Abstract
Although there is evidence that sphingosine-1-phosphate receptor-1 (S1P1) activation occurs following experimental brain injury, there is little information about its metabolic pathway in cerebral ischemia. The purpose of this study was to evaluate the role of the sphingosine metabolic pathway including S1P1, sphingosine kinases 1 (SphK1), and 2 (SphK2) in transient middle cerebral artery occlusion (MCAO). Fifty-eight male Sprague-Dawley rats were used to asses temporal profiles of S1P1, SphK1 and 2 on neurons in infarct and periinfarct cortices at pre-infarct state, 6, and 24 hours after MCAO. The animals were then treated with vehicle and 0.25mg/kg FTY720, which is an agonist of S1P receptors, and evaluated regarding neurological function, infarct volume, and S1P1 expression on neurons at 24 hours after MCAO. The expressions of S1P1, SphK1, and SphK2 were significantly decreased after MCAO. Labeling of all markers were reduced in the infarct cortex but remained present in the periinfarct cortex, and some were found to be on neurons. Significant improvements of neurological function and brain injury were observed in the FTY720 group compared with the vehicle and untreated groups, although S1P1 expression on neurons was reduced in the FTY720 group compared with the vehicle group. We demonstrated that S1P1, SphK1, and SphK2 were downregulated in the infarct cortex, whereas they were preserved in the periinfarct cortex where FTY720 reduced neuronal injury possibly via S1P1 activation. Our findings suggest that activation of the sphingosine metabolic pathway may be neuroprotective in cerebral ischemia.
Keywords: sphingosine 1-phosphate receptor-1, sphingosine kinase 1, sphingosine kinase 2, FTY720, transient middle cerebral artery occlusion
Introduction
Experimental transient middle cerebral artery occlusion (MCAO) is widely approved as a representative model for cerebral ischemia in humans [1]. MCAO in rodent animal modeling produces an infarct core which is defined as necrosis, and a penumbra (periinfarct area) in the frontoparietal cortex, in which apoptotic pathways are mainly implicated [2,3]. Neurons in the infarct core are undergoing a sudden failure of cellular energy, swelling and rupture of the organelles, whereas neurons in the periinfarct area are viable targets as they can be rescued with sutable treatments including antiapoptotic drugs [4,5].
Sphingosine 1-phosphate (S1P) is one of the sphingolipid metabolites generated by sphingosine kinases (SphKs) 1 and 2 from sphingosine. Endogenous S1P levels are controlled by the expression and activity of SphKs, and S1P is recognized as an important bioactive mediator that regulates many cellular and physiological processes [6]. S1P mediates its effects via a family of G protein-coupled receptors, including S1P receptor-1 to -5 (S1P1-5) [7]. It is previously reported that S1P1 activation could reduce brain injuries in various types of experimental stroke models including transient MCAO [8], intracerebral hematoma [9], subarachnoid hemorrhage [10], and neonatal hypoxia-ischemia [11], most of which revealed that S1P1 activation mediated antiapoptotic pathway. Therefore, S1P1 activation is thought to be a good candidate subject for stroke research. Although increasing evidence supports the beneficial effect of S1P1 activation, there is little information about the role of the sphingosine metabolism pathway including S1P1, SphK1, and SphK2 on neurons following cerebral ischemia.
The aim of this study was to determine the temporal profile of changes of the sphingosine pathway (S1P1, SphK1, and SphK2) in the early phase after experimental cerebral ischemia, and to examine the effects of S1P1 activation by FTY720, which is a S1P agonist, on neurons after 2 hour transient MCAO in rats.
Materials and Methods
Experimental animals
All experiments were approved by the institutional Animal Care and Use Committee of Loma Linda University. Fifty-eight male Sprague-Dawley rats (Harlan, Indianapolis, Ind) weighing 330–380g were divided into the following groups: preoperation (healthy control; n=8), sham-operation (n=6), MCAO (control group; n=24), MCAO treated with dimethyl sulfoxide (DMSO) as a vehicle (vehicle group; n=10), MCAO with 0.25mg/kg FTY720 (FTY group; n=10).
Transient MCAO model
Anesthesia was induced with 4% isoflurane and maintained with 2.5% isoflurane, 30% oxygen, and 70% medical air via face mask. Arterial blood gas analysis, mean arterial blood pressure, heart rate, and blood glucose level before, during, and after MCAO were analyzed through the femoral artery. Transient focal ischemia was induced by occluding the MCA using the intraluminal technique [8]. Briefly, under the monitoring of body temperature and physiological conditions, the left common carotid artery (CCA) was exposed and a heat-blunted 3-0 nylon suture coated with poly-L-lysine was introduced into the left internal carotid artery through the CCA. After 2 hours the suture was withdrawn to allow MCA reperfusion. For sham MCAO, vessels were visualized but no additional manipulations were made.
Administration of drugs
Two groups of rats with transient MCAO received DMSO and 0.25mg/kg FTY720 in DMSO [8] intraperitoneally immediately after reperfusion. DMSO (1.1g/mL/kg) at no more than ≤0.4mL was used [8].
Western blot analysis
The left cerebral cortex in the MCA region (MCA cortex; Fig. 1A, black area), which is considered as a dying area over 24 hours after transient MCAO (sometimes involving the area perfused by the posterior cerebral artery), was isolated and collected at preoperation, 6 (4 hours after reperfusion) or 24 (22 hours after reperfusion) hours after MCAO (n=5, respectively). Protein concentration was determined using DC protein assay (Bio-Rad, Hercules, CA). SDS-polyacrylamide gel electrophoresis was performed as previously described [8] using the following primary antibodies: 1) anti-S1P1 (1:200, Cayman Chemical, Ann Arbor, MI), 2) anti-SphK1 (1:200, Abgent, San Diego, CA), and 3) anti-SphK2 (1:200, Lifespan Biosciences Inc., Seattle, WA). Changes in expressions of S1P1, SphK1, and SphK2 were measured as a percentage of the preoperative level. β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as an internal control for every experiment.
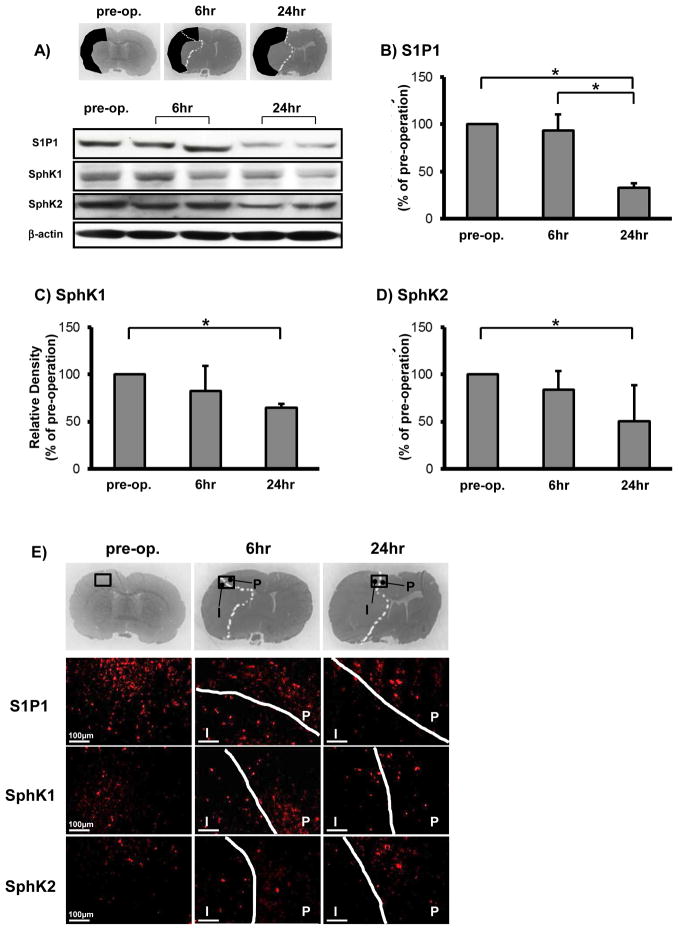
Fig. 1.
Changes in sphingosine-1-phosphate receptor-1 (S1P1), sphingosine kinase 1 (SphK1), and sphingosine kinase 2 (SphK2) expressions in the middle cerebral artery (MCA) region including infarct (I) and periinfarct (P) cortices at preoperation (pre-op.), 6 and 24 hours after MCA occlusion (n=5). Representative changes of the infarct MCA region and Western blots (A), and temporal profiles of quantitative analysis of S1P1 (B), SphK1 (C), and SphK2 (D). The band density values were calculated as a ratio of that of β-actin, and the values from the preoperation were used as 100%. Immunofluorescence of S1P1, SphK1, and SphK2 showed that the expressions were reduced in the infarct cortex, but they were preserved in periinfarct cortex (E). Values are expressed as mean±SD; *P<0.05, ANOVA.
Histology and immunofluorescence staining
Samples from preoperation, 6 or 24 hours after MCAO in control, vehicle, and FTY groups were used for experiments (n=3, respectively). The rat brains were fixed by cardiovascular perfusion with phosphate-buffered saline and 10% paraformaldehyde. The brains were quickly removed and postfixed in 10% paraformaldehyde followed by 30% sucrose for 3 days. Ten-micron-thick coronal sections at the level of bregma +1 mm (rostrally) were cut on cryostat (Leica Microsystems LM3050S) and mounted on poly-L-lysine-coated slides. The stainings of double fluorescence labeling and terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling (TUNEL) were performed on the samples as described previously [8,12] without using Triton X-100 which can modulate the activity of SphK [13]. The following primary antibodies were used: anti-S1P1 (1:100, Cayman Chemical), anti-SphK1 (1:100, Abgent), anti-SphK2 (1:100, Lifespan Biosciences Inc.), and anti-NeuN (1:200; Chemicon International, Temecula, CA). The specimens were then subjected to TUNEL staining procedure with an in situ cell death detection kit (Roche Inc., Mannheim, Germany). For negative controls the primary antibodies were omitted and the same staining procedures were followed.
Measurement of neurological function
Three neurological scoring tests were carried out at 24 hours after MCAO among the groups. Firstly, rats were evaluated regarding composite sensorimotor scoring (3 to 18) described by Garcia et al. (18-point composite score) [2] : (1) spontaneous activity, (2) symmetry in limb movement, (3) forepaw outstretching, (4) climbing, (5) body proprioception, and (6) response to vibrissae touch. Higher scores indicate greater function. Next, another sensorimotor scoring (0–3) using the beam walking apparatus, described by Goldstein et al. [14] with modification, was measured in all animals (beam walking score). The rat’s performance was scored as follows; 0 or 1 for rats which fell off or stood stationary on the beam, and 2 or 3 indicated that the animals could walk on the beam less than or beyond 20cm during 1 min. The highest score of three trials was scored for each rat. Finally, 4-point paralytic scoring described by Bederson et al. [15] with slight modification was evaluated (paralytic score). The rat’s performance was scored as follows; 0 was assigned to comatose animals, 1 and 2 when there was decreased resistance to lateral push in the presence or absence of circling, 3 in the presence of forelimb flexion, and 4 was recorded in the absence of observable deficits.
Measurement of infarct volume
The animals were euthanized 24 hours after MCAO (immediately after evaluating neurological function). Their brains were sliced into 2 mm-thick coronal slices with a rodent brain matrix. Six selected sections (±5 mm, ±3 mm, and ±1 mm from the bregma) were stained for 10 min in a 2% solution of 2,3,5-triphenyltetrazolium chloride. The volume of total, cortex, and subcortex ischemic brain injuries was measured separately as previously described [8].
Statistical analysis
All values are expressed as the mean±SD. Statistical differences among the various groups were assessed with one-way analysis of variance followed by a Tukey-Kramer post hoc analysis. The differences between the two groups were compared using an unpaired t test. Differences of p<0.05 were considered significant.
Results
Mortality
The mortality rate was as follows: 8.3% (2 of 24 rats) in the control group, and 10% (1 of 10 rats) in both of the vehicle and FTY groups. No rats in the sham-operation group died. No statistical differences were observed among the groups with regard to physiological parameters (data not shown).
The temporal profile of S1P1, SphK1, and SphK2 expressions
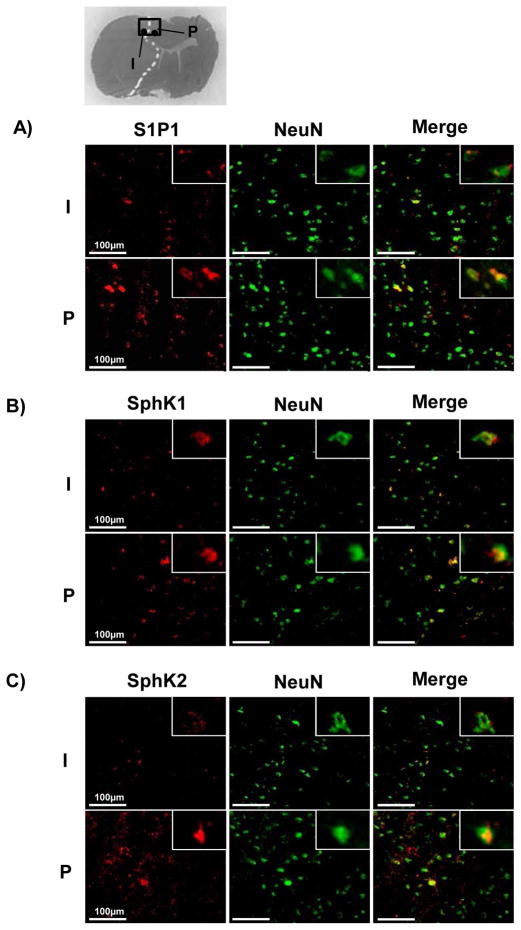
We examined the temporal profiles in the expressions of S1P1, SphK1, and SphK2 in MCA cortex (Fig. 1A) after transient MCAO. Their expressions were significantly decreased at 24 hours after MCAO (Fig. 1A–D). Immunofluorescence showed that the expressions of all targets were reduced in infarct cortex, but were preserved in periinfarct cortex, where some neurons co-expressed them (Fig. 1E and 2A–C).
Fig. 2.
Representative immunofluorescence of colocalizations (arrow) between neuronal nuclei (NeuN) and sphingosine-1-phosphate receptor-1 (S1P1; A), sphingosine kinase 1 (SphK1; B), or sphingosine kinase 2 (SphK2; C) in infarct (I) and periinfarct (P) cortices at 24 hours after middle cerebral artery occlusion (n=3). Scale bar: 100μm.
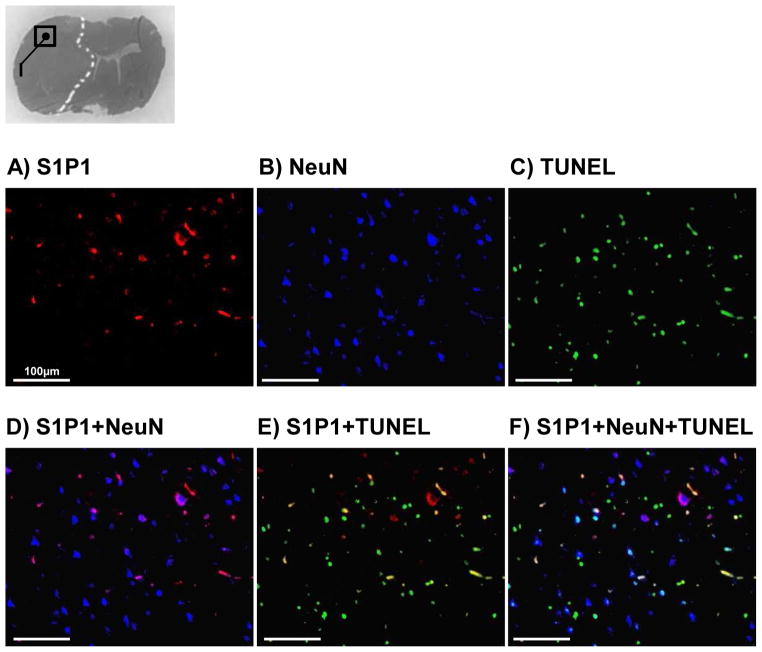
Immunohistochemical evaluations of S1P1 and TUNEL on neurons
Next, we focused on the expression of S1P1 and examined whether the receptor was observed on dead or surviving neurons in the infarct cortex, thought to be an area progressively dying (Fig. 3A–F). Immunofluorescence staining showed that S1P1 was expressed on some neurons in the infarct cortex (Fig. 3D). Additionally, TUNEL-S1P1 double positive cells were also seen in the area (Fig. 3E) but only a few dead neurons revealed S1P1 expression (Fig. 3F).
Fig. 3.
Representative immunofluorescence of sphingosine-1-phosphate receptor-1 (S1P1; A), neuronal nuclei (NeuN; B), terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling (TUNEL; C), and colocalizations of S1P1+NeuN (arrow; D), S1P1+TUNEL (arrow; E), and S1P1+NeuN+TUNEL (arrowhead; F) in the infarct MCA cortex at 24 hours after middle cerebral artery occlusion (n=3). Scale bar: 100μm.
The effect of S1P1 activation by FTY720 on neurons in MCAO
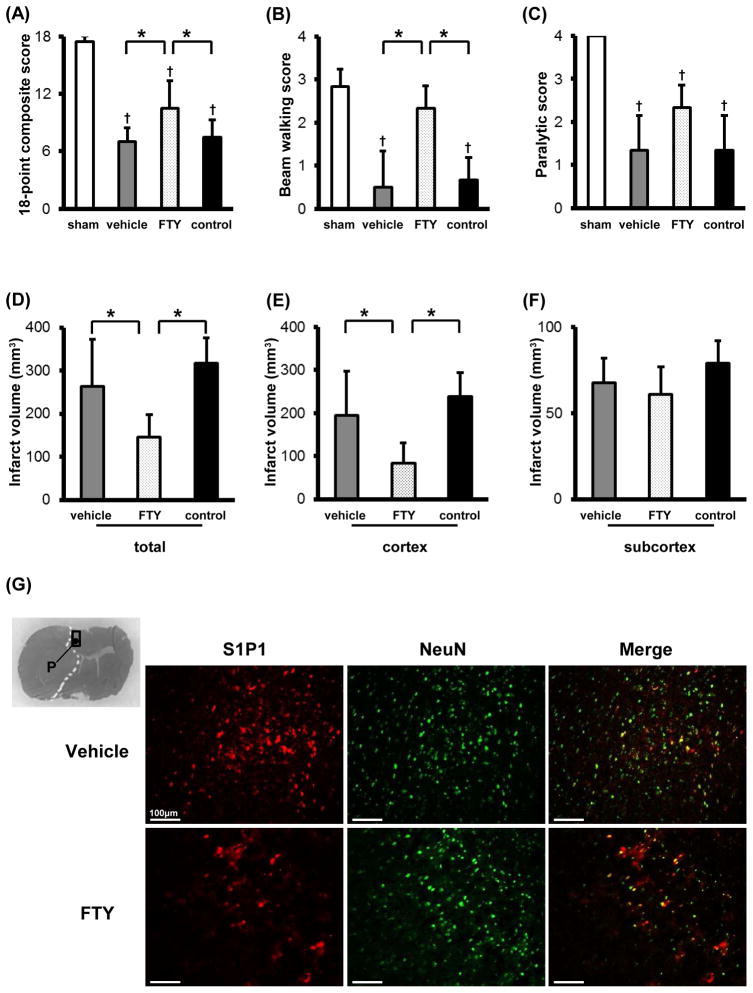
Significant improvements in sensorimotor tests such as the 18-point composite and beam walking scores were observed in the FTY group compared with the vehicle and control groups at 24 hours after MCAO (Fig. 4A and B), although there was no significant difference in paralytic score among the groups (Fig. 4C).
Fig. 4.
Quantitative analysis of neurological function (A, 18-point composite score; B, beam walking score; C, paralytic score) and infarct volume (D, total; E, cortex; F, subcortex) in groups treated with dimethyl sulfoxide (vehicle), 0.25mg/kg of FTY720 (FTY), no-treatment (control), and/or sham-operation at 24 hours after middle cerebral artery occlusion (n=6). Representative immunofluorescence colocalizations (arrow) between sphingosine-1-phosphate receptor-1 (S1P1) and neuronal nuclei (NeuN) treated with vehicle and FTY720 (G). Values are the mean±SD; †P<0.05 vs. sham, *P<0.05 vs. FTY, ANOVA. Scale bar: 100μm.
The total infarct volume in the FTY group (145 ± 53 mm3) was significantly smaller than in the vehicle (263 ± 110 mm3) and control (317 ± 58 mm3) groups at 24 hours after MCAO (Fig. 4D). This reduction in infarct size was derived from reduced infarct volume in the cortex, but not in the subcortex (Fig. 4E and F).
To examine whether S1P1 expression on neurons was modulated by FTY720 after transient MCAO, the region in periinfarct cortex was immunostained with anti-NeuN and anti-S1P1 antibodies. Although S1P1 expressions were seen on neurons in both the vehicle and FTY groups, the expressions were less observed in the FTY group (Fig. 4G).
Discussion
The present study showed that S1P1, SphK1, and SphK2 were decreased in the infarct cortex, whereas they were preserved in the periinfarct cortex after transient MCAO. We also demonstrated that FTY720, an S1P receptor agonist, acting upon S1P1 on neurons [8], reduced infarct volume in the cortex but not the subcortex, improving sensorimoter function at 24 hours after MCAO in rats.
SphK1 has an 80% sequence homology to SphK2 [13] and both produce physiological concentrations of S1P if either is expressed, indicating that they can compensate for each other [16]. The brain contains the largest amount of S1P [17] and neurons are the candidate cells for this significant source of S1P. The expressions of SphK2 and S1P1 mRNA were quickly upregulated (within 30 min) after experimental MCAO [18,19]. Ipsilateral SphK2 mRNA was found to be decreased in the striatum, which can be considered an ischemic core, and preserved in the cortex (penumbra) at 24 hours after 2 hour transient MCAO in mice [19]. In this study, Western blotting and immunofluorescence analyses demonstrated that S1P1, SphK1, and SphK2 were decreased in the infarct cortex but preserved in the periinfarct cortex after transient MCAO. It is reasonable that protein synthesis within dead regions would be exhausted after cerebral ischemia, due to a cerebral blood flow (CBF) reduction and related ATP depletion. Furthermore, neurons in the periinfarct cortex should be dying as CBF is decreased. Molecular signaling pathways in the periinfarct cortex occurring after transient MCAO such as S1P1, SphK1, and SphK2, are supposed to have important roles in cell death and thought to be therapeutic targets in cerebral ischemia. Although endogenous S1P may act as a neuroprotectant in cerebral ischemia, it was reported that S1P gradually decreased in the ischemic brain over 3 days after photochemical-induced permanent cerebral infarction [20]. Therefore, we suggested that pharmacological activation of the sphingosine signaling pathway may be a fruitful area of research for neuroprotection in cerebral ischemia.
FTY720 is derived from the fungal metabolite ISP-1 (myriocin; 2-amino-2-[2-(4-octylphenyl)ethyl] propane-1,3-diol) showed clinical efficacy in phase III clinical trials for multiple sclerosis [21] and was recently approved by the FDA for clinical use. The drug is rapidly phosphorylated in vivo to form FTY720 phosphate, which behaves as a full agonist on S1P1, S1P4, and S1P5 at low nanomolar concentration [22]. Although SphK1 is the major SphK isoform in many tissues and both endogenous SphK1 and SphK2 are capable of phosphorylating FTY720 in vivo, SphK2 is predominantly responsible for FTY720 phosphorylation [19,23]. In fact, it was reported that the well-established neuroprotective effect of FTY720 was preserved in SphK1−/− mice but lost in SphK2−/− mice [24]. On the other hand, the well-known biological effect of FTY720 is a depletion of immune cells, in particular lymphocytes by preventing their egress from lymph nodes via a mechanism which involves a downregulation of lymphocyte S1P1 [25]. The reduction of circling lymphocytes by FTY720 prevented the infiltration at the sites of inflammation in autoimmune disease and hepatic ischemia/reperfusion injury [26,27]. Moreover, increasing evidences support that cerebral invasion by lymphocytes is a significant pathogenesis in the neuroinflammation against cerebral ischemia [28,29]. Therefore, immunosuppression by FTY720 is thought to be a major neuroprotective action against ischemic stroke [30–32]. However, FTY720, which can cross the blood-brain barrier, may have additional beneficial effects via direct regulation of S1P receptors on brain cells [7]. Previously, we showed that FTY720 had a neuroprotective effect through antiapoptosis after transient MCAO [8]. The current study also demonstrated that FTY720 did not show a protective effect in the subcortex (mostly a sight of necrosis) but did provide protection in the periinfarct cortex at 24 hours after MCAO, suggesting that FTY720 directly acts in the periinfarct cortex where S1P1 expressions can be preserved, and induce its antiapoptotic effects. Again, FTY720 needs to be phosphorylated by SphK2 to acquire its bioactive roles [15] and local SphK activity is needed for phosphorylation of FTY720 [23]. In this study, we demonstrated that S1P1 and SphK2 were preserved in the periinfarct cortex at least 6 hours after MCAO. Therefore, we believe FTY720 could be phosphorylated by local SphK2 and act as a neuroprotectant in this region. Also, we speculate that FTY720 may inhibit the gradual delayed infarct expansion by activation of S1P1 in the periinfarct cortex. Another finding in this study is that S1P1 expression in the periinfarct cortex of the FTY720 group was less preserved compared with the vehicle group. Although it was reported that the function of FTY720 on neurons might be limited [33], our findings support the possible direct effect of FTY720 on neurons.
It is quite difficult to rescue neurons in the ischemic core after cerebral ischemia. In this study, we observed some TUNEL-negative neurons, which expressed S1P1 in the infarct cortex at 24 hours after MCAO. It was reported that S1P1 plays a role in neurogenesis and that endogenous S1P was upregulated in chronic phase after cerebral ischemia [20]. Although the chronic time course of S1P1 expression is unclear in the infarct cortex, we speculate that the neuronal S1P1 may have a beneficial role by promoting neurogenesis after cerebral ischemia. In this regard, further studies are needed.
Assessment of functional recovery is important to evaluate the efficacy of therapies to protect neurons in brain injuries including cerebral ischemia [34]. In this study, neurological evaluation, using an 18-point composite neuroscore and beam walking scores, demonstrated the neuroprotective effect of FTY720, but paralytic score failed to do so. These data support that the sensorimotor tests might be more sensitive indicators for neuronal dysfunction than the standard neurological test in experimental stroke models [35].
Considering the translation of these discoveries to clinical treatments, long-term behavioral and pathological outcomes should be evaluated [36,37]. Also, we did not evaluate the therapeutic time window of FTY720 even if S1P1 and SphKs were preserved in periinfarct cortex at least 6 hours after MCAO. We plan on addressing these issues in our next projects.
In conclusion, we showed that S1P1, SphK1, and SphK2 were downregulated in the infarct cortex, whereas they were preserved in the periinfarct cortex during the early phase of cerebral ischemia. FTY720 reduced infarct size in the cortex and improved neurological function after transient MCAO. We believe that FTY720 could be a novel compound available for treatment of stroke patients.
Acknowledgments
Acknowledgements and Funding
This study was partially supported by a grant (NS053407) from the National Institutes of Health to J.H.Z.
Footnotes
The authors report no conflicts of interest.
Conflict of Interest
none
References
- 1.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–42. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 2.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 3.Lapchak PA. Emerging Therapies: Pleiotropic Multi-target Drugs to Treat Stroke Victims. Transl Stroke Res. 2011;2:129–135. doi: 10.1007/s12975-011-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–39. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 5.Ford AL, An H, Vo KD, Lin W, Lee JM. Defining the Ischemic Penumbra Using Hyperacute Neuroimaging: Deriving Quantitative Ischemic Thresholds. Transl Stroke Res. 2012;3:198–204. doi: 10.1007/s12975-012-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 7.Dev KK, Mullershausen F, Mattes H, Kuhn RR, Bilbe G, Hoyer D, Mir A. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–74. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolland WB, 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, Zhang JH. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2011;111:213–7. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med. 2012;40:1908–13. doi: 10.1097/CCM.0b013e3182474bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–7. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrowski RP, Schulte RW, Nie Y, Ling T, Lee T, Manaenko A, Gridley DS, Zhang JH. Acute Splenic Irradiation Reduces Brain Injury in the Rat Focal Ischemic Stroke Model. Transl Stroke Res. 2012;3:473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–20. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB, Davis JN. Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury. J Neurosci Methods. 1990;31:101–7. doi: 10.1016/0165-0270(90)90154-8. [DOI] [PubMed] [Google Scholar]
- 15.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 16.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu, Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–28. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 17.Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–6. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 19.Blondeau N, Lai Y, Tyndall S, Popolo M, Topalkara K, Pru JK, Zhang L, Kim H, Liao JK, Ding K, Waeber C. Distribution of sphingosine kinase activity and mRNA in rodent brain. J Neurochem. 2007;103:509–17. doi: 10.1111/j.1471-4159.2007.04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–7. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 21.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 23.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–15. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 24.Pfeilschifter W, Czech-Zechmeister B, Sujak M, Mirceska A, Koch A, Rami A, Steinmetz H, Foerch C, Huwiler A, Pfeilschifter J. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochem Biophys Res Commun. 2011;413:212–7. doi: 10.1016/j.bbrc.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, Chiba K. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–48. [PubMed] [Google Scholar]
- 27.Martin M, Mory C, Prescher A, Wittekind C, Fiedler M, Uhlmann D. Protective effects of early CD4(+) T cell reduction in hepatic ischemia/reperfusion injury. J Gastrointest Surg. 2010;14:511–9. doi: 10.1007/s11605-009-1104-3. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–12. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 29.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251–6. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- 31.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–50. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, Liao JK, Lo EH, Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–29. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod) J Neurol Sci. 2008;274:13–7. doi: 10.1016/j.jns.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 34.Hartman RE. A Brief History of Behavioral Assessment Following Experimental Traumatic Brain Injury in Juveniles. Transl Stroke Res. 2011;2:433–437. doi: 10.1007/s12975-011-0132-y. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J Neurol Sci. 2000;174:141–6. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 36.Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, Shamloo M. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Methods. 2011;196:247–57. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winters A, Taylor JC, Ren M, Ma R, Liu R, Yang SH. Transient Focal Cerebral Ischemia Induces Long-Term Cerebral Vasculature Dysfunction in a Rodent Experimental Stroke Model. Transl Stroke Res. 2012;3:279–285. doi: 10.1007/s12975-012-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]