Abstract
PURPOSE
On June 7th, 2000, President Clinton issued an executive memorandum directing Medicare payment for routine patient care in qualifying clinical trials. Our objective was to estimate the proportion of older prostate cancer patients who were examined as part of a qualifying clinical trial and the association between their participation and patient characteristics.
MATERIALS and METHODS
An observational study using the Surveillance, Epidemiology and End Results Medicare database was conducted to determine participation in qualifying clinical trials from a sample of 37 216 men aged 66 and older, enrolled in Medicare, and diagnosed with prostate cancer between September 2000 and December 2002.
RESULTS
Within 3 years of diagnosis, 211 men (0.567%) received routine patient care in a qualifying clinical trial. These participants were more likely to be under 70 years in age (odds ratio [OR] 1.687; confidence interval [CI] 1.27–2.24) and less likely to be less-educated and reside in low-income, metropolitan neighborhoods. White men were more likely to participate in clinical trials (OR 1.426; CI 0.97–2.09) than nonwhites; however this association is not statistically significant. Participation varied significantly by registry site (0% to 1.2%), but not by tumor grade, stage, and prostate-specific antigen status.
CONCLUSION
Few older prostate cancer patients participated in qualifying trials between 2000 and 2002, and those who participated were not representative of the general population of older prostate cancer patients. Greater efforts are required to expand trial enrollment and reduce disparities in research participation.
Keywords: Neoplasms, Oncology, Access, Healthcare Disparities, Underserved
INTRODUCTION
Under-representation of certain subgroups has long been a concern in clinical trials. Despite the existence of federal laws designed to promote representative participation by women and minorities, evidence is mixed regarding how successful these laws have been.1–6
Patients over age 65 are another subgroup often under-represented in clinical trials. For cancer trials, older patients account for over half of cancer cases, but only about one-quarter of trial participants.4, 6–8 The oft-cited statistic, dating back to 1988, maintains that “three percent or less of adult cancer patients participate in studies of cancer treatments, or drug regimens.”7, 9, 10 Two decades later, there are no signs of improvement.4, 7, 10, 11
On June 7th, 2000, President Clinton issued an executive memorandum directing Medicare payment for routine patient care in qualifying clinical trials. Senators Connie Mack (R-FL) and Jay Rockefeller (D-WV) orchestrated this expansion to promote clinical trial participation.12,13 Although the policy remains in effect, it has yet to be codified into law and could be overturned at any time. Unger et al. showed a positive impact of the 2000 policy change; however, trial participation increased only among Medicare beneficiaries with Medicare coverage and private supplemental insurance.14 Therefore, the policy has had little impact on trial participation in older adults with Medicare coverage alone.14, 15 Dhruva and Redberg considered it “imperative that evidence applicable to Medicare beneficiaries” become available, since the Centers for Medicare and Medicaid Services (CMS) determine coverage of “products and services” in published literature.16
Evidence of low participation is noteworthy among men with prostate cancer, since efficacy of screening and invasive procedures remains unclear.17, 18 Prostate cancer patients are generally older, so trial participation of Medicare beneficiaries is particularly relevant. The purpose of this study is to examine trial participation among older men who were enrolled in Medicare and diagnosed with prostate cancer since the 2000 Medicare expansion. This study extends the literature on trial participation among older adults by addressing 2 questions: (1) what proportion of older Medicare prostate cancer patients received routine care as part of a qualifying trial between 2000 and 2002 in comparison to the generally quoted; and (2) how do the characteristics of these participants compare to older prostate cancer patients who did not participate?
MATERIALS and METHODS
Data Source
The Surveillance, Epidemiology and End Results (SEER) program is a national population-based tumor registry that collects information on incident cancer cases, representing 26% of the United States’ population. Of the persons age 65 and older diagnosed with cancer, as reported by SEER, 93% were matched with Medicare enrollment records as previously described.19
Study Population
For inclusion, a male patient must have: (1) been diagnosed with prostate cancer between 2000 and 2002; (2) been continuously enrolled in traditional Medicare for a year prior to and 3 years after diagnosis; and (3) resided in the continental United States (US), Alaska, or Hawaii. Furthermore, we excluded persons whose cancer was diagnosed only on a death certificate or by autopsy (N=43). The resulting analytical sample included 37 216 older men with prostate cancer.
Variable Definitions
Median income and the proportion of the adult population at each education level were assigned based on the 2000 US census using respondent zip-codes. Based on the interquartile ranges of the zip-code-specific measures, we categorized patient neighborhoods as: (1) low-socioeconomic status (SES), when the zip-code was in the first quartile in both income and college education, (2) high SES, when the zip-code was in the fourth quartile for both measures, and (3) middle SES for all zip-codes other than high- and low-SES zip-codes. A small proportion of the sample (4%) did not have complete zip-codes (N=1485); univariate analyses of the income and education variables excluded these individuals. Aside from zip-code-level socioeconomic characteristics, SEER describes place of residence along the US Department of Agriculture’s rural-urban continuum (Metropolitan, Urban, Rural) and by registry and census region (Northeast, South, Midwest, West).
SEER data include tumor grade, prostate-specific antigen (PSA) status, and stage. Tumor grade is described as the following Gleason Scores: well differentiated (GS: 2–4), moderately differentiated (GS: 5–7), or poorly differentiated (GS: 8–10). PSA status is described as normal, borderline, or elevated based on laboratory-specific determinations of normal range. Tumor grade and PSA status were unavailable for some patients (1507 and 7838, respectively). Tumor stage is described by local/regional, distant, and unstaged. However, local/regional tumors may be further separated into categories put forth by the American Joint Committee on Cancer (AJCC I, II, III, IV, unknown).
Using 2 protocols developed by the National Cancer Institute (NCI), the number of Charlson comorbid conditions at diagnosis were calculated by reviewing all National Claims History, Medicare Provider Analysis and Review, and outpatient claims relating to services delivered within the 12 months before the month of diagnosis and were recoded into 4 categories (0, 1, 2, ≥3). 20
Healthcare Common Procedure Coding System (HCPCS) codes are used to identify and reimburse clinical trial-related services. Three years of post-diagnosis Medicare claims were searched for indicators of participation in an eligible trial.
On September 19th, 2000, CMS introduced the HCPCS modifier QV (item or service provided as routine care in a Medicare-qualifying clinical trial) and diagnostic code V70.5 (health examination of a defined subpopulation) to identify trial items.21 Effective January 2nd, 2002, CMS discontinued the diagnostic code V70.5, leaving only the procedure modifier QV. At the same time, CMS introduced a new diagnosis code (V70.7, examination of participant in a clinical trial) to represent trial items provided to healthy participants in diagnostic trial control groups.22 Although these codes may have appeared multiple times within 3 years after a participant’s diagnosis, the presence of any trial-related code (QV, V70.7, V70.5) was used to identify trial participation (participant, nonparticipant).
Statistical Analysis
Univariate analyses were conducted to test crude differences between participant and nonparticipant demographic and disease characteristics. We estimated the medians and interquartile ranges of the continuous variables (ie, age, zip-code-level median income, and the zip-code-level proportion of college-educated adults) and tested the equivalence in variable distributions between participants and nonparticipants using a Wilcoxon-Mann-Whitney U test. For the remaining categorical variables, the frequency of each category was presented, and the equivalence of category frequencies between participants and nonparticipants was tested using a chi-squared test.
In the multivariable analysis, a logistic regression model of participation on respondent characteristics was estimated using maximum likelihood estimation, and the results presented as odds ratios with 95% confidence intervals. Univariate odds ratios were also estimated and presented for comparison.
Database management was conducted in SAS 9.1.3; however, the statistical analysis was conducted using STATA MP 9.0.23, 24 SEER-Medicare data do not contain sample weights that represented the target population.
RESULTS
Univariate Analysis
Only 0.567% (211 out of 37 216) of the prostate cancer patients diagnosed after age 65 who were included in this study participated in a Medicare-eligible clinical trial within 3 years of diagnosis. This estimate equates to an accrual rate of 0.19% per year for a cohort of older men (−ln(100%-0.567%)/3 years)). This is substantially lower than NCI’s 2001 estimate that “less than 5 percent of adults diagnosed with cancer each year will get treated through enrollment in a clinical trial.” 25
Comparing the demographic characteristics of participants and nonparticipants, participants were younger (Table 1). In terms of race, the proportion of white patients was higher among participants as compared to the proportion of nonparticipants, but the difference between the 2 proportions (85% and 80%) was not statistically significant at a 5% significance level. Similarly, marital status and place of residence were not significantly associated with trial participation.
Table 1.
Demographic and Residential Characteristics
| Patient Characteristics | Participants (%) | NonParticipants (%) | P-value* |
|---|---|---|---|
| Number of Patients | 211(100) | 37005(100) | |
| Median Age in Years (IQR) | 72 (68–76) | 73 (69–78) | <0.001 |
| Race/Ethnicity | |||
| White | 180(85) | 29707(80) | 0.314 |
| Black | 13(6) | 3350(9) | |
| Hispanic | 8(4) | 1638(4) | |
| Other | 10(5) | 2310(6) | |
| Marital Status | |||
| Single | 11(5) | 2371(6) | 0.447 |
| Married | 154(73) | 25469(69) | |
| Separated, Divorced, or Widowed | 22(10) | 5071(14) | |
| Unknown | 24(11) | 4094(11) | |
| Place of Residence | |||
| Metropolitan | 177(84) | 31230(84) | 0.253 |
| Urban | 27(13) | 5103(14) | |
| Rural | 7(3) | 672(2) | |
| Zip-code of Residence | |||
| Unknown | 11(5) | 1474(4) | 0.363 |
| Known | 200(95) | 35531(96) | |
| Median Income within Zip-code (IQR) | 51656 (38763–69754) | 46723 (35351–61363) | <0.001 |
| Median Education within Zip-code (IQR) | |||
| Less than High School | 0.129 (0.075–0.193) | 0.150(0.095–0.246) | 0.001 |
| High School Graduate | 0.254 (0.184–0.345) | 0.266(0.197–0.336) | 0.731 |
| Some College | 0.263 (0.207–0.304) | 0.274(0.227–0.328) | 0.001 |
| College Graduate | 0.256 (0.171–0.458) | 0.232(0.143–0.381) | 0.003 |
P-value based on either a chi-squared test or Wilcoxon-Mann-Whitney U test.
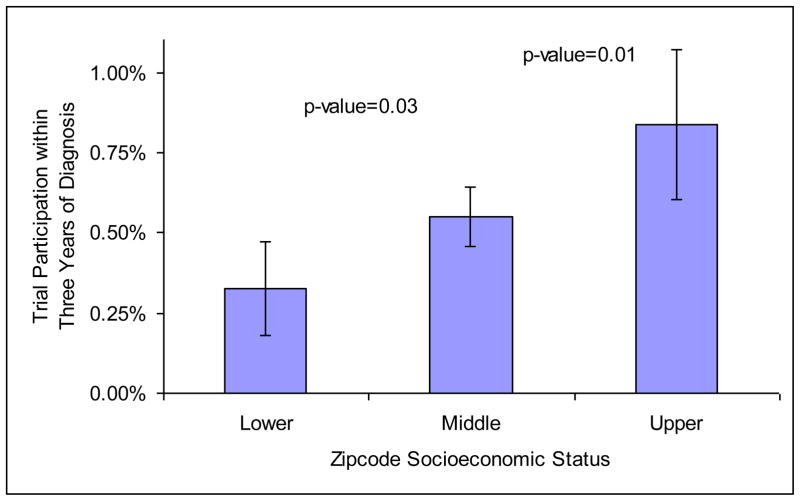
Participants were more likely to reside in zip-codes with higher median income and education than nonparticipants (Table 1). Figure 1 shows the proportion of trial participants by zip-code-level SES. Respondents in the high- or low-SES zip-codes compose one-third of the sample (16.35% each), and the remaining two-thirds were included in the middle-SES category. Men who resided in upper-SES neighborhoods were more likely to participate in a clinical trial than men who resided in lower-SES neighborhoods (odds ratio [OR] 2.594; confidence interval [CI] 95% 1.525–4.411).
Figure 1.
Trial Participation by Zip-code Socioeconomic Status
Trial participation was significantly associated with registry (Table 2). A disproportionately large amount of participants reside in the Northeast, namely Connecticut (12% versus 6%) and New Jersey (33% versus 19%), and in the Midwest, specifically Iowa (12% versus 7%). In contrast, registries in the South and West were under-represented, except for Los Angeles, California (11% versus 7%).
Table 2.
Regional Characteristics
| Region and Registry | Participants (%) | NonParticipants (%) |
|---|---|---|
| All Registries | 211(100) | 37005(100) |
| Northeast | ||
| Connecticut | 26(12) | 2166(6) |
| New Jersey | 70(33) | 7096(19) |
| South | ||
| Atlanta, Georgia | 1(0) | 1051(3) |
| Rural Georgia | 1(0) | 83(0) |
| Kentucky | 10(5) | 2827(8) |
| Louisiana | 9(4) | 2561(7) |
| Midwest | ||
| Iowa | 26(12) | 2513(7) |
| Detroit, Michigan | 8(4) | 3545(10) |
| West | ||
| Hawaii | 0(0) | 552(1) |
| New Mexico | 3(1) | 940(3) |
| Seattle, Washington | 6(3) | 2112(6) |
| Utah | 7(3) | 1282(3) |
| California | ||
| San Francisco | 1(0) | 1164(3) |
| San Jose-Monterey | 0(0) | 790(2) |
| Los Angeles | 23(11) | 2527(7) |
| Greater California | 20(9) | 5796(16) |
In terms of tumor characteristics and comorbidities, participants and nonparticipants were surprisingly similar (Table 3). Participants were more likely to have unstaged tumors than nonparticipants (8% versus 3%). After excluding this category, the differences between the frequencies of the staged categories were not statistically significant (p-value=0.732). Tumor grade, PSA status, and comorbid condition number were not significantly associated with trial participation.
Table 3.
Tumor Characteristics and Comorbidities
| Tumor Characteristics | Participants (%) | NonParticipants (%) | Chi-squared P-value |
|---|---|---|---|
| Number of Patients | 211(100) | 37005(100) | |
| Tumor Grade | |||
| Well Differentiated | 8(4) | 1225(3) | 0.827 |
| Moderately Differentiated | 150(71) | 25506(69) | |
| Poorly Differentiated | 46(22) | 8774(24) | |
| Unknown | 7(3) | 1500(4) | |
| Stage | |||
| Local/regional | 0.007 | ||
| AJCC I | 73(35) | 14590(39) | |
| AJCC II | 25(12) | 3786(10) | |
| AJCC III | 13(6) | 1765(5) | |
| AJCC IV | 4(2) | 841(2) | |
| Unknown | 73(35) | 13484(36) | |
| Distant | 7(3) | 1431(4) | |
| Un-staged | 16(8) | 1108(3) | |
| Prostate-specific Antigen Status | |||
| Normal | 130(62) | 24838(67) | 0.081 |
| Borderline | 11(5) | 2583(7) | |
| Elevated | 11(5) | 1805(5) | |
| Unknown | 59(28) | 7779(21) | |
| Charlson Comorbidity Index | |||
| None | 164(78) | 27590(75) | 0.402 |
| 1 | 31(15) | 6437(17) | |
| 2 | 13(6) | 1941(5) | |
| 3 or more | 3(1) | 1037(3) | |
Abbreviations: AJCC, American Joint Committee on Cancer.
Odds of Participating in a Medicare-qualifying Clinical Trial
In the univariate logistic regression analysis, variables significantly associated with the odds of trial participation included age at diagnosis, the proportion of college-educated adults by zip-code, and median income by zip-code (Table 4). With each year of age, the odds of participating decreased by around 5%. Increasing the proportion of college-educated adults by 10% or median income by $10 000 within a zip-code increased the odds of trial participation of Medicare enrollees by more than 10%.
Table 4.
Odds of Trial Participation
| Unadjusted | Adjusted | |
|---|---|---|
| Patient and Diagnostic Characteristics | OR (95% CI) | OR (95% CI) |
| Age at Diagnosis in Years | 0.952 (0.928–0.976) | 0.948 (0.924–0.974) |
| Race | ||
| White | 1.00 | 1.00 |
| Black | 0.640 (0.364–1.126) | 0.808 (0.452–1.443) |
| Hispanic | 0.806 (0.396–1.639) | 0.896 (0.436–1.839) |
| Other | 0.714 (0.377–1.352) | 0.776 (0.408–1.474) |
| Married | 1.224 (0.902–1.660) | 1.112 (0.813–1.522) |
| Resides in Metropolitan Area | 0.963 (0.666–1.391) | 0.710 (0.475–1.061) |
| Zip-code Unknown | 1.326 (0.721–2.438) | 2.740 (1.385–5.420) |
| College Education in Zip-code* | 1.135 (1.051–1.226) | 0.948 (0.825–1.089) |
| Median Income in Zip-code† | 1.141 (1.085–1.200) | 1.180 (1.078–1.292) |
| Tumor Grade | ||
| Well Differentiated | 1.00 | 1.00 |
| Moderately Differentiated | 1.191 (0.86–1.648) | 1.062 (0.763–1.478) |
| Poorly Differentiated | 0.699 (0.302–1.615) | 0.690 (0.298–1.597) |
| Unknown | 0.859 (0.463–1.592) | 0.929 (0.500–1.725) |
| Stage | ||
| Local/Regional | 1.00 | 1.00 |
| Distant Stage | 1.245 (0.652–2.374) | 1.341 (0.695–2.586) |
| Un-staged | 0.901 (0.441–1.838) | 0.878 (0.428–1.802) |
| PSA Status | ||
| Normal | 1.00 | 1.00 |
| Borderline | 0.803 (0.378–1.705) | 0.856 (0.400–1.831) |
| Elevated | 0.715 (0.258–1.976) | 0.573 (0.204–1.606) |
| Unknown | 0.897 (0.421–1.911) | 1.172 (0.539–2.549) |
Abbreviations: CI, confidence interval; OR, odds ratio; PSA, prostate specific antigen.
College education is in units of 10 percent.
Median income is in units of $10,000 dollars.
The results changed in the multivariate analysis, where all ratios were estimated simultaneously. The odds ratios for age at diagnosis and median income were similar to the univariate results. However, the odds ratio for the proportion of college-educated adults became insignificant, and the odds ratio for missing zip-code became statistically significant. The odds ratio associated with residing in a metropolitan area (0.710) is nearly significant (95% CI 0.475 to 1.061) after the addition of the control variables. Otherwise, the estimated odds ratios of the multivariate and the univariate analyses were similar.
DISCUSSION
Few older men who were enrolled in Medicare and have been diagnosed with prostate cancer participate in eligible trials, and their likelihood of participation decreases with age. Trial participation is more prevalent among residents of wealthy, college-educated neighborhoods, particularly along the northeast coast of the US; participation is not shown to be associated with tumor characteristics or number of comorbidities prior to diagnosis.
Although the age gap is minimal in terms of an absolute age difference, it is consistent with findings from other studies of other diseases, revealing that older individuals are infrequently included in clinical trials.15, 16 In the US, the median age of prostate cancer diagnosis is 69 in whites and 66 in blacks; therefore, excluding potential study subjects according to age is impractical.26 However, the evidence is not clear as to whether there is favoritism towards offering trials to younger aged patients or if uptake of trials is lower among older aged patients. Beyond the setting of clinical trials, evidence suggests older patients are less likely to receive similar care when compared to their younger counterparts.27 For men with prostate cancer, participation in a clinical trial may provide them an opportunity to receive newer treatments.
Multiple factors may contribute to the higher prevalence of trial participants from higher-income, more-educated neighborhoods. First, residents of such neighborhoods may have evaluate the benefits of trial participation more highly (or the costs of trial participation less) than residents of neighborhoods with lower-income, less-educated levels. Second, the geographic locations of recruitment sites for clinical trials may disproportionately favor neighborhoods with larger pools of patients with higher incomes and more education. Third, the inclusion (exclusion) criteria (eg, transportation, supplemental health insurance, care-giver support) of clinical trials may be more (less) frequently met in higher-income, more-educated neighborhoods. In this analysis, we were unable to separate patient-level SES from neighborhood-level SES, which may be examined in future research.
In this analysis, clinical differences between the prostate cancer patients’ typical disease characteristics (eg, disease grade, stage, PSA) were not apparent, although a significantly greater proportion of participants were unstaged compared to nonparticipants. While the importance of this finding is unclear, the similarity of participants to nonparticipants is notable, particularly in settings in which clinical trials target at-risk populations. Despite a number of available clinical trials in adjuvant, neoadjuvant, and metastatic disease settings, men in high-risk groups were not more likely to participate in clinical trials than those with lower-risk, low-stage disease.
Limitations and Future Research
Health factors, not described in SEER, may be important in determining candidacy for trial participation, so the lack of significant associations between health and participation may be a limitation of the registry information.15 Targeted participation in clinical trials has become increasingly common.28,29 In prostate cancer, risk factors, such as a family history of cancer or being of African descent may trigger clinical trial design, translating into targeted participation. The effect of targeting subsets of potential trial participants results in a narrowed study population and a potential reduction in clinical trial opportunities for larger segments of affected individuals.
The claims analysis may underestimate overall trial participation. Some respondents may have participated in eligible trials without the Medicare covered services, or in ineligible trials. “Deemed” trials funded by the federal government automatically qualify based on a set of requirements and desirable criteria.21, 30 CMS intended to implement a self-certification process for private studies; however, it never materialized.
Furthermore, we acknowledge that the claim modifiers may be absent from Medicare records due to inadequacy of claims management. To identify the extent of this potential limitation, we contacted all NCI-designated cancer centers that ran clinical trials between 2000 and 2002 (total of 58). Ten centers could not be reached for comment and of the remaining 48 centers, 41 (85%) verified CMS modifier use. Seven centers reported not using the modifier for various reasons: children’s cancer center (1), does not enroll Medicare beneficiaries in trials (1), does not use codes or modifiers (1), initiation of modifiers and/or establishing a system was in process (4). These findings suggest prevalent use of trial modifiers among NCI-designated cancer centers. However, to the extent that incomplete use (or missing data of any kind) is differentially associated with confounder levels, the results with respect to the direction and significance of the predictors could be biased.
CONCLUSION
Our findings show that Medicare beneficiaries with prostate cancer have a lower representation in qualifying trials than the oft-cited 3%.7, 9, 10 This lower rate may be attributed to the 7-fold difference between new cases and mortality in prostate cancer. Slow progression to terminal disease may detract from the immediacy of trial participation in prostate cancer. Dr. Richard Schilsky, an oncologist at the University of Chicago and immediate past president of the American Society of Clinical Oncology, alternatively ascribes poor CMS reimbursement for low participation, “A lot of doctors say, ‘This is not worth it [offering trials to their patients].’ ”10 While low reimbursement may be a primary deterrent, low participation is multifactorial. Future analysis may extend this study beyond prostate cancer, investigate the effects of additional reimbursement or other policy options to motivate trial participation, or examine the cost and health outcomes between participants and nonparticipants.
Acknowledgments
We would like to thank Riddhi Patel (Research Assistant at Moffitt Cancer Center), Kelly Willenberg (Health Care Compliance Advocate at Synergism) and Karen Mottola (Clinical Research Compliance Officer at Huntsman Cancer Institute) for their insights on CMS coverage policy, healthcare compliance, and other support.
Acknowledgement of Research Support: Dr Craig and the coauthors have no conflicts of interest, and received no funding or support for this particular project; however, Dr Craig is supported by a K25 Career Development Award from the National Cancer Institute.
Contributor Information
Benjamin M. Craig, Health Outcomes & Behavior Program, Moffitt Cancer Center, Tampa, FL. Associate Professor, Department of Economics, University of South Florida, Tampa, FL.
Scott M. Gilbert, Departments of Urology & Epidemiology & Health Policy Research, University of Florida, Gainesville, FL.
Jill Boylston Herndon, Department of Epidemiology & Health Policy Research, Institute for Child Health Policy, University of Florida, Gainesville, FL.
Bruce Vogel, Department of Epidemiology & Health Policy Research, University of Florida, Gainesville, FL. Research Health Scientist & Director, Rehabilitation Outcomes Research Center, North Florida/South Georgia Veterans Health System, Gainesville, FL.
Gwendolyn P. Quinn, Department of Oncologic Sciences, University of South Florida College of Medicine, Tampa, FL. Associate Member, Health Outcomes & Behavior Program, Moffitt Cancer Center, Tampa, Florida.
References
- 1.Catania C, De Pas T, Goldhirsch A, et al. Participation in clinical trials as viewed by the patient: understanding cultural and emotional aspects which influence choice. Oncology. 2008;74:177. doi: 10.1159/000151365. [DOI] [PubMed] [Google Scholar]
- 2.Eggly S, Albrecht TL, Harper FW, et al. Oncologists’ recommendations of clinical trial participation to patients. Patient Educ Couns. 2008;70:143. doi: 10.1016/j.pec.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 4.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 5.Outlaw FH, Bourjolly JN, Barg FK. A study on recruitment of black Americans into clinical trials through a cultural competence lens. Cancer Nurs. 2000;23:444. doi: 10.1097/00002820-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JH, Bertoni AG, Staten JL, et al. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14:3328. doi: 10.1245/s10434-007-9500-y. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65:2376. doi: 10.1002/1097-0142(19900515)65:10+<2376::aid-cncr2820651504>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Kolata G. Forty Years’ War: Lack of Study Volunteers Hobbles Cancer Fight The New York Times. 2009 [Google Scholar]
- 11.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 12.Clinton tells Medicare to pay for clinical trials. Oncology-New York. 2000;14:1109. [Google Scholar]
- 13.Berlyn D. Routine patient care in clinical trials: whose cost is it anyway? J Law Health. 2001;16:77. [PubMed] [Google Scholar]
- 14.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 15.Gross CP, Wong N, Dubin JA, et al. Enrollment of older persons in cancer trials after the medicare reimbursement policy change. Arch Intern Med. 2005;165:1514. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 16.Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168:136. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 17.The Prostate Cancer Muddle. The New York Times; 2009. [Google Scholar]
- 18.Couzens GS. Detection Comes Earlier, and So Do Tough Questions. The New York Times; 2007. [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A New Method of Classifying Prognostic Co-Morbidity in Longitudinal -Studies - Development and Validation. Journal of Chronic Diseases. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Medicare Coverage~ Clinical Trials: Program Memorandum. CMS. 2000 [Google Scholar]
- 22.Revised Guidelines for Processing Claims for Clinical Trial Routine Care Services. CMS Program Memorandum Intermediaries/Carriers, Transmittal AB-01-142. 2001 [Google Scholar]
- 23.Stata Statistical Software Revision 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- 24.SAS, 9.1.3. Cary, NC, USA: SAS Institute Inc; 2008. [Google Scholar]
- 25.Doctors, Patients Face Different Barriers to Clinical Trials. Vol. 2009. National Cancer Institute; 2001. [Google Scholar]
- 26.Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29. doi: 10.1016/j.cdp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kane RL, Priester R, Neumann D. Does Disparity in the Way Disabled Older Adults Are Treated Imply Ageism? Gerontologist. 2007;47:271. doi: 10.1093/geront/47.3.271. [DOI] [PubMed] [Google Scholar]
- 28.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 30.Hamill JA. Medicare: coverage approved for participation in clinical trials. J Law Med Ethics. 2000;28:317. doi: 10.1111/j.1748-720x.2000.tb00682.x. [DOI] [PubMed] [Google Scholar]