Abstract
Background
There are limited data about the role of chemotherapy in patients with
advanced chondrosarcomas.
Methods
The medical charts of 180 patients with advanced chondrosarcomas having received chemotherapy in 15 participating institutions between 1988 and 2011 were reviewed.
Results
Median age was 52 years. Sixty-three percent of patients had conventional chondrosarcoma and 88% had metastatic disease. Combination chemotherapy was delivered in 98 cases (54.5%). One hundred and thirty-one patients (73%) received an anthracycline-containing regimen. Using RECIST, the objective response rate was significantly different according to histological subtype, being 31% for mesenchymal chondrosarcoma, 20.5% for dedifferentiated chondrosarcoma, 11.5% for conventional chondrosarcoma and 0% for clear-cell chondrosarcoma (P = 0.04). Median progression-free survival (PFS) was 4.7 months [95% confidence interval (CI) 3–6.5]. Performance status (PS) ≥2, number of metastatic sites ≥1 and single-agent regimen were independently associated with poor PFS. Median overall survival (OS) was 18 months (95% CI 14.5–21.6). PS, number of metastatic sites and palliative surgery were independently associated with OS.
Conclusions
Conventional chemotherapy have very limited efficacy in patients with advanced chondrosarcoma, the highest benefit being observed in mesenchymal and dedifferentiated chondrosarcoma. These data should be used as a reference for response and outcome in the assessment of investigational drugs in advanced chondrosarcoma.
Keywords: chondrosarcoma, chemotherapy, prognosis, treatment
introduction
Chondrosarcoma is the most common bone sarcoma in adults. Several histological subtypes have been described, including conventional, dedifferentiated, mesenchymal and clear-cell chondrosarcoma [1].
Conventional chondrosarcomas represent ∼85% of all chondrosarcomas and can be categorized according to their location in bone into primary central and secondary peripheral chondrosarcomas. Histological grade is the most powerful prognostic factor for metastatic relapse which is observed in about 70% of cases of grade 3 [1, 2]. Moreover, recurrent case may exhibit a higher grade of malignancy than the original lesion increasing therefore the risk of metastasis.
Dedifferentiated chondrosarcoma represent 9%–10% of all chondrosarcomas and is characterized from a histological point of view by conventional low-grade chondrosarcoma with abrupt transition to foci that have dedifferentiated into a higher grade, more aggressive component [1]. Patients with dedifferentiated chondrosarcoma are older than those with conventional lesions. Metastatic relapse is very frequent and the prognosis is poor with a 5-year survival rate <10%–25% [1–3].
Mesenchymal chondrosarcoma and clear-cell chondrosarcoma represent two rare variants of chondrosarcoma occurring in younger patients than for conventional or dedifferentiated chondrosarcoma [1]. Local and distant recurrences of mesenchymal chondrosarcomas are frequent while clear-cell chondrosarcoma is a low-grade malignant tumor with unfrequent metastatic relapse.
Surgery is the cornerstone of the primary management of chondrosarcoma. There is no standard treatment of patients with advanced disease. In fact, due to the rarity of this condition, data regarding the outcome of patients with advanced disease and the potential impact of chemotherapy are almost nonexistent. The aim of this multi-institutional, retrospective study was to address these specific points.
methods and patients
patients
This study was based on retrospectively retrieved data from medical records of patients with unresectable and/or metastatic chondrosarcoma who were treated at 15 European and American institutions. These institutions are major referral centers for sarcomas and agreed to cooperate for this study. Inclusion criteria were: age ≥18 years, histologically proven conventional, dedifferentiated, mesenchymal or clear-cell chondrosarcoma, unresectable and/or metastatic disease, and first-line systemic treatment with a cytotoxic agent started between 1988 and 2011. In all cases, the histological diagnosis was established according to the World Health Organization Classification of Tumours [1] by an expert pathologist.
treatments and evaluation
The best response to treatment was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) [4]. Routine follow-up was similar across all centers. Progression-free survival (PFS) was defined as the time from the start of treatment until disease progression, death or last patient contact. Overall survival (OS) was defined as the time from the start of treatment until death or last patient contact.
statistical analysis
The cut-off date for statistical analysis of baseline demographic data and clinical outcome was the 31 August 2012. Survival rates were estimated using the Kaplan–Meier method. Descriptive statistics were used to show the distribution of variables in the population. Differences between groups were evaluated by Chi square test or Fisher's exact test for categorical variables and Student's test for continuous variables. Prognostic factors were planned to be identified by univariate and multivariate analyses using a Cox regression model. Variables tested in univariate analysis included: age, gender, histological subtype, grade, number of metastatic sites, performance status (PS), single-agent versus combination therapy, anthracycline-containing regimen versus anthracycline-free regimen and surgical resection of metastatic disease. Variables associated with PFS and OS with a P-value <0.05 in the univariate analysis were planned to be included in the multivariate analysis. Analyses were carried out using SPSS 18.0 statistical software (IPSS, Inc., Chicago, IL, USA). All statistical tests were two sided, and P < 0.05 indicated statistical significance.
results
patients
One hundred eighty patients were included in this study. Their characteristics are described in Table 1.
Table 1.
Patients' characteristics (n = 180)
| n | % | |
|---|---|---|
| Age at diagnosis (years) | ||
| Median | 52 | |
| Range | 17–84 | |
| Gender | ||
| Male | 112 | 62.0 |
| Female | 68 | 28.0 |
| Histological subtype | ||
| Conventional chondrosarcoma | 113 | 63.0 |
| Dedifferentiated chondrosarcoma | 42 | 23.5 |
| Mesenchymal chondrosarcoma | 17 | 9.5 |
| Clear-cell chondrosarcoma | 2 | 1.0 |
| Unknown | 6 | 3.0 |
| Grade | ||
| 1 | 15 | 8.5 |
| 2 | 86 | 46.0 |
| 3 | 62 | 36.0 |
| Unknown | 17 | 9.0 |
| PS ECOG | ||
| 0 | 52 | 29.0 |
| 1 | 71 | 39.5 |
| 2 | 26 | 14.5 |
| 3 | 1 | 0.5 |
| Unknown | 30 | 16.5 |
| Number of metastatic sites | ||
| 0 | 22 | 12.0 |
| 1 | 106 | 59.0 |
| ≥2 | 52 | 29.0 |
| Sites of metastasis | ||
| Lung | 135 | 75.0 |
| Bone | 40 | 22.0 |
| Liver | 8 | 4.5 |
| Other | 39 | 21.5 |
treatments
Combination chemotherapy was delivered in 98 cases (54.5%) and single agent in 82 cases (45.5%), respectively. One hundred thirty-one patients (73%) received an anthracycline-containing regimen. Chemotherapy regimens are described in Table 2.
Table 2.
Chemotherapy regimens in patients with chondrosarcomas (n = 180)
| Protocol | Drugs | n (%) |
|---|---|---|
| Anthracycline-containing regimen (n = 131) | ||
| Doxorubicin | Doxorubicin 50–75 mg/m2 21 days cycle | 32 (18) |
| Pegylated liposomal doxorubicin | Pegylated liposomal doxorubicin 40–50 mg/m2 28 days cycle | 9 (5) |
| AI | Doxorubicin 20–25 mg/m2 (d1–d3) Ifosfamide 2.5–3 g/m2 (d1–d3) 21 days cycle | 34 (19) |
| API | Doxorubicin 60 mg/m2 (d1) Ifosfamide 5 g/m2 (d1) Cisplatin 100 mg/m2 (d2) 21 days cycle | 20 (11) |
| AP | Doxorubicin 60 mg/m2 (d1) Cisplatin 80–100 mg/m2 (d1–d3) 21 days cycle | 21 (11.5) |
| Others regimens | Doxorubicin + miscellaneous cytotoxic or investigational agent | 15 (8.5) |
| Nonanthracycline regimen (n = 49) | ||
| Ifosfamide | Ifosfamide 5–9 g/m2(d1) 21 days cycle | 8 (4.5) |
| IP | Ifosfamide 2.5 g/m2/day (d1–d3) Cisplatin 80–100 mg/m2 (d1) 21 days cycle | 6 (3) |
| Gemcitabine | Gemcitabine 1000 mg/m2 d1, d8, d15 28 days cycle | 8 (4.5) |
| Others regimens | Imatinib, sirolimus-cyclophosphamide, trabectedin, docetaxel–gemcitabine or investigational agents | 27 (15) |
response
One hundred sixty-three patients were assessable for response. Using RECIST, 2 patients (1%) had complete response, 22 (14%) had partial response and 67 (41%) had stable disease. Progressive disease was observed in 72 (45%) patients. No objective response was observed in patients with grade 1 disease. Moreover, the objective response rate was significantly different according to histological subtype, being 31% for mesenchymal chondrosarcoma, 20.5% for dedifferentiated chondrosarcoma, 11.5% for conventional chondrosarcoma and 0% for clear-cell chondrosarcoma (P = 0.04). The objective response rate was also higher for patients treated with combination therapy than for patients treated with single agent, although this difference did not reach significance (20% versus 11%, P = 0.09).
progression-free survival
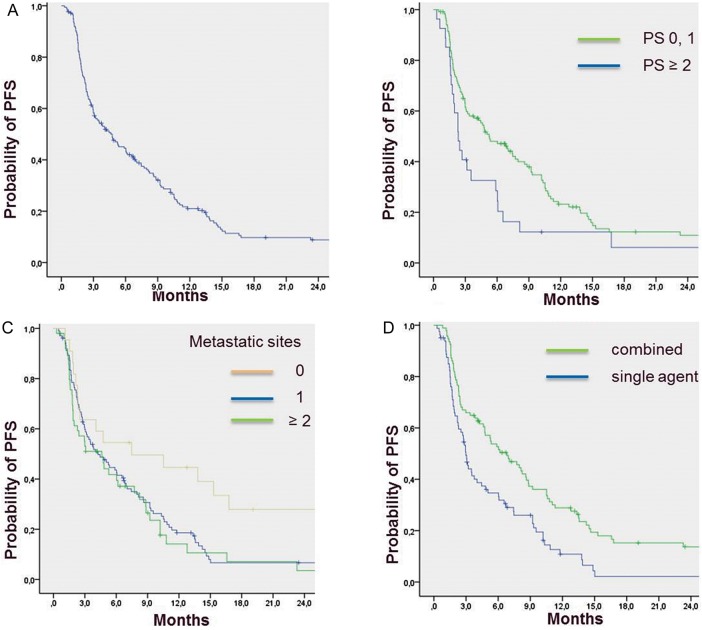
The median follow-up was 38 months (range 28–48 months). Median PFS was 4.7 months [95% confidence interval (CI) 3–6.4] (Figure 1). The 3-month, 6-month and 1-year PFS rates were 58% (95% CI 51–65), 44.5% (95% CI 37–52) and 21% (95% CI 15–27), respectively. On univariate analysis, age, number of metastatic sites, PS and type of chemotherapy (combination therapy versus single agent) were significantly associated with PFS (Table 3). On multivariate analysis, PS, number of metastatic sites and combination therapy remained independently associated with PFS (Table 4, Figure 1).
Figure 1.
Progression-free survival of the entire cohort of patients (A), and according to performance status (PS) (B), number of metastatic sites (C) and type of chemotherapy regimen (D).
Table 3.
Significant prognostic factors on progression-free survival and overall survival (univariate analysis n = 180)
| Variables | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Median (months) | 95% CI | P | Median (months) | 95% CI | P | |
| Whole population | ||||||
| 4.7 | [3–6.5] | – | 18 | [14.5–21.6] | – | |
| Age (years) | ||||||
| ≤52 | 7.7 | [4.5–11] | 0.002 | 22.3 | [18.1–26.6] | 0.04 |
| >52 | 3.1 | [2.3–4] | 13.7 | [10.8–16.6] | ||
| Grade | ||||||
| 1 | – | – | NS | 27.8 | [8–47.7] | 0.006 |
| 2 | 22.3 | [17.4–27.3] | ||||
| 3 | 12.2 | [8.8–15.7] | ||||
| Number of metastatic sites | ||||||
| 0 | 7.5 | [0–16.9] | 0.02 | 35.5 | [3.6–67.4] | 0.006 |
| 1 | 4.3 | [2.5–6] | 19.9 | [14.7–25.1] | ||
| ≥2 | 4.6 | [2.2–7] | 10.4 | [5–15.8] | ||
| Performance status | ||||||
| ≤1 | 5.2 | [3.1–7.4] | 0.04 | 23.4 | [19.1–27.7] | <0.0001 |
| ≥2 | 2.3 | [1.7–2.9] | 8.6 | [2.3–15] | ||
| Combined chemotherapy | ||||||
| Yes | 6.8 | [4.4–9.2] | <0.0001 | – | – | NS |
| No | 3 | [2.6–3.4] | ||||
| Palliative surgery | ||||||
| Yes | – | – | – | 27.8 | [15.2–40.5] | 0.01 |
| No | 15.1 | [11–19.2] | ||||
CI, confidence interval; NS, not significant.
Table 4.
Significant prognostic factors on progression-free survival and overall survival (multivariate analysis n = 180)
| Variables | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Performance status | ||||||
| ≤1 | 1 | 0.02 | 1 | <0.0001 | ||
| ≥2 | 1.7 | [1.1–2.8] | 3.1 | [1.9–5.2] | ||
| Number of metastatic sites | ||||||
| 0 | 1 | 1 | ||||
| 1 | 2.3 | [1.2–4.3] | 0.008 | 3.7 | [1.7–7.8] | 0.001 |
| 2 | 2.5 | [1.3–4.9] | 0.007 | 5 | [2.4–10.6] | <0.0001 |
| Combined chemotherapy | ||||||
| Yes | 1 | 0.006 | – | – | – | |
| No | 1.7 | [1.2–2.4] | ||||
| Palliative surgery | ||||||
| Yes | – | – | – | 1 | 0.004 | |
| No | 1.9 | [1.2–3.1] | ||||
CI, confidence interval; HR, hazard ratio; NS, not significant.
overall survival
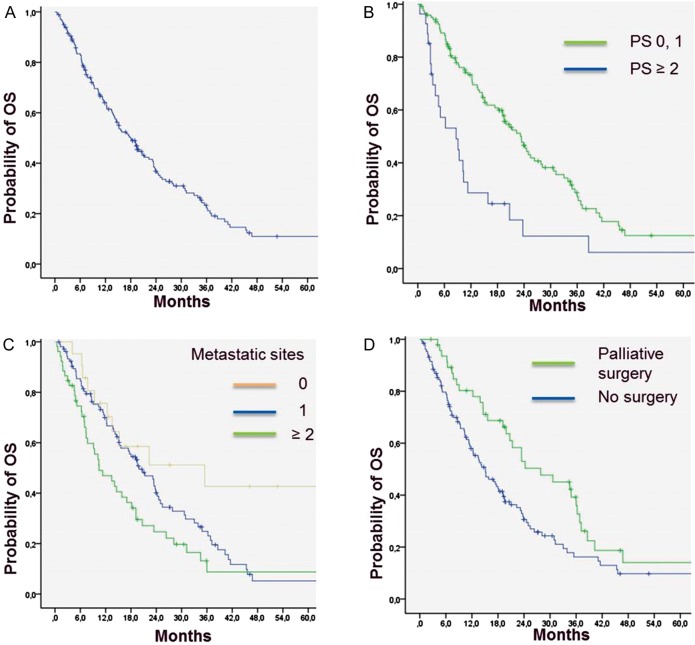
At the time of analysis, 133 patients (74%) had died and 47 (26%) were still alive. The median OS for the entire cohort of patients was 18 months (95% CI 14.5–21.5). The 6-month, 1-year and 2-year OS rates were 83% (95% CI 77–88), 64% (95% CI 57–71) and 37% (95% CI 30–44), respectively. On univariate analysis, age, PS, grade, number of metastatic sites and surgery of metastases were significantly associated with OS (Table 3). On multivariate analysis, PS, number of metastatic sites and surgery of metastases remained independently associated with OS (Table 4, Figure 2).
Figure 2.
Overall survival of the entire cohort of patients (A), and according to performance status (PS) (B), number of metastatic sites (C) and palliative surgery (D).
patient care after first-line chemotherapy
After completion of first-line chemotherapy, 116 patients (88 patients in the anthracycline group, 18 in the nonanthracycline group) received at least one new line of chemotherapy. Several types of chemotherapy regimen were used. Nine of these patients (five in the anthracycline group) had objective response on second-line therapy. None of them had objective response on first-line treatment. Regimens associated with objective response on second-line treatment were gemcitabine-based combination (n = 3), dacarbazine (n = 2), oral etoposide (n = 1), ifosfamide (n = 1), ifosfamide-etoposide (n = 1) and temozolomide-irinotecan (n = 1).
discussion
We report here the first large series of chondrosarcoma patients treated with chemotherapy in the advanced setting.
In this study, the treatment characteristics are dominated by a very large majority of anthracycline-containing regimen administered (73%). The second most frequently used agent was cisplatin. Preclinical data regarding the sensitivity of chondrosarcoma cells to anticancer agents are scarce. A recent study has shown that although some chondrosarcoma cell lines may display some sensitivity to doxorubicin, resistance to cisplatin was the rule [5]. Interestingly, chondroid matrix does not hinder doxorubicin from entering the nuclei of the tumor cells. In our series, we did not find a significant difference in terms of objective response rate between patients treated with or without anthracyclines. However, none of the patients of the nonanthracyline group received cisplatin as a single agent. Indeed, a majority of them were managed with a regimen containing drugs that have shown activity in other soft-tissue or bone sarcomas such as ifosfamide or gemcitabine. There are no preclinical data regarding the activity of these two drugs in chondrosarcoma. Our results showing the occurrence of objective response in the first-line setting as well as in the second-line setting in patients with primary resistance to anthracyclines suggest that they may have a potential although modest clinical activity.
Our results suggest that chemotherapy may be more effective in mesenchymal chondrosarcoma, and in dedifferentiated chondrosarcoma than in conventional chondrosarcoma. The response rate we found in dedifferentiated chondrosarcomas was similar to that observed in a previous series of patients treated in the neoadjuvant setting (15%) [6]. However, the objective response rate we observed in patients with mesenchymal chondrosarcoma was somewhat lower than that reported by the CWS and COSS study groups showing an objective tumor response in four of seven children and young adult patients treated also in the neoadjuvant setting [7]. In these small studies as well as in our series, various combinations of various drugs were prescribed. We found that combination chemotherapy was associated with a higher objective response rate and PFS, but did not improve OS. This in line with what is also reported in the field of soft-tissue sarcomas [8–13]. This underscores the crucial need for further collaborative efforts in order to identify the most effective chemotherapy regimens for patients with advanced chondrosarcoma and particularly those with potentially chemosensitive subtypes.
The median OS of patients with advanced chondrosarcoma included in our series was poor (18 months) indicating that such a disease is not an indolent one. Interestingly, we found that surgery of metastases was significantly associated with improved outcome. This is in line with several reports suggesting that resection of pulmonary and extrapulmonary metastases is associated with long-term survival in soft-tissue sarcoma patients [14]. Surgery or alternative locoregional treatments of metastases are now considered as an option in selected patients with advanced soft-tissue sarcomas [15]. Our results suggest that this may represent also a therapeutic approach in chondrosarcoma patients with resectable metastasis. However, as for soft-tissue sarcomas, the design of our study is not randomized. Therefore, we cannot exclude that survival improvement was not related to metastasectomy per se but to disease's biology and natural history.
One other aim of this study was to provide data that could be used as a reference for response and outcome in the assessment of investigational drugs in chondrosarcomas. There is indeed an urgent need for improved therapeutics in chondrosarcomas. One strategy consists in targeting the hedgehog pathway. Indeed, several studies have shown activation of hedgehog signaling in chondrosarcoma. Two in vitro and in vivo studies showed that hedgehog inhibitors (triparanol and IPI926) blocked chondrosarcoma cell proliferation in cell culture and also in xenografted human chondrosarcoma cell tumors [16, 17]. These results represented the rationale for one phase II trial we have conducted on behalf on the French Sarcoma Group and which assessed the activity of the hedgehog inhibitor GDC-0449 in patients with advanced chondrosarcoma [18]. GDC-0449 did not meet the primary endpoint of this trial. However, results suggested some activity in a subset of patients with progressive grade 1 or 2 conventional chondrosarcoma. Further studies assessing its role in combination with chemotherapy are warranted. Several other therapeutic targets are currently under investigation. For instance, mTOR inhibitors have shown preclinical efficacy in an orthotopic rat grade II chondrosarcoma model [19]. A clinical trial is under discussion. Another strategy may consist in interacting with the peroxisome proliferator-activated receptor-γ (PPARγ), which is highly expressed in chondrosarcoma cells. Preclinical data have shown that PPAR-γ activation results in the suppression of chrondrosarcoma proliferation and induction of apoptosis by regulating the Bcl-2 family of proapoptotic and antiapoptotic proteins [20]. New generation of PPAR-γ agonist is currently under investigation in early phase clinical trials. Such drugs may be worth for testing in chondrosarcoma patients. Other studies have suggested also that chondrosarcomas may be sensitive to hormonal therapy [21], histone deacetylase inhibitors [22] or angiogenesis inhibitors [23]. These preliminary but highly interesting data warrant further studies.
In conclusion, we showed here that conventional chemotherapy have very limited efficacy in patients with advanced chondrosarcoma, the highest benefit being observed in mesenchymal and dedifferentiated chondrosarcoma. On multivariate analysis, the number of chemotherapeutic agents used in a regimen (single agent versus combination) did not correlate with improved OS. Our results should serve as a reference for response and outcome in the assessment of new investigational drugs.
funding
This work was supported by French National Cancer Institute (INCa) (INCa-DGOS-Inserm 6046).
disclosure
The authors have declared no conflicts of interest.
references
- 1.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2002. pp. 247–258. [Google Scholar]
- 2.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri M, Picci P, Campanacci L, et al. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995;24:409–416. doi: 10.1007/BF00941235. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.van Oosterwijk JG, Herpers B, Meijer D, et al. Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann Oncol. 2012;23:1617–1626. doi: 10.1093/annonc/mdr512. [DOI] [PubMed] [Google Scholar]
- 6.Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Dantonello TM, Int-Veen C, Leuschner I, et al. CWS study group; COSS study group. Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer. 2008;112(11):2424–2431. doi: 10.1002/cncr.23457. [DOI] [PubMed] [Google Scholar]
- 8.Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 9.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285. doi: 10.1200/JCO.1993.11.7.1276. [DOI] [PubMed] [Google Scholar]
- 10.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 11.Schoenfeld DA, Rosenbaum C, Horton J, et al. A comparison of adriamycin versus vincristine and adriamycin, and cyclophosphamide versus vincristine, actinomycin-D, and cyclophosphamide for advanced sarcoma. Cancer. 1982;50:2757–2762. doi: 10.1002/1097-0142(19821215)50:12<2757::aid-cncr2820501211>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Borden EC, Amato DA, Edmonson JH, et al. Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer. 1990;66:862–867. doi: 10.1002/1097-0142(19900901)66:5<862::aid-cncr2820660509>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 14.Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open. 2012;2(5):pii: e001736. doi: 10.1136/bmjopen-2012-001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii92–vii99. doi: 10.1093/annonc/mds253. [DOI] [PubMed] [Google Scholar]
- 16.Tiet TD, Hopyan S, Nadesan P, et al. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am J Pathol. 2006;168:321–330. doi: 10.2353/ajpath.2006.050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell V, Puviindran Nadesan P, Wang Y, et al. Direct targeting of the hedgehog pathway in primary chondrosarcoma xenografts with the Smoothened inhibitor IPI-926. Cancer Res. 2011;71(Suppl 1) AM2011-LB-380. [Google Scholar]
- 18.Italiano A, Le Cesne A, Bellera C, et al. GDC-0449 In Patients With Advanced Chondrosarcomas: A French Sarcoma Group/ US And French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol. 2013;24:2922–2926. doi: 10.1093/annonc/mdt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez J, Decouvelaere AV, Pointecouteau T, et al. Inhibition of chondrosarcoma growth by mTOR inhibitor in an in vivo syngeneic rat model. PLoS One. 2012;7:e32458. doi: 10.1371/journal.pone.0032458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida K, Kunisada T, Shen ZN, et al. Chondrosarcoma and peroxisome proliferator-activated receptor. PPAR Res. 2008;2008:250568. doi: 10.1155/2008/250568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleton-Jansen AM, van Beerendonk HM, Baelde HJ, et al. Estrogen signaling is active in cartilaginous tumors: implications for antiestrogen therapy as treatment option of metastasized or irresectable chondrosarcoma. Clin Cancer Res. 2005;11:8028–8035. doi: 10.1158/1078-0432.CCR-05-1253. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Tanaka K, Sakimura R, et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 2008;28:1585–1591. [PubMed] [Google Scholar]
- 23.Klenke FM, Abdollahi A, Bertl E, et al. Tyrosine kinase inhibitor SU6668 represses chondrosarcoma growth via antiangiogenesis in vivo. BMC Cancer. 2007;7:49. doi: 10.1186/1471-2407-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]