of print 3 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00919-13.
REFERENCES
- 1.Jennison AV, Verma NK. 2004. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28:43–58 [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77:651–666 [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222 [DOI] [PubMed] [Google Scholar]
- 4.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159:1126–1128 [DOI] [PubMed] [Google Scholar]
- 5.Sansonetti PJ, Kopecko DJ, Formal SB. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, Sansonetti P, Allaoui A. 2001. Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol. Microbiol. 39:652–663 [DOI] [PubMed] [Google Scholar]
- 7.High N, Mounier J, Prevost MC, Sansonetti PJ. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, Sansonetti PJ. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619–627 [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Sasakawa C, Yoshikawa M. 1988. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kilodalton VirF protein. Mol. Microbiol. 2:589–597 [DOI] [PubMed] [Google Scholar]
- 10.Tran CN, Giangrossi M, Prosseda G, Brandi A, Di Martino ML, Colonna B, Falconi M. 2011. A multifactor regulatory circuit involving H-NS, VirF and an antisense RNA modulates transcription of the virulence gene icsA of Shigella flexneri. Nucleic Acids Res. 39:8122–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobe T, Yoshikawa M, Mizuno T, Sasakawa C. 1993. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by VirF and repression by H-NS. J. Bacteriol. 175:6142–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai T, Sasakawa C, Makino S, Kamata K, Yoshikawa M. 1986. Molecular cloning of a genetic determinant for Congo red binding ability which is essential for the virulence of Shigella flexneri. Infect. Immun. 51:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. 1972. Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J. Infect. Dis. 125:5–11 [DOI] [PubMed] [Google Scholar]
- 14.Mel DM, Terzin AL, Vuksic L. 1965. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull. World Health Organ. 32:647–655 [PMC free article] [PubMed] [Google Scholar]
- 15.Sansonetti PJ, Arondel J, Fontaine A, d'Hauteville H, Bernardini ML. 1991. ompB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine 9:416–422 [DOI] [PubMed] [Google Scholar]
- 16.Dentchev V, Marinova S, Vassilev T, Bratoyeva M, Linde K. 1990. Live Shigella flexneri 2a and Shigella sonnei I vaccine candidate strains with two attenuating markers. II. Preliminary results of vaccination of adult volunteers and children aged 2–17 years. Vaccine 8:30–34 [DOI] [PubMed] [Google Scholar]
- 17.Pickering LK. 2004. Antimicrobial resistance among enteric pathogens. Semin. Pediatr. Infect. Dis. 15:71–77 [DOI] [PubMed] [Google Scholar]
- 18.Walsh C. 2003. Where will new antibiotics come from? Nat. Rev. Microbiol. 1:65–70 [DOI] [PubMed] [Google Scholar]
- 19.Coates A, Hu Y, Bax R, Page C. 2002. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 1:895–910 [DOI] [PubMed] [Google Scholar]
- 20.Projan SJ, Bradford PA. 2007. Late stage antibacterial drugs in the clinical pipeline. Curr. Opin. Microbiol. 10:441–446 [DOI] [PubMed] [Google Scholar]
- 21.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668 [DOI] [PubMed] [Google Scholar]
- 22.Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9:117–128 [DOI] [PubMed] [Google Scholar]
- 23.Blaser M. 2011. Antibiotic overuse: stop the killing of beneficial bacteria. Nature 476:393–394 [DOI] [PubMed] [Google Scholar]
- 24.Champion GA, Neely MN, Brennan MA, DiRita VJ. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323–331 [DOI] [PubMed] [Google Scholar]
- 25.Coburn PS, Baghdayan AS, Dolan GT, Shankar N. 2008. An AraC-type transcriptional regulator encoded on the Enterococcus faecalis pathogenicity island contributes to pathogenesis and intracellular macrophage survival. Infect. Immun. 76:5668–5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casaz P, Garrity-Ryan LK, McKenney D, Jackson C, Levy SB, Tanaka SK, Alekshun MN. 2006. MarA, SoxS and Rob function as virulence factors in an Escherichia coli murine model of ascending pyelonephritis. Microbiology 152:3643–3650 [DOI] [PubMed] [Google Scholar]
- 27.Frota CC, Papavinasasundaram KG, Davis EO, Colston MJ. 2004. The AraC family transcriptional regulator Rv1931c plays a role in the virulence of Mycobacterium tuberculosis. Infect. Immun. 72:5483–5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser AR, Kang PJ, Engel JN. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807–818 [DOI] [PubMed] [Google Scholar]
- 29.Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibarra JA, Perez-Rueda E, Segovia L, Puente JL. 2008. The DNA-binding domain as a functional indicator: the case of the AraC/XylS family of transcription factors. Genetica 133:65–76 [DOI] [PubMed] [Google Scholar]
- 31.Flashner Y, Mamroud E, Tidhar A, Ber R, Aftalion M, Gur D, Lazar S, Zvi A, Bino T, Ariel N, Velan B, Shafferman A, Cohen S. 2004. Generation of Yersinia pestis attenuated strains by signature-tagged mutagenesis in search of novel vaccine candidates. Infect. Immun. 72:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes D. 2003. Exploiting genomics, genetics and chemistry to combat antibiotic resistance. Nat. Rev. Genet. 4:432–441 [DOI] [PubMed] [Google Scholar]
- 33.Knowles DJ. 1997. New strategies for antibacterial drug design. Trends Microbiol. 5:379–383 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt FR. 2004. The challenge of multidrug resistance: actual strategies in the development of novel antibacterials. Appl. Microbiol. Biotechnol. 63:335–343 [DOI] [PubMed] [Google Scholar]
- 35.Buchrieser C, Glaser P, Rusniok C, Nedjari H, D'Hauteville H, Kunst F, Sansonetti P, Parsot C. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760–771 [DOI] [PubMed] [Google Scholar]
- 36.Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17:7033–7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobe T, Nagai S, Okada N, Adler B, Yoshikawa M, Sasakawa C. 1991. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol. Microbiol. 5:887–893 [DOI] [PubMed] [Google Scholar]
- 38.Prosseda G, Fradiani PA, Di Lorenzo M, Falconi M, Micheli G, Casalino M, Nicoletti M, Colonna B. 1998. A role for H-NS in the regulation of the virF gene of Shigella and enteroinvasive Escherichia coli. Res. Microbiol. 149:15–25 [DOI] [PubMed] [Google Scholar]
- 39.Porter ME, Dorman CJ. 1997. Differential regulation of the plasmid-encoded genes in the Shigella flexneri virulence regulon. Mol. Gen. Genet. 256:93–103 [DOI] [PubMed] [Google Scholar]
- 40.Durand JM, Dagberg B, Uhlin BE, Björk GR. 2000. Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Mol. Microbiol. 35:924–935 [DOI] [PubMed] [Google Scholar]
- 41.Porter ME, Dorman CJ. 1994. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J. Bacteriol. 176:4187–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. 1993. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J. Bacteriol. 175:2334–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorman CJ. 1992. The VirF protein from Shigella flexneri is a member of the AraC transcription factor superfamily and is highly homologous to Rns, a positive regulator of virulence genes in enterotoxigenic Escherichia coli. Mol. Microbiol. 6:1575–1576 [DOI] [PubMed] [Google Scholar]
- 44.Dorman CJ, Porter ME. 1998. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol. Microbiol. 29:677–684 [DOI] [PubMed] [Google Scholar]
- 45.Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Miki H, Takenawa T, Sasakawa C. 1998. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17:2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, Sasakawa C. 2002. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell. Microbiol. 4:223–233 [DOI] [PubMed] [Google Scholar]
- 48.Goldberg MB. 1997. Shigella actin-based motility in the absence of vinculin. Cell Motil. Cytoskeleton 37:44–53 [DOI] [PubMed] [Google Scholar]
- 49.Beloin C, Dorman CJ. 2003. An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol. Microbiol. 47:825–838 [DOI] [PubMed] [Google Scholar]
- 50.Kane CD, Schuch R, Day WA, Jr, Maurelli AT. 2002. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J. Bacteriol. 184:4409–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blocker A, Gounon P, Larquet E, Niebuhr K, Cabiaux V, Parsot C, Sansonetti P. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran Van Nhieu G, Caron E, Hall A, Sansonetti PJ. 1999. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 18:3249–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853–3860 [PMC free article] [PubMed] [Google Scholar]
- 54.Ménard R, Sansonetti P, Parsot C. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. 2005. Escape of intracellular Shigella from autophagy. Science 307:727–731 [DOI] [PubMed] [Google Scholar]
- 56.Kayath CA, Hussey S, El hajjami N, Nagra K, Philpott D, Allaoui A. 2010. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 12:956–966 [DOI] [PubMed] [Google Scholar]
- 57.Sasakawa C, Kamata K, Sakai T, Makino S, Yamada M, Okada N, Yoshikawa M. 1988. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J. Bacteriol. 170:2480–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills JA, Venkatesan MM, Baron LS, Buysse JM. 1992. Spontaneous insertion of an IS1-like element into the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect. Immun. 60:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurt JK, McQuade TJ, Emanuele A, Larsen MJ, Garcia GA. 2010. High-throughput screening of the virulence regulator VirF: a novel antibacterial target for shigellosis. J. Biomol. Screen. 15:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skredenske JM, Koppolu V, Kolin A, Deng J, Kettle B, Taylor B, Egan SM. 2013. Identification of a small molecule inhibitor of bacterial AraC family activators. J. Biomol. Screen. 18:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart GS, Lubinsky-Mink S, Jackson CG, Cassel A, Kuhn J. 1986. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid 15:172–181 [DOI] [PubMed] [Google Scholar]
- 62.Backman K, Chen Y-M, Magasanik B. 1981. Physical and genetic characterization of the glnA-glnG region of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. U. S. A. 78:3743–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 64.Bhende PM, Egan SM. 1999. Amino acid-DNA contacts by RhaS: an AraC family transcription activator. J. Bacteriol. 181:5185–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottesman ME, Yarmolinsky MB. 1968. The integration and excision of the bacteriophage lambda genome. Cold Spring Harbor Symp. Quant. Biol. 33:735–747 [DOI] [PubMed] [Google Scholar]
- 66.Jubete Y, Maurizi MR, Gottesman S. 1996. Role of the heat shock protein DnaJ in the Lon-dependent degradation of naturally unstable proteins. J. Biol. Chem. 271:30798–30803 [DOI] [PubMed] [Google Scholar]
- 67.Wickstrum JR, Skredenske JM, Balasubramaniam V, Jones K, Egan SM. 2010. The AraC/XylS family activator RhaS negatively autoregulates rhaSR expression by preventing cyclic AMP receptor protein activation. J. Bacteriol. 192:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jullien N, Herman JP. 2011. LUEGO: a cost and time saving gel shift procedure. Biotechniques 51:267–269 [DOI] [PubMed] [Google Scholar]
- 69.Pantoliano MW, Petrella EC, Kwasnoski JD, Lobanov VS, Myslik J, Graf E, Carver T, Asel E, Springer BA, Lane P, Salemme FR. 2001. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 6:429–440 [DOI] [PubMed] [Google Scholar]
- 70.Ericsson UB, Hallberg BM, Detitta GT, Dekker N, Nordlund P. 2006. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 357:289–298 [DOI] [PubMed] [Google Scholar]
- 71.Senisterra GA, Finerty PJ., Jr 2009. High throughput methods of assessing protein stability and aggregation. Mol. Biosyst. 5:217–223 [DOI] [PubMed] [Google Scholar]
- 72.Runyen-Janecky L, Dazenski E, Hawkins S, Warner L. 2006. Role and regulation of the Shigella flexneri sit and mntH systems. Infect. Immun. 74:4666–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broach WH, Egan N, Wing HJ, Payne SM, Murphy ER. 2012. VirF-independent regulation of Shigella virB transcription is mediated by the small RNA RyhB. PLoS One 7:e38592. 10.1371/journal.pone.0038592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bore E, Hebraud M, Chafsey I, Chambon C, Skjaeret C, Moen B, Moretro T, Langsrud O, Rudi K, Langsrud S. 2007. Adapted tolerance to benzalkonium chloride in Escherichia coli K-12 studied by transcriptome and proteome analyses. Microbiology 153:935–946 [DOI] [PubMed] [Google Scholar]
- 75.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 76.Klimpel GR, Shaban R, Niesel DW. 1990. Bacteria-infected fibroblasts have enhanced susceptibility to the cytotoxic action of tumor necrosis factor. J. Immunol. 145:711–717 [PubMed] [Google Scholar]
- 77.Kapasi K, Inman RD. 1992. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J. Immunol. 148:3554–3559 [PubMed] [Google Scholar]
- 78.Okamura N, Nakaya R. 1977. Rough mutant of Shigella flexneri 2a that penetrates tissue culture cells but does not evoke keratoconjunctivitis in guinea pigs. Infect. Immun. 17:4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sandlin RC, Goldberg MB, Maurelli AT. 1996. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol. Microbiol. 22:63–73 [DOI] [PubMed] [Google Scholar]
- 80.Elsinghorst EA. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405–420 [DOI] [PubMed] [Google Scholar]
- 81.Willard HH, Merritt LL, Dean JA. 1965. Instrumental methods of analysis, 4th ed, p 94–95 Van Nostrand, Princeton, NJ [Google Scholar]
- 82.Rees DC, Congreve M, Murray CW, Carr R. 2004. Fragment-based lead discovery. Nat. Rev. Drug Discov. 3:660–672 [DOI] [PubMed] [Google Scholar]
- 83.Nguyen CC, Saier MH., Jr 1995. Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett. 377:98–102 [DOI] [PubMed] [Google Scholar]
- 84.Swint-Kruse L, Matthews KS. 2009. Allostery in the LacI/GalR family: variations on a theme. Curr. Opin. Microbiol. 12:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Körner H, Sofia HJ, Zumft WG. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559–592 [DOI] [PubMed] [Google Scholar]
- 86.Niesen FH, Berglund H, Vedadi M. 2007. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2:2212–2221 [DOI] [PubMed] [Google Scholar]
- 87.Lo MC, Aulabaugh A, Jin G, Cowling R, Bard J, Malamas M, Ellestad G. 2004. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 332:153–159 [DOI] [PubMed] [Google Scholar]
- 88.Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. 2012. Implications of binding mode and active site flexibility for inhibitor potency against the salicylate synthase from Mycobacterium tuberculosis. Biochemistry 51:4868–4879 [DOI] [PubMed] [Google Scholar]
- 89.Hau JC, Fontana P, Zimmermann C, De Pover A, Erdmann D, Chene P. 2011. Leveraging the contribution of thermodynamics in drug discovery with the help of fluorescence-based thermal shift assays. J. Biomol. Screen 16:552–556 [DOI] [PubMed] [Google Scholar]
- 90.Allaoui A, Menard R, Sansonetti PJ, Parsot C. 1993. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect. Immun. 61:1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. 2006. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 25:1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. 2002. Conversion of PtdIns(4,5)P2 into PtdIns(5)P by the Shigella flexneri effector IpgD reorganizes host cell morphology. EMBO J. 21:5069–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakayama GR, Caton MC, Nova MP, Parandoosh Z. 1997. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 204:205–208 [DOI] [PubMed] [Google Scholar]
- 94.Maloy SR, Stewart VJ, Taylor RK. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 95.Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 21:134–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuch R, Sandlin RC, Maurelli AT. 1999. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol. Microbiol. 34:675–689 [DOI] [PubMed] [Google Scholar]
- 97.Casadaban M. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555 [DOI] [PubMed] [Google Scholar]
- 98.Silber KR, Keiler KC, Sauer RT. 1992. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc. Natl. Acad. Sci. U. S. A. 89:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.