Abstract
Background
Vitamin D deficiency is common in HIV infection and has been associated with advanced disease. This study investigated whether vitamin D related genetic variants were associated with disease progression in HIV-infected children.
Methods
The Fok-I (C/T), Bsm-I (G/A), GC (A/C), DHCR7 (G/T) and CYP2R1 (G/A) genetic variants were detected by RT-PCR in HIV-infected children who participated in the PACTG P152 and P300 protocols which pre-dated the availability of effective combination antiretroviral therapy. The primary endpoints included time to progression to the first HIV-related disease end-point (≥2 OI's, weight-growth failure) or death, which constituted the progression-free-survival. Analyses were performed for age >2 years and ≤2 years separately adjusting for race and treatment effect.
Results
Of the 998 children evaluated, 139 experienced HIV disease progression. For children >2 years, rapid disease progression was associated with the DHCR7 G allele compared to the T allele (G/G vs. T/T: HR=5.0, p=0.035, G/T vs. T/T: HR=4.5, p=0.042, G/G+G/T vs. T/T: HR=4.8, p=0.036), and the Bsm-I A allele compared to the G allele (A/G vs. G/G: HR=2.2, p=0.014 and A/G+A/A vs. G/G: HR=2.0, p=0.026). In children ≤2 years, the Bsm-I A allele increased the risk of disease progression in Hispanics (A/A vs. G/A+G/G: HR=2.8, p=0.03; A/A vs. G/G: HR=2.8, p=0.046) and whites (A/A vs. G/G: HR=6.6, p=0.025; A/A vs. G/A+G/G: HR=3.6, p=0.038).
Conclusions
Vitamin D related host genetic variants that alter the availability and activity of vitamin D are associated with risk of HIV disease progression in children, and may vary by age and race.
Keywords: Vitamin D, Human immunodeficiency virus, HIV, Vitamin D receptor, pediatric AIDS, host genetics
Vitamin D is traditionally associated with calcium homeostasis and bone mineralization, but recent work has shed light on its role as an important regulator of both innate and adaptive immunity (1-3). The immune modulating effects of vitamin D are mediated by the binding of biologically active 1,25 dihydroxy (OH) vitamin D to vitamin D receptors (VDRs) within macrophages, dendritic cells, neutrophils, B cells, and activated T lymphocytes (4,5). Vitamin D regulates the release of specific cytokines, modifies T lymphocyte proliferation and function, and increases the production of antimicrobial peptides like cathelicidin (3,6-8). Furthermore, vitamin D has been shown to increase autophagy, a cellular process utilized by immune cells to kill intracellular pathogens through increased phago-lysosomal fusion (9,10). In vitro studies have demonstrated that autophagy induced by physiological concentrations of 1,25(OH) vitamin D, leads to inhibition of human immunodeficiency virus type-1 (HIV) replication in HIV-infected macrophages (11). Low levels of vitamin D have been associated with increased susceptibility to several infectious diseases including HIV, and have been associated with worse outcomes in these patients (12-14).
Children, adolescents and adults who are infected with HIV have been reported to have a high prevalence of vitamin D deficiency (15-23). In large studies of European and North American HIV-infected adults, low levels of vitamin D (defined as 25 hydroxy vitamin D levels <30 ng/ml) were found in 89% and 70.3% of patients, respectively (15,16). Children infected with HIV were found to have a similarly high prevalence of vitamin D insufficiency and deficiency (17,20). Low levels of both 25(OH) vitamin D and biologically active 1,25(OH) vitamin D have been associated with advanced clinical stage of HIV infection, lower CD4 counts and increased mortality (16,23,24). In HIV infected pregnant women not receiving HAART, vitamin D deficiency was associated with progression to World Health Organization HIV stage III or greater, severe anemia and all cause mortality (13). Infants born to these mothers had a significantly higher risk of acquiring HIV infection during the perinatal and postnatal period, and were more likely to die during follow-up regardless of HIV infection status (25). Furthermore, these infants had an increased risk of stunting and being underweight (26).
Factors associated with low vitamin D levels in HIV infection include obesity, black or Hispanic race, exposure to HIV drugs like efavirenz, renal insufficiency, darker skin pigmentation, higher latitude, inadequate vitamin D dietary intake and lower exposure to ultraviolet light (15,19,20,23,27). Host genetic variants associated with low serum 25(OH) vitamin D levels have been described in large genome-wide association studies but not in HIV-infected persons (28-30). Genetic variants that lead to altered activity of vitamin D, however, have been reported in adult HIV-infected intravenous drug users and include single nucleotide polymorphisms (SNPs) in the vitamin D receptor (VDR) gene (31-34). Taken together these findings suggest that factors that alter the availability or function of biologically active vitamin D are important in determining susceptibility to HIV infection and in predicting the rate of progression to advanced disease.
The present study investigated the role of five vitamin D related host genetic variants (GC [group-specific component (vitamin D binding) protein], Fok-I, Bsm-I, DHCR7 and CYP2R1) in HIV disease progression in a cohort of HIV-infected children who participated in the Pediatric AIDS Clinical Trials Group (PACTG) P152 and P300 protocols that pre-dated the availability of effective combination antiretroviral therapy (35,36).
Methods
Patient population
The PACTG protocols, P152 and P300, were multicenter, prospective, randomized, double blind placebo controlled studies that assessed the efficacy of mono- or dual-nucleoside reverse transcriptase inhibitor (NRTI) treatment regimens in symptomatic HIV infected children in North America, prior to the availability of effective combination therapy (35,36). Subjects were eligible to participate if they had received less than 8 weeks of prior anti-retroviral therapy (ART). The P300 protocol assessed the efficacy and safety of combination zidovudine/lamuvudine compared with either didanosine (ddI) monotherapy or combination zidovudine/ddI. The P152 protocol assessed the efficacy of treatment with zidovudine alone compared to either ddI monotherapy or combination zidovudine/ddI. Stored DNA samples from children in both studies (n=998) were screened for the presence of vitamin D related genetic variants.
Baseline characteristics of the subjects, eligibility criteria, study end points, markers of disease progression, and neuropsychological tests used have been previously described (35,36). Informed consent was obtained from the parents or legal guardians of study participants, and the study followed the human experimentation guidelines of the US Department of Health and Human Services and of the institutional review board of the University of California, San Diego. The use of specimens for genetic testing was approved by the IMPAACT Network. All specimens were given a patient identification number during the original studies and were processed without any access to or knowledge of study participants.
Genotyping
Five vitamin D related single nucleotide polymorphisms (SNPs) were detected using real-time PCR with melting curve analysis (Lightcycler; Roche, Indianapolis, Indiana) as described previously (37). Two SNPs within the vitamin D receptor gene rs1544410 (Bsm-I G/A) and rs2228570 (Fok-I C/T) were investigated. These SNPs were selected for their identified functional effects on VDR transcriptional efficiency (rs2228570) and VDR messenger RNA stability (rs1544410). Furthermore, these SNPS have been associated with increased susceptibility to HIV infection and rapid HIV disease progression in adults (31-34). Three additional SNPs that influence vitamin D synthesis: rs12785878 (DHCR7/NADSYN1 G/T), vitamin D transport: rs2282679 (GC A/C) and vitamin D hydroxylation: rs10741657 (CYP2R1 G/A) were also studied (28-30).
The Fok-I SNP which occurs in the translation initiation codon of the vitamin D receptor gene was detected using a 338 base pair (bp) fragment that was amplified by the forward primer 5′-CCAGCTATGTAGGGCGAATC-3′ and the reverse primer 5′-CCTTCACAGGTCATAGCATTGA-3′. The fluorescein sensor probe sequence was 5′-TTCTTACAGGGACGGAGGCA-3′ and the LCR640 (Light Cycler Red 640 fluorophore) anchor probe sequence was 5′-TGGCGGCCAGCACTTCCCTG-3′. The Bsm-I SNP which occurs in the 3′ untranslated region (UTR) of the vitamin D receptor gene was detected using a 150 bp fragment that was amplified by the forward primer 5′-TAGGGGGGATTCTGAGGAACTA-3′ and the reverse primer 5′-AGTTTTGTACCCTGCCCGC-3′. The fluorescein sensor probe sequence was 5′-AGTATTGGGAATGCGCAGGCC-3′ and the LCR640 anchor probe (LCR640 – Light Cycler Red 640 fluorophore) sequence was 5′-TCTGTGGCCCCAGGAACCCTG-3′. The GC (rs2282679) SNP on chromosome 4p12 was detected using a 192 bp fragment that was amplified by the forward primer 5′-TGACCTTGTGATCCACCC-3′ and the reverse primer 5′-CTGATTCTACACTGTGAGCCA-3′. The fluorescein sensor probe sequence was 5′-ACCTGGCTTTGTGAGATAATTAAGAG-3′ and the LCR640 anchor probe sequence was 5′-CAGAGATTTGCTGGGCATGGTG-3′. The rs10741657 SNP located near the CYP2R1 gene on chromosome 11p15 was detected using a 279 bp fragment that was amplified by the forward primer 5′-AGAGGGAAGAGCAATGACATG-3′ and the reverse primer 5′-TTAAGCCATCAGATTGGTGG-3′. The fluorescein sensor probe sequence was 5′-ACAGCCCTCGCCTGCTAAAG-3′ and the LCR640 anchor probe sequence was 5′-ATCTCCCCAACCACCAGGCGTTT-3′. The rs12785878 SNP which is located within an intron in the DHCR7/NADSYN1 locus on chromosome 11q12 was detected using a 128 bp fragment that was amplified by the forward primer 5′-TCACCTAAGTGCCAAGGGA-3′ and the reverse primer 5′-GTCAGGCTCACGAGACGAT-3′. The fluorescein sensor probe sequence was 5′-CTTCTATCCTCTCCTGGCCCCG-3′ and the LCR640 anchor probe sequence was 5′-GGCCGGATCTTCTCCTGGGCT-3′.
Statistical Analysis
The primary end points for the analyses were either time to progression to the first clinical HIV-related disease end-point or death, which constituted the progression-free-survival (PFS). Criteria for disease progression included weight-growth failure and ≥2 opportunistic infections (OI). In the P152 study, weight-growth failure was defined as a weight-growth velocity under the third percentile at week 24 or two consecutive 6-month growth velocities under the third percentile after more than 24 weeks of study follow-up. In the P300 study, weight growth failure was defined as three consecutive months with less than the third percentile for age-specific and gender-specific 6-month weight growth velocities. Children with an acute illness or explainable cause of abrupt weight loss (overweight children on a diet, cases of neglect or food deprivation) during the week preceding the time of weight calculation were not assigned this endpoint. A significant number of children who reached the weight-growth failure endpoint before the other endpoints (death, OI) died shortly afterwards. All-cause mortality and weight-growth failure were investigated because vitamin D deficiency has been associated with increased mortality (25), stunting and growth failure (26) in HIV infected children. A summary of end points by age group (age >2 years and ≤2 years) is listed in Table 1.
Table 1. Baseline Characteristics and Outcome by Age.
| Characteristic | Total (N=998) |
Age ≤2 (N=461) |
Age >2 (N=537) |
P-Value | |
|---|---|---|---|---|---|
| Gender | male | 455 (46%) | 213 (46%) | 242 (45%) | 0.750* |
| female | 543 (54%) | 248 (54%) | 295 (55%) | ||
| Race | Black | 607 (61%) | 284 (62%) | 323 (60%) | 0.430* |
| Hispanic | 247 (25%) | 106 (23%) | 141 (26%) | ||
| White/Other | 144 (14%) | 71 (15%) | 73 (14%) | ||
| Age (years) at randomization | Mean (s.d.) | 3.78 (3.84) | 0.90 (0.49) | 6.25 (3.75) | |
| Min, Max | 0.12, 17.98 | 0.12, 1.99 | 2.00, 17.98 | ||
| Median | 2.34 | 0.78 | 5.24 | ||
| baseline CD4 count/mm3 | Mean (s.d.) | 978 (838) | 1,391 (997) | 627 (430) | <.001** |
| Median | 775 | 1,259 | 583 | ||
| baseline CD4 percent | Mean (s.d.) | 23.7 (12.0) | 26.7 (12.4) | 21.2 (11.0) | <.001** |
| Median | 24.0 | 26.8 | 22 | ||
| baseline plasma HIV RNA copies/ml | Mean (s.d.) | 865,899 (2,458,260) | 1,669,496 (3,410,391) | 177,358 (490,909) | <.001** |
| Median | 139,738 | 470,317 | 53,709 | ||
| Months of follow-up for progression | Mean (s.d.) | 19.36 (10.61) | 19.23 (10.35) | 19.47 (10.84) | 0.868** |
| Min, Max | 0.23, 46.71 | 0.23, 46.71 | 0.33, 45.92 | ||
| Median | 18.36 | 18.19 | 18.39 | ||
| Primary endpoint = 1, else 0 | 0 | 859 (86%) | 372 (81%) | 487 (91%) | <.001* |
| 1 | 139 (14%) | 89 (19%) | 50 (9%) | ||
| Primary end points(death, opportunistic infection (OI), weight growth failure) | Death | 48 (5%) | 44 (10%) | 4 (1%) | |
| ≥2 OIs | 8 (1%) | 4 (1%) | 4 (1%) | ||
| Weight Growth Failure | 83 (8%) | 41 (9%) | 42 (8%) |
Fisher's Exact Test
Wilcoxon Test
The analyses of the association between the vitamin D related genetic variants and time to disease progression (i.e. PFS) were performed using Cox Proportional Hazards models. As age and race are known to be associated with serum vitamin D levels (38,39), the analyses were done for age >2 years and ≤2 years separately adjusting for race and treatment effect. Baseline variables like CD4 count, CD4 percentage and HIV viral load were not included in the adjusted analyses as vitamin D has been reported to modulate innate immune responses to HIV infection and decrease HIV viral replication (11). For allelic variants with significant genotype*race interaction (p<0.1), individual Cox proportional hazard models were fitted to each race separately to evaluate the hazard ratios between genotypes/genotype combinations.
Results
Characteristics of the children
Of the 998 children included in the analysis, 543 (54%) were female. The majority were classified as non-Hispanic black (61%), followed by Hispanic (25%) and non-Hispanic white (14%). The subjects' ages ranged from 1 month to 18 years with a median age of 2.3 years. Median baseline CD4 count was 775 cell/mm3 (1259 cell/mm3 for age ≤2 years and 583 cell/mm3 for age >2 years, P<0.001) and median CD4 percent was 24% (27% for age ≤2 years and 22% for age >2 years, P<0.001). The median baseline log HIV RNA viral load was 5.15 copies/ml (5.67 copies/ml for age ≤2 years and 4.73 copies/ml for age >2 years, P<0.001). Children were followed for signs of disease progression over a median period of 18.3 months (range 0.2 months to 4 years). During this time, 139 (14%) children developed a study defined clinical endpoint; (89 (19%) in children age ≤2 years and 50 (9%) in those >2 years). Weight-growth failure which is an important clinical sign of HIV disease progression in children, accounted for a significant number of the clinical endpoints reached, especially in children >2 years of age (84%). Characteristics of the children are summarized in Table 1.
Distribution of Vitamin D genotypes
The distributions of vitamin D related genotypes were similar between sex and age groups (≤2 years and >2 years), but not race/ethnicity. In children >2 years, black children were more likely to be GG homozygous for Bsm-I (rs1544410), GG homozygous for DHCR7 (rs12785878), AA homozygous for GC (rs2282679), and CC homozygous for Fok-I (rs2228570) than Hispanic and white children. In children ≤2 years, blacks and Hispanics were more likely to be GG homozygous for CYP2R1 (rs10741657), GG homozygous for DHCR7, and AA homozygous for GC (rs2282679) than white children. Apart from the higher prevalence of the AA genotype reported for the GC (rs2282679) SNP, all the other genotypes with high allelic frequencies in black and Hispanic children have been associated with either low vitamin D levels (28-30), or increased risk of HIV disease progression (31-34). Genotype distributions by age and race are presented in Table 2.
Table 2. Genotype Distributions for the Vitamin D SNPs.
| Age ≤2 years | Age >2 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | All | Hispanic | Black | White/Other | All | Hispanic | Black | White/Other | |||
| SNP | Minor Allele (Frequency) | Genotype | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) |
| All Subjects | 988 (100) | 461(46.2) | 106(23.0) | 284(61.6) | 71(15.4) | 537(53.8) | 141(26.3) | 323(60.1) | 73(13.6) | ||
| Bsm-I b | A (31.8%) | A/A | 102(10.2) | 48(10.4) | 12(11.3) | 23(8.1) | 13(18.3) | 54(10.1) | 17(12.1) | 26(8.1) | 11(15.1) |
| rs1544410 | G/A | 431(43.3) | 194(42.1) | 45(42.5) | 123(43.3) | 26(36.6) | 237(44.3) | 66(46.8) | 125(38.9) | 46(63.0) | |
| G/G | 463(46.5) | 219(47.5) | 49(46.2) | 138(48.6) | 32(45.1) | 244(45.6) | 58(41.1) | 170(53.0) | 16(21.9) | ||
| CYP2R1 a | A (31.5%) | A/A | 127(12.7) | 52(11.3) | 10(9.4) | 30(10.6) | 12(16.9) | 75(14.0) | 19(13.5) | 43(13.4) | 13(17.8) |
| rs10741657 | A/G | 375(37.6) | 171(37.1) | 41(38.7) | 94(33.1) | 36(50.7) | 204(38.1) | 58(41.1) | 114(35.4) | 32(43.8) | |
| G/G | 495(49.6) | 238(51.6) | 55(51.9) | 160(56.3) | 23(32.4) | 257(47.9) | 64(45.4) | 165(51.2) | 28(38.4) | ||
| DHCR7 a b | T (34.7%) | G/G | 443(44.4) | 218(47.3) | 46(43.4) | 164(57.7) | 8(11.3) | 225(42.0) | 39(27.7) | 179(55.6) | 7(9.6) |
| rs12785878 | G/T | 416(41.7) | 183(39.7) | 45(42.5) | 101(35.6) | 37(52.1) | 233(43.5) | 67(47.5) | 125(38.8) | 41(56.2) | |
| T/T | 138(13.8) | 60(13.0) | 15(14.2) | 19(6.7) | 26(36.6) | 78(14.6) | 35(24.8) | 18(5.6) | 25(34.2) | ||
| Fok-I b | T (28.4%) | C/C | 516(51.7) | 246(53.4) | 52(49.1) | 164(57.7) | 30(42.3) | 270(50.3) | 57(40.4) | 189(58.5) | 24(32.9) |
| rs2228570 | C/T | 398(39.9) | 179(38.8) | 42(39.6) | 104(36.6) | 33(46.5) | 219(40.8) | 63(44.7) | 119(36.8) | 37(50.7) | |
| T/T | 84(8.4) | 36(7.8) | 12(11.3) | 16(5.6) | 8(11.3) | 48(8.9) | 21(14.9) | 15(4.6) | 12(16.4) | ||
| GC a b | C (14.6%) | A/A | 734(73.7) | 337(73.3) | 72(67.9) | 234(82.7) | 31(43.7) | 397(74.1) | 94(66.7) | 266(82.6) | 37(50.7) |
| rs2282679 | A/C | 233(23.4) | 112(24.3) | 33(31.1) | 44(15.5) | 35(49.3) | 121(22.6) | 39(27.7) | 51(15.8) | 31(42.5) | |
| C/C | 29(2.9) | 11(2.4) | 1(0.9) | 5(1.8) | 5(7.0) | 18(3.4) | 8(5.7) | 5(1.6) | 5(6.8) | ||
P<.05 (Chi-square test) for the difference of the proportions in race/ethnicity in age ≤2 years
P<.05 (Chi-square test) for the difference of the proportions in race/ethnicity in age >2 years
Association between disease status at study entry and Vitamin D related polymorphisms
At study entry, the DHCR7 G allele (related to lower serum vitamin D levels) was associated with lower HIV log10 RNA levels than the T allele (5.49 vs. 5.88 copies/ml, p=0.020) in all children ≤2 years of age. In all children >2 years of age, the GC (rs2282679) A allele was associated with higher HIV log10 RNA levels compared to the C allele (4.74 vs. 4.46 copies/ml, p=0.005) but this was not clinically significant. There were no significant associations between genotypes and other baseline variables including CD4+ lymphocyte count or CD4+ percentage. Additionally, no associations were found between genotypes and neurocognitive status at baseline or during follow-up.
Association between HIV disease progression and Vitamin D related polymorphisms in children ≤2 years
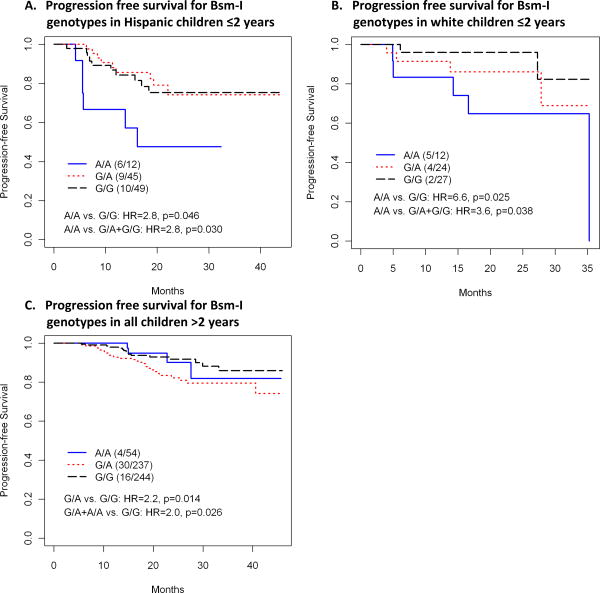
The Bsm-I A allele (associated with increased susceptibility to HIV infection) demonstrated an association with disease progression based on the identified race of the study participants. Due to the marginally significant genotype*race interaction (p=0.08), an individual Cox proportional model was fitted for each race. For children who were identified as Hispanic, an increased risk of disease progression was observed (A/A vs. G/G: HR=2.8, p=0.046; A/A vs. G/A+G/G: HR=2.8, p=0.03) for the A allele. Similarly, for children identified as white, the Bsm-I A allele was associated with disease progression (A/A vs. G/G: HR=6.6, p=0.025; A/A vs. G/A+G/G: HR=3.6, p=0.038) (Figure 1). In contrast, in children identified as black, non-Hispanic the A allele did not show an increased risk of disease progression.
Figure 1.
Kaplan Meier plots illustrating progression free survival for Bsm-I genotypes in (A) Hispanic children ≤2 years, (B) white children ≤2 years and (C) all children >2 years.
Association between HIV disease progression and Vitamin D related polymorphisms in children >2 years
In children >2 years the incidence of disease progression or death was lower than in children <2 years of age (9% of subjects versus 19% of subjects) (Table 1). Despite fewer endpoints, an effect was still observed for two of the SNPs, Bsm-I (rs1544410) and DHCR7 (rs12785878). The genotype*race interactions were not significant (P>0.1) for either of the two SNPS so an overall effect of the specific genotypes for each SNP was investigated, separately adjusting for race. In all children >2 years the Bsm-I A allele was associated with more rapid disease progression than the G allele (G/A vs. G/G: HR=2.2, p=0.014, and G/A+A/A vs. GG: HR=2.0, p=0.026) (Figure 1). The DHCR7 G allele (associated with lower serum vitamin D levels) was associated with rapid HIV disease progression compared to the T allele (G/G vs. T/T: HR=5.0, p=0.035; G/T vs. T/T: HR=4.5, p=0.042 and G/G+G/T vs. T/T: HR=4.8, p=0.036) (Figure 2).
Figure 2.
Kaplan Meier plots illustrating progression free survival for DHCR7 genotypes in (A) all children ≤2 years and (B) all children >2 years.
Discussion
Vitamin D insufficiency and deficiency is common in HIV infection and is associated with worse outcomes in adults and children. The causes of low vitamin D levels in HIV infection are multifactorial and include host genetic factors, factors directly associated with HIV infection and factors related to HIV therapy (15,23). In patients with low serum vitamin D levels, genetic variants that alter the availability of vitamin D or the function of VDR may further diminish the critical immune regulating effects of this hormone. To our knowledge, this is the first study that has investigated the role of vitamin D related genetic variants on HIV disease progression in children. Furthermore, we are unaware of any studies that have examined the effect of genetic variants that alter serum 25(OH) vitamin D levels on HIV disease progression.
The VDR gene is known to have over 60 single nucleotide polymorphisms (SNPs) affecting the promoter, coding and 3′ untranslated region (UTR) with functional effects (31,40). The VDR Fok-I T→C polymorphism alters the translation initiation codon site of the VDR gene and results in a shorter VDR that is thought to have increased transcriptional activity (4). The Fok-I polymorphism in the VDR gene is not in linkage disequilibrium with any other VDR polymorphism and is therefore considered an independent marker. When biologically active 1,25(OH) vitamin D binds to VDR the vitamin D/VDR complex acts as a transcription factor that directly interacts with the promoter regions of 1,25(OH) vitamin D responsive target genes (1,5). The Fok-I SNP has been associated with bone mineral density, prostate cancer risk, susceptibility to autoimmune disease and viral infections including HIV (41,42,31). Heterozygosity (C/T) at the Fok-I site has been associated with rapid HIV-1 disease progression (32) in HIV infected injection drug users, while the C allele has been associated with lower serum 25(OH) vitamin D levels (30,43). In the current study however, no significant associations between the Fok-I SNP and HIV disease progression in children were found.
In this study the Bsm-I A allele was associated with rapid HIV disease progression in all children older than 2 years of age and in Hispanic and white children <2 years of age. This finding is consistent with adult studies and suggests that the Bsm-I A allele is an important marker of HIV disease progression in patients of all ages and across different racial groups. The Bsm-I A allele did not appear to be associated with increased risk of pediatric HIV infection when allele frequencies were examined, but no comparison with an HIV uninfected cohort was made. The exact mechanism by which the Bsm-I G allele confers protection and the Bsm-I A allele increases risk of disease progression, is not well understood. The Bsm-I G→A polymorphism occurs in the 3′ UTR of the VDR gene and is thought to alter VDR messenger RNA stability and has been associated with increased susceptibility to HIV infection and higher rate of HIV disease progression in Caucasian adult injection drug users (31-34). Bsm-I is in strong linkage disequilibrium with other 3′ UTR polymorphisms (Apa-I, Taq-I) which have also been associated with HIV disease progression (40). As the Bsm-I polymorphism is a synonymous mutation, it is possible that linkage disequilibrium with one or more functional polymorphisms at other sites in the VDR gene may explain the associations observed (40). However, synonymous mutations rather than being “silent” may cause changes in protein expression, conformation and function; thus, the Bsm-1 polymorphisms could also directly alter the vitamin D receptor (44). The finding that the Bsm-I A allele was associated with disease progression in children older than 2 years but not in black, non-Hispanic children <2 years of age might be explained by differences in serum vitamin D levels. Infants and children <2 years of age were likely to have had higher vitamin D levels as they received formula fortified with vitamin D during the first year of life to avoid exposure to maternal breast-milk. Any effect of a genetic variant that lowered vitamin D levels or altered its function would be expected to be less pronounced in patients with higher vitamin D levels. Another factor to consider is that individual functional polymorphisms like Fok-I are expected to have similar functional effects in different ethnic groups while non-functional polymorphisms like Bsm-I may be in linkage disequilibrium with different functional alleles that may vary by race (40). This might explain some of the differences seen between black, white and Hispanic patients for the different genetic variants examined.
In addition to the VDR genetic variants, three SNPs identified in genome-wide association studies that were linked to low serum 25(OH) vitamin D levels were also studied. The presence of all three SNPS (GC (rs2282679), CYP2R1 and DHCR7) more than doubled the risk of vitamin D insufficiency in a large Caucasian cohort (28). A major limitation of the present study was that information on vitamin D consumption and nutritional intake was not available. Furthermore, patient serum was not available to measure vitamin D levels. Thus, the role of these genetic variants on serum vitamin D levels and the association between serum vitamin D levels and HIV disease progression could not be assessed. The weight-growth failure endpoint, which was reached by a significant number of children, required that a child be less than the third percentile for an extended period of time. While malnutrition may have contributed to this endpoint, it is more likely that advanced HIV disease caused this degree of failure to thrive especially given the high rate of subsequent mortality in these children.
The GC (rs2282679) A→C polymorphism in the vitamin D binding protein gene leads to lower serum vitamin D binding protein levels and may alter the affinity of the protein for vitamin D metabolites (28-30). No significant associations between the GC (rs2282679) SNP and HIV disease progression in children were found. The CYP2R1 G→A polymorphism is thought to alter the 25 hydroxylation of vitamin D with the G allele associated with lower serum 25(OH) vitamin D levels (28,29). There were no significant associations between this genetic variant and HIV disease progression in children.
The DHCR7 gene encodes the enzyme 7-dehydrocholesterol (DHC) reductase which converts 7-DHC to cholesterol. This activity removes the substrate (7-DHC) from the vitamin D synthetic pathway (28). The DHCR7 G allele is thought to lower concentrations of the precursor 7-DHC and has been associated with lower 25(OH) vitamin D levels (28,29). In all subjects ≤2 years of age the G allele was associated with marginally lower baseline HIV log RNA levels (5.49 versus 5.88). In children >2 years of age the G allele was significantly associated with rapid HIV disease progression, especially in Hispanic children. As mentioned earlier, children older than 2 years of age were more susceptible to having lower serum vitamin D levels than infants who received formula fortified with vitamin D during the first year of life. Therefore, the effect of the G allele would be more pronounced in older children who presumably had lower vitamin D levels.
The findings of this study and others demonstrate that vitamin D related genetic factors are important determinants of HIV related disease progression in adults and children. The exact mechanisms by which these genetic variants alter the availability and function of biologically active vitamin D are not fully understood, but likely impact both innate and adaptive immune responses to HIV infection. The recent discovery that physiological levels of 1,25 dihydroxy vitamin D increase autophagy in HIV infected macrophages with inhibition of HIV replication in a dose-dependent manner suggests that this critical innate defense mechanism might be sensitive to serum vitamin D levels (11). In a recently published prospective study, multivitamin supplementation (excluding vitamin D) of HIV exposed infants in Tanzania was not associated with an improvement in infant mortality, hospitalizations, clinic visits or diarrheal disease suggesting that associated micronutrient deficiencies may not be as important as vitamin D in contributing to somatic growth and other health outcomes in HIV exposed infants (45). This study provides additional support to the hypothesis that supplemental vitamin D may be of benefit to HIV-infected individuals with low serum vitamin D levels. Moreover, host genetics may play an important role in the optimal utilization of vitamin D and in determining the ideal serum concentration of vitamin D in individuals of different racial groups.
Acknowledgments
The authors would like to thank Rodney Trout and Justine Wong for their assistance with genotyping.
Sources of Support: This research was supported in part by National Institutes of Health grants R21AI084573, R01MH095585, the Thrasher Research Foundation and the International Maternal Perinatal Adolescent AIDS Clinical Trials (IMPAACT) Network. [Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).]
Footnotes
Conflicts of Interest: The authors have no conflicts of interest or funding to disclose Presented in part at CROI 2012, 19th Conference on Retroviruses and Opportunistic Infections, Seattle, March 5-8, 2012
References
- 1.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helming L, Bose J, Ehrchen J, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 4.van Etten E, Verlinden L, Giulietti A, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37(2):395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 5.Carlberg C, Polly P. Gene regulation by vitamin D3. Crit Rev Eukaryot Gene Expr. 1998;8(1):19–42. doi: 10.1615/critreveukargeneexpr.v8.i1.20. [DOI] [PubMed] [Google Scholar]
- 6.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49(1):26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 7.Rook GA, Steele J, Fraher L, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 8.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 9.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12(8):1026–1035. doi: 10.1111/j.1462-5822.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 11.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286(21):18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5(1):e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–446. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 15.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52(3):396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 16.Viard JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 17.Kakalia S, Sochett EB, Stephens D, et al. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159(6):951–957. doi: 10.1016/j.jpeds.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Vescini F, Cozzi-Lepri A, Borderi M, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58(2):163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 19.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24(11):1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 20.Stephensen CB, Marquis GS, Kruzich LA, et al. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83(5):1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 21.Haug C, Muller F, Aukrust P, et al. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J Infect Dis. 1994;169(4):889–893. doi: 10.1093/infdis/169.4.889. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez M, Daniels B, Gunawardene S, et al. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retroviruses. 2009;25(1):9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 23.Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24(8):1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 24.Haug CJ, Aukrust P, Haug E, et al. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab. 1998;83(11):3832–3838. doi: 10.1210/jcem.83.11.5270. [DOI] [PubMed] [Google Scholar]
- 25.Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200(7):1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein JL, Mehta S, Duggan C, et al. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatr Infect Dis J. 2012;31(2):171–175. doi: 10.1097/INF.0b013e318245636b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123(1):e121–6. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath JJ, Saha S, Burne TH, et al. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121(1-2):471–477. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 31.de la Torre MS, Torres C, Nieto G, et al. Vitamin D receptor gene haplotypes and susceptibility to HIV-1 infection in injection drug users. J Infect Dis. 2008;197(3):405–410. doi: 10.1086/525043. [DOI] [PubMed] [Google Scholar]
- 32.Nieto G, Barber Y, Rubio MC, et al. Association between AIDS disease progression rates and the Fok-I polymorphism of the VDR gene in a cohort of HIV-1 seropositive patients. J Steroid Biochem Mol Biol. 2004;89-90(1-5):199–207. doi: 10.1016/j.jsbmb.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 33.Barber Y, Rubio C, Fernandez E, et al. Host genetic background at CCR5 chemokine receptor and vitamin D receptor loci and human immunodeficiency virus (HIV) type 1 disease progression among HIV-seropositive injection drug users. J Infect Dis. 2001;184(10):1279–1288. doi: 10.1086/324000. [DOI] [PubMed] [Google Scholar]
- 34.Alagarasu K, Selvaraj P, Swaminathan S, et al. 5′ regulatory and 3′ untranslated region polymorphisms of vitamin D receptor gene in south Indian HIV and HIV-TB patients. J Clin Immunol. 2009;29(2):196–204. doi: 10.1007/s10875-008-9234-z. [DOI] [PubMed] [Google Scholar]
- 35.McKinney RE, Jr, Johnson GM, Stanley K, et al. A randomized study of combined zidovudine-lamivudine versusdidanosine monotherapy in children with symptomatic therapy-naive HIV-1 infection. The Pediatric AIDS Clinical Trials Group Protocol 300 Study Team. J Pediatr. 1998;133(4):500–508. doi: 10.1016/s0022-3476(98)70057-5. [DOI] [PubMed] [Google Scholar]
- 36.Englund JA, Baker CJ, Raskino C, et al. Zidovudine, didanosine, or both as the initial treatment for symptomatic HIV-infected children. AIDS Clinical Trials Group (ACTG) Study 152 Team. N Engl J Med. 1997;336(24):1704–1712. doi: 10.1056/NEJM199706123362403. [DOI] [PubMed] [Google Scholar]
- 37.Singh KK, Barroga CF, Hughes MD, et al. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188(10):1461–1472. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- 38.Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 39.Yetley EA, Pfeiffer CM, Schleicher RL, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140(11):2030S–45S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Gross C, Eccleshall TR, Malloy PJ, et al. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican-American women. J Bone Miner Res. 1996;11(12):1850–1855. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- 42.Chokkalingam AP, McGlynn KA, Gao YT, et al. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2001;61(11):4333–4336. [PubMed] [Google Scholar]
- 43.Smolders J, Damoiseaux J, Menheere P, et al. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol. 2009;207(1-2):117–121. doi: 10.1016/j.jneuroim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- 45.Duggan C, Manji KP, Kupka R, et al. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo controlled clinical trial. Am J Clin Nutr. 2012;96(6):1437–1446. doi: 10.3945/ajcn.112.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]