Abstract
The Shu complex, consisting of Rad51 paralogues, is an important regulator of homologous recombination, an error-free DNA repair pathway. Consequently, when members of this complex are disrupted, cells exhibit a mutator phenotype, sensitivity to DNA damage reagents and increased gross chromosomal rearrangements. Previously, we found that the Shu complex plays an important role in ribosomal DNA (rDNA) recombination when the Upstream Activating Factor (UAF) protein Uaf30 is disrupted. UAF30 encodes a protein needed for rDNA transcription and when deleted, rDNA recombination increases and the rDNA expands in a Shu1-dependent manner. Here we find using the uaf30-sensitized background that the central DNA repair protein Rad52, which is normally excluded from the nucleolus, frequently overlaps with the rDNA. This close association of Rad52 with the rDNA is dependent upon Shu1 in a uaf30 mutant. Previously, it was shown that in the absence of Rad52 sumoylation, Rad52 foci mislocalize to the nucleolus. Interestingly, here we find that using the uaf30 sensitized background the ability to regulate Rad52 sumoylation is important for Shu1 dependent rDNA recombination as well as Rad52 close association with rDNA. Our results suggest that in the absence of UAF30, the Shu complex plays a central role in Rad52 rDNA localization as long as Rad52 can be sumoylated. This discrimination is important for rDNA copy number homeostasis.
Keywords: DNA repair, UAF complex, Shu complex, Rad52, Rad52 sumoylation, rDNA recombination
1. Introduction
Accurate repair of DNA damage is essential to prevent mutations and genomic rearrangements and perturbations of this process are observed in cancer. Repair of repetitive DNA sequences are particularly difficult since their mis-repair can lead to expansions or contractions. One such element is the rDNA, whose organization is a multiple tandem array, a defining feature in most eukaryotic cells [1]. Mis-regulation of rDNA recombination has been implicated in both aging and cancer [1]. Maintenance of rDNA copy number is critical for genomic integrity. For example, decreases in rDNA repeats are associated with sensitivity to DNA damaging agents [2]. Therefore, cells have developed mechanisms to repair rDNA breaks to maintain rDNA copy number.
In yeast when the number of rDNA repeats are limiting, the replication fork block located within the rDNA itself can mediate double-strand break (DSB) formation and subsequent recombination for repair [1]. Each rDNA repeat is 9.1 kb long and present in approximately 100-200 copies [3-5]. Unequal sister chromatid exchange between the rDNA repeats can result in expansions as well as contractions of the rDNA [6, 7]. The UAF complex, which contains Rrn5, Rrn9, Rrn10, Uaf30, and histones H3 and H4, is important for promoting rDNA transcription by RNA polymerase I [8, 9]. Absence of UAF complex proteins results in slow growth due to rDNA being transcribed by RNA polymerase II transcription and subsequent increases in repeat number [8]. We previously described how disruption of the UAF complex protein uaf30 results in increased rDNA recombination that is mediated through the homologous recombination (HR) repair pathway [10]. Interestingly, we find that the hyper-rDNA recombination observed in uaf30Δ cells is dependent upon the Shu complex [10], a group of four proteins (Shu1, Shu2, Psy3, and Csm2) that promotes error-free DNA repair through HR [11-15].
Many components of the HR machinery are important for rDNA repair, including the central DNA repair protein Rad52. Surprisingly Rad52 is excluded from the nucleolus, but yet is still crucial for rDNA repair. Therefore a model has been proposed where Rad52-mediated recombination at the rDNA is regulated by moving the rDNA broken ends out of the nucleolus where Rad52 repair centers, observed as foci, can be visualized [16]. In addition, HR at the rDNA is largely dependent upon Rad52 sumoylation and the Smc5-Smc6 complex [16].
Here we report that the absence of UAF30, which alters rDNA structure, leads to the close association of Rad52 to the rDNA, a region of the nucleus from which it is normally excluded. We show that in uaf30 mutant cells, Shu1 promotes Rad52 association with the rDNA. Importantly, Shu1 is critical in regulating Rad52 localization with respect to the rDNA as long as Rad52 can be sumoylated. Furthermore, the ability of Shu1 to regulate rDNA recombination is contingent upon Rad52 localization. Our results suggest a model where the Shu complex promotes rDNA recombination by enabling Rad52 to access the rDNA.
2. Material and methods
2.1. Strains, plasmids, and media
The strains used in this study are listed in Table 1. They are isogenic with W303 and derived from the RAD5+ strains W1588-1C and W5909-1B [17, 18]. Standard procedures were used for making crosses, tetrad dissection, and yeast transformation (LiOAc method) [19]. The media was prepared as described, except twice the amount of leucine was added [19].
Table 1. Yeast Strains and Plasmids.
| Name | Description |
|---|---|
| W5909-1B | MATa TRP1 ADE2 his3-11,15 leu2-3,112 ura3-1 lys2Δ RAD5 |
| W1588-4A | MATα leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1 LYS2 MET14 RAD5 |
| W4314-2C | MATα rDNA(∷)ADE2-CAN1 |
| W8284-3D | MATα uaf30(∷)URA3 rDNA(∷)ADE2-CAN1 |
| W8284-18B | MATα uaf30(∷)URA3 shu1(∷)HIS3 rDNA(∷)ADE2-CAN1 |
| W8284-9B | MATα shu1(∷)HIS3 rDNA(∷)ADE2-CAN1 |
| W5204-1B | MATa FOB1-YFP RAD52-CFP ADE2 bar1(∷)LEU2 |
| W8442-18A | MATa uaf30(∷)URA3 FOB1-YFP RAD52-CFP bar1(∷)LEU2 |
| W9073-16C | MATa uaf30(∷)URA3 shu1(∷)HIS3 FOB1-YFP RAD52-CFP bar1(∷)LEU2 |
| W9137-14D | MATa shu1(∷)HIS3 bar1(∷)LEU2 FOB1-YFP RAD52-CFP |
| W9069-4B | MATa rad52-K43R,K44R,K253-YFP FOB1-CFP bar1(∷)LEU2 |
| W9069-4D | MATα rad52-K43R,K44R,K253R-YFP FOB1-CFP uaf30(∷)URA3 shu1(∷)HIS3 |
| W9069-9D | MATα rad52-K43R,K44R,K253R-YFP FOB1-CFP shu1(∷)HIS3 |
| W9069-22B | MATα rad52-K43R,K44R,K253R-YFP FOB1-CFP uaf30(∷)URA3 |
| W9085-17D | MATa rDNA(∷)ADE2-CAN1 rad52-K43R,K44R,K253R-YFP |
| W9085-8D | MATα rDNA(∷)ADE2-CAN1 rad52-K43R,K44R,K253R-YFP shu1(∷)HIS3 |
| W9085-17A | MATα rDNA(∷)ADE2-CAN1 rad52-K43R,K44R,K253R-YFP uaf30(∷)URA3 |
| W9085-3D | MATa rDNA(∷)ADE2-CAN1 rad52-K43R,K44R,K253R-YFP shu1(∷)HIS3 uaf30(∷)URA3 |
2.2. Microscopy
Cells were grown overnight in 5 ml cultures of SC medium plus 100 mg/L adenine at 23°C, and harvested for microscopy, as previously described [20]. Images were captured under a 100× magnification oil immersion objective (1.46NA) on a Leica DM5500B upright microscope (Leica Microsystems) illuminated with a 100W mercury arc lamp using high efficiency YFP and CFP filter cubes. The images were captured with a Hamamatsu Orca AG cooled digital CCD camera, operated by Volocity software (Improvision). Stacks of 11 0.3 micron sections were captured using the following channels and exposure times: DIC (15 ms), Fob1-YFP (800 ms), Fob1-CFP (800 ms), Rad52-CFP (800 ms), and Rad52-KR3-YFP (800 ms). Images were processed and enhanced identically using the Volocity software and analyzed for localization.
2.3. Recombination assays
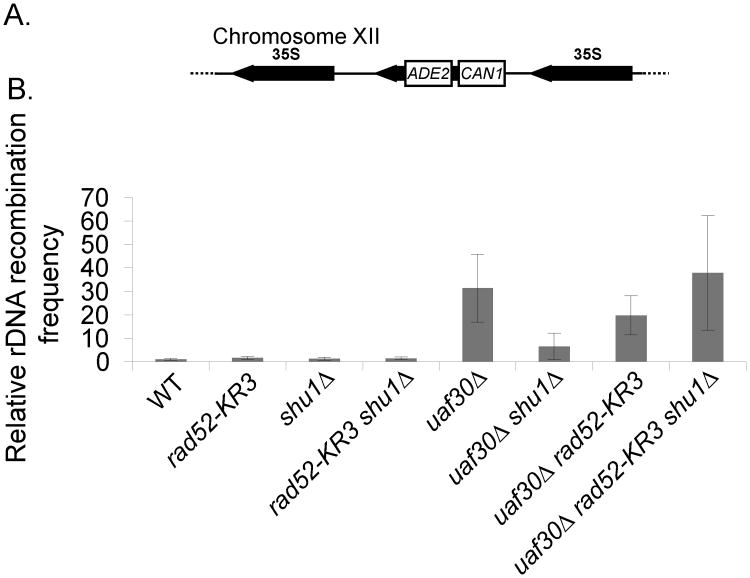
The rDNA recombination assay was performed by analyzing cells for loss of the ADE2/CAN1 markers inserted into one of the rDNA repeated sequences, as described [21] see Fig. 3. A wild-type strain harboring this assay (W4314-2C) was crossed to the mutant strains. Segregants that contained both the rDNA assay and the deletion of the gene of interest were analyzed as described [10]. Each recombination frequency was normalized to WT, which was set to one.
Fig. 3.
Recombination rates remain elevated in a uaf30Δ shu1Δ strain in Rad52-KR3. (A) Schematic of rDNA recombination assay used [21]. (B) The frequency of rDNA recombinants (CANR, ade2) was measured in WT, rad52-KR3, shu1Δ, rad52-KR3 shu1Δ, uaf30Δ, uaf30Δ shu1Δ, uaf30Δ rad52-KR3 and uaf30Δ rad52-KR3 shu1Δ yeast strains with standard deviations plotted.
3. Results and discussion
3.1. Rad52 localization near rDNA is altered in uaf30Δ cells in a Shu1-dependent manner
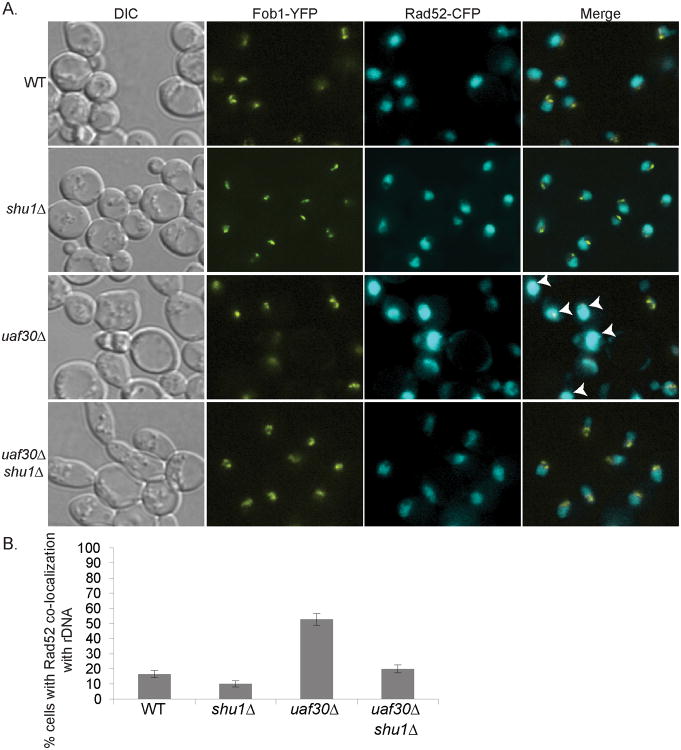
Rad52 plays a central role in rDNA repair despite its exclusion from the nucleolus [16]. During rDNA repair, the broken ends of the rDNA leave the nucleolus where they have access to the DNA repair machinery, including Rad52 as observed after induction of a single DSB in the rDNA [16]. We asked whether the 30-50 fold increase in rDNA recombination seen in the absence of UAF30 would use a similar mechanism for repair [10]. Wild-type or uaf30Δ cells were examined for the sub-nuclear localization of Rad52-CFP with respect to a protein that marks the rDNA, Fob1-YFP. Consistent with previous reports [16], Fob1 and Rad52 do not co-localize in the parental strain; instead, the two proteins are often visibly separated (Fig. 1A). In contrast, uaf30Δ cells often exhibit Fob1 signal that partially overlaps with Rad52 (Fig. 1A, white arrows). This result suggests that when UAF30 is absent, Rad52 can be more closely associated with the rDNA to aid in the recombinational repair of damage. Perhaps this association is due to the fact that loss of uaf30 causes the rDNA to be disorganized, which may alter the interface between the nucleolus and the nucleus making it more accessible to Rad52. Consistent with the idea that uaf30 loss leads to an increased demand for recombination, disruption of uaf30Δ leads to synthetic lethality when combined with mutations in many DSB repair genes (RAD52, MRE11-RAD50-XRS2, TOP3, RMI1, TOP1; Table 2). Interestingly unlike its synthetic effect with rad52Δ, uaf30Δ cells do not exhibit synthetic sickness when combined with rad51Δ or rad54Δ (Table 2). Perhaps other Rad51-independent pathways, such as single-strand annealing, can also be utilized for rDNA repair in uaf30 mutants.
Fig. 1.
Rad52 localization near rDNA is altered in uaf30Δ cells in a Shu1-dependent manner. (A) Wild-type (WT), uaf30Δ, uaf30Δ shu1Δ, and shu1Δ strains were analyzed for Fob1-YFP and Rad52-CFP co-localization (Merge). In WT and shu1Δ cells, there is a separation between the bulk of the Rad52 fluorescence from that of Fob1. In uaf30Δ, the demarcation between the two proteins is diminished (p ≤ 0.005) and is quantitated in (B). Arrows indicate the absence of an interface between Rad52 and Fob1 signals in the cell.
Table 2. uaf30Δ synthetic sick/lethal interacting genes.
| Mutation | Growth |
|---|---|
| Recombination: | |
| top1Δ | − |
| rmi1Δ | − |
| top3Δ | − |
| mre11Δ | − |
| xrs2Δ | − |
| rad50Δ | − |
| rad52Δ | − |
| shu1Δ | + |
| srs2Δ | + |
| rad51Δ | + |
| rad54Δ | + |
| rdh54Δ | + |
| Checkpoint: | |
| rad9Δ | + |
| mec1Δ sml1Δ | + |
| tof2Δ | + |
| mad2Δ | + |
All strains were combined with a uaf30Δ. The (-) sign indicates a lack of cell growth where the (+) sign indicates wild-type growth.
Hyper-recombination and rDNA expansion observed in uaf30Δ cells are largely suppressed by disrupting SHU1 [10]. Therefore, we tested whether Rad52 localization with respect to the rDNA would be restored by a shu1Δ mutation. In Fig. 1A, uaf30Δ and uaf30Δ shu1Δ cells were monitored for Fob1-YFP and Rad52-CFP co-localization and compared to the parental strain (WT). Unlike that observed in uaf30Δ mutants, Rad52 does not overlap with Fob1 in a uaf30Δ shu1Δ double mutant, more closely resembling the association seen in WT strains (Fig. 1A and 1B). These data suggests that shu1Δ suppresses uaf30Δ rDNA recombination by preventing Rad52-mediated repair in this array.
3.2. Rad52 sumoylation is required for Shu1-dependent Rad52 localization near the rDNA in uaf30Δ cells
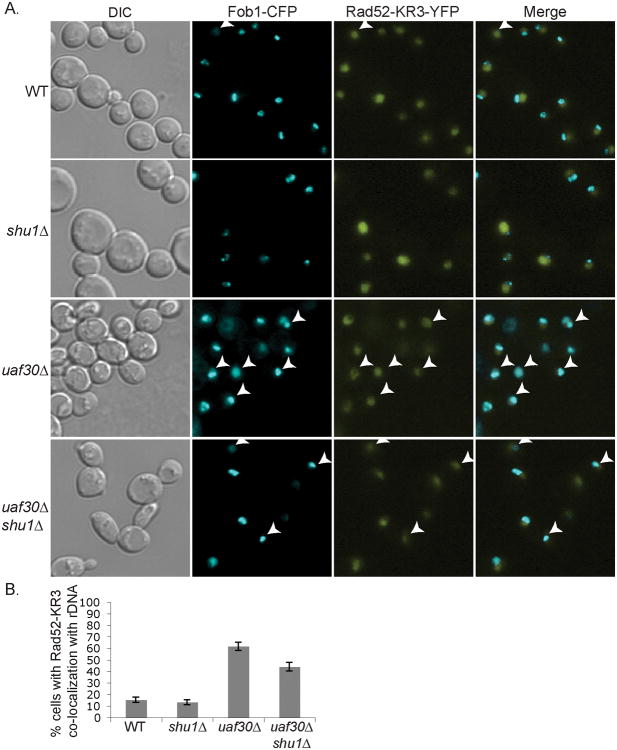
Sumoylation, an ubiquitin-like modifier, has been previously shown to be important for nuclear organization [22, 23]. Repair of rDNA damage is mediated by non-sumoylated Rad52 [16]. Therefore, we determined whether Rad52 localization, with respect to rDNA, is altered in uaf30Δ cells based upon the sumoylation status of Rad52 (Fig. 2A). The rad52- K43R, K44R, K253R (rad52-KR3) allele, which has three sumoylation sites of Rad52 mutated, was introduced into our strains [16, 24]. Similar to wild-type Rad52, we find that in uaf30Δ cells, the mutant rad52-KR3 protein is closely associated with the rDNA (p ≤ 0.005; Fig. 2A and 2B). In contrast to what is observed with wild-type Rad52, the rDNA localization of the rad52-KR3 SUMO mutant protein, is largely independent of SHU1 (p ≤ 0.005; Fig. 2A and 2B), although there is a small, but significant, restoration of its localization in uaf30Δ shu1Δ cells (p ≤ 0.005). These results suggest that the Shu complex plays a key role in Rad52 rDNA localization when Rad52 can be sumoylated.
Fig. 2.
Localization of Rad52-KR3 near the rDNA is largely Shu1-independent in uaf30Δ cells. (A) Wild-type, shu1Δ uaf30Δ, and uaf30Δ shu1Δ strains were analyzed for Fob1-CFP and Rad52-KR3-YFP co-localization (labeled as Merge). (B) Quantitation of the co-localization of Fob1-CFP and Rad52-KR3-YFP with standard errors plotted.
3.3. Rad52 sumoylation regulates Shu1-dependent rDNA recombination
We previously showed that uaf30Δ cells exhibit increased rDNA recombination frequencies, which are largely dependent upon Shu1 in a Rad52 wild-type background [10]. rDNA recombination was measured in an assay where the ADE2 and CAN1 genes were inserted into one of the 100-200 rDNA repeats and recombination frequencies are calculated by determining the simultaneous loss of both markers (Fig. 3A). Consistent with our previous studies, the hyper-recombination observed in uaf30Δ cells is largely alleviated by disrupting shu1Δ (Fig. 3B; compare uaf30Δ to uaf30Δ shu1Δ)[10]. When the three lysines of Rad52 in the rad52-KR3 allele cannot be sumoylated, its localization is comparatively unaffected by absence of SHU1 (Fig. 2A and 2B). Therefore, we hypothesized that since unsumoylated Rad52 is still closely associated with the rDNA, then the increased rDNA recombination frequency observed in a uaf30Δ cell would not be alleviated by SHU1 disruption. Indeed, we find uaf30Δ rad52-KR3 double mutant cells maintain increased recombination frequencies, even in the absence of SHU1 (p≤ 0.1; Fig. 3B, compare uaf30Δ to rad52-KR3 uaf30Δ shu1Δ). Altogether, these results suggest that Shu1 plays a key role in regulating rDNA recombination as well as Rad52 localization as long as Rad52 can be sumoylated.
3.4. Concluding remarks
The Shu complex, consisting of Shu1, Shu2, Csm2, and Psy3, promotes homologous recombination while suppressing error-prone repair [11, 12, 15, 25]. Here we characterize a novel role for Shu1 in the repair of uaf30Δ-induced rDNA recombination through its role in the localization of Rad52. When rDNA is destabilized in uaf30 mutants, we find that Shu1 promotes Rad52/rDNA co-localization. Interestingly, Shu1-dependent regulation of the Rad52 association with rDNA is largely dependent upon Rad52 sumoylation at lysines 43, 44 and 253. The rDNA can be repaired through multiple mechanisms such as sister-chromatid exchange and single-strand annealing, which both utilize Rad52. Since uaf30Δ cells exhibit increased rDNA copy number [10], unequal sister chromatid gene conversion is likely the predominant mechanism in mediating the rDNA expansions observed [7].
Acknowledgments
We thank Michael Mihalevic and Christina Hornack for helpful comments and careful reading of the manuscript. This study was supported by National Institute Health grants GM50237 and GM67055 to RR, and GM078840 and GM088413 to KAB.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi T. A new role of the rDNA and nucleolus in the nucleus--rDNA instability maintains genome integrity. Bioessays. 2008;30:267–272. doi: 10.1002/bies.20723. [DOI] [PubMed] [Google Scholar]
- 2.Ide S, Miyazaki T, Maki H, Kobayashi T. Abundance of ribosomal RNA gene copies maintains genome integrity. Science. 2010;327:693–696. doi: 10.1126/science.1179044. [DOI] [PubMed] [Google Scholar]
- 3.Chindamporn A, Iwaguchi S, Nakagawa Y, Homma M, Tanaka K. Clonal size-variation of rDNA cluster region on chromosome XII of Saccharomyces cerevisiae. J Gen Microbiol. 1993;139:1409–1415. doi: 10.1099/00221287-139-7-1409. [DOI] [PubMed] [Google Scholar]
- 4.Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson JB, Kohn LM. Evolution of drug resistance in experimental populations of Candida albicans. J Bacteriol. 2000;182:1515–1522. doi: 10.1128/jb.182.6.1515-1522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rustchenko EP, Curran TM, Sherman F. Variations in the number of ribosomal DNA units in morphological mutants and normal strains of Candida albicans and in normal strains of Saccharomyces cerevisiae. J Bacteriol. 1993;175:7189–7199. doi: 10.1128/jb.175.22.7189-7199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szostak JW, Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980;284:426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- 7.Gangloff S, Zou H, Rothstein R. Gene conversion plays the major role in controlling the stability of large tandem repeats in yeast. EMBO J. 1996;15:1715–1725. [PMC free article] [PubMed] [Google Scholar]
- 8.Keys DA, Lee BS, Dodd J, Nguyen TT, Vu L, Fantino E, Burson LM, Nogi Y, Nomura M. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 1996;10:887–903. doi: 10.1101/gad.10.7.887. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqi IN, Dodd JA, Vu L, Eliason K, Oakes ML, Keener J, Moore R, Young MK, Nomura M. Transcription of chromosomal rRNA genes by both RNA polymerase I and II in yeast uaf30 mutants lacking the 30kDa subunit of transcription factor UAF. EMBO J. 2001;20:4512–4521. doi: 10.1093/emboj/20.16.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein KA, Reid RJ, Sunjevaric I, Demuth K, Burgess RC, Rothstein R. The Shu complex, which contains Rad51 paralogues, promotes DNA repair through inhibition of the Srs2 anti-recombinase. Mol Biol Cell. 2011;22:1599–1607. doi: 10.1091/mbc.E10-08-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shor E, Weinstein J, Rothstein R. A genetic screen for top3 suppressors in Saccharomyces cerevisiae identifies SHU1, SHU2, PSY3 and CSM2: four genes involved in error-free DNA repair. Genetics. 2005;169:1275–1289. doi: 10.1534/genetics.104.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mankouri HW, Ngo HP, Hickson ID. Shu proteins promote the formation of homologous recombination intermediates that are processed by Sgs1-Rmi1-Top3. Mol Biol Cell. 2007;18:4062–4073. doi: 10.1091/mbc.E07-05-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball LG, Zhang K, Cobb JA, Boone C, Xiao W. The yeast Shu complex couples error-free post-replication repair to homologous recombination. Mol Microbiol. 2009;73:89–102. doi: 10.1111/j.1365-2958.2009.06748.x. [DOI] [PubMed] [Google Scholar]
- 14.Sasanuma H, Tawaramoto MS, Lao JP, Hosaka H, Sanda E, Suzuki M, Yamashita E, Hunter N, Shinohara M, Nakagawa A, Shinohara A. A new protein complex promoting the assembly of Rad51 filaments. Nat Commun. 2013;4:1676. doi: 10.1038/ncomms2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin S, Wier A, Kabbinavar F, Bratton-Palmer DS, Ghodke H, Van Houten B, Vandemark AP, Bernstein KA. The Shu complex interacts with Rad51 through the Rad51 paralogues Rad55-Rad57 to mediate error-free recombination. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Rosell J, Sunjevaric I, De Piccoli G, Sacher M, Eckert-Boulet N, Reid R, Jentsch S, Rothstein R, Aragon L, Lisby M. The Smc5-Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat Cell Biol. 2007;9:923–931. doi: 10.1038/ncb1619. [DOI] [PubMed] [Google Scholar]
- 17.Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- 19.Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 20.Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci U S A. 2001;98:8276–8282. doi: 10.1073/pnas.121006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritze CE, Verschueren Strich, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Blobel G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci U S A. 2005;102:4777–4782. doi: 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai S, Dubrana K, Tsai-Pflugfelder M, Davidson MB, Roberts TM, Brown GW, Varela E, Hediger F, Gasser SM, Krogan NJ. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacher M, Pfander B, Hoege C, Jentsch S. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol. 2006;8:1284–1290. doi: 10.1038/ncb1488. [DOI] [PubMed] [Google Scholar]
- 25.Huang ME, Rio AG, Nicolas A, Kolodner RD. A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc Natl Acad Sci U S A. 2003;100:11529–11534. doi: 10.1073/pnas.2035018100. [DOI] [PMC free article] [PubMed] [Google Scholar]