Abstract
In order to reduce calories in foods and beverages, the food industry routinely uses non-nutritive sweeteners. Unfortunately, many are synthetically derived, and many consumers have a strong preference for natural sweeteners, irrespective of the safety data on synthetic non-nutritive sweeteners. Additionally, many non-nutritive sweeteners elicit aversive side tastes such as bitter and metallic in addition to sweetness. Bitterness thresholds of acesulfame-K (AceK) and saccharin are known to vary across bitter taste receptors polymorphisms in TAS2R31. RebA has shown to activate hTAS2R4 and hTAS2R14 in vitro. Here we examined bitterness and sweetness perception of natural and synthetic non-nutritive sweeteners. In a follow-up to a previous gene-association study, participants (n=122) who had been genotyped previously rated sweet, bitter and metallic sensations from rebaudioside A (RebA), rebaudioside D (RebD), aspartame, sucrose and gentiobiose in duplicate in a single session. For comparison, we also present sweet and bitter ratings of AceK collected in the original experiment for the same participants. At similar sweetness levels, aspartame elicited less bitterness than RebD, which was significantly less bitter than RebA. The bitterness of RebA and RebD showed wide variability across individuals, and bitterness ratings for these compounds were correlated. However, RebA and RebD bitterness did not covary with AceK bitterness. Likewise, single nucleotide polymorphisms (SNPs) shown previously to explain variation in the suprathreshold bitterness of AceK (rs3741845 in TAS2R9 and rs10772423 in TAS2R31) did not explain variation in RebA and RebD bitterness. Because RebA activates hT2R4 and hT2R14, a SNP in TAS2R4 previously associated with variation in bitterness perception was included here; there are no known functional SNPs for TAS2R14. In present data, a putatively functional SNP (rs2234001) in TAS2R4 did not explain variation in RebA or RebD bitterness. Collectively, these data indicate the bitterness of RebA and RebD cannot be predicted by AceK bitterness, reinforcing our view that bitterness is not a simple monolithic trait that is high or low in an individual. This also implies consumers who reject AceK may not find RebA and RebD aversive, and vice versa. Finally, RebD may be a superior natural non-nutritive sweetener to RebA, as it elicits significantly less bitterness at similar levels of sweetness.
Keywords: bitterness, non-nutritive sweetener, rebaudioside A, rebaudioside D, genetics, taste phenotype, Project GIANT-CS
1. Introduction
Rebaudioside A and Rebaudioside D are glycosides extracted from Stevia rebaudiana (Bertoni) Bertoni, a perennial shrub native to Paraguay and Brazil. The Guaraní Paraguayan Indians and Mestizos have used Stevia leaves for centuries to sweeten teas (Bertoni, 1905), beer and tobacco (Melville, 1941) (see (Lewis 1992) for a review). The sweetness is elicited by diterpenic ent-kaurene glycosides, each exhibiting a steviol aglycone (13-hydroxykaur-16-en-18-oic acid). The Joint FAO/WHO Expert Committee on Food Additives reported that 9 glycosides are found in Stevia leaves (JECFA 2010); however, since publication of this report, an additional 26 glycosides have been identified (Ohta et al. 2010) (see (Wölwer-Rieck 2012) for a comprehensive list). These glycosides differ greatly in concentration in the leaf and are also influenced by the plants’ genotype and growing conditions. Typically, stevioside and rebaudioside A (RebA) are the two most abundant glycosides (Ohta et al. 2010). Currently, purified RebA, purified stevioside, and purified RebA/stevioside blends have GRAS (generally recognized as safe) status in the United States, while whole leaf Stevia and crude Stevia extracts are not approved for use in foods. It is likely additional purified steviol glycosides will be approved in the near future.
These glycosides differ in their sensory properties, as shown by (DuBois et al. 1991; Hellfritsch et al. 2012; Phillips 1989; Schiffman et al. 1995a; Schiffman et al. 1995b). Although Stevia leaves and its glycoside extracts are known for their sweetness, many also elicit undesirable side-tastes, including bitterness (DuBois et al. 1991; Hellfritsch et al. 2012; Schiffman et al. 1995b).
The ability to detect bitterness from various bitter compounds differs greatly across individuals. Much of the variation can be explained by polymorphisms in bitter taste receptors (Dotson et al. 2012; Duffy et al. 2004; Duffy et al. 2010; Feeney 2011; Hayes et al. 2011; Pronin et al. 2007; Roudnitzky et al. 2011; Wang et al. 2007). Additional variance can be attributed to age (Mennella et al. 2010), and fungiform papillae density (Bartoshuk et al. 1994; Duffy and Bartoshuk 2000) as well as other factors (Tepper et al. 2003). Specific to non-nutritive sweeteners, variation in bitter taste receptor genes can explain differences in the bitterness arising from these sweeteners.
Kuhn and colleagues (Kuhn et al. 2004) showed saccharin and AceK activate bitter taste receptors hT2R31 and hT2R43 (encoded by the TAS2R31 and TAS2R43 genes near chromosome 12p13.2) in vitro. Subsequently, Pronin and colleagues found polymorphisms within TAS2R31, specifically the Arg35Trp allele predicted bitterness perception of sampled saccharin in 55 subjects (Pronin et al. 2007). Presumably, these SNPs might also influence AceK bitterness, as earlier psychophysical evidence suggested saccharin and AceK bitterness covary across individuals (Horne et al. 2002). Roudnitzky (Roudnitzky et al. 2011) confirmed this, showing that the bitterness of saccharin and AceK is associated with several polymorphisms in TAS2R31, with the greatest variation explained by the Arg35Trp allele. Individuals who expressed Trp at position 35 perceived greater bitterness than those with an Arg at this residue. This finding was confirmed in vitro, where taste receptor cells expressing the Arg35 variant show little to no activation when exposed to saccharin or AceK (Roudnitzky et al. 2011). They also identified eight additional SNPs that further modify the activation of TAS2R31 in varying amounts (D45H, M162L, Q127E, A226V, L237F, V240I, P276R and W281C) (Roudnitzky et al. 2011).
Elsewhere, Dotson et al. (Dotson et al. 2008) reported that the Ala187Val polymorphism in TAS2R9 (chromosome 12p13) influenced the activation of cells exposed to three pharmaceuticals commonly described as bitter: ofloxacin, procainamide and pirenzapine. The Val187 allele was associated with limited to no activation, compared to the Ala187 allele. Data from our laboratory indicates this SNP significantly predicts the bitterness from AceK (Allen et al. 2013). Unexpectedly, the pattern that was observed is the opposite of that seen in vitro by Dotson et al. for different stimui; our data suggest the Val197 allele is the high function allele, at least with respect to AceK (Allen et al. 2013).
Recently, Hellfritsch and colleagues (Hellfritsch et al. 2012) expressed all 25 bitter taste receptors in cell culture and treated them with 1mM stevioside. Two of these receptors, hT2R4 and hT2R14, were activated by stevioside. Subsequently, these two receptors were tested with 7 additional glycosides (RebA, RebB, RebC, RebD, dulcoside A, steviolbioside and rubusoside). All of the steviol glycosides tested activated these receptors, except steviolbioside, which did not activate hT2R14, and RebD which did not activate either receptor hT2R4 or hT2R14 (Hellfritsch et al. 2012). There are no polymorphisms reported to be functional at a behavioral level for TAS2R14, while published (Hayes et al. 2011) and unpublished reports (Alarcon et al. 2008) suggest the Val96Leu allele in TAS2R4 may associate with differences in bitterness perception.
Few studies have compared bitterness and sweetness across multiple non-nutritive sweeteners (Kamerud and Delwiche 2007). Here, we report the sweetness and bitterness intensity of two natural non-nutritive sweeteners (RebA and RebD), as well as two commonly used synthetic non-nutritive sweeteners (AceK and Aspartame).
Multiple studies indicate polymorphisms in bitter taste receptor genes (TAS2Rs) influence the perception of bitterness from synthetic sulfonyl amide sweeteners like saccharin and AceK. Likewise, anecdotal reports suggest the bitterness from natural Stevia derived sweeteners is not experienced universally. However, it remains unknown whether a) the bitterness of natural Stevia derived sweeteners, specifically rebaudioside A and rebaudioside D, covary with the bitterness of sulfonyl amide sweeteners across individuals, and b) whether variable bitterness in rebaudioside A and D might be explained by genetic polymorphisms in TAS2R genes. Here, we compare the sensory profiles of Stevia derived glycosides, describe the variation in the bitterness of these stimuli across individuals, and explore whether this variability can be predicted by polymorphisms in genes previously implicated in the bitterness of other non-nutritive sweeteners (i.e. TAS2R9, and TAS2R31).
2. Materials and Methods
2.1 Overview
Here, we present data from a follow-up study to a larger project on the genetics of oral sensation (Project GIANT-CS; (Allen et al. 2013; Byrnes and Hayes 2013)). Participants who had completed the main study and had been genotyped were invited to return to our laboratory to taste a variety of tastants not originally included in the main study, including multiple non-nutritive sweeteners and disaccharides. 122 participants returned to complete the follow-up study. The study was conducted in the Sensory Evaluation Center at the Pennsylvania State University in individual testing test booths under white light. After participants were re-consented in writing for the follow-up study, they participated in a 3-minute training session in a multifunction space in our facility prior to entering the test booths. All procedures for the main project and follow-up study were approved by the Pennsylvania State University Institutional Review Board (protocol numbers #33176 and #40921). In isolated testing booths, participants rated bitter, sweet, and metallic sensations on a computerized general Labeled Magnitude Scale (gLMS).
2.2 Participants
Participants were screened prior to the start of the main study. Eligibility criteria included: between 18–45 years old, not pregnant or breastfeeding, non-smoker (had not smoked in the last 30 days), no known defects of smell or taste, no lip, cheek or tongue piercings, no history of any condition involving chronic pain, not currently taking any prescription pain medication, no reported history of choking or difficulty swallowing and no history of thyroid disease.
Here, we report data from 122 participants (44 men), with a mean age 27.7 (±7.89) years. Self-reported race and ethnicity was collected based on criteria provided by the 1997 OMB Directive 15. This population is largely of European ancestry (n=88), with marginal representation from other ancestry, African (n=2) and Asian (n=21), with 9 individuals choosing to not disclose their ancestry.
2.3 Training Session
Participants were asked to rate sweetness, bitterness and metallic on a gLMS. Due to potential confusion between bitter and metallic, a brief training session was implemented to provide individuals examples of these three taste qualities. After consent was obtained, participants went through a short 2 to 3 minute training session from project staff individually or in small groups (no more than 3 to a group). Four 10mL samples were presented labeled samples as exemplars of specific taste qualities: ‘sweet’ = 250mM sucrose (Domino), ‘bitter’= 0.05mM quinine (SAFC), ‘bitter and sweet’= 263mM sucrose and 0.5mM quinine and ‘metallic’= 1.0mM FeSO4 (J.T. Baker). The concentrations of the training samples were chosen to be similar to the test samples, eliciting sweet, bitter and mixture and metallic (all well above threshold values). The presentation order of the training stimuli was fixed (sweet, bitter, sweet and bitter, and metallic). At the start of the training session, participants were given a spit cup and a water cup. Participants were provided the following instructions: “This training session is to help familiarize you with the taste qualities you will be asked to rate in this study. The three qualities are sweetness, bitterness and metallic”. The researcher then provided instructions on how to taste the samples: “Put the whole sample in your mouth, swish for 3 seconds and spit it out. Then rinse with water”. The samples were presented in a clear plastic medicine cup with the respective labels (sweet, bitter, sweet and bitter, and metallic). The researcher presented each sample one at a time and participants were told the taste quality(s). After tasting, participants were told, that if they experience this taste they would rate the perceived intensity on the respective scale. A mixture of sucrose and quinine was provided to give an example of a sample that may have multiple taste qualities (sweet and bitter). Prior to entering the testing booths, participants were asked to rinse with water to remove any lingering sensations.
2.4 Psychophysical Scaling of Test stimuli
Participants reported perceived intensity of the test stimuli by rating on a general Labeled Magnitude Scale (gLMS). The gLMS anchors are 0 (‘no sensation’) to 100 (’the strongest imaginable sensation of any kind’), with descriptors at 1.4 (‘barely detectable’), 6 (‘weak’), 17 (‘moderate’), 35 (‘strong’) and 51 (‘very strong’). All participants had been trained to use the gLMS in the main study, and they were reoriented to the scale before tasting test stimuli. This was done by asking participants to rate 6 imagined or remembered sensations on the gLMS. The orientation included both oral and non-oral items to promote ratings to be in context of all items, not just taste (Hayes et al. 2013). Data were collected using Compusense five, version 5.2 (Guelph, Ontario, Canada). We considered collecting bitter recognition thresholds (e.g. (Hellfritsch et al. 2012)), but we chose to focus on suprathreshold measures here because they are more relevant to food sensations and intake (e.g. (Duffy et al. 2004), (Hayes et al. 2010), (Lucas et al. 2011)) and for greater efficiency (see (Genick et al. 2011) for a discussion of threshold versus suprathreshold phenotyping methods in genetic association studies).
2.5 Test Stimuli
Five food grade test samples were presented: 219mM sucrose (Domino), 20mM gentiobiose (Biosynth), 6.8mM aspartame (Spectrum), 1.65mM rebaudioside A (Enliten from Corn Products International), 1mM rebaudioside D (kindly donated by J. Delwiche at PepsiCo). Concentrations of the non-nutritive sweeteners were selected to be above bitterness threshold and have similar sweetness. RebD was near saturation at 1mM, however we chose to use a higher concentration of RebA because this was a gene-association study. That is, we wanted to maximize bitterness to avoid potential floor effects that might obscure any associations between perceived bitterness and candidate polymorphisms. Sucrose and gentiobiose were included as purely sweet and purely bitter controls. All were prepared using reverse osmosis (RO) water. Test stimuli (10mL) were presented at room temperature under white lighting. Stimuli were presented in duplicate in clear plastic medicine cups labeled with a three-digit blinding code. Presentation order was counterbalanced in a blocked Williams Design, with each stimulus being presented once before a duplicate was presented. Participants had a 30 second break enforced between samples, and they rinsed with RO water as needed.
During the main study (Project GIANT-CS), participants sampled (in the same manner as above) a 25mM concentration of Acesulfame K (Spectrum), among other stimuli (see (Allen et al. 2013; Byrnes and Hayes 2013)). Participants rated sweetness, bitterness, burning/stinging, sourness, savory/umami, and saltiness of the sample on a separate gLMS for each quality. AceK ratings from the participants (n=122) who completed the follow-up study were reanalyzed here to facilitate direct comparisons across stimuli within a single group of individuals. (Because of participant overlap, these data should not be taken as independent replication of our prior report (Allen et al. 2013). Although the total number of attributes rated differed across sessions, sweet and bitter, the primary attributes of interest where provided in each session.
2.5 Genetic Analysis
As reported elsewhere (Allen et al. 2013), DNA was collected using Oragene saliva collection kits according to manufacturer instructions (Genotek Inc, Ontario, Canada). Genotypes for selected SNPs (single nucleotide polymorphisms) in TAS2R4 and TAS2R38 on chromosome 7 and TAS2R9 and TAS2R31 on chromosome 12 were determined using Sequenom MassARRAY technology (Sequenom, San Diego, CA). Primers were acquired from Integrated DNA Technologies (Coralville, Iowa, USA). Genotypes were assigned automatically via MassARRAY software (Sequenom) and inspected by two technicians. 15% of samples are randomly selected and subjected to a secondary analysis to ensure accuracy.
A tag SNP approach was used due our inability to obtain functional primers for the Arg35Trp (rs10845295) allele in TAS2R31. Repeated attempts were made on multiple platforms (Sequenom MassARRAY and custom TaqMan assays) and these attempts all failed vendor quality control standards, requiring us to use rs10772423 as a tag SNP instead.
2.6 Statistical Analysis
Analyses were performed using SAS 9.2 (Cary, NC). SNP analyses were performed individually using analysis of variance (ANOVA) via proc mixed. Posthoc comparisons were made via the Tukey-Kramer method.
3. Results
Comparing sweetness and bitterness ratings of sweeteners
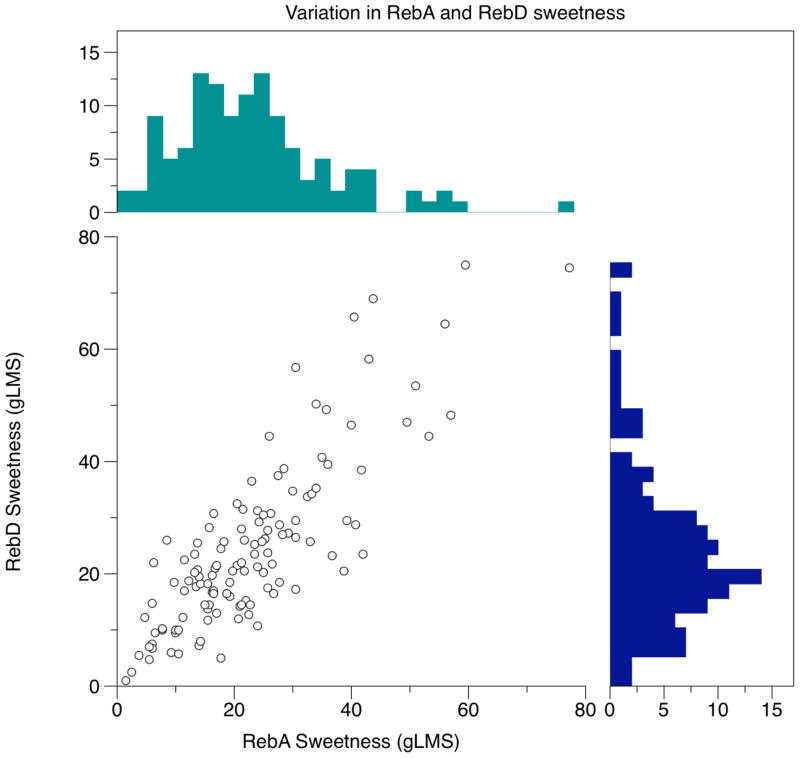
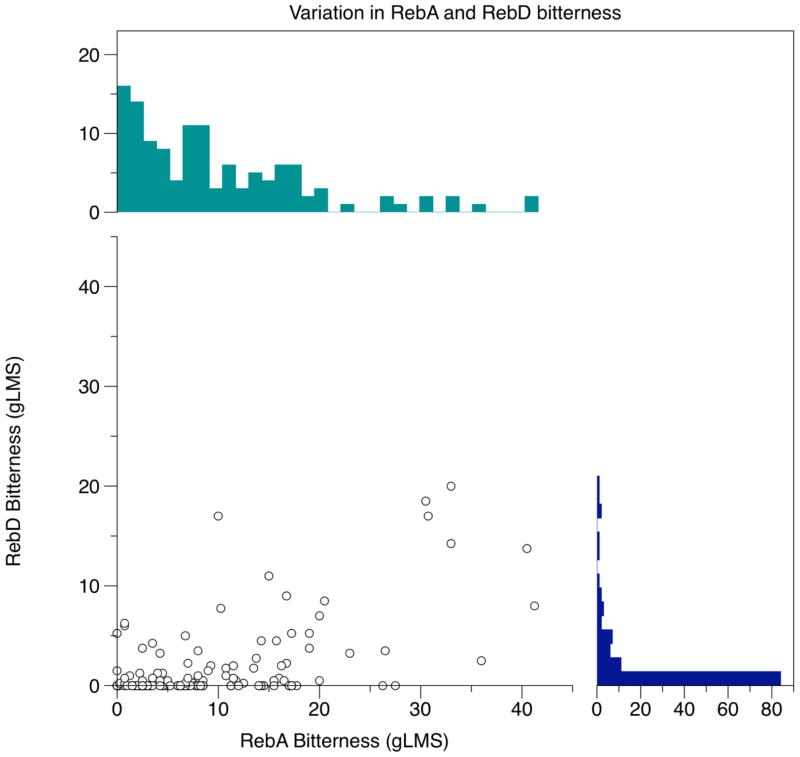
Many non-nutritive sweeteners elicit both sweetness and bitterness (DuBois et al. 1991; Hellfritsch et al. 2012; Kamerud and Delwiche 2007; Schiffman et al. 1995b). Of these reports, many compare the sweetness and bitterness to a fixed concentration of reference stimuli. However, use of a fixed modulus (reference stimulus) may minimize real individual differences across people; thus we used a generalized scale (i.e. a gLMS) here to better reflect differences across individuals, similar to work by Kamerud and Delwiche (Kamerud and Delwiche 2007). Here, we measure the variation the sweetness and bitterness of RebA and RebD at suprathreshold concentrations, using a gLMS (without using reference samples) (Figure 1). By plotting sweetness of RebA and RebD simultaneously, we observed substantial variation in the perceptual sweetness intensity (Figure 1a). Reported sweetness of RebA ranges from 0 to 41.25 and RebD sweetness ranges from 1 to 75. Figure 1b shows variation bitterness ratings of RebA and RebD, with bitterness of RebA ranging from 1.5 to 77.25 while RebD ranges from 0 to 20 on a gLMS. Here, we document the wide range of individual variation in RebA and RebD sweetness and bitterness in a large genetically informed cohort.
Figure 1.
Scatter plots showing (a) sweetness and (b) bitterness intensities for RebA and RebD to illustrate covariation of these compounds within an individual, and histograms are shown along the axes to illustrate the substantial amount of variation found across individuals. Mean sweetness was not significantly different between RebA and RebD, although both show substantial individual differences. Mean bitterness differed, with RebD showing significantly less bitterness at the concentrations tested here. Again, substantial individual differences in bitterness were observed for these sweeteners.
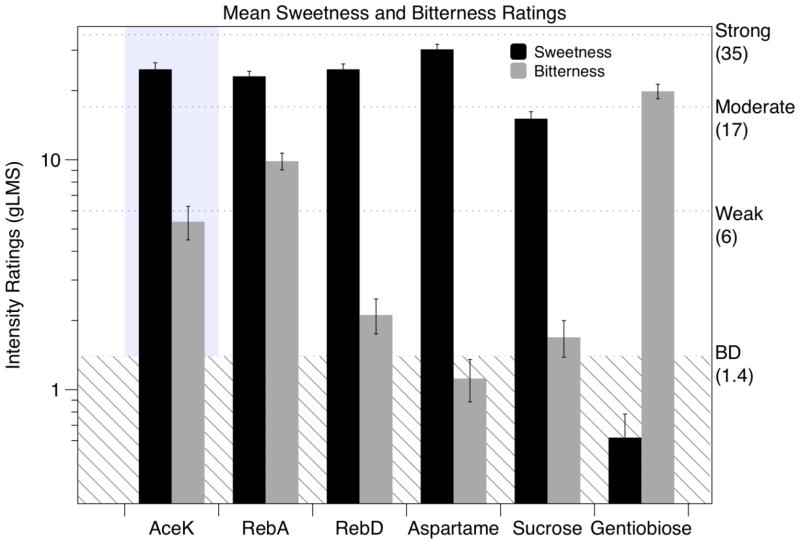
To allow comparison across non-nutritive sweeteners, AceK ratings from the same individuals (n=122) from day 1 of the main study are also shown here (gray box in Figure 2). In ANOVA comparing the non-nutritive sweeteners (i.e. excluding the disaccharides sucrose and gentiobiose), mean bitterness differed across stimuli [F (3,363) = 37.20; p <0.0001]. Bitterness from RebA (9.9±0.8 SEM) was greater than AceK (5.4±0.9), RebD (2.1±0.4)) and Aspartame (1.1±0.2; all Tukey p’s <0.0001). AceK was more bitter than RebD (p = 0.002) and aspartame (p < 0.0001). There was not sufficient evidence to conclude that RebD was more bitter than aspartame (p = 0.70). Figure 2 also shows as expected that Gentiobiose, a natural disaccharide containing a β-(1–6) linkage, was predominantly bitter with little to no sweetness, while the reverse was true for sucrose.
Figure 2.
Mean (±Std Error) gLMS ratings for bitterness and sweetness of AceK (collected in a separate test session: indicated by the gray box), RebA, RebD, aspartame, sucrose and gentiobiose are reported here. The sweetness ratings for AceK, RebA and RebD were not statistically different while bitterness was significantly different across the four non-nutritive sweeteners (see text). Adjectives refer to semantic labels on a general Labeled Magnitude Scale (gLMS). BD refers to ‘barely detectable’.
The mean sweetness for the four non-nutritive sweeteners fell between moderate and strong on a gLMS. In ANOVA, the sweetness ratings differed across stimuli [F(3,363) = 4.53; p = 0.004], and this effect was driven by the ratings for aspartame (30.2±1.6 SEM), which was sweeter than RebA, RebD and AceK (all p’s <.05). Notably, the sweetness of AceK, RebA and RebD (24.7±1.68, 23.1±1.2, and 24.8±1.36, respectively) were not statistically different from one another (all p’s > 0.8), which is notable given that they did differ with regard to bitterness.
Figure 3 shows correlations for the bitterness of RebA with the bitterness of RebD, AceK and Aspartame. The bitterness of RebA and RebD were significantly correlated with each other (R2=0.33; p<0.0001). Notably, RebA bitterness is not significantly correlated with bitterness of AceK (R2=0.03; p=0.055). Although the association between RebA and Asp was significant, the amount of variance explained was low (R2=0.08; p<0.0013).
Figure 3.
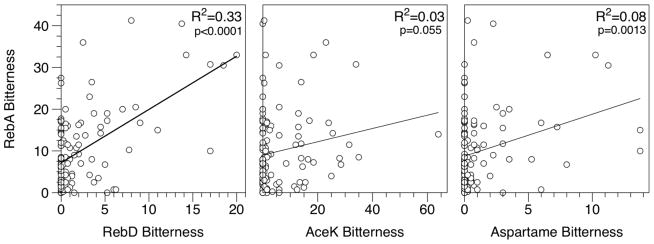
Correlations of bitterness ratings between RebA and RebD, AceK and aspartame.
TAS2R9 SNP explains AceK bitterness but not bitterness of RebA and RebD
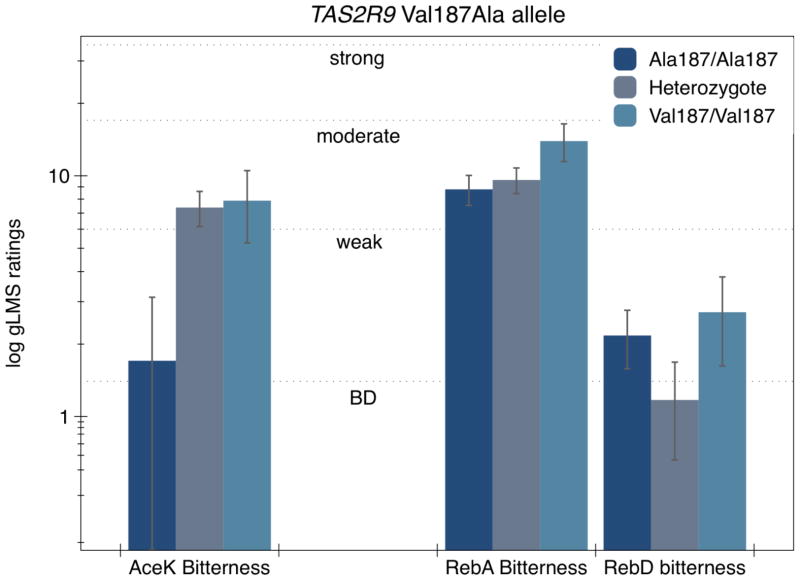
Previously, bitterness ratings of AceK collected from day 1 (Allen et al. 2013) were associated with a SNP in TAS2R9, (Val187Ala, rs374184; chr. 12). Here, to allow direct comparisons for these same polymorphisms across different non-nutritive sweeteners, we have reanalyzed the AceK ratings taken from Day 1 for the 122 individuals reported here. (Because of participant overlap, these data should not be taken as independent replication of our prior report (Allen et al. 2013); they are included here to facilitate comparisons with RebA and RebD). Figure 4 shows sweet and bitter ratings for AceK, RebA and RebD as a function of the Val187Ala SNP in TAS2R9. Here, a primarily European mixed ancestry cohort contained 44 Ala homozygotes, 59 heterozygtoes and 13 Val homozygtoes (genotype frequencies did not vary from Hardy Weinberg equilibrium chi-sq = 1.06 p = 0.30). The bitterness from AceK was predicted by the amino acid at position 187, with Val homozygotes and heterozygotes perceiving significantly more bitterness than Ala homozygotes (7.88±2.6, 7.38±1.2 and 1.70±1.4 respectively) [F(2,113) = 5.10; p = 0.0076]. There was no significant difference between Val homozygotes and heterozygotes (Tukey p = 0.98). Ala homozygotes had lower mean bitterness compared to heterozygotes (Tukey p = 0.009) and a similar trend was observed between Ala homozygotes and Val homozygotes (p = 0.10). AceK sweetness did not differ by Val187Ala genotype [F(2,113) = 0.91; p=0.40].
Figure 4.
Effect of the TAS2R9 Val187Ala polymorphism on the bitterness of AceK, RebA and RebD. As expected, the bitterness of AceK (collected on a separate day) was significantly different across genotype for these individuals; conversely, no effect of genotype was observed for RebA or RebD (see text).
In this cohort, the bitterness of RebA and RebD were not explained by Val187Ala allele. RebA bitterness did not differ across genotypes with Val homozygotes rating 13.9±2.5, heterozygotes 9.6±1.2, and Ala homozygotes 8.8±1.4 [F(2,113) = 1.68; p = 0.19]. Similarly, RebD bitterness did not differ [F(2,113) = 0.41; p = 0.66] across Val homozygotes, heterozygotes and Ala homozygotes (2.7±1.1, 1.7±0.5 and 1.2±0.6 respectively).
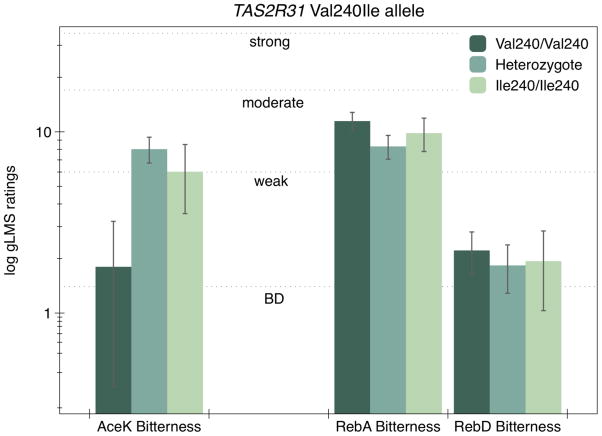
TAS2R31 SNPs explain AceK bitterness but not bitterness of RebA and RebD
Polymorphisms in TAS2R31 (chr. 12) have shown to mediate the bitterness response to sulfonyl amide sweeteners (Allen et al. ; Pronin et al. 2007; Roudnitzky et al. 2011). Figure 5 shows the bitterness ratings of RebA, RebD and AceK across Val240Ile genotypes. Here, Val240Ile (rs10772423) failed to explain variation in the bitterness of RebA [F(2,113) = 1.49; p = 0.23]. Similarly, Val240Ile did not explain variation in RebD bitterness [F(2,113) = 0.12; p = 0.89], which was quite low (1.9±0.9, 1.8±0.5 and 2.2±0.6, for Ile homozygotes, heterozygotes and Val homozygotes, respectively). AceK ratings from day 1 were included to make the point that Val240Ile genotypes explained the bitterness of AceK in these same individuals [F(2,113) = 5.33; p = 0.006]. Ile homozygotes (n=19) and heterozygotes (n=52) perceived greater bitterness compared to the non-functioning, Val homozygotes (n=45) (genotype frequencies did not vary from Hardy Weinberg equilibrium X2 = 0.141 p = 0.71). Conversely, AceK sweetness ratings did not differ across genotype [F(2,113) = 0.52; p = 0.60].1
Figure 5.
Same as Figure 4, except for the Val240Ile polymorphism in TAS2R31. AceK bitterness ratings were significantly different across genotype, as expected, while there was no evidence of a effect for RebA and RebD (see text).
TAS2R4 SNP does not explain bitterness of RebA and RebD
Previously, RebA was shown to activate hT2R4 in vitro (Hellfritsch et al. 2012). A polymorphism (rs2234001) in TAS2R4 (chr. 7) which reportedly explains variation in coffee bitterness (Hayes et al. 2011), was tested here to determine if this SNP can explain variable bitterness from RebA. The Val96Leu allele in TAS2R4 failed to explain variation in the bitterness of RebA [F(2,107) = 1.79; p = 0.17] nor RebD bitterness [F(2,107) = 0.38; p = 0.68].
4. Discussion
Although the main function of non-nutritive sweeteners is to provide sweetness with negligible calories, they often elicit undesirable bitter and metallic sensations (DuBois et al. 1991; Ellis 1995; Helgren et al. 1955), limiting their utility. Moreover, many non-nutritive sweeteners are synthetic which are less desirable to many consumers, resulting in greater interest in natural non-nutritive sweeteners like Stevia and Monkfruit extracts. Currently, purified RebA extract (from the leaves of Stevia rebaudiana) is approved for use in foods in the United States as a GRAS ingredient. However, other glycosides within Stevia also elicit a wide range of sweet and bitter sensations (Hellfritsch et al. 2012). Hellfritsch and colleagues recently described sweetness and bitterness response functions for 9 different Stevia glycosides. To generate these response curves, individuals were trained using sweet and bitter references (sucralose and rubusoside) at fixed concentrations. Their data suggested RebD might be a superior sweetener to RebA, as it has a steeper sweetness response curve with lower bitterness compared to RebA. Here, we confirm these data using a different psychophysical approach (i.e. measuring individual perceived intensities without providing a standard reference). We did not generate a dose response function; rather, a concentration was selected to provide at least moderate sweetness on a gLMS as well as some bitterness. At isosweet concentrations, RebD elicited significantly less bitterness than RebA. However, we should note that because the concentrations of RebA and RebD in our study differed, it remains possible that when tested at the same concentration (rather than equivalent sweetness), RebA may not be significantly more bitter than RebD. Still, our data, coupled with that of Hellfritsch et al. suggest it may be desirable to commercialize RebD as a GRAS ingredient, or to selectively breed for Stevia plants with higher RebD content.
Many non-nutritive sweeteners exhibit a hyperbolic dose response function, which limits their maximal sweetness. This is why we use the phrase non-nutritive sweeteners rather than ‘high-intensity sweeteners’; the later is misleading given the linear dose-response function for bulk mono- and disaccharides (DuBois et al. 1991; Hayes 2008). At the concentration used here for RebA (1.65 mM), we are in the flat region of the sweetness response curve (DuBois et al. 1991). Conversely, the bitterness response curve for RebA continues to increase linearly. That is, it may be possible to use a lower dose and still achieve the desired level of sweetness with lower levels of bitterness. However, because we were specifically interested in the covariation of bitterness across sweeteners, we intentionally selected concentrations that would ensure participants perceived some bitterness. Overall, aspartame bitterness was very low, consistent with previous reports (DuBois et al. 1991; Kamerud and Delwiche 2007).
Roudnitzky and colleagues (Roudnitzky et al. 2011) showed that AceK bitterness recognition thresholds are associated with polymorphisms in TAS2R31. Subsequently, we extended this work using a tag SNP approach, showing that TAS2R31 polymorphisms could also explain variation in the suprathreshold bitterness of AceK (Allen et al. 2013). Here, we show that a tag SNP thought to be in linkage disequilibrium with the functional Arg35Trp allele in TAS2R31 does not explain variability in the bitterness perception of RebA or RebD. While activation of TAS2R31 with AceK has been well documented (Kuhn et al. 2004; Meyerhof et al. 2010; Roudnitzky et al. 2011), we are unaware of any reports showing RebA and RebD activate TAS2R31 in vitro or in vivo. Likewise, putatively functional polymorphisms in TAS2R9 did not predict the bitterness of RebA or RebD. The inability of TAS2R31 and TAS2R9 SNPs to predict RebA and RebD bitterness would be expected given the absence of a relationship between AceK bitterness and RebA/RebD bitterness. It is likely that the bitterness from RebA and RebD in vivo occur via other bitter taste receptors, such as hT2R4 and hT2R14 (Hellfritsch et al. 2012). Additional work is needed to determine if allelic variations alter the function of hT2R14, and thus might explain variable bitterness from RebA and RebD. Here, we tested one putatively functional SNP in TAS2R4 and did not find any evidence of a relationship; however, full sequencing of this gene would be required to rule out an effect of TAS2R4 SNPs on RebA bitterness. Future work should explore potential relationships between additional TAS2R4 and TAS2R14 polymorphisms in relation to steviol glycoside recognition thresholds and suprathreshold bitterness.
5. Conclusions
Here we show that steviol glycoside extracts from Stevia differ in their bitterness at suprathreshold concentrations that are isosweet. Purified RebD may be a superior natural non-nutritive sweetener compared to RebA, as it elicits less bitterness than RebA. Functional SNPs in TAS2R31 known to explain variation in AceK and saccharin bitterness were unable to explain variability bitterness of RebA or RebD. This is consistent with psychophysical data showing that AceK bitterness was not correlated with the bitterness of RebA or RebD. However, we also note that AceK data were collected in a separate testing context on a different day, which may have attenuated our ability to observe a potential relationship. Nonetheless, taken together, the psychophysical data and genetic data both suggest the absence of a relationship between AceK bitterness and RebA and RebD bitterness. Collectively, these data are consistent with our position that bitterness is not a simple monolithic trait that is high or low in an individual (Hayes et al. 2008; Hayes et al. 2011). Practically, this suggests consumers who report aversive sensations from RebA and RebD may not report aversive sensations from AceK and vice versa. Whether variable bitterness in Stevia extracts can be explained by polymorphisms in other bitter taste genes (TAS2Rs) remains to be determined.
Acknowledgments
Funding
This work was partially supported by a National Institutes of Health grant from the National Institute National of Deafness and Communication Disorders [DC010904] to the corresponding author, United States Department of Agriculture Hatch Project PEN04332 funds, funds from the Pennsylvania State University and a VA shared equipment grant to JEM.
This manuscript was completed in partial fulfillment of the requirements for a Master of Science degree at the Pennsylvania State University by the first author. The authors would like to thank Dr. Emma L. Feeney, Nadia K. Byrnes, Meghan Kane and Rachel J. Primrose for collecting psychophysical data, and leading training sessions, and Samantha M. Bennett for assistance with protocol development, and Kayla Beaucage for genotyping our DNA samples. We also thank our study participants for their time and participation.
Footnotes
Previously, we reported a second SNP on TAS2R31, Ala227Val (rs10845293) also associated with the bitterness of AceK in a European-American cohort (Allen et al. 2013). The Ala227Val SNP was not associated with the bitterness ratings of RebA [F(1,108) = 1.25; p = 0.29] or RebD [F(2,108) = 0.18; p = 0.84] here.
Conflict of interest
The corresponding author has been paid for developing and delivering educational presentations on taste perception and taste biology for the food industry; his laboratory also conducts routine consumer acceptability tests for the food industry to facilitate practical training for students. None of these companies have provided any direct or indirect support for the work described here. The RebD was kindly donated by Dr. Jeannine Delwiche at PepsiCo; PepsiCo did not provide any funding for this project, and was not involved in the design of the experiment or the decision to publish this work. The authors declare no other relationships or activities that may have influenced the work described here.
References
- Alarcon S, Tharp A, Tharp C, Breslin PA. The effect of polymorphisms in 4 hTAS2R genes on PROP bitterness perception. Chem Senses. 2008;33(8):S43. [Google Scholar]
- Allen AL, McGeary JE, Hayes JE. Bitterness of the non-nutritive sweetener Acesulfame Potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem Senses. 2013 doi: 10.1093/chemse/bjt017. Epub 18 April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: Anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56(6):1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Byrnes NK, Hayes JE. Personality factors predict spicy food liking and intake. Food Qual Pref. 2013;28(1):213–221. doi: 10.1016/j.foodqual.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Wallace MR, Bartoshuk LM, Logan HL. Variation in the Gene TAS2R13 is Associated with Differences in Alcohol Consumption in Patients with Head and Neck Cancer. Chem Senses. 2012;37(8):737–744. doi: 10.1093/chemse/bjs063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AET, Choi HJ, Shaw H, Egan JM. Bitter taste receptors influence glucose homeostasis. PloS one. 2008;3(12):e3974. doi: 10.1371/journal.pone.0003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois GE, Walters DE, Schiffman SS, Warwick ZS, Booth BJ, Pecore SD, Gibes K, Carr BT, Brands LM. Concentration-response relationships of sweeteners. In: Walters DE, Orthoeter FT, DuBois GE, editors. Discovery, Molecular Design and Chemoreception. American Chemical Society; Washington, D.C: 1991. pp. 261–276. [Google Scholar]
- Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. Journal of the American Dietetic Association. 2000;100(6):647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, Reed DR, Snyder DJ, Bartoshuk LM. Bitter Receptor Gene (TAS2R38), 6-n-Propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcoholism: Clinical and Experimental Research. 2004;28(11):1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB, Hayes JE, Davidson AC, Kidd JR, Kidd KK, Bartoshuk LM. Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosensory perception. 2010:1–12. doi: 10.1007/s12078-010-9079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JW. Overview of sweeteners. Journal of Chemical Education. 1995;72(8):671. [Google Scholar]
- Feeney E. The impact of bitter perception and genotypic variation of TAS2R38 on food choice. Nutrition Bulletin. 2011;36(1):20–33. [Google Scholar]
- Genick UK, Kutalik Z, Ledda M, Destito MCS, Souza MM, Cirillo CA, Godinot N, Martin N, Morya E, Sameshima K. Sensitivity of genome-wide-association signals to phenotyping strategy: the PROP-TAS2R38 taste association as a benchmark. PloS One. 2011;6(11):e27745. doi: 10.1371/journal.pone.0027745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE. Transdisciplinary perspectives on sweetness. Chemosensory Perception. 2008;1(1):48–57. [Google Scholar]
- Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS) Food Qual Pref. 2013;28(1):36–44. doi: 10.1016/j.foodqual.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP Bitterness Depends on More Than the TAS2R38 Gene. Chem Senses. 2008;33(3):255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Sullivan BS, Duffy VB. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol Behav. 2010;100(4):369–380. doi: 10.1016/j.physbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, Duffy VB. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses. 2011;36(3):311–9. doi: 10.1093/chemse/bjq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgren FJ, Lynch MJ, Kirchmeyer F. A taste panel study of the saccharin “off-taste”. Journal of the American Pharmaceutical Association. 1955;44(6):353–355. doi: 10.1002/jps.3030440613. [DOI] [PubMed] [Google Scholar]
- Hellfritsch C, Brockhoff A, Staehler F, Meyerhof W, Hofmann T. Human Psychometric and Taste Receptor Responses to Steviol Glycosides. Journal of Agricultural and Food Chemistry. 2012;60(27):6782–6793. doi: 10.1021/jf301297n. [DOI] [PubMed] [Google Scholar]
- Horne J, Lawless HT, Speirs W, Sposato D. Bitter taste of saccharin and acesulfame-K. Chem Senses. 2002;27(1):31–38. doi: 10.1093/chemse/27.1.31. [DOI] [PubMed] [Google Scholar]
- JECFA. Steviol glycosides. Compendium of Food Additive Specifications; 73th Meeting; Rome. FAO; 2010. pp. 17–22. FAO JECFA Monographs 10. [Google Scholar]
- Kamerud JK, Delwiche JF. Individual differences in perceived bitterness predict liking of sweeteners. Chem Senses. 2007;32(9):803–810. doi: 10.1093/chemse/bjm050. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. The Journal of Neuroscience. 2004;24(45):10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WH. Early uses of Stevia rebaudiana (Asteraceae) leaves as a sweetener in Paraguay. Economic Botany. 1992;46(3):336–337. [Google Scholar]
- Lucas L, Riddell L, Liem G, Whitelock S, Keast R. The influence of sodium on liking and consumption of salty food. J Food Sci. 2011;76(1):S72–S76. doi: 10.1111/j.1750-3841.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- Mennella J, Pepino MY, Duke F, Reed D. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genetics. 2010;11(1):60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35(2):157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Ohta M, Sasa S, Inoue A, Tamai T, Fujita I, Morita K, Matsuura F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. Journal of Applied Glycoscience. 2010:57. [Google Scholar]
- Phillips K. Stevia: steps in developing a new sweetener. In: Grenby TH, editor. Developments in sweeteners. Elsevier Applied Science; 1989. pp. 1–43. [Google Scholar]
- Pronin AN, Xu H, Tang H, Zhang L, Li Q, Li X. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Current Biology. 2007;17(16):1403–1408. doi: 10.1016/j.cub.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Roudnitzky N, Bufe B, Thalmann S, Kuhn C, Gunn HC, Xing C, Crider BP, Behrens M, Meyerhof W, Wooding SP. Genomic, genetic and functional dissection of bitter taste responses to artificial sweeteners. Human Molecular Genetics. 2011;20(17):3437–3449. doi: 10.1093/hmg/ddr252. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Booth B, Carr B, Losee M, Sattely-Miller E, Graham B. Investigation of synergism in binary mixtures of sweeteners. Brain Research Bulletin. 1995a;38(2):105–120. doi: 10.1016/0361-9230(95)00062-j. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Research Bulletin. 1995b;36(5):505–513. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Keller KL, Ullrich NV. Challenges in Taste Chemistry and Biology. ACS Publications; 2003. Genetic variation in taste and preferences for bitter and pungent foods: implications for chronic disease risk; pp. 60–74. ACS Symposium Series. [Google Scholar]
- Wang JC, Hinrichs AL, Bertelsen S, Stock H, Budde JP, Dick DM, Bucholz KK, Rice J, Saccone N, Edenberg HJ. Functional Variants in TAS2R38 and TAS2R16 Influence Alcohol Consumption in High-Risk Families of African-American Origin. Alcoholism: Clinical and Experimental Research. 2007;31(2):209–215. doi: 10.1111/j.1530-0277.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Wölwer-Rieck U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: a review. Journal of Agricultural and Food Chemistry. 2012;60(4):886–895. doi: 10.1021/jf2044907. [DOI] [PubMed] [Google Scholar]