Abstract
Type I collagen is the most abundant protein in the human body and is composed of two α1(I) and one α2(I) polypeptides which assemble into a triple helix. For the proper assembly of the collagen triple helix, the individual polypeptides must be translated in coordination. Here, we show that serine-threonine kinase receptor-associated protein (STRAP) is tethered to collagen mRNAs by interaction with LARP6. LARP6 is a protein which directly binds the 5′ stem-loop (5′SL) present in collagen α1(I) and α2(I) mRNAs, but it interacts with STRAP with its C-terminal domain, which is not involved in binding 5′SL. Being tethered to collagen mRNAs, STRAP prevents unrestricted translation, primarily that of collagen α2(I) mRNAs, by interacting with eukaryotic translation initiation factor 4A (eIF4A). In the absence of STRAP, more collagen α2(I) mRNA can be pulled down with eIF4A, and collagen α2(I) mRNA is unrestrictedly loaded onto the polysomes. This results in an imbalance of synthesis of α1(I) and α2(I) polypeptides, in hypermodifications of α1(I) polypeptide, and in inefficient assembly of the polypeptides into a collagen trimer and their secretion as monomers. These defects can be partially restored by supplementing STRAP. Thus, we discovered STRAP as a novel regulator of the coordinated translation of collagen mRNAs.
INTRODUCTION
Collagen type I is a heterotrimeric protein composed of two α1 and one α2 polypeptides which are folded into a triple helix and secreted into the extracellular matrix (1). Type I collagen forms fibrils and provides strength and elasticity to connective tissues (2) and is normally expressed at a high level in bone, tendon, skin, and arterial adventitia (1). Fibroproliferative disorders are characterized by excessive and persistent synthesis of collagen I in parenchymal organs. Excessive deposition of type I collagen forms a fibrotic scar, resulting in organ failure. Fibroproliferative disorders are a major medical problem, associated with 45% of deaths in the United States (3, 4). Conversely, lack of type I collagen expression is incompatible with life, and an impaired ability to assemble collagen I heterotrimer leads to osteogenesis imperfecta and Ehlers-Danlos syndromes (5, 6). Currently, there is no cure for fibroproliferative diseases due to the lack of understanding of complex mechanism of collagen I biosynthesis.
Expression of type I collagen is predominantly regulated at the posttranscriptional level. Stabilization of collagen mRNAs is one of the mechanisms by which collagen-producing cells increase collagen expression upon stimulation (7–13). Additionally, collagen expression is regulated by coordinating translation of collagen α1(I) and α2(I) mRNAs. Translation of collagen mRNAs is regulated by a unique structural element in the 5′ untranslated regions (UTRs) of collagen mRNAs, the 5′ stem-loop (5′SL) (10, 11, 14–16). La ribonucleoprotein domain family member 6 (LARP6) binds 5′SL with high affinity and specificity (17, 18). Knockdown of LARP6 or mutation of 5′SL reduces type I collagen expression and prevents development of hepatic fibrosis (17), suggesting the importance of interaction between these two molecules in type I collagen expression. LARP6 mediates association of collagen I mRNAs with filaments composed of nonmuscle myosin (19) and with intermediate filaments composed of vimentin (9, 20). Binding of collagen mRNAs to nonmuscle myosin is required for coordinated translation of collagen I mRNAs (19), while binding to vimentin filaments prolongs the half-life of these mRNAs (9). In addition, LARP6 recruits RNA helicase A (RHA) to collagen mRNAs. RHA stimulates translation initiation, presumably by unwinding the 5′SL and exposing the start codon for the initiation of translation (21–23).
Collagen I mRNAs are translated on the membrane of the endoplasmic reticulum (ER) (24). Collagen propolypeptides undergo cotranslational hydroxylation of lysyl and prolyl residues, followed by glycosylation of the hydroxylysines of the C-terminal domains (25, 26). The heterotrimer is formed by disulfide bonding of the C-terminal domains of two α1(I) chains and one α2(I) chain (27–29), and the process is facilitated by the action of several chaperons in the ER (30, 31). Some evidence suggests that heterotrimer formation occurs while α1(I) and α2(I) chains are still associated with polysomes on the membrane of ER. Mutations of collagen genes that impair the recognition between collagen α1(I) and α2(I) polypeptides result in hypermodification of the polypeptides and synthesis of aberrant collagen (5, 32). This indicates that the rate of posttranscriptional modifications and the rate of assembly into the heterotrimer are coordinated and that synthesis of collagen polypeptides is not random. We have shown that type I collagen synthesis is concentrated at discrete regions of the ER and postulated that such focal synthesis increases local concentration of the chains to facilitate folding. When 5′SL was mutated, type I collagen was synthesized in a diffuse pattern, suggesting that LARP6 coordinates the spatial translation of collagen I α1 and α2 mRNAs (17).
Serine-threonine kinase receptor-associated protein (STRAP; also known as unrip) was initially identified as a protein interacting with proteins Unr and Gemin 7, suggesting that STRAP is involved in regulation of cap-independent translation, as well as in assembly of snRNPs (33, 34). STRAP lacks kinase activity but has 7 WD domains, which are responsible for protein-protein interactions. There are also reports that STRAP inhibits transforming growth factor (TGF) signaling by interacting with TGF-β receptor and maintains the mesenchymal morphology of fibroblasts (35–37). STRAP is ubiquitously expressed, and STRAP−/− mice are embryonically lethal (38).
Here, we report that STRAP interacts with LARP6 through the STRAP C-terminal domain but not through the WD repeats. This interaction recruits STRAP to collagen mRNAs and prevents default translation, primarily that of collagen α2(I) mRNA. STRAP knockout mouse embryonic fibroblasts overproduce collagen polypeptides, and these polypeptides are hypermodified and not assembled into the heterotrimer. We propose a model whereby STRAP, through its interaction with LARP6, restricts random synthesis of collagen I polypeptides and regulates polysomal loading of collagen I α1 and α2 mRNAs for coordinated synthesis of the corresponding polypeptides.
MATERIALS AND METHODS
Cells.
Mouse embryonic fibroblasts (MEFs) were isolated from wild-type (WT) and two different STRAP knockout mice and were a kind gift of P. Soriano (38). Human lung fibroblasts, immortalized by expression of telomerase reverse transcriptase, have been described previously (17). HEK293 cells have been described before (39). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Valley Biomedical) or with 10% FetalPlus serum for HEK293 cells and were maintained for up to 10 passages.
Plasmids and adenovirus construction.
The hemagglutinin (HA)-tagged full-size LARP6, the N-terminal part of LARP6, and the C-terminal part of LARP6 were described previously (17, 21). To create LARP6 constructs lacking only the last 20 amino acids (LARP6-ΔSBS and C-terminal LARP6-ΔSBS), a stop codon was created after amino acid 439 by site-directed mutagenesis. Plasmid expressing Flag-tagged STRAP (pCS2-STRAP-Flag) was constructed by PCR amplification of the human STRAP cDNA and cloning the PCR product into PCS2-Flag vector with the Flag-tag at the N terminus. Strap-ΔCI and Strap-ΔCII were constructed by introducing a stop codon at the indicated positions by site-directed mutagenesis. Plasmids containing mouse eukaryotic translation initiation factor 4A (eIF4A)-HA, mouse eIF4A-Flag, and mouse eIF4E-HA were a kind gift from N. Sonenberg (40).
Adenoviruses were constructed by recloning LARP6 and N-terminal LARP6 constructs from the pCDNA3 vector into the pAdCMVTRACK vector, followed by recombination with the pAdEasy vector, described previously (17, 41). The same procedure was used for STRAP and STRAP-ΔCII adenoviruses. All LARP6 adenovirus constructs retained the HA tag, and all STRAP constructs retained the Flag tag at the N terminus. All adenoviruses also express green fluorescent protein (GFP) from a separate transcription unit, which was used to monitor transduction efficiency (41). A control adenovirus used in this study expressed only GFP. Expression of each construct was confirmed by Western blotting.
Transfections and transductions.
HEK293 cells were seeded on a 35-mm dish and transfected with 1 to 2 μg plasmid/dish using 293TransIT reagent (Mirus). The cells were harvested 48 h after the transfection.
Lung fibroblasts were seeded on a 35-mm dish and transduced with adenoviruses at a multiplicity of infection (MOI) of 100. This MOI resulted in 95 to 100% of cells infected, as determined by expression of the viral marker, GFP. The same procedure was used for MEFs. The cells were harvested 48 to 72 h after the transduction.
RT-PCR analysis.
Total cellular RNA was isolated using an RNA isolation kit (Sigma). Reverse transcription (RT)-PCRs were performed with 100 ng of total RNA and by using rTth reverse transcriptase (Boca Scientific, Boca Raton, FL), according to the published procedures (11, 14, 16, 21). The primers used are listed in Table 1. For analysis of total RNA, 18 to 20 PCR cycles were used, while for the analysis of polysomal fractions, 25 to 30 PCR cycles were used, to maintain the reaction in the linear range. [α-32P]dATP was included in PCR amplifications to label the products, which were resolved on 6% sequencing gels and detected by autoradiography. Quantification of PCR products was performed on a Typhoon phosphoimager or by densitometric scanning of the bands on autoradiography films and quantification by NIH Image J software. The results are shown as integrated density, which represents the intensity of collagen signal normalized to the intensity of the corresponding GAPDH signal. The standard error of the means (SEM) for normalized values and graphics were calculated using the biostatic program GraphPad Prism 3.02. The one-way analysis of variance (ANOVA) method, followed by Tukey's multiple comparison test, was used to determine significant differences (P < 0.05). The P values were calculated by the Student t test.
Table 1.
Primers used for RT-PCR and qRT-PCR
| Primera | Sequence |
|
|---|---|---|
| Forward | Reverse | |
| h-collagen α1(I) | AGAGGCGAAGGCAACAGTCG | GCAGGGCCAATGTCTAGTCC |
| h-collagen α2(I) | CTTCGTGCCTAGCAACATGC | TCAACACCATCTCTGCCTCG |
| h-actin | GTGCGTGACATTAAGGAGAAG | GAAGGTAGTTTCGTGGATGCC |
| h-fibronectin | ACCAACCTACGGATGACTCG | GCTCATCATCTGGCCATTTT |
| m-collagen α1(I) | GAGCGGAGAGTACTGGATCG | TACTCGAACGGGAATCCATC |
| m-collagen α2(I) | CTTCGTGCCTAGCAACATGC | TCAACACCATCTCTGCCTCG |
| m-fibronectin | AATGGAAAAGGGGAATGGAC | CTCGGTTGTCCTTCTTGCTC |
| m-actin | CGTGCGTGACATCAAAGAGAAGC | TGGATGCCACAGGATTCCATACC |
| h-GAPDH | ACCGGTTCCAGTAGGTACTG | CTCACCGTCACTACCGTACC |
h, human; m, mouse.
qRT-PCR analysis.
Total cellular RNA was isolated using an RNA isolation kit (Sigma) and treated with DNase I to remove contaminating DNA. Equal amounts of RNA were used in a reverse transcription reaction. The cDNA was synthetized using SuperScript II RT (Invitrogen) by following the manufacturer's protocol. Five percent of total cDNA was used in 40 cycles of quantitative real-time PCR (qRT-PCR) in a Bio-Rad IQ5 thermocycler, as described previously (21). The primers used in qRT-PCR are presented in Table 1. The qRT-PCRs were performed in duplicate, and the threshold cycle (CT) was calculated using standard curves constructed for each set of primers with a stepwise dilution of input DNA and IQ5 software (Bio-Rad). The total collagen α1(I) and α2(I) mRNA levels were standardized to the actin level and the negative control (empty vector). Statistical analysis of the results was done as described above.
Immunoprecipitations (IP).
Cells were lysed in buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40 (Calbiochem), 1 mM phenylmethylsulfonyl fluoride (PMSF) (Pierce), and 1× protease inhibitors (Sigma). After removal of nuclei by centrifugation, lysate was precleared with 20 μl of equilibrated protein A/G-agarose beads (Santa Cruz Biotechnology) for 1 h at 4°C. After removal of the beads by centrifugation, 1 μg of antibody was added to clear lysate and was incubated for 1 h at 4°C. A total of 20 μl of equilibrated protein A/G beads was added, and incubation continued for an additional 3 h. The A/G beads were washed five times with lysis buffer, and samples were dissolved in SDS-PAGE loading dye and analyzed by Western blotting. When pulldown of mRNA was analyzed, the RNA was extracted from the beads using an RNA extraction kit (Sigma).
Nuclear and cytosolic extracts were prepared by the method of Dignam et al. (42). When RNase A treatment was done, 1 μg of RNase A was added to 250 μl of extract and incubated at room temperature for 30 min. This resulted in complete degradation of RNA.
Western blots.
Cellular proteins were isolated by lysing the cells in the IP buffer. After removal of nuclei and insoluble material by centrifugation, the protein concentration of the clear lysate was measured using a Bradford assay. Typically, 50 to 70 μg of total proteins was loaded on 7.5% or 10% SDS-PAGE gels under reducing or nonreducing conditions, as indicated. For Western blot analysis of type I collagen secreted into the cellular medium, 0.5 × 106 to 1 × 106 cells were seeded into 35-mm dishes. A total of 24 to 48 h after the seeding, cells were washed and 0.5 ml of serum-free medium was added, and incubation continued for an additional 3 h. The serum-free medium was collected, and 50 μl was analyzed directly by Western blotting (19). When collagen was analyzed in the medium of cells that produce smaller amounts of type I collagen (WT MEFs and STRAP−/− MEFs), the secreted proteins were precipitated with 6.5% trichloroacetic acid (TCA) (Fisher Biotech) and 0.05% Na deoxycholate (DOC) (Fisher Biotech). The precipitated proteins were dissolved in 0.1% SDS and 10 mM Tris (pH 6.8) and loaded on the gel. Western blots were imaged with the Odyssey infrared imaging system or by chemiluminescence.
The following antibodies were used: anti-collagen α1(I) polypeptide antibody from Rockland; anti-collagen α2(I) polypeptide antibody from Santa Cruz Biotechnology; antifibronectin antibody from BD Transduction Laboratories; anti-α/β tubulin antibody from Cell Signaling; antiactin and anti-LARP6 antibodies from Abnova; anti-HA, anti-Flag, and anti-GFP antibodies from Sigma; anti-STRAP antibodies from BD Transduction Laboratories; anti-RNA helicase A (RHA) and anti-eIF4A from Abcam; and anti-eIF4E from Cell Signaling. The secondary antibodies were IRDye 800CW goat anti-mouse antibody, IRDye 800CW donkey anti-goat antibody, and IRDye 680LT goat anti-rabbit antibody (Li-COR).
Gel mobility shift assay.
WT 5′ stem-loop RNA probe was synthesized and radiolabeled by in vitro transcription from the 68-nucleotide (nt) template cloned into pGEM3 vector (Promega), as previously described (17). Lung fibroblasts were transduced with adenoviruses expressing HA-LARP6 or Flag-STRAP, and 40 μg of total cytosolic proteins was incubated with 20 fM RNA probe in the presence of 10 μg tRNA for 10 min on ice. The samples were run on 6% native polyacrylamide gels and visualized by autoradiography. For supershift assays, 2 μl of anti-HA antibody or anti-Flag antibody was added prior to the addition of RNA probes (17). The input of expressed proteins was determined by Western blotting.
Isolation and fractionation of polysomes.
Polysomes were isolated from 3 × 107 cells treated with cycloheximide (100 μg/ml) for 1 h prior to lysis. The cells were lysed in 10 mM Tris-HCl (pH 8), 150 mM NaCl, 5 mM MgCl2, 1% NP-40 (Calbiochem), 40 mM dithiothreitol (DTT), 1 mM PMSF. To verify which fractions represent polysomes, in some experiments, 50 mM EDTA was added to the lysis buffer to disrupt polysomes. Cell lysate was laid on top of a linear 15 to 45% sucrose gradient and centrifuged at 38,000 × g for 2 h in a Beckman Ti41 rotor (17, 21, 43). Sixteen 500-μl fractions were collected, and the optical density at 260 nm (OD260) of each fraction was measured. Total RNA was isolated from each fraction by phenol-chloroform extraction and isopropanol precipitation. RNA samples were analyzed on a 1% agarose gel to determine distribution of rRNA in the fractions. The amount of collagen α1(I) and α2(I) mRNAs in the fractions was analyzed by RT-PCR. GAPDH mRNA was analyzed as the control. Protein levels of LARP6 and STRAP in each fraction were analyzed by Western blotting, after the precipitation of total proteins from the fractions by 6.5% TCA and 0.05% DOC.
2D SDS electrophoresis.
After lysis of cells in IP buffer, cellular proteins were precipitated with 9 volumes of ethanol and recovered by centrifugation (44). The protein pellet was solubilized in 150 μl of rehydration buffer, and Immobiline DryStrip strips (7 cm, pH 3 to 10; GE Healthcare) and an Ettan IPGphor 3 instrument (GE Healthcare) were used for first-dimension separation, according to standard protocol (45, 46). The second-dimension (2D) separation was by SDS-PAGE using 7.5% gels, and the collagen α1 and α2 polypeptide chains were visualized by Western blotting. Glycosylated proteins were detected by incubating the membranes after blotting in solution of biotinylated concanavalin A (Vector Laboratories), at dilution of 1:1,000 for 3 h, followed by incubation in a 1:10,000 dilution of IRDye 800CW-streptavidin (LI-COR), as described previously (47). The blot was imaged with the Odyssey infrared imaging system.
RESULTS
Interaction of STRAP with LARP6.
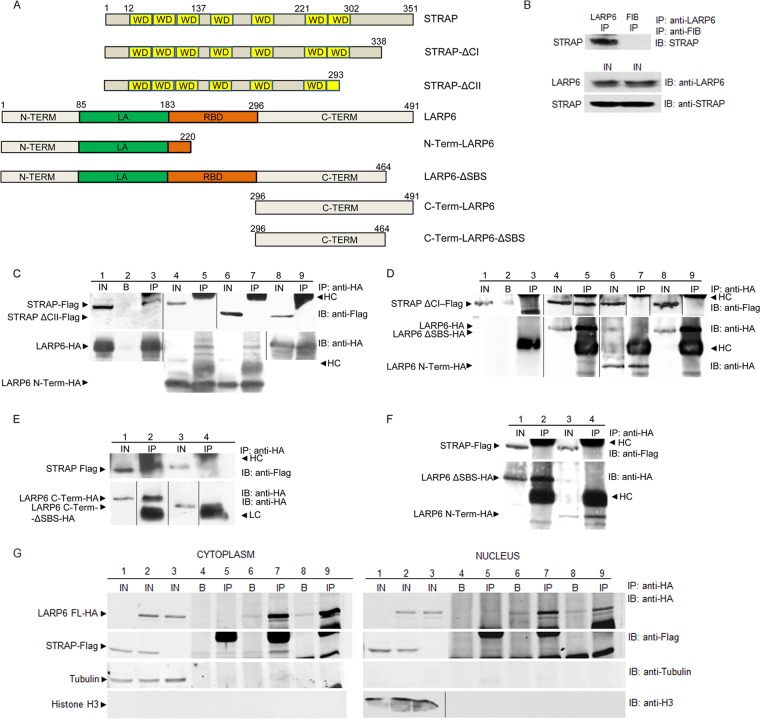
To identify the proteins that interact with LARP6, we expressed HA-tagged LARP6 in human lung fibroblasts (HLFs) and performed immunoprecipitation (IP). STRAP was identified by liquid chromatography tandem mass spectrometry (LC-MS/MS) as one of the proteins that specifically interacted with LARP6. To verify this result, a series of IP experiments was performed (Fig. 1). First, the interaction between the endogenous STRAP and LARP6 was analyzed in HLFs. Anti-LARP6 antibody efficiently pulled down endogenous STRAP from HLF lysates (Fig. 1B), suggesting that these proteins interact in the cell. To map the protein domains involved in this interaction, we coexpressed STRAP-Flag and LARP6-HA in HEK293 cells. When the IP was done with anti-HA antibody, the STRAP-Flag was pulled down (Fig. 1C, lanes 1 to 3), while the control IP was negative (1C, lane 2), suggesting a specific interaction between the tagged proteins as well. Then, we created deletion mutants of the tagged proteins (shown in Fig. 1A). Two deletion mutants of STRAP lacking the C-terminal domain, STRAP-ΔCI (amino acids [aa] 1 to 338) and STRAP-ΔCII (aa 1 to 293), were constructed. STRAP-ΔCI interacted with LARP6 in the IP experiments (Fig. 1D, lanes 4 and 5); however, STRAP-ΔCII failed to interact (Fig. 1C, lanes 8 and 9), suggesting that aa 294 to 338 of STRAP are indispensable for the interaction. To assess if the C-terminal aa 294 to 338 of STRAP are also sufficient for the interaction with LARP6, we overexpressed this short protein, but it failed to interact with LARP6 (not shown). Thus, the C-terminal domain of STRAP is necessary but not sufficient for the interaction with LARP6.
Fig 1.
Interaction of STRAP and LARP6. (A) Schematic representation of STRAP and LARP6 constructs. Amino acid numbering is on the top. WD, Trp-Asp repeats; N-TERM, N-terminal domain; LA, La homology domain; RBD, RNA binding domain; C-TERM, C-terminal domain. (B) Interaction of endogenous STRAP and LARP6. IP was done with anti-LARP6 antibody (Ab) (lane 1) or antifibronectin Ab (FIB) (lane 2), and Western blotting was with anti-STRAP Ab. Bottom, expression of the proteins in the input (IN). (C) STRAP-Flag immunoprecipitates with LARP6-HA. Tagged proteins were coexpressed in HEK293 cells, and IP was done with anti-HA Ab (lanes 3, 5, 7, and 9) and Western blotting with anti-HA Ab. Lanes 1, 4, 6, and 8 are analyses of 10% of the input material, and lane 2 is IP using only protein A/G beads. HC, antibody heavy chain. (D) STRAP-ΔCI interacts with LARP6 but not with LARP6-ΔSBS. Experiment as described for panel B, except STRAP-ΔCI-Flag and LARP6-ΔSBS-HA were used. (E) C-terminal LARP6 is sufficient for interaction with STRAP. Experiments as described for panel B, except C-Term-LARP6-HA and C-Term-LARP6-ΔSBS-HA were used. (F) STRAP does not interact with full-size LARP6 lacking the SBS. Experiment as described for panel B, except LARP6-ΔSBS-HA was used. (G) STRAP interacts with LARP6 in the cytosol. STRAP-Flag and LARP6-HA were expressed in HLFs and cytosolic (left) and nuclear (right) extract were used in IP with anti-HA Ab. Tubulin is shown as a marker of cytosolic fraction and histone H3 as a marker of nuclear fraction. IN, 10% of input (lanes 1 to 3); B, A/G beads only (lanes 4, 6, and 8); IP, immunoprecipitations (lanes 5, 7, and 9).
To assess which part of LARP6 is required for the interaction with STRAP, we created several deletion mutants of LARP6 (Fig. 1A). When LARP6 containing the first 220 aa (N-terminal LARP6) was coexpressed with STRAP (Fig. 1C, lanes 4 and 5) or STRAP-ΔCI (Fig. 1D, lanes 6 and 7), these STRAP constructs were not pulled down. As a negative control, we used construct STRAP-ΔCII, which does not interact with LARP6 (Fig. 1C, lanes 6 and 7). Next, we tested the constructs containing only the C-terminal domain of LARP6. STRAP coimmunoprecipitated with the C-terminal domain of LARP6 containing aa 296 to 491 (Fig. 1E, lanes 1 and 2), but it failed to coimmunoprecipitate if the last 27 aa of this domain were deleted (C-terminal LARP6-ΔSBS) (Fig. 1E, lanes 3 and 4). This revealed that the last 27 aa of LARP6, termed the STRAP binding sequence (SBS), are essential for the interaction with STRAP. To further confirm this, we made the full-size LARP6 lacking only the terminal 27 aa (LARP6-ΔSBS). This construct also failed to pull down STRAP (Fig. 1F, lanes 1 and 2) or STRAP-ΔCI (Fig. 1D, lanes 8 and 9). This result confirmed that the SBS domain of LARP6 is critical for its interaction with STRAP. Interestingly, STRAP has been shown previously to interact with Unr and Gemin 7 (33, 34), which share a conserved stretch of amino acids, termed here the SBS (Table 2). Therefore, it is likely that the SBS in the other two STRAP binding proteins also binds STRAP, although the SBS is not at the C terminus of Gemin 7. A BLAST search using the SBS consensus sequence did not identify additional proteins that have a highly similar sequence.
Table 2.
Homology of the SBS between three STRAP binding proteins
| Protein | Sequence | Amino acids |
|---|---|---|
| LARP6 | VLRLPRGPD-NTRGF | 465–481 |
| Gemin 7 | VLRLPRGPDGFSRGF | 11–25 |
| Unr | VLRQPRGPD-NSMGF | 744–757 |
As STRAP (36) and LARP6 (17) are both cytosolic and nuclear proteins, we tested if STRAP and LARP6 can interact in nuclear extracts. Cellular extracts of HLFs expressing STRAP-Flag and LARP6-HA were separated into nuclear and cytosolic fractions (42). STRAP-Flag and LARP6-HA were expressed in both the cytoplasm (Fig. 1G, left, lanes 1 to 3) and the nucleus (right, lanes 1 to 3). The interaction between STRAP and LARP6 was detected only in the cytoplasmic fraction (Fig. 1G, left, lanes 6 and 7) but could not be detected in the nuclear fraction (right, lanes 6 and 7). Analysis of tubulin and histone H3, as cytosolic and nuclear markers, respectively, revealed that there was no cross-contamination of the fractions. These results suggested that STRAP and LARP6 interact in the cytoplasm and that the interaction may be involved in regulation of translation of collagen mRNAs.
STRAP is recruited to collagen α1(I) and α2(I) mRNAs by LARP6.
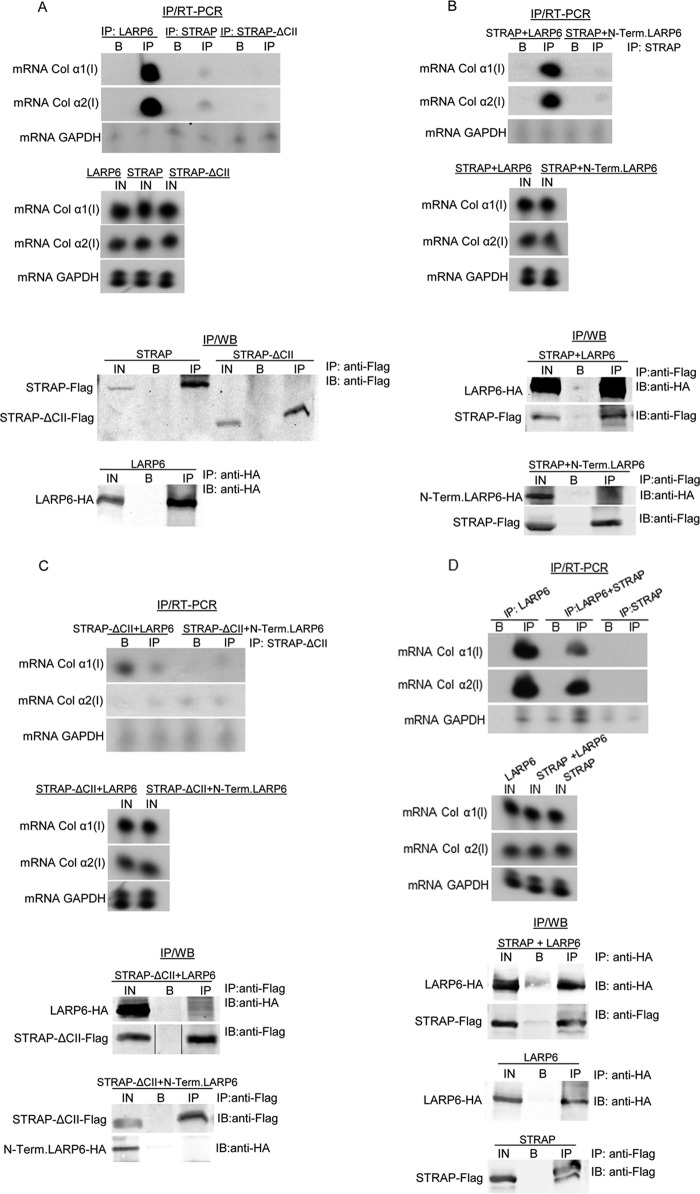
To regulate translation of collagen mRNAs, we surmised that STRAP must be recruited to these mRNAs. Since STRAP interacted with LARP6, which binds both collagen mRNAs with high affinity (17, 21), we tested if STRAP and LARP6 can interact by protein-protein interactions or only in an RNA-dependent manner. We performed IP of the proteins after treatment of the cell lysate with RNase A. LARP6 pulled down STRAP regardless of the RNase A treatment, suggesting that the LARP6 and STRAP interaction is not RNA dependent (Fig. 2A).
Fig 2.
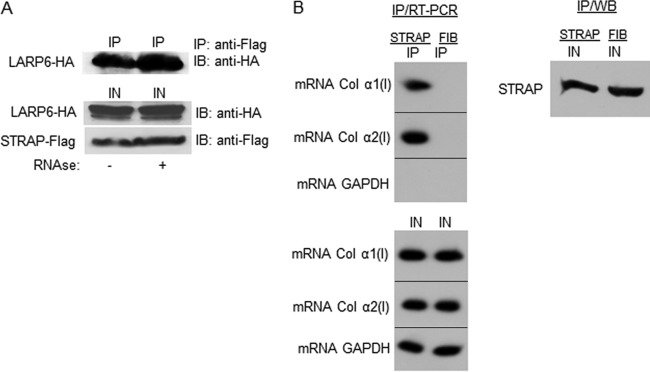
RNA-independent interaction of STRAP and LARP6. (A) IP of STRAP and LARP6 from extracts treated with RNase A. Top, STRAP-Flag and LARP6-HA were coexpressed in HEK293 cells, and IP was done with anti-Flag Ab and Western blotting with anti-HA Ab. Lane 1, extract without RNase A treatment; lane 2, extract after RNase A treatment. Bottom, expression of the proteins in the input (IN). (B) Endogenous STRAP associates with collagen mRNAs. Top left, IP was done with anti-STRAP Ab (lane 1) or control antifibronectin Ab (FIB; lane 2), and collagen α1(I) and α2(I) mRNAs were analyzed in the immunoprecipitated material by RT-PCR. GAPDH mRNA was analyzed as the control. Bottom, expression of mRNAs in the input (IN). Right, expression of STRAP in the input analyzed by Western blotting.
To analyze if STRAP is tethered to collagen mRNAs in vivo, we immunoprecipitated the endogenous STRAP and analyzed for pulldown of collagen α1(I) and α2(I) mRNAs (Fig. 2B). Both collagen mRNAs were pulled down with anti-STRAP antibody but not with control antibody, indicating association of the endogenous STRAP with type I collagen mRNAs.
Next, we tested if overexpressing LARP6 can stimulate association of STRAP with collagen mRNAs. We overexpressed STRAP-Flag, STRAP-ΔCII-Flag, or LARP6-HA alone or in combinations in HLFs, immunoprecipitated the proteins, and analyzed the pulldown of collagen mRNAs in the immunoprecipitated material. When LARP6-HA alone was overexpressed, anti-HA antibody immunoprecipitated a large amount of collagen α1(I) and α2(I) mRNAs, as expected for the collagen mRNA binding protein. When STRAP-Flag alone was overexpressed, a small amount of collagen mRNAs was pulled down with anti-Flag antibody, suggesting that STRAP alone cannot bind collagen mRNAs and that the pulldown was limited by the amount of endogenous LARP6 (Fig. 3A, top). The expression of LARP6, STRAP, and STRAP-ΔCII did not change the input levels of collagen mRNAs (Fig. 3A, middle). Next, we tested if an excess of LARP6 can increase the association of STRAP with collagen mRNAs. To this goal, we coexpressed STRAP-Flag and LARP6-HA and repeated IP of collagen mRNAs. In the presence of excess LARP6, STRAP pulled down much more collagen mRNA. When STRAP was coexpressed with N-terminal LARP6, the mutant that cannot bind collagen mRNAs (17) or STRAP, no stimulation was seen (Fig. 3B, top). These results strongly suggest that STRAP is recruited into a complex with collagen mRNAs by its interaction with LARP6. To further corroborate these results, STRAP-ΔCII was coexpressed with LARP6 or with N-terminal LARP6, as the control. STRAP-ΔCII, which cannot interact with LARP6, did not pull down collagen mRNAs even in the presence of excess LARP6 (Fig. 3C). This confirmed that STRAP is brought into a complex with collagen mRNAs only if it can interact with LARP6.
Fig 3.
STRAP is tethered to collagen mRNAs by binding to LARP6. (A) Pulldown of collagen mRNAs with LARP6-HA and STRAP-Flag. Top, LARP6-HA, STRAP-Flag, or STRAP-ΔCII-Flag was expressed in HLFs, IP was done with anti-HA or anti-Flag antibodies, and the IP material was analyzed by RT-PCR for pulldown of collagen mRNAs. GAPDH mRNA was analyzed as the control. B, A/G beads; IP, immunoprecipitations. Middle, expression of collagen mRNAs in the input (IN); bottom, presence of the expressed proteins in input (IN) and in IP. B, A/G beads only; IP, immunoprecipitations. (B) Excess LARP6 stimulates association of STRAP with collagen mRNAs. Top, experiment as described for panel A, except STRAP-Flag and LARP6-HA or LARP6 N-Term-HA were coexpressed and IP was done with anti-Flag Ab; middle, collagen mRNAs in the input analyzed by RT-PCR; bottom, analysis of the expressed proteins in the input (IN) and in IP by Western blotting. (C) STRAP-ΔCII does not bind collagen mRNAs even in the presence of excess LARP6. Experiment as described for panel B, except STRAP-ΔCII-Flag was coexpressed with LARP6-HA or N-Term-LARP6-HA. (D) STRAP minimally affects binding of LARP6 to collagen I mRNAs. Top, LARP6-HA and STRAP-Flag were expressed alone or in combination and IP was done with anti-HA Ab or anti-Flag Ab. Pulled-down collagen mRNAs were analyzed by RT-PCR. Middle, expression of collagen mRNAs in the input (IN); bottom, proteins in IP and in input by Western blotting.
Next, we reversed the experiment and tested if STRAP affects the association of LARP6 and collagen mRNAs. An excess of STRAP had only a minimal effect on the association LARP6 with collagen α1(I) and α2(I) mRNAs (Fig. 3D). Thus, STRAP is not inhibitory to binding of LARP6 to collagen mRNAs.
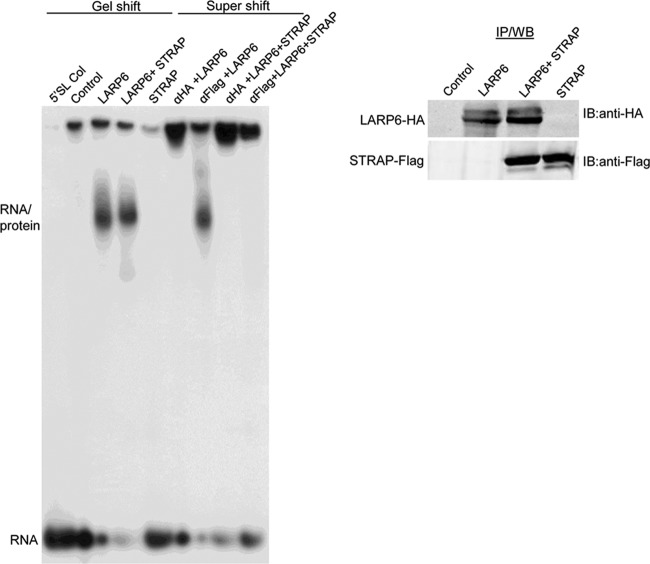
To provide biochemical evidence for the formation of the LARP6-STRAP-5′SL complex, we performed gel mobility shift assays. Extracts of HLFs expressing LARP6-HA or STRAP-Flag, alone or in combination, were incubated with 5′SL RNA probe, and the complexes were resolved on a native acrylamide gel. Overexpression of LARP6 alone or with STRAP resulted in formation of an RNA-protein complex. The electrophoretic mobility of this 5′SL-LARP6 complex was slightly higher than the mobility of the complex formed when STRAP was also present, but the intensities of the protein-RNA complexes were equal (Fig. 4, left). When STRAP alone was overexpressed, no complex formation with 5′SL RNA was seen. These results demonstrated that STRAP cannot bind 5′SL RNA but that it may be integrated into the complex when LARP6 is present. To verify the presence of LARP6 and STRAP in the complexes, we performed a supershift assay by adding anti-HA or anti-Flag antibody to the binding reactions. When LARP6 was expressed alone, anti-HA antibody caused a supershift of the complex into the slot of the gel, verifying the presence of LARP6. When anti-Flag was added, there was no supershift. However, when STRAP was coexpressed with LARP6, the anti-Flag antibody did supershift the complex, suggesting that now it contains STRAP in addition to LARP6 (Fig. 4, left). These results confirm that STRAP and LARP6 can be found in the complex with 5′SL RNA, where STRAP is recruited by LARP6.
Fig 4.
STRAP and LARP6 are found in complex with 5′SL RNA. Left, gel mobility shift assay with 5′SL RNA probe and extracts of HLFs expressing LARP6-HA and STRAP-Flag, alone or in combination. Anti-HA or anti-Flag Ab was added to the binding reactions as indicated. Migration of the RNA-protein complex and free 5′SL mRNA (I) is indicated. The control is HLF lysate without adenovirus infection and 5′SL is the probe alone. Right, expression of LARP6 and STRAP in input by Western blotting.
Excessive synthesis of collagen monomers in the absence of STRAP.
To assess if STRAP has an impact on synthesis of collagen polypeptides, we analyzed type I collagen assembly of WT and STRAP knockout mouse embryonic fibroblasts (STRAP−/− MEFs, two independent isolates, 1110 and 1108) by Western blotting under reducing and nonreducing conditions (Fig. 5). Type I collagen is a heterotrimer of disulfide-bonded α1(I) and α2(I) chains and resolves under nonreducing conditions as an ∼550-kDa protein, while under reducing conditions monomers of both α1(I) and α2(I) chains are detected as heterogenous molecular species of ∼160 to 180 kDa (25, 48), because the antibody used recognizes both polypeptides. When analyzed under reducing conditions, the STRAP−/− MEFs accumulated larger amounts of collagen monomers than WT MEFs (Fig. 5A, left). However, when the total intracellular level of collagen I was analyzed under nonreducing conditions, almost exclusive formation of the trimer was observed in WT MEFs, with only trace amounts of unassembled monomers present (Fig. 5A, middle). In both isolates of STRAP−/− MEFs, in addition to the trimer, a significant accumulation of collagen monomers was observed. This indicates excessive synthesis of one or both collagen polypeptides that surpassed the cell capacity to fold them into a trimer. The total levels of collagen α1(I) and α2(I) mRNAs in WT and STRAP−/− MEFs were similar (see Fig. S1 in the supplemental material), suggesting that an excessive translation or slower degradation of collagen polypeptides in STRAP−/− MEFs may have contributed to the effect. In this experiment, WT and STRAP−/− MEFs were seeded at 3 different cell densities (0.25 × 106/well to 1 × 106/well), and the same phenomenon was observed. To verify that the high-molecular-weight collagen secreted by MEFs is indeed a trimer, we analyzed in parallel type I collagen extracted from rat tail (Fig. 5A, right, tissue col). In tissues, only the fully processed heterotrimeric type I collagen is found. The tissue-extracted collagen migrated slightly faster than the trimer produced by WT MEFs, suggesting that MEFs secreted collagen trimer in its precursor (procollagen) form.
Fig 5.
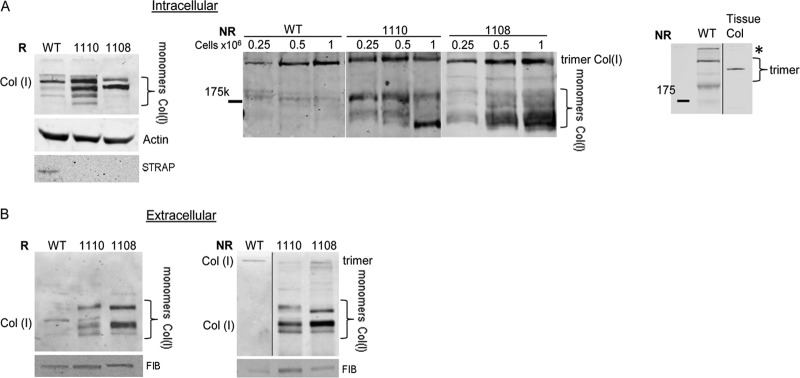
STRAP is necessary for proper secretion of the collagen trimer. (A) STRAP−/− MEFs accumulate excessive amounts of collagen monomers. The cellular level of collagen polypeptides was analyzed by Western blotting in WT and STRAP−/− MEFs (1110, 1108) under reducing conditions (R; left) and nonreducing conditions (NR; middle). Actin and STRAP served as loading controls for reducing conditions. Three different cell densities (indicated on the top) were used for nonreducing conditions. Migration of individual collagen polypeptides (monomers) and assembled trimer is indicated. Right, Western blot of collagen trimer from MEFs (WT) and trimeric collagen extracted from rat tail (tissue col), run in parallel. Migration of the mature collagen trimer and procollagen trimer is shown by brackets. *, the top of the gel. (B) STRAP−/− MEFs are deficient in secreting the collagen trimer. The medium of WT and STRAP−/− MEFs was analyzed by Western blotting under reducing conditions (R; left) and nonreducing conditions (NR; right). Fibronectin (FIB) was used as the loading control.
We also analyzed type I collagen secreted into the medium of WT and STRAP−/− MEFs. Secretion of fibronectin (FIB) was analyzed as the loading control in these experiments. Under reducing conditions, a larger amount of total monomers was secreted from STRAP−/− MEFs than from WT MEFs (Fig. 5B, left). Analysis under nonreducing conditions revealed that most collagen secreted from WT MEFs was in the trimeric form, while STRAP−/− MEFs secreted predominantly collagen monomers. This result again pointed to the unrestricted collagen synthesis in STRAP−/− cells, which overwhelms the capacity of cells to fold type I collagen. We concluded that in the absence of STRAP, a coordinated translation of type I collagen mRNAs is perturbed, resulting in overproduction of individual polypeptides.
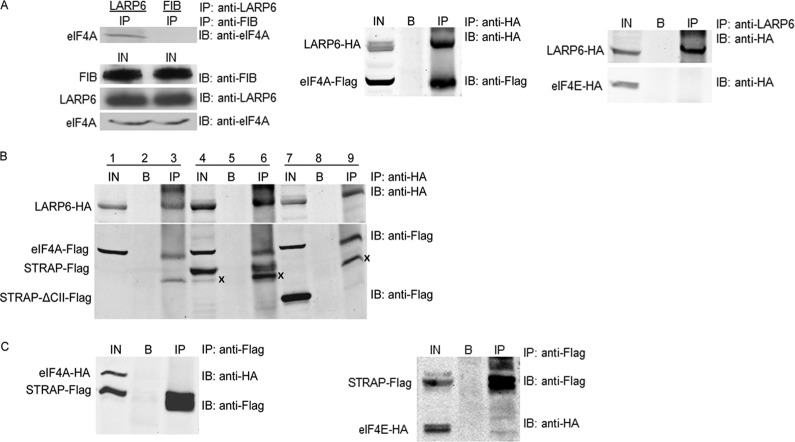
Interaction of STRAP and LARP6 with eIF4A.
We hypothesized that STRAP together with LARP6 can coordinate production of type I collagen polypeptides by regulating the initiation of translation. To assess if STRAP and LARP6 interact with translation initiation factors eIF4A and eIF4E (49, 50), we performed IP of the endogenous proteins. LARP6 pulled down eIF4A (Fig. 6A, left) but not eIF4E (see Fig. S2B in the supplemental material). Likewise, overexpressed LARP6-HA pulled down eIF4A (Fig. 6A, middle). When STRAP-Flag was coexpressed with LARP6-HA and eIF4A, immunoprecipitation of LARP6 pulled down both STRAP and eIF4A, suggesting that all three proteins can form a complex (Fig. 6B). In a similar experiment, STRAP-ΔCII-Flag could not be pulled down (Fig. 6B). Because this mutant does not interact with LARP6, we concluded that the interaction of STRAP and LARP6 brought STRAP into a complex with eIF4A. When STRAP and eIF4A were expressed without LARP6, these two proteins did not interact (Fig. 6C, left), confirming the above-described result.
Fig 6.
Binding of STRAP and LARP6 to eIF4A. (A) LARP6 interacts with eIF4A but not with eiF4E. Left, interaction of endogenous LARP6 and eIF4A. IP was done with anti-LARP6 Ab (lane 1) or antifibronectin Ab (FIB; lane 2), and Western blotting was with anti-eIF4A Ab. Bottom, expression of the proteins in the input (IN). Middle, eIF4A-Flag and LARP6-HA were coexpressed in HEK293 cells, and IP was done with anti-HA Ab, followed by Western blotting with anti-HA and anti-Flag antibodies. Right, LARP6-HA and eIF4E-HA were coexpressed, and IP was done with anti-LARP6 Ab, followed by Western blotting with anti-HA Ab. IN, 10% of the cell lysate used for IP; B, protein A/G beads only; IP, immunoprecipitation. (B) LARP6 brings STRAP into complex with eIF4A. eIF4A-Flag, LARP6-HA, and eIF4A-Flag were coexpressed, and IP was done with anti-HA Ab. The Western blot was probed with anti-HA and anti-Flag antibodies (IP; lanes 3, 6, and 9). Lanes 1, 4, and 7 show 10% of the input, and lanes 2, 5, and 8 show protein A/G beads alone as the control. X marks a nonspecific band. (C) STRAP does not interact with eIF4A or eIF4E. The same experiment as described for panel A, except eIF4A-HA and STRAP-Flag were coexpressed (left) or eiF4E-HA and STRAP-Flag were coexpressed (right).
Collagen mRNAs are likely to be translated in a cap-dependent manner, because both collagen mRNAs can be immunoprecipitated with anti-eIF4E antibody (see Fig. S2A in the supplemental material) (24, 51). However, we could not pull down eIF4E with LARP6 even when both proteins were overexpressed (Fig. 6A, right). STRAP-Flag also did not pull down eIF4E (Fig. 6C, right). The lack of pulldown of eIF4E with STRAP or LARP6 does not implicate a cap-independent regulation of translation by these proteins. It may simply reflect weak association of eIF4E in the complex. Cap-dependent translation of collagen mRNAs is strongly supported by the immunoprecipitation of collagen mRNAs with anti-eIF4E antibody (see Fig. S2A in the supplemental material).
STRAP restricts binding of eIF4A to collagen α2(I) mRNA.
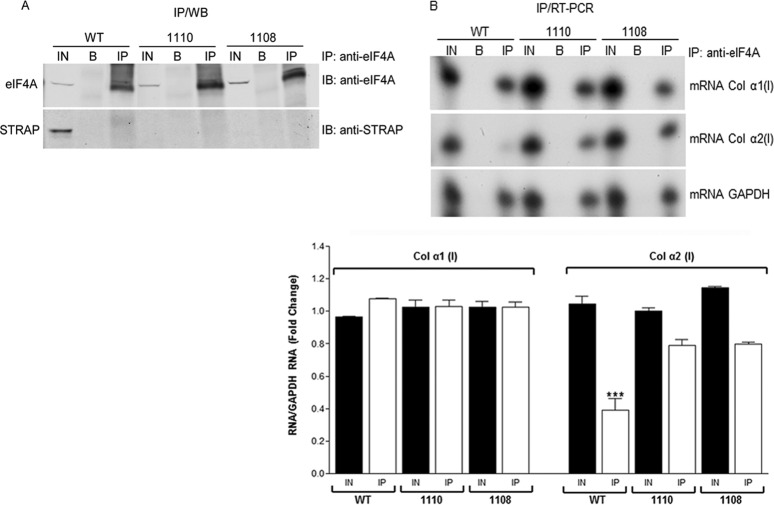
So far, we have shown that STRAP is found in the complex with LARP6 and collagen mRNAs (Fig. 2, 3, and 4) and in complex with LARP6 and eIF4A (Fig. 6). Therefore, we wanted to analyze if STRAP could affect the recruitment of eIF4A to collagen mRNAs. We compared IP of collagen mRNAs with anti-eIF4A antibody in WT and STRAP−/− MEFs (38). Pulldown of GAPDH mRNA was analyzed as a positive control. Figure 7A shows that eIF4A could be immunoprecipitated with similar efficiency from WT and 1110 and 1108 STRAP−/− MEFs (top) and that STRAP could be detected only in WT MEFs (bottom). Figure 7B shows the pulldown of collagen mRNAs. The expression of collagen α1(I) and α2(I) mRNA in WT and STRAP−/− MEFs was similar (Fig. 7B, compare the IN lanes). Anti-eIF4A antibody coimmunoprecipitated the equivalent amount of collagen α1(I) mRNA from WT and STRAP−/− MEFs (Fig. 7B, top, IP lanes). However, much smaller amounts of collagen α2(I) mRNA were pulled down from WT MEFs than from both STRAP−/− MEFs (middle, IP lanes). GAPDH was immunoprecipitated with equal efficiency in all MEFs (bottom). The bottom panel of Fig. 7B shows quantitative real-time PCR analysis of the pulled down RNAs, clearly indicating weaker IP of α2(I) mRNA in WT MEFs. This suggested that STRAP specifically inhibited the interaction of collagen α2(I) mRNA with eIF4A and that it may be a negative regulator of the polysomal loading of collagen α2(I), acting at the level of translation initiation.
Fig 7.
Association of eIF4A with collagen mRNAs. (A) IP of endogenous eIF4A from WT and STRAP−/− MEFs (1110 and 1108). Top, IP was done with anti-eIF4A Ab and the blot was probed with the same antibody. Blotting with anti-STRAP Ab is shown as the control. IN, 10% input; B, protein A/G beads only; IP, immunoprecipitations. (B) Pulldown of collagen mRNAs with anti-eIF4 Ab. Pulled-down RNA from samples in panel A was analyzed for collagen α1(I) and α2(I) mRNAs by RT-PCR. GAPDH mRNA was analyzed as the control. Bottom, quantitative real-time RT-PCR analysis of collagen mRNA from panel B, normalized to GAPDH mRNA. The mean and SEM values from three independent experiments are shown. ***, P < 0.01.
STRAP regulates polysomal loading of collagen mRNAs.
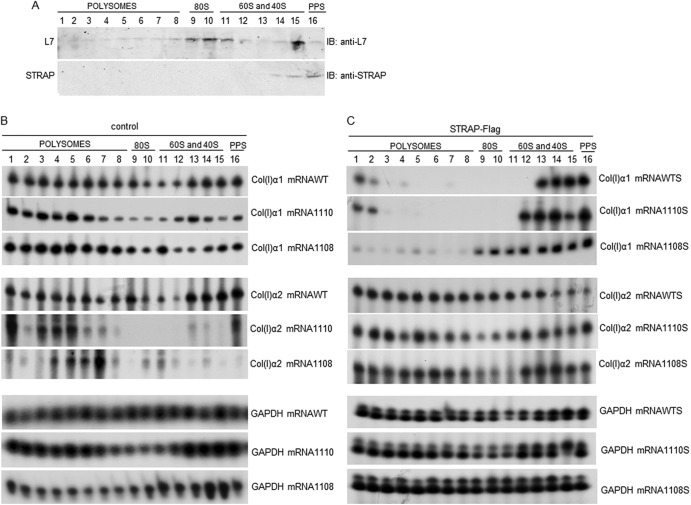
To assess if STRAP regulates polysomal loading of collagen mRNAs, we fractionated polysomes from WT and STRAP−/− MEFs on sucrose gradients (a typical polysomal profile is shown in Fig. S3A and B in the supplemental material). Fractionation in the presence of EDTA additionally confirmed that fractions 1 to 8 contained polysomes and collagen mRNA (see Fig. S2B and C, right, in the supplemental material). Endogenous STRAP was found only in the fractions containing free ribosomal subunits (Fig. 8A, fractions 14 and 15) or in the postpolysomal supernatant (Fig. 8A, fraction 16), indicating that STRAP does not associate with polysomes. Next, total RNA was isolated from each fraction of WT and STRAP−/− MEFs and analyzed for the presence of collagen α1(I) and α2(I) mRNAs by RT-PCR. In WT MEFs, collagen α1(I) and α2(I) mRNAs were found on polysomes (fractions 1 to 8), as well as in fractions containing ribosomal subunits and in postpolysomal supernatant (Fig. 8B; for quantification, see Fig. S4 in the supplemental material). We verified this distribution of collagen mRNAs by quantitative real-time PCR (see Fig. 4C), and the results of quantitative RT-PCR were in good agreement with the results of our radiolabeled RT-PCR method. Such distribution of collagen mRNA was reported before (17, 19, 21). In STRAP−/− MEFs, the distribution of collagen α1(I) mRNA was similar to that in WT MEFs, suggesting that the efficacy of collagen α1(I) translation is not dramatically changed in the absence of STRAP. However, in STRAP−/− MEFs, collagen α2(I) mRNA was found almost exclusively in polysomal fractions and disappeared from the nonpolysomal fractions (Fig. 8B, middle; see also Fig. S4A in the supplemental material). This suggested that in the absence of STRAP, translation of collagen α2(I) mRNA is enhanced, and most available α2(I) mRNA is engaged in translation. The polysomal profiles of GAPDH mRNA were similar in STRAP−/− MEFs and WT MEFs (Fig. 8B, bottom; see also Fig. S4A and C in the supplemental material), indicating that STRAP specifically restricts translation of collagen α2(I) mRNA. This result is in agreement with a stronger interaction of eIF4A and collagen α2(I) mRNA in the absence of STRAP (Fig. 7B). We concluded from these experiments that STRAP restricts translation initiation of collagen α2(I) mRNA.
Fig 8.
Excessive translation of collagen α2(I) mRNA in the absence of STRAP. (A) STRAP does not associate with polysomes. Polysomes were isolated from WT MEFs, and fractions containing polysomes, monoribosome (80S), ribosomal subunits (60S and 40S), and postpolysomal supernatant (PPS) are indicated. The fractions were probed for the presence of endogenous STRAP and ribosomal protein L7 by Western blotting. (B) Excessive polysomal loading of collagen α2(I) mRNA in STRAP knockout cells. Polysomal fractions were isolated from WT and STRAP−/− MEFs (1110 and 1108) and were analyzed by RT-PCR for the presence of collagen α1(I) and α2(I) mRNA. GAPDH mRNA was analyzed as the control. (C) Supplementing STRAP reduces translation of collagen mRNAs in STRAP−/− MEFs. STRAP-Flag was expressed in WT and STRAP−/− MEFs using adenoviral transfer, and polysomal fractions were analyzed by RT-PCR for collagen α1(I), collagen α2(I), and GAPDH mRNAs.
If STRAP negatively regulates translation of collagen α2(I) mRNA, then reexpression of STRAP in the STRAP−/− cells should shift collagen mRNAs from polysomes into nonpolysomal fractions. Therefore, we supplemented STRAP in STRAP−/− MEFs and reanalyzed the polysomal profiles of collagen mRNAs (Fig. 8C; see also Fig. S4B in the supplemental material). The level of STRAP that was restored in the STRAP−/− MEFs was about 2-fold higher than its level in the WT MEFs (see Fig. S5 in the supplemental material), suggesting that close to WT conditions were established. Reexpression of STRAP in STRAP−/− MEFs resulted in redistribution of collagen α1(I) mRNA from polysomes into fractions containing ribosomal subunits and into postpolysomal supernatant. STRAP overexpression in WT MEFs also resulted in a shift of collagen α1(I) mRNA from polysomes (Fig. 8C, top; see also Fig. S4B in the supplemental material). Polysomal loading of collagen α2(I) mRNA in WT MEFs was unchanged by overexpression of STRAP and was similar to that in cells expressing normal levels of STRAP (WT; Fig. 8B and C, middle). However, in STRAP−/− MEFs, reexpression of STRAP shifted a significant fraction of collagen α2(I) mRNA into nonpolysomal fractions, indicating a partial inhibition of translation (Fig. 8C, middle; see also Fig. S4B in the supplemental material). The polysomal profile of GAPDH mRNA was not changed upon overexpression of STRAP (Fig. 8C, bottom). The polysomal profiles of collagen mRNAs and GAPDH mRNA were not changed upon transduction of MEFs with the control virus expressing GFP (see Fig. S3B and C in the supplemental material).
Altogether, these results indicate that STRAP, which is not associated with polysomes, restricts translation of collagen α2(I) mRNA in WT cells. This is consistent with the interpretation that STRAP regulates translation of collagen mRNAs by primarily inhibiting a default synthesis of α2(I) polypeptide.
Hyperglycosylation of collagen α1(I) polypeptide in the absence of STRAP.
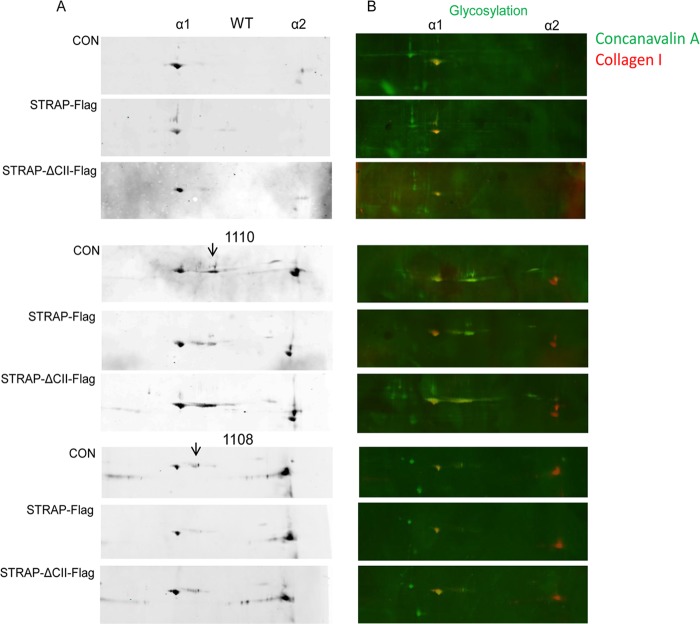
If synthesis of collagen α1(I) and α2(I) polypeptides is not strictly coordinated, the rate of folding of the polypeptides into a triple helix is limited by diffusion, and the individual polypeptides get hypermodified (32). This includes excessive hydroxylations and glycosylations of hydroxy lysines (52). We surmised that excessive accumulation of collagen polypeptides in the absence of STRAP may result in their hypermodifications. To analyze modifications of collagen polypeptides, we employed 2D SDS-PAGE, followed by Western blotting. Collagen α1(I) and α2(I) polypeptides have very different pIs (5 and 9, respectively), allowing their separation by 2D SDS-PAGE. When cellular extracts of WT MEFs were analyzed under reducing conditions, the Western blot revealed a single species representing α1(I) polypeptide and α2(I) polypeptide (Fig. 9A, top panel, top blot). The relative intensity of the signals was close to 2:1, as expected for the 2:1 ratio of the polypeptides in normal heterotrimeric type I collagen (53). The signals were distributed over a narrow pI range (dot-like), suggesting that collagen polypeptides are homogeneous molecular species. When STRAP was overexpressed in WT MEFs, expression of collagen polypeptides was reduced, but no change in the pI of collagen polypeptides was seen (Fig. 9A, top panel, middle blot). Overexpression of STRAP-ΔCII was used as the negative control, and the pI and expression of collagen polypeptides in STRAP-ΔCII cells were similar to that in control cells (Fig. 9A, top panel, bottom blot).
Fig 9.
Collagen modifications analyzed by 2D SDS-PAGE. (A) Intracellular collagen in WT and STRAP−/− MEFs (1110 and 1108). MEFs were transduced with control adenovirus (CON) or with adenovirus expressing STRAP-Flag or STRAPΔCII-Flag, and collagen was analyzed by 2D SDS-PAGE and Western blotting under reducing conditions. Positions of collagen α1(I) and α2(I) polypeptides are indicated at the top. Hypermodified collagen α1(I) polypeptides are indicated by arrows. (B) The blots in panel A were reprobed with biotin-labeled concanavalin A and IRDye-labeled streptavidin (green signal). This signal was overlaid over the Western blot signal (red for collagen). Yellow color indicates colocalization of collagen polypeptides and glycosylated proteins.
However, when either isolate of STRAP−/− MEFs (Fig. 8A, 1110 [middle] and 1108 [bottom]) was analyzed, α1(I) polypeptide was resolved as at least two species that differed in pI by approximately 1.3 pH units, indicating two different populations of molecules with the same molecular weight but different modifications (28). One population had a pI identical to that in WT cells, suggesting that they represent normal polypeptides; the other population was shifted to the more basic pI, suggesting excessive glycosylations (Fig. 9A, middle and bottom panels, top blots). It is known that glycosylations shift pI of a protein to the more basic region (54). In addition, expression of collagen α2(I) polypeptide was dramatically increased, and the ratio of α1(I) and α2(I) polypeptides was now inverted to 1:2. This increased expression of α2(I) polypeptide is consistent with unrestricted loading of collagen α2(I) mRNA on polysomes in STRAP−/− MEFs (Fig. 8B). We concluded from these results that without STRAP, collagen α2(I) polypeptide is overproduced and that the imbalance between synthesis of α1(I) and α2(I) polypeptides results in inefficient folding into the triple helix and in excessive posttranslational modifications of α1(I) polypeptide. This supports the hypothesis that STRAP is required for coordinated translation of collagen polypeptides.
If the observed changes in collagen modifications are due to lack of STRAP, then it should be possible, at least partially, to restore the normal collagen synthesis by supplementing STRAP in the STRAP knockout cells. Therefore, we expressed STRAP in STRAP−/− MEFs by adenoviral transfer and reanalyzed collagen polypeptides by 2D SDS-PAGE (Fig. 9A, middle and bottom panels, middle blots). When STRAP was reexpressed, the amount of hypermodified collagen α1(I) polypeptides was reduced by about 50% in both isolates of STRAP−/− cells. This indicated that it is possible to partially restore normal synthesis of α1(I) polypeptide by supplementing STRAP. However, the excessive synthesis of α2(I) polypeptide could not be corrected. This has already been hinted by experiments shown in Fig. 8C, middle, where reexpressing STRAP in STRAP−/− MEFs did not restrict polysomal loading of collagen α2(I) mRNA. As a control, we supplemented STRAP-ΔCII, the mutant which cannot interact with LARP6. This mutant could not correct the hypermodifications of the α1(I) polypeptide, as they were similar to that in nonsupplemented STRAP−/− cells (Fig. 9A, middle and bottom panels, bottom blots). The total levels of collagen α1(I), collagen α2(I), and GAPDH mRNAs in WT MEFs overexpressing STRAP were similar, confirming that STRAP affects translation and not the steady-state levels of collagen mRNAs (see Fig. S1 in the supplemental material).
To verify that hypermodifications of α1(I) polypeptide are glycosylations, we reprobed the blot with concanavalin A, a protein that binds carbohydrates (47). The western blot detecting collagen signal (red) was overlaid with concanavalin A signal (green). This analysis showed that collagen α1 polypeptide is glycosylated (yellow signal) and that the shift in pI of this peptide to the more basic region in STRAP−/− cells may have been due to excessive glycosylations (Fig. 9B, right). We could not detect glycosylations of the collagen α2(I) polypeptide. Either this polypeptide is not glycosylated in MEFs or concanavalin A cannot bind to the carbohydrates in this peptide due to a steric hindrance.
STRAP enhances secretion of the collagen trimer by STRAP−/− cells.
We have observed inefficient secretion of the collagen trimer into the cellular medium by STRAP−/− cells (Fig. 5B). To assess if STRAP can correct the defect in secretion of trimeric collagen from STRAP−/− MEFs, we compared the amounts of secreted collagen trimers from STRAP−/− MEFs and from the same cells supplemented with STRAP (Fig. 10). While WT MEFs exclusively secreted trimeric collagen, both isolates of STRAP−/− MEFs secreted only trace amounts of the trimer but large amounts of monomers. When STRAP was reexpressed, the amount of trimers secreted was dramatically increased, although the monomers were still present. As a control, STRAP-ΔCII was unable to stimulate secretion of the trimers (Fig. 10, top). Overexpression of STRAP did not affect the secretion of fibronectin, suggesting that STRAP specifically enhanced secretion of the trimeric type I collagen.
Fig 10.
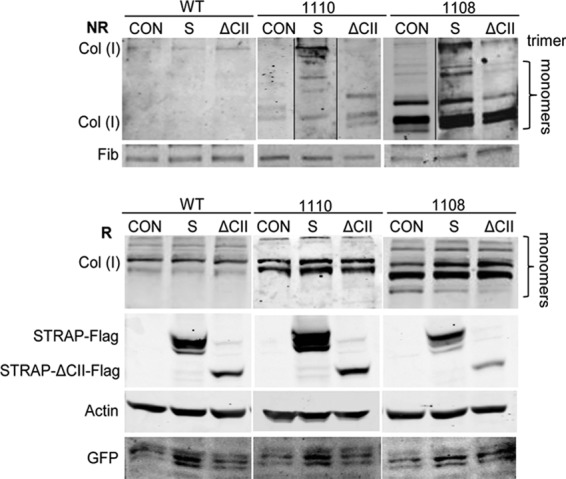
STRAP rescues secretion of the collagen trimer from STRAP−/− MEFs. STRAP-Flag (S) or STRAP-ΔCII-Flag (ΔCII) was overexpressed in WT and STRAP−/− MEFs (1110 and 1108) by adenoviral transfer, and the cellular medium was analyzed by Western blotting under nonreducing conditions (NR; top). CON are cells infected with the control virus. Migration of collagen trimer and monomers is indicated. Fibronectin (FIB) was used as the loading control. Bottom, expression of collagen and transfected proteins in the cellular extract, analyzed by Western blotting under reducing conditions (R). Loading control, actin; GFP, marker of equal adenoviral infection.
Taken together, these results suggest that STRAP is an integral component of the mechanism that regulates coordinated translation of collagen α1(I) and α2(I) mRNAs and that ensures proper modifications of polypeptides and their efficient folding and secretion as a triple helix.
DISCUSSION
Biosynthesis of type I collagen is unique among the synthesis of complex proteins, because it requires balanced production of two polypeptides and their folding in the ratio of 2:1 into a heterotrimeric molecule (2). At the same time, folding of collagen homotrimers must be prevented, because collagen α1(I) polypeptides have a natural propensity to form homotrimers (24, 27, 53). In addition, individual polypeptides have to be posttranslationally modified, and the rate of modifications is in a dynamic equilibrium with the rate of folding (28, 30). A highly coordinated collagen biosynthetic pathway appeared in evolution with the appearance of vertebrates to enable the synthesis of strictly heterotrimeric type I collagen (1). The key regulatory element in this process is the 5′SL, which is highly conserved in all vertebrate collagen α1(I) and α2(I) mRNAs (11, 14). 5′SL binds LARP6 in a sequence-specific manner, and LARP6, by interacting with other proteins, integrates the mechanism of coordinated production of collagen polypeptides (9, 17, 19, 21, 24, 55). Here, we report that (i) LARP6 interacts with STRAP and this interaction requires the last 27 amino acids of LARP6, termed the STRAP binding sequence (SBS), (ii) STRAP does not directly bind collagen mRNAs, but it is tethered to collagen mRNAs by LARP6, (iii) STRAP restricts uncontrolled translation of collagen α2(I) mRNA by decreasing its association with eIF4A, (iv) STRAP knockout cells hypermodify collagen α1(I) polypeptide and synthesize disproportional amount of α2(I) polypeptide, which can be partially corrected by supplementing STRAP, and (v) poor secretion of collagen trimers from STRAP knockout cells can also be rescued by supplementing STRAP. Together, these results show that STRAP is involved in coordinating translation of collagen α1(I) and α2(I) mRNAs to ensure production of correctly modified and folded type I collagen.
STRAP has been found in two ribonucleoprotein complexes containing either Unr or Gemin 7 (33, 34, 56). Here, we describe that it is also found in a ribonucleoprotein complex containing collagen mRNAs. All three proteins that interact with STRAP, LARP6, Unr, and Gemin 7, share a conserved stretch of amino acids, the SBS (Table 2). We could not identify other proteins with a highly similar motif. Therefore, it is likely that the SBS sequence in the other two STRAP binding proteins also binds STRAP. LARP6 binds STRAP directly, as suggested by cloning STRAP using the yeast two-hybrid screen.
LARP6 (17) interacts with eIF4A but not with eIF4E (Fig. 6A; see also Fig. S2B in the supplemental material), while STRAP is recruited to the complex of LARP6 and eIF4A via LARP6 (Fig. 6B and C). STRAP is not found on polysomes (Fig. 8A) but associates with collagen mRNAs in vivo and is found in a complex with LARP6 and 5′SL RNA in vitro (Fig. 2, 3, and 4). This indicates that a ternary complex of LARP6, STRAP, and collagen α1(I) or α2(I) mRNA can form. This probably takes place prior to or during translation initiation. The LARP6-STRAP complex specifically inhibits recruitment of eIF4A to collagen α2(I) mRNAs, because in the cells lacking STRAP, α2(I) mRNA immunoprecipitates more efficiently with anti-eIF4A antibody (Fig. 7B) and is better loaded onto the polysomes (Fig. 8B). There is little effect of STRAP on the interaction of collagen α1(I) mRNA with eIF4A. The reason for this is not entirely clear but may be due to binding of another RNA binding protein, αCP, to the C-rich sequence in the 3′ UTR of α1(I) mRNA. This sequence is absent in α2(I) mRNA. Binding of αCP stabilizes collagen α1(I) mRNA (15), but αCP can also stimulate translation (57, 58). Thus, having two cis-acting sequences, the 5′SL and the C-rich 3′ UTR sequence, recruitment of eIF4A to collagen α1(I) mRNA may depend less on the STRAP activity.
When STRAP is reexpressed in STRAP knockout cells, a fraction of both collagen mRNAs shifts from polysomes into nonpolysomal fractions (Fig. 8C), supporting the conclusion that STRAP restricts translation of collagen mRNAs. The unrestricted translation of collagen mRNAs in STRAP−/− cells results in hypermodifications of collagen α1(I) polypeptides and disproportionally increased synthesis of collagen α2(I) polypeptide (Fig. 9A and B). In addition, STRAP−/− cells poorly secrete collagen trimer but secrete an excessive amount of collagen monomers (Fig. 5B). This points to a lack of coordination in synthesis of α1(I) and α2(I) polypeptides (15, 31). All these defects can be partially corrected by supplementing STRAP in the STRAP knockout cells (Fig. 9 and 10). Since STRAP regulates translation initiation on collagen mRNAs by modulating the amount of available eIF4A, it appears that translation regulation is the critical step (40) that determines if the cells will excrete normal type I collagen or the hypermodified individual collagen polypeptides. LARP6 serves as an adapter protein in this process and STRAP as an effector protein.
The N-terminal domain of STRAP, which contains the WD repeats, interacts with TGF-β receptor I, while its C-terminal domain is phosphorylated by the TGF-β receptor II (36). TGF-β is the major profibrotic cytokine, which stimulates type I collagen synthesis at transcriptional and posttranscriptional levels (7, 59). It remains to be investigated how the phosphorylation of STRAP by the TGF-β receptor relates to collagen synthesis in fibrosis, but our work suggests that TGF-β may regulate translation of collagen mRNAs through phosphorylation of STRAP by the activated TGF-β receptor II.
In conclusion, we have identified a novel interaction of LARP6 and STRAP. This interaction tethers STRAP to the 5′ UTR of collagen mRNAs, where STRAP restricts the association of eIF4A with collagen mRNAs and their random translation. This mechanism is critical for synthesis of properly modified collagen polypeptides and their productive folding and excretion as the type I collagen heterotrimer. These findings provide a new insight into the complex biosynthesis of this essential protein.
Supplementary Material
ACKNOWLEDGMENTS
Special thanks go to P. Soriano for kindly providing us with WT and STRAP−/−MEFs, to N. Sonenberg for generously providing eIF4A- and eIF4E-containing plasmids, to Y. Kato for appreciated assistance with qRT-PCR, to V. Marin for technical support with 2D SDS electrophoresis, to A. Challa, R. Rizkalah, L. Stefanovic, and Y. Zhang for laboratory help and valuable discussions of the manuscript, and to T. Megraw for valuable comments on the manuscript.
This work was supported by National Institutes of Health grant 2R01DK059466-07A2 to B.S.
Footnotes
Published ahead of print 5 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00195-13.
REFERENCES
- 1.Gelse K, Poschl E, Aigner T. 2003. Collagens—structure, function, and biosynthesis. Adv. Drug Deliver. Rev. 55:1531–1546 [DOI] [PubMed] [Google Scholar]
- 2.Shoulders MD, Raines RT. 2009. Collagen structure and stability. Annu. Rev. Biochem. 78:929–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitterman PB, Henke CA. 1991. Fibroproliferative disorders. Chest 99:S81–S84 [DOI] [PubMed] [Google Scholar]
- 4.Huang CY, Ogawa R. 2012. Fibroproliferative disorders and their mechanobiology. Connect. Tissue Res. 53:187–196 [DOI] [PubMed] [Google Scholar]
- 5.Forlino A, Cabral WA, Barnes AM, Marini JC. 2011. New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 7:540–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parapia LA, Jackson C. 2008. Ehlers-Danlos syndrome—a historical review. Br. J. Haematol. 141:32–35 [DOI] [PubMed] [Google Scholar]
- 7.Penttinen RP, Kobayashi S, Bornstein P. 1988. Transforming growth factor-beta increases messenger-RNA for matrix proteins both in the presence and in the absence of changes in messenger-RNA stability. Proc. Natl. Acad. Sci. U. S. A. 85:1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, Shegogue D, Hatamochi A, Yamazaki S, Trojanowska M. 2004. Lysophosphatidic acid inhibits TGF-beta-mediated stimulation of type I collagen mRNA stability via an ERK-dependent pathway in dermal fibroblasts. Matrix Biol. 23:353–361 [DOI] [PubMed] [Google Scholar]
- 9.Challa AA, Stefanovic B. 2011. A novel role of vimentin filaments: binding and stabilization of collagen mRNAs. Mol. Cell. Biol. 31:3773–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. 1997. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol. Cell. Biol. 17:5201–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanovic B, Hellerbrand C, Brenner DA. 1999. Regulatory role of the conserved stem-loop structure at the 5′ end of collagen alpha 1(I) mRNA in hepatic stellate cells. Mol. Cell. Biol. 19:4334–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rippe RA, Stefanovic B. 2005. Methods for assessing the molecular mechanisms controlling gene regulation. Methods Mol. Med. 117:141–160 [DOI] [PubMed] [Google Scholar]
- 13.Rippe RA, Brenner DA. 2004. From quiescence to activation: gene regulation in hepatic stellate cells. Gastroenterology 127:1260–1262 [DOI] [PubMed] [Google Scholar]
- 14.Stefanovic B, Hellerbrand C, Brenner DA. 1999. Regulatory role of the conserved stem-loop structure at the 5 ′ end of collagen alpha 1(I) mRNA. Mol. Cell. Biol. 19:4334–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanovic B, Lindquist J, Brenner DA. 2000. The 5′ stem-loop regulates expression of collagen alpha1(I) mRNA in mouse fibroblasts cultured in a three-dimensional matrix. Nucleic Acids Res. 28:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanovic B, Schnabl B, Brenner DA. 2002. Inhibition of collagen alpha 1(I) expression by the 5 ′ stem-loop as a molecular decoy. J. Biol. Chem. 277:18229–18237 [DOI] [PubMed] [Google Scholar]
- 17.Cai L, Fritz D, Stefanovic L, Stefanovic B. 2010. Binding of LARP6 to the conserved 5′ stem-loop regulates translation of mRNAs encoding type I collagen. J. Mol. Biol. 395:309–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolin SL, Cedervall T. 2002. The La protein. Annu. Rev. Biochem. 71:375–403 [DOI] [PubMed] [Google Scholar]
- 19.Cai L, Fritz D, Stefanovic L, Stefanovic B. 2010. Nonmuscle myosin-dependent synthesis of type I collagen. J. Mol. Biol. 401:564–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Challa AA, Vukmirovic M, Blackmon J, Stefanovic B. 2012. Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS One 7:e42989. 10.1371/journal.pone.0042989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manojlovic Z, Stefanovic B. 2012. A novel role of RNA helicase A in regulation of translation of type I collagen mRNAs. RNA 18:321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada Y, Mudryj M, de Crombrugghe B. 1983. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J. Biol. Chem. 258:14914–14919 [PubMed] [Google Scholar]
- 23.Kozak M. 1987. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 196:947–950 [DOI] [PubMed] [Google Scholar]
- 24.Lindquist JN, Marzluff WF, Stefanovic B. 2000. Fibrogenesis. III. Posttranscriptional regulation of type I collagen. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G471–G476 [DOI] [PubMed] [Google Scholar]
- 25.Kivirikko KI, Myllyharju J. 1998. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 16:357–368 [DOI] [PubMed] [Google Scholar]
- 26.Eberhardt ES, Panasik N, Raines RT. 1996. Inductive effects on the energetics of prolyl peptide bond isomerization: implications for collagen folding and stability. J. Am. Chem. Soc. 118:12261–12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veis A, Brownell AG. 1977. Triple-helix formation on ribosome-bound nascent chains of procollagen: deuterium-hydrogen exchange studies. Proc. Natl. Acad. Sci. U. S. A. 74:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck K, Boswell BA, Ridgway CC, Bachinger HP. 1996. Triple helix formation of procollagen type I can occur at the rough endoplasmic reticulum membrane. J. Biol. Chem. 271:21566–21573 [DOI] [PubMed] [Google Scholar]
- 29.Bulleid NJ, Dalley JA, Lees JF. 1997. The C-propeptide domain of procollagen can be replaced with a transmembrane domain without affecting trimer formation or collagen triple helix folding during biosynthesis. EMBO J. 16:6694–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamande SR, Bateman JF. 1999. Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Semin. Cell Dev. Biol. 10:455–464 [DOI] [PubMed] [Google Scholar]
- 31.Stefanovic B, Stefanovic L, Schnabl B, Bataller R, Brenner DA. 2004. TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis. Mol. Cell. Biol. 24:1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman JF, Golub SB. 1994. Deposition and selective degradation of structurally abnormal type I collagen in a collagen matrix produced by osteogenesis imperfecta fibroblasts in vitro. Matrix Biol. 14:251–262 [DOI] [PubMed] [Google Scholar]
- 33.Hunt SL, Hsuan JJ, Totty N, Jackson RJ. 1999. Unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Gene Dev. 13:437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa C, Usui K, Ito F, Itoh M, Hayashizaki Y, Suzuki H. 2009. Role of survival motor neuron complex components in small nuclear ribonucleoprotein assembly. J. Biol. Chem. 284:14609–14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halder SK, Anumanthan G, Maddula R, Mann J, Chytil A, Gonzalez AL, Washington MK, Moses HL, Beauchamp RD, Datta PK. 2006. Oncogenic function of a novel WD-domain protein, STRAP, in human carcinogenesis. Cancer Res. 66:6156–6166 [DOI] [PubMed] [Google Scholar]
- 36.Datta PK, Moses HL. 2000. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol. Cell. Biol. 20:3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashikar ND, Reiner J, Datta A, Datta PK. 2010. Serine threonine receptor-associated protein (STRAP) plays a role in the maintenance of mesenchymal morphology. Cell. Signal. 22:138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WSV, Delrow J, Corrin PD, Frazier JP, Soriano P. 2004. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat. Genet. 36:304–312 [DOI] [PubMed] [Google Scholar]
- 39.Yamada NA, Castro A, Farber RA. 2003. Variation in the extent of microsatellite instability in human cell lines with defects in different mismatch repair genes. Mutagenesis 18:277–282 [DOI] [PubMed] [Google Scholar]
- 40.Fukao A, Sasano Y, Imataka H, Inoue K, Sakamoto H, Sonenberg N, Thoma C, Fujiwara T. 2009. The ELAV protein HuD stimulates cap-dependent translation in a poly(A)- and eIF4A-dependent manner. Mol. Cell 36:1007–1017 [DOI] [PubMed] [Google Scholar]
- 41.He TC, Zhou SB, da Costa LT, Yu J, Kinzler KW, Vogelstein B. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 95:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CY, Xu N, Shyu AB. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choe LH, Dutt MJ, Relkin N, Lee KH. 2002. Studies of potential cerebrospinal fluid molecular markers for Alzheimer's disease. Electrophoresis 23:2247–2251 [DOI] [PubMed] [Google Scholar]
- 45.Gorg A, Weiss W, Dunn MJ. 2004. Current two dimensional electrophoresis technology for proteomics. Proteomics 4:3665–3685 (Erratum, 5:826–827, 2005) [DOI] [PubMed] [Google Scholar]
- 46.Gorg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. 2000. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 21:1037–1053 [DOI] [PubMed] [Google Scholar]
- 47.Champoux JA, Ambroz KLH, Hwang JB, Volcheck WM, Schutz-Geshwender AR. 2006. Glycoprotein detection with the Odyssey infrared imaging system. LI-COR Biosciences, Lincoln, NE [Google Scholar]
- 48.Stefanovic B, Brenner DA. 2003. 5′ Stem-loop of collagen alpha 1(I) mRNA inhibits translation in vitro but is required for triple helical collagen synthesis in vivo. J. Biol. Chem. 278:927–933 [DOI] [PubMed] [Google Scholar]
- 49.Gingras AC, Raught B, Sonenberg N. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913–963 [DOI] [PubMed] [Google Scholar]
- 50.Jackson RJ, Hellen CUT, Pestova TV. 2010. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Bio. 11:113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prevot D, Darlix JL, Ohlmann T. 2003. Conducting the initiation of protein synthesis: the role of eIF4G. Biol. Cell 95:141–156 [DOI] [PubMed] [Google Scholar]
- 52.Tajima S, Takehana M, Azuma N. 1994. Production of overmodified type I procollagen in a case of osteogenesis imperfecta. J. Dermatol. 21:219–222 [DOI] [PubMed] [Google Scholar]
- 53.Veis A, Kirk TZ. 1989. The coordinate synthesis and cotranslational assembly of type I procollagen. J. Biol. Chem. 264:3884–3889 [PubMed] [Google Scholar]
- 54.Packer NH, Ball MS, Devine PL. 1999. Glycoprotein detection of 2-D separated proteins. Methods Mol. Biol. 112:341–352 [DOI] [PubMed] [Google Scholar]
- 55.Cai L, Fritz D, Stefanovic L, Stefanovic B. 2009. Coming together: liver fibrosis, collagen mRNAs and the RNA binding protein. Expert Rev. Gastroenterol. Hepatol. 3:1–3 [DOI] [PubMed] [Google Scholar]
- 56.Grimmler M, Otter S, Peter C, Muller F, Chari A, Fischer U. 2005. Unrip, a factor implicated in cap-independent translation, associates with the cytosolic SMN complex and influences its intracellular localization. Hum. Mol. Genet. 14:3099–3111 [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Kiledjian M, Weiss IM, Liebhaber SA. 1995. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol. Cell. Biol. 15:1769–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paulding WR, Czyzyk-Krzeska MF. 1999. Regulation of tyrosine hydroxylase mRNA stability by protein-binding, pyrimidine-rich sequence in the 3′-untranslated region. J. Biol. Chem. 274:2532–2538 [DOI] [PubMed] [Google Scholar]
- 59.Bhogal RK, Stoica CM, McGaha TL, Bona CA. 2005. Molecular aspects of regulation of collagen gene expression in fibrosis. J. Clin. Immunol. 25:592–603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.