Abstract
The aim of this study was to examine the contribution of sugar, organic acid, neutral phenol, and anthocyanin fractions and added ascorbic acid to grape and pomegranate-nectarine juice total phenol, ORAC, FRAP, and DPPH values. Neutral phenol and anthocyanin fractions contributed ≥75% of the total antioxidant capacity (TAC) for both juices. Intrinsic synergy and antagonism among the fractionated constituents occurred inconsistently in each assay. Sugars and organic acids antagonized pomegranate juice neutral phenols and anthocyanins in the DPPH assay by 50% and the grape juice ORAC value by 21%, but were synergistic to the grape juice FRAP value. The added ascorbic acid was dose-dependently synergistic with pomegranate and grape juice total phenol, DPPH, and FRAP assays, but less so in the ORAC assay. Thus, the interactions between grape and pomegranate juice constituents determine TAC and total phenol values, and synergy in these assays could not be attributed solely to polyphenols.
Keywords: ascorbic acid, polyphenol, pomegranate, grape, antioxidant, synergy, antagonism, juice, ORAC, DPPH
INTRODUCTION
Observational studies have suggested that increased consumption of dietary antioxidants may have health benefits. Particularly, increased consumption of nutrients with in vitro antioxidant activity is associated with reductions in the risk for cancer and cardiovascular disease risk, and other chronic diseases (Hollman et al., 2011; Stoner et al., 2008). The utility of using in vitro antioxidant assays to predict health outcomes is limited due to bioavailability, metabolism, and relevance of antioxidant probe mechanisms in vivo (Hollman et al., 2011). However, in vitro antioxidant assays could still be useful to assess chemistry relevant to product shelf life or as a simple test for bioactive components such as polyphenols (Mermelstein, 2010).
The total antioxidant capacity (TAC) of foods, beverages, botanicals, and spices has been assessed by variety of methods. TAC assays include the DDPH (2,2-diphenyl-1-picrylhydrazyl), FRAP (ferric reducing antioxidant power), ORAC (oxygen radical absorbance capacity), among others. TAC values are moderately correlated between assays. For example, FRAP values correlated with ORAC values (r = 0.86, P < 0.01) for legume extracts, but not grape extracts (Lutz et al., 2011; Xu et al., 2007). Different assay mechanisms, conditions, or extract phytochemical content may cause divergent TAC values (Huang et al., 2005). For example, in a study of 12 polyphenol-rich beverages, Concord grape juice had the highest ORAC ranking, while pomegranate juice was ranked the highest for FRAP, DPPH, and Trolox-equivalent antioxidant capacity (TEAC) assays (Seeram et al., 2008). As pomegranate and grape juices are both rich in polyphenols, including neutral phenols and anthocyanins, their contents may contribute to the observed differential TAC (Seeram et al., 2008). For example, pomegranate punicalagins are more potent DPPH scavengers than its anthocyanins (Gil et al., 2000). Individual anthocyanins present in grape juice display potent ORAC values (Wang et al., 1997). However, the basis for the differential behavior of polyphenols and other antioxidants in TAC assays is not well understood.
Interactions among juice constituents can contribute to differential TAC values of different assays. Recently, we reported that polyphenol-rich juices have variable TAC values depending on their dilution factors employed in the assays (Bolling et al., 2012), implicating that the degree of interactions between constituents may be altered by their assay concentrations. Also, citrus polyphenols in combinations are synergistic or antagonistic in the ORAC assay (Freeman et al., 2010).
The TAC of beverages can also be changed by fortification. Additives such as ascorbic acid, citric acid, caramel color, and fruit extracts may substantially alter the TAC rank order for a juice. Among these additives, ascorbic acid has been identified as a major contributor to the TAC of some juices (Gardner et al., 2000; Miller & Rice-Evans, 1997). We have noted that polyphenols have synergistic TAC with ascorbic acid in the FRAP, DPPH, and superoxide scavenging assays, but are antagonistic or synergistic in the ORAC assay depending on polyphenol composition (Chen & Blumberg, 2008). As ascorbic acid may have a larger ORAC to FRAP ratio (ORAC:FRAP) than intrinsic constituents in juices, i.e., the flavonol isorhamnetin (Chen & Blumberg, 2008), fortifying polyphenol-rich juices with ascorbic acid may modulate ORAC:FRAP and consequent rank order of different TAC assays.
The aim of this study was to investigate the underlying mechanisms for differential TAC values in polyphenol-rich grape and pomegranate juices. We hypothesized that polyphenol profile, ascorbic acid content, and their interaction would have a significant impact on fruit juice TAC values and consequent ranking order.
MATERIALS AND METHODS
Materials
POM Wonderful, Inc. provided commercial samples of pomegranate-nectarine juice (100% juice without added ascorbic acid, POM Wonderful, Inc.) and organic Concord grape juice (100% juice without added ascorbic acid, Trader Joe’s). The reagent AAPH, 2-2′-azobis (2-amidinopropionamidine) dihydrochloride was from Wako Chemicals USA (Richmond, VA). Hydrochloric acid, HPLC-grade methanol, and sodium hydroxide were from Fisher Scientific (Pittsburg, PA). All other chemicals and reagents were acquired from Sigma-Aldrich (St. Louis, MO).
Fractionation of antioxidants in juice
Sugar, organic acid, neutral phenol, and anthocyanin fractions of commercial juices were collected using a 500 mg C18 Sep-Pak Vac 10 mL mini-cartridge (Waters Corporation, Milford, MA) by the method of Giusti and Wrolstad with slight modifications (Giusti & Wrolstad, 1996). Cartridges were first activated by sequential additions of 10 mL ethyl acetate, methanol, and water. A 250-μL aliquot of juice was then applied to the cartridge and eluted without addition to collect the sugar fraction (F1), followed by elution of the organic acid fraction (F2) with 4 mL of 0.01 N HCl. After complete removal of water under vacuum with ambient air, fractions containing neutral phenols (F3) and anthocyanins (F4) were stepwise eluted with 20 mL ethyl acetate and 4 mL of acidified methanol (0.01% HCl), respectively. Fractions 3 and 4 were dried under nitrogen gas at ambient temperature. All fractions were stored at −20°C in darkness until analysis within 14 d. Fractions were reconstituted or diluted with the appropriate assay buffers or solvents prior to analysis. For total phenol and TAC analyses, dilutions were adjusted to enable raw assay values to fall approximately midpoint of the linear assay ranges since dilution is known to affect final TAC and total phenol values (Bolling et al., 2012).
Total phenol
The Folin method as described by Singleton and other (Singleton et al., 1999) was used to determine juice total phenol values. Results were expressed as gallic acid equivalents (GAE), using a standard curve ranging from 16.125 to 500 mg GAE/mL. Intra- and inter-assay relative standard deviations (RSD) were 1.6% and 4.9%, respectively.
TAC assays
DPPH radical scavenging was assessed as previously described (Benzie & Strain, 1996; Bolling et al., 2012). Briefly, methanol-diluted juice, fractions or standards were added to 10 μmol/L DPPH (final concentration) in methanol. Following 30 min incubation at room temperature in the dark, absorbance was measured at 520 nm. Intra- and inter-assay RSD were 1.4% and 7.6%, respectively.
FRAP was determined as previously described (Benzie & Strain, 1996; Bolling et al., 2012). Briefly, juice, fractions or standards were incubated for 1 h at room temperature with Fe3+-2,4,6-tri-pyridyl-S-triazine. FRAP values were determined using standard curves of 31.25 to 500 μmol Trolox/L. Intra- and inter-assay RSD were 0.7 and 4.2%, respectively.
ORAC values were determined using AAPH generation of peroxyl radicals as previously described (Bolling et al., 2012; Ou et al., 2001). Briefly, juice, fractions, or standards were diluted in 750 mM phosphate buffer (pH 7.0) and incubated with AAPH and fluorescein for 70 min. Fluorescence was monitored at 485 nm excitation and 520 nm emission using a FLUOstar OPTIMA plate reader (BMG LABTECH Inc., Cary, N.C., USA). Standard curves of 5 to 50 μmol Trolox/L were used to determine ORAC values, based on area under the curve of sample responses. Intra- and inter-assay RSD were 3.0 and 7.3%, respectively.
Interaction of added ascorbic acid and antioxidants in juice
Ascorbic acid was added to pomegranate-nectarine and grape juices and their respective fractions to determine TAC interactions. Concentrations of 50, 100, and 200% of the daily value (DV) for vitamin C in final products were prepared, where 100% DV was 60 mg per 240 mL juice. The TAC of fortified juices was determined immediately after the addition of ascorbic acid.
Vitamin C analysis
Reduced (ascorbic acid) and oxidized (dehydroascorbic acid) vitamin C were determined by HPLC according to Gokmen et al. (2000). Briefly, ascorbic acid in fresh juice samples was determined at 254 nm after isocratic elution by 0.2 M KH2PO4 in HPLC grade water at pH 2.4 on a Zorbax C18 column (4.6 μm × 250 mm). The limit of quantification for ascorbic acid was 10 μg/mL juice.
Statistical analysis
Data are presented as mean ± SD of at least duplicate determinations. Interactions were calculated by the percent difference between the observed vs. expected TAC as previously described (Chen & Blumberg 2008), where % interaction = 100*(activityactual − activityexpected)/activityexpected. Thus, positive percentages represent synergistic interactions, whereas negative percentages represent antagonistic interactions. Statistical significance was determined at the P ≤0.05 level first by a two-tailed t-test between expected and experimental values, by two-way ANOVA, followed by one-way ANOVA and Tukey’s HSD multiple comparison test among groups. Pearson’s correlation of vitamin C interactions was performed using Graphpad Prism software (GraphPad Software, Inc.).
RESULTS AND DISCUSSION
Commercial grape and pomegranate juices have similar ORAC values (Seeram et al., 2008). However, pomegranate juice has greater total phenol, DPPH and FRAP values than grape juice. The objective of this study was to characterize the contributions of polyphenols (neutral phenols and anthocyanins) and ascorbic acid to TAC variability, particularly by investigating the ORAC:FRAP in pomegranate-nectarine and grape juices. We hypothesized that ascorbic acid content, polyphenol profile, and their interaction would contribute to differential TAC ranking of two juices.
Pomegranate-nectarine juice had 22% more total phenol, a 5% greater DPPH value, and a 12% greater FRAP value than grape juice (Table 1). Grape juice had 53% greater ORAC value than pomegranate-nectarine juice. Thus, grape juice had a 125% higher ORAC:FRAP than pomegranate-nectarine juice. This is in agreement with Seeram et al. (2008), where grape juice had a 74% greater ORAC:FRAP than pomegranate juice.
Table 1.
Total antioxidant capacity and total phenol content of fractionated, recombined, and whole juices.
| Juice | Fraction | Total Phenols | DPPH | ORAC | FRAP | ORAC/FRAP |
|---|---|---|---|---|---|---|
| mM GAE (% F1-4) | mM TE (% F1-4) | mM TE (% F1-4) | mM TE (% F1-4) | ratio | ||
| pomegranate-nectarine | F1 | <0.016 | 0.02 (0.2)a | <0.1 | 0.05 (0.3)a | |
| F2 | 0.16 (10.1)a | 1.90 (18.8)c | 2.00 (10.0)a | 1.88 (11.8)b | 1.1 | |
| F3 | 0.69 (43.4)c | 4.65 (45.9)f | 9.90 (49.5)b | 7.89 (49.4)g | 1.3 | |
| F4 | 0.74 (46.5)e | 3.55 (35.1)e | 8.10 (40.5)b | 6.15 (38.5)f | 1.3 | |
| sum:F1+F2+F3+F4 | 1.59 (100)h | 10.12 (100)h | 20.02 (100)d | 15.97 (100)i | 1.3 | |
| whole juice | 1.87 (117.4)i | 15.99 (158)j | 21.16 (106)d | 18.39 (115)k | 1.2 | |
| grape | F1 | <0.016 | 0.02 (0.4)a | <0.1 | 0.06 (0.5)a | |
| F2 | 0.36 (18.1)b | 1.36 (25.2)b | 2.20 (6.5)a | 3.07 (25.3)c | 0.7 | |
| F3 | 0.92 (45.9)f | 1.39 (25.8)b | 13.80 (40.4)bc | 3.28 (27.0)d | 4.2 | |
| F4 | 0.73 (36.1)d | 2.61 (48.5)d | 18.13 (53.1)cd | 5.73 (47.2)e | 3.2 | |
| sum:F1+F2+F3+F4 | 2.01 (100)j | 5.38 (100)g | 34.14 (100)e | 12.15 (100)h | 2.8 | |
| whole juice | 1.53 (76.0)g | 15.52 (288)i | 44.99 (132)f | 16.45 (135)j | 2.7 |
Data within columns bearing different letters are significantly different by ANOVA and Tukey’s HSD multiple comparison test, P <0.05. Values in parentheses are % of the sum of F1+F2+F3+F4 TAC values.
In order to determine the individual contributions of juice constituents to TAC, juices were fractionated using a C18 cartridge into predominately sugar (F1), organic acid (F2), neutral phenol (F3), and anthocyanin (F4) isolates. Fractions were then assayed in the total phenol, DPPH, FRAP and ORAC assays and used to compare the value of whole juice to the calculated sum of the 4 fractions, which represents their additive total phenol and TAC values without accounting for interactions between fractions (Table 1).
We observed that neutral phenols and anthocyanins of pomegranate-nectarine juice contributed to 90% of the total phenol value, and their contributions were nearly equivalent with only a 3% difference. In contrast, grape juice neutral phenols contributed 31% more than anthocyanins to the total phenol value. The grape and pomegranate-nectarine juice organic acid fractions contributed 18.1 and 10.1% of total phenol values, respectively. Ascorbic acid was present in the organic acid fraction of grape juice (223 ± 2 μg/mL), but not of pomegranate-nectarine juice (<10 μg/mL). The overall contribution of ascorbic acid to total phenol values was very negligible (0.006%), as ascorbic acid had an equivalency of 0.13-fold gallic acid. The sugar fraction did not contribute to total phenol values for either juice, in agreement with a previous study where glucose, fructose, and sucrose had no reactivity with the Folin reagent (Everette et al., 2010).
In TAC assays, grape juice anthocyanins contributed 47 to 53% of DPPH, FRAP, and ORAC values. Grape juice neutral phenols contributed 40% of ORAC values, but only 26 and 27% of DPPH and FRAP values, respectively. Grape juice organic acids contributed ~25% of DPPH and FRAP values, but only 6.5% to the ORAC value. For pomegranate-nectarine juice, neutral phenol and anthocyanin fractions contributed 80 to 88% of the DPPH, ORAC, and FRAP values. The pomegranate-nectarine juice organic acids fraction contributed approximately 10% of the ORAC and FRAP values, and to 18.8% of DPPH values. The descending contribution order for pomegranate-nectarine juice fractions to TAC was consistent for the DPPH, FRAP, and ORAC assays, where F3 >F4 >F2 >F1. Similarly, the descending order of grape juice fractions was consistent for all TAC assays, where F4 >F3 >F2 >F1. Thus, polyphenol fractions contributed the greatest to juice TAC values.
It has been well appreciated that the mechanisms of some TAC assays differ, possibly due to the type of assay probe, interaction between antioxidants, differential redox potential between antioxidant and the assay probe and/or oxidant, and assay conditions (Huang et al., 2005). Nevertheless, our results implicated that the FRAP and DPPH assays might have a common mechanism because the contribution of pomegranate-nectarine and grape juice fractions to both TAC assays were comparable. However, the ORAC:FRAP of juices and fractions emphasized the disparity of the ORAC and FRAP assay mechanisms (Table 1). While the ORAC:FRAP for pomegranate-nectarine juice fractions were similar, the ORAC:FRAP of grape juice anthocyanins and neutral phenols were 19 and 56% greater than that of whole juice, respectively. Thus, the capacity to scavenge peroxyl radicals was greater than the ability to reduce ferric ions for grape juice polyphenols, but less so for pomegranate-nectarine juice polyphenols.
Ascorbic acid and ellagic acid TAC was also dependent on assay (data not shown). Ellagic acid had an ORAC:FRAP of 4.4, in contrast to 0.86 for ascorbic acid. The ORAC:FRAPs of the organic acid fraction of pomegranate-nectarine and grape juices at 1.06 and 0.70 were comparable to ascorbic acid. Thus, while added ascorbic acid may contribute fruit juice TAC, its potential impact on ORAC:FRAP may be less than polyphenols. The ellagic acid ORAC:FRAP was closer to the grape juice polyphenol ORAC:FRAPs than to pomegranate-nectarine juice polyphenol ORAC:FRAPs. We speculate constituents other than ellagic acids may account for the low pomegranate-nectarine juice ORAC:FRAP. Similarly, a previous study found ellagic acid from commercial pomegranate juice, present at 121 mg/L, accounted for <3% of DPPH activity, whereas the hydrolyzable tannins, punicalagins, and anthocyanins were the major contributors (Gil et al., 2000). Wang et al. (1997) also reported cyanidin-3-glucoside, a Concord grape anthocyanin, had an ORAC potency 3.5-fold of Trolox, while other anthocyanins were in the range of 1.1- to 2.9-fold Trolox. Concord grape juice has a diverse anthocyanin profile, including glucosides and coumaroylglucosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin (Stalmach et al., 2011). Other Concord grape juice neutral phenols include hydroxycinnamic acids (mainly trans-caftaric acid), stilbenes, catechins, and proanthocyanidins (Stalmach et al., 2011). More work is warranted to examine the contribution of individual polyphenols to differential ORAC:FRAPs of juices. This information will help to enhance our understanding of the divergent TAC rank orders among different assays.
Juice TAC values are a complex result of additive, synergistic, and antagonistic interactions among whole-juice constituents. Thus, it is unclear if testing fractionated antioxidants represents whole juice TAC. Surprisingly, the total phenol value of grape juice was less than the fraction sum, perhaps a consequence of antagonism occurring in the whole juice. The sums of fractionated TAC also diverged from whole juice TAC. Total phenol, ORAC, and FRAP values of whole juice were 76 to 135% of the calculated sum, but DPPH value of grape juice was 1.8-fold greater than the sum of its fractions. Potential TAC losses of the calculated sum could arise from either the degradation of phenolic constituents or loss of synergism among constituents.
To account for potential losses during fractionation, we recombined fractions at whole-juice equivalent concentrations and then assessed TAC and total phenol values (Table 2). Recombination of all pomegranate-nectarine juice fractions was antagonistic in the total phenol and DPPH assays. Particularly, the recombined pomegranate-nectarine juice fractions displayed only half the potency of the expected DPPH values. Further, such reductions in total phenol and DPPH values could not be accredited to the antagonism between neutral phenol and anthocyanin fractions, as each had only 3% antagonism. However, there was 27% synergism of the ORAC value between pomegranate-nectarine juice neutral phenol and anthocyanin fraction, but this was negated by the addition of sugar and organic acid fractions. Overall, sugar and organic acid fractions antagonized total phenol, DPPH, and ORAC values of pomegranate-nectarine juice, but not FRAP values. For grape juice, recombination of all its fractions was synergistic for the DPPH and FRAP assays. Recombination of all grape juice fractions was antagonistic in the total phenol assay, solely attributed to sugars and organic acids fractions as there was synergism in the recombined polyphenol fractions. In the ORAC assay, recombination of all grape juice fractions were 21% antagonistic, as a consequence of combining F1 and F2. Thus, for both juices, sugar and organic acids fractions antagonized polyphenols for all test assays, except FRAP.
Table 2.
Interactions of antioxidants between juice fractions depend upon assay and juice.
| Juice | Recombined Fractions | Total phenol | DPPH | ORAC | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| mM GAE | % Interactiona | mM TE | % Interactiona | mM TE | % Interaction | mM TE | % Interaction | ||
| pomegranate-nectarine | F(1+2+3+4) | 1.30±0.01* | −18 | 5.07±0.02* | −50 | 18.92±0.65 | −8 | 15.82±0.03* | −1 |
| F(3+4) | 1.38±0.00* | −3 | 7.96±0.04* | −3 | 23.78±0.17* | 27 | 13.51±0.06* | −4 | |
| grape | F(1+2+3+4) | 1.66±0.01* | −17 | 6.31±0.05* | 17 | 26.58±0.70* | −21 | 14.49±0.10* | 19 |
| F(3+4) | 2.58±0.00* | 30 | 4.65±0.04* | 16 | 31.13±0.33 | −2 | 10.03±0.06* | 11 | |
% interaction = 100*(activityactual − activityexpected)/activityexpected, with positive values as synergy and negative values as antagonism. Expected values are sum of F1, F2, F3, and F4 or sum of F3 and F4 in Table 1.
P <0.05 by two-tailed t-test compared to expected values.
Previous studies have demonstrated that recombined isolated polyphenols and sugars can be synergistic, antagonistic, or additive to TAC. In the TEAC assay, the anthocyanin cyanidin-3-rutinoside worked synergistically with isorhamnetin-3-rutinoside but antagonistically with kaempferol (Kirakosyan et al., 2010). In the ORAC assay, myricetin and naringenin was antagonistic, but the addition of hesperidin to this combination resulted in synergy (Freeman et al., 2010). Cherry anthocyanins were antagonistic in the TEAC assay and bilberry anthocyanins synergistic toward peroxynitrite scavenging (Kirakosyan et al., 2010; Rahman et al., 2006). These findings are in agreement with our study that TAC assay and polyphenol composition both affect juice TAC interactions. A glucose, sucrose, and fructose solution contributed to synergy between rutin and p-coumaric acid in the ORAC assay (Parker et al., 2010), which conflicted with our finding that sugars and organic acids together interfered action of antioxidants in pomegranate-nectarine and grape juices in TAC and total phenol assays. It is worth noting that we did not test the interaction using the sugar fraction alone in the study. Taken together, minor compositional differences between polyphenol-rich juices can affect TAC values through complicated interactions among constituents with and without significant antioxidant actions.
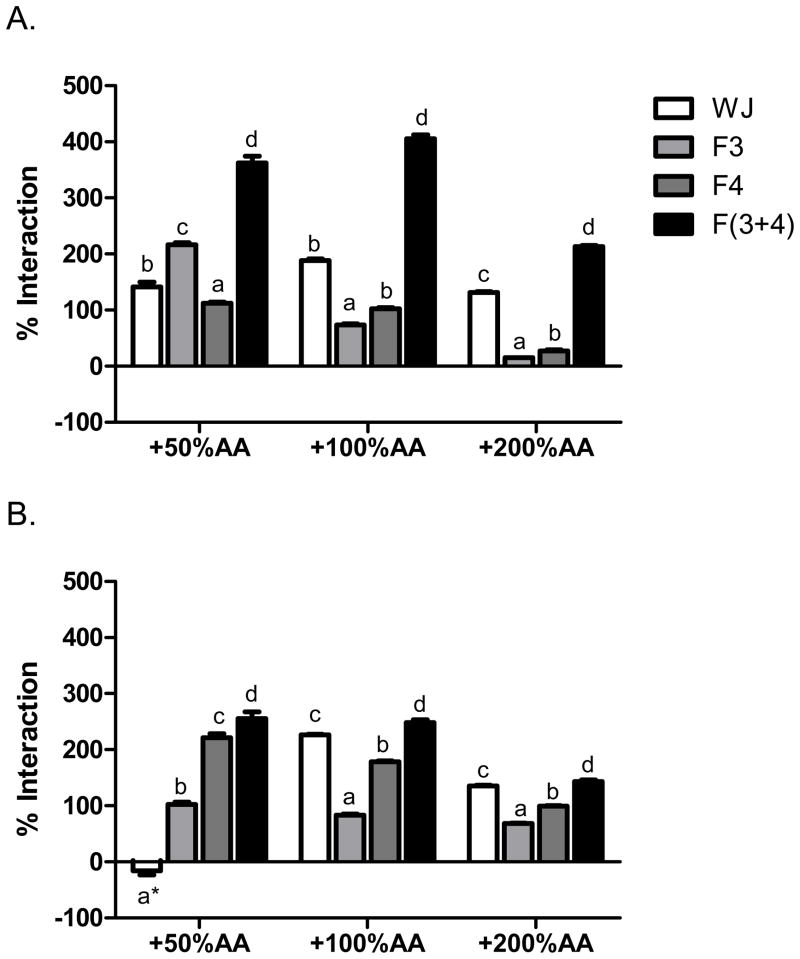
Ascorbic acid is commonly added to juices and can contribute greatly to TAC values in a diverse manner, depending on ascorbic acid amount, juice type, and assay. In this study, additional ascorbic acid at 50, 100, and 200% DV was synergistic with pomegranate-nectarine juice in the total phenol assay and synergistic with grape juice at two larger doses (Fig. 1). Juice and ascorbic acid synergy was dose independent as the synergy of 200% DV ascorbic acid was less than 100% DV for both juices. This may be caused in part by decreasing total phenol synergy of pomegranate-nectarine and grape juice neutral phenols and grape juice anthocyanins, with increasing ascorbic acid concentration. Our results support that the ascorbic acid in polyphenol-rich beverages may lead to an overestimation of total phenol values. For example, at 100% DV, the actual contribution of ascorbic acid is ~2-fold that of the expected value for pomegranate-nectarine and grape juices. Future efforts reporting total phenol values should consider ascorbic acid synergism with polyphenols.
Figure 1.
Interactions between ascorbic acid (AA) and whole juice, neutral phenols (F3), anthocyanins (F4), and recombined neutral phenols and anthocyanins F(3+4) from A. pomegranate nectarine juice and B. grape juice in the total phenol assay. Data are % interaction = 100*(activityactual − activityexpected)/activityexpected, with positive values as synergy and negative values as antagonism. The changes in expected values were 0.069, 0.162, and 0.346 mg gallic acid equivalents/mL juice for 50, 100, and 200 % daily values of added AA respectively. By 2-way ANOVA, both pomegranate and grape juice data were P <0.0001 for juice and fractions, P <0.0001 for AA concentrations, and P <0.0001 for their interaction. Within groups, bars marked with different letters are significantly different by 1-way ANOVA and Tukey’s HSD multiple comparison test, P <0.05. Bars marked with asterisk (*) AA were not significantly different from expected values by a one sample, two-tailed t-test (P >0.05).
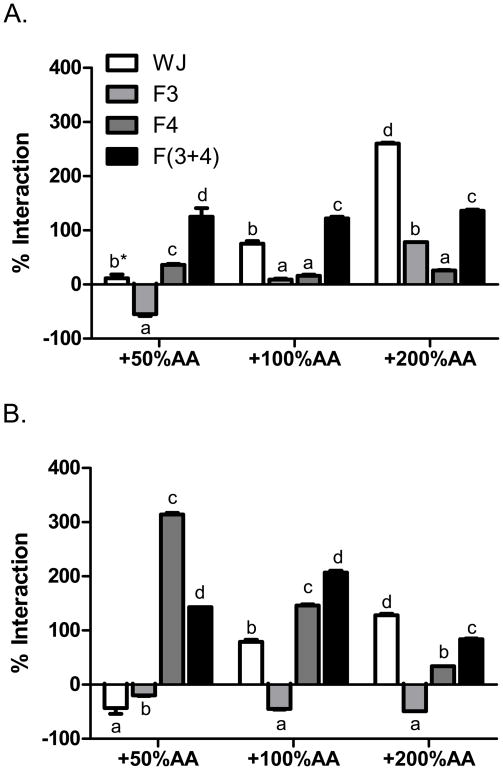
The interaction of ascorbic acid with juices and their fractions were very mixed in three TAC assays. In the DPPH assay, the interaction of ascorbic acid and juices was more complicated than the total phenol assay. The synergy of ascorbic acid with pomegranate-nectarine juices and their neutral phenol fractions increased proportionally with its concentration (Fig. 2). However, the interaction of ascorbic acid with other juice fractions was inconsistent. The synergy of 200% DV ascorbic acid and pomegranate-nectarine juice could not be readily attributed to its interaction with polyphenols alone because neutral phenols, anthocyanins, and their recombination were divergent from whole juice values. In contrast, additional ascorbic acid was highly synergistic with grape juice anthocyanins at 50% ascorbic acid, but this effect declined at 100 and 200% DV and did not lead to synergy between ascorbic acid and grape juice. Further, grape juice neutral phenols were 20 to 49 % antagonized in the DPPH assay at each level of added ascorbic acid. Therefore, non-polyphenol constituents may be responsible for the ascorbic acid-juice synergy in the DPPH assay.
Figure 2.
Interactions between ascorbic acid (AA) and whole juice, neutral phenols (F3), anthocyanins (F4), and recombined neutral phenols and anthocyanins F(3+4) from A. pomegranate nectarine juice and B. grape juice in the DPPH assay. Data are % interaction = 100*(activityactual − activityexpected)/activityexpected, with positive values as synergy and negative values as antagonism. The changes in expected values were 0.75, 1.5, and 3.0 mM Trolox equivalents for 50, 100, and 200% daily values of added AA respectively. By 2-way ANOVA, both pomegranate and grape juice data were P <0.0001 for juice and fractions, P <0.0001 for AA concentrations, and P<0.0001 for their interaction. Within groups, bars marked with different letters are significantly different by 1-way ANOVA and Tukey’s HSD multiple comparison test, P <0.05. Bars marked with asterisk (*) AA were not significantly different from expected values by a one sample, two-tailed t-test (P >0.05).
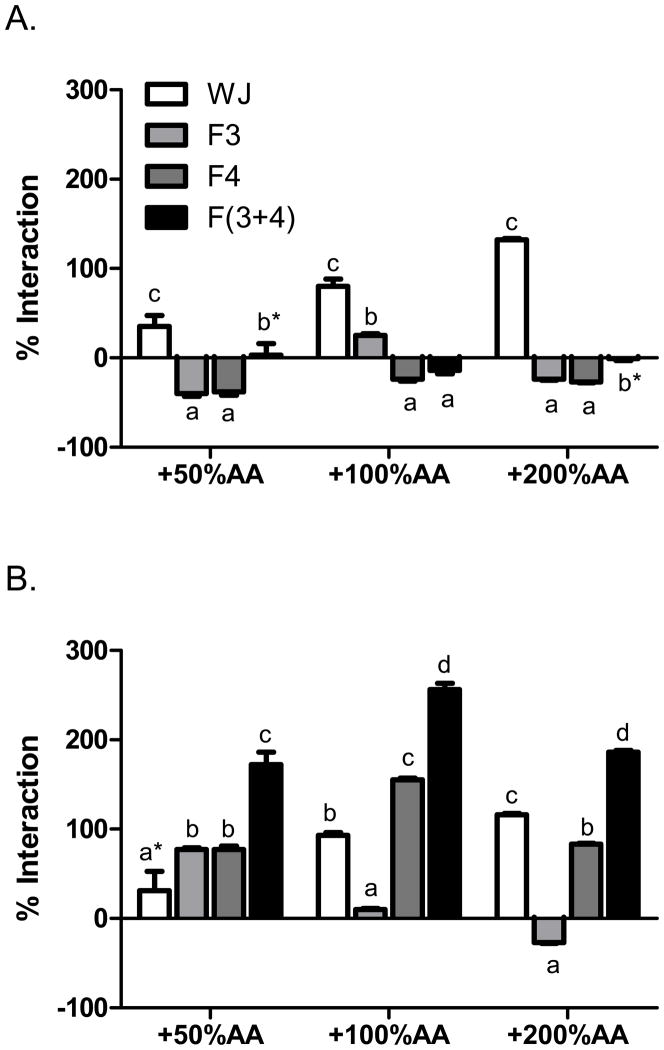
In the FRAP assay, there was dose-dependent ascorbic acid synergy with both juices (Fig. 3). The constituents in pomegranate-nectarine juice that interacted with ascorbic acid in the FRAP assay most likely were not polyphenols since either small synergy or antagonism occurred between ascorbic acid and neutral phenols and anthocyanins. In contrast, recombination of grape juice neutral phenol and anthocyanin fractions had 70 to 163% more synergy with ascorbic acid than juice alone. The grape juice neutral phenol fraction synergism with ascorbic acid was inversely proportional to ascorbic acid concentration and the interaction changed to antagonism at 200% DV ascorbic acid. Thus, anthocyanins may be responsible for the increasing synergy among ascorbic acid and grape juice in the FRAP assay. Constituents other than polyphenols may account for FRAP synergy between ascorbic acid and pomegranate-nectarine juice.
Figure 3.
Interactions between ascorbic acid (AA) and whole juice, neutral phenols (F3), anthocyanins (F4), and recombined neutral phenols and anthocyanins F(3+4) from A. pomegranate nectarine juice and B. grape juice in the FRAP assay. Data are % interaction = 100*(activityactual − activityexpected)/activityexpected, with positive values as synergy and negative values as antagonism. The changes in expected values were 0.91, 1.9, and 3.7 mM Trolox equivalents for 50, 100, and 200 % daily values of added AA respectively. By 2-way ANOVA, both pomegranate and grape juice data were P <0.0001 for juice and fractions, P <0.0001 for AA concentrations, and P <0.0001 for their interaction. Within groups, bars marked with different letters are significantly different by 1-way ANOVA and Tukey’s HSD multiple comparison test, P < 0.05. Bars marked with asterisk (*) AA were not significantly different from expected values by a one sample, two-tailed t-test (P >0.05).
In the ORAC assay, the ascorbic acid interactions with both juices, and their fractions were inconsistent (Table 3). Anthocyanins from both juices were synergistic with 100% DV ascorbic acid. Grape juice neutral phenols were antagonistic at 100% DV ascorbic acid. Nonetheless, ascorbic acid interactions with juice ORAC values were not statistically different from expected values. Thus, ORAC interactions are ascorbic acid dose and polyphenol- dependent. Likewise, Parker et al. demonstrated that ascorbic acid was not synergistic with sugar and p-coumaric acid in the ORAC assay, but was synergistic with abscisic acid (Parker, Miller, Myers, Miguez, & Engeseth, 2010). However, the present study did not provide evidence for sugar and ascorbic acid interactions in the ORAC assay. Thus, more research is necessary to determine why ascorbic acid-polyphenol interactions in the ORAC assay are concentration- dependent.
Table 3.
Interactions between ascorbic acid and whole juice, neutral phenols (F3), anthocyanins (F4), and recombined neutral phenols and anthocyanins F(3+4) from pomegranate-nectarine and grape juices in the ORAC assay.
| Juice | Component | % Interaction | ||
|---|---|---|---|---|
|
| ||||
| Added ascorbic acid, % Daily Value
|
||||
| 50 | 100 | 200 | ||
| pomegranate-nectarine | whole juice | −475 ± 101 | −58 ± 13a | 60 ± 27 |
| F3 | −51 ± 50 | −21 ± 18a | 49 ± 15 | |
| F4 | −16 ± 141 | 103 ± 10*b | 8.6 ± 94 | |
| F(3+4) | −169 ± 153 | −47 ± 8a | 98 ± 23 | |
| grape | whole juice | 166 ± 227 | 20 ± 23b | 7.7 ± 9.0a |
| F3 | −124 ± 139 | −42 ± 0*a | 12 ± 41a | |
| F4 | −158 ± 88 | 101 ± 11*c | 151 ± 38b | |
| F(3+4) | −241 ± 159 | 12 ± 9ab | 44 ± 11ab | |
Data are % interaction = 100*(activityactual − activityexpected)/activityexpected, with positive values as synergy and negative values as antagonism. The changes in expected values were 0.80, 1.6, and 3.2 mM Trolox equivalents for 50, 100, and 200 % daily values of added ascorbic acid respectively. By 2-way ANOVA, pomegranate data were P = 0.0062 for juice and fractions, P = 0.0050 for AA concentrations, and P = 0.0001 for their interaction, and grape data were P = 0.1035 for juice and fractions, P = 0.0259 for AA concentration, and P = 0.0456 for their interaction. Within groups, values marked with different letters are significantly different by 1-way ANOVA and Tukey’s HSD multiple comparison test, P <0.05. Values bearing asterisks (*) are significantly different from expected values by a one sample, two-tailed t-test (P <0.05).
Grape and pomegranate-nectarine juice interactions with ascorbic acid were similar in the total phenol, DPPH, and FRAP assays, with a correlation of 0.39 (P = 0.006). The interactions of ascorbic acid with juice TAC values were highly dose- and assay-dependent. In the DPPH and FRAP assays, synergy increased at least 3-fold for both juices at 200% DV ascorbic acid relative to 50% DV. The dose-dependent nature of juice- ascorbic acid interactions may be related in-part to the prooxidant effects of ascorbic acid. Ascorbic acid was increasingly antagonistic in the presence of catechins in a chemiluminescent peroxyl-scavenging assay (Choueiri et al., 2012). In the same study, quercetin and ascorbic acid were synergistic, but did not display the same dose-dependency of ascorbic acid. Therefore, modeling with individual polyphenols alone is not predicative of TAC interactions in juices.
Fruit juices are typically fortified to provide 100% DV of ascorbic acid. Not only can fortification of polyphenol-rich juices increase TAC and total phenol values, but also alter their rank order of TAC. In our study, fortifying grape juice with 100% ascorbic acid added 3.6 mmol/L to FRAP potency and 1.9 mmol/L ORAC potency, representing increase of 20 and 4% of the original grape juice potency, respectively. Similarly, fortification raised FRAP value of pomegranate-nectarine juice by 14% and ORAC value by 3%. Therefore, fortification at 100% DV likely changes the rank orders of commercial beverages in TAC assays because juice TAC value differences can be small. For example, the four juices with the most potent ORAC values varied by only 4.5% activity (Seeram et al., 2008). Consequently, fortification of ascorbic acid to juice modifies rank order of juices through additive, antagonistic or synergistic interaction between added antioxidant and intrinsic constituents.
CONCLUSION
We investigated the basis for differential TAC and total phenol values between grape and pomegranate-nectarine juices, and their interaction with ascorbic acid. Pomegranate-nectarine and grape juice anthocyanin and neutral phenol fractions contributed the most to TAC and total phenol values when tested in isolation. The higher ORAC:FRAP for grape compared to pomegranate-nectarine juice was attributed to its polyphenols, rather than ascorbic acid content. Complex intrinsic synergy and antagonism among constituents contributed to both total phenol and TAC values, including a 27% synergy of pomegranate-nectarine juice neutral phenols and anthocyanins in the ORAC assay. Fortification of juices with ascorbic acid also produced synergy or antagonism in TAC assays, and was highly dependent on juice, ascorbic acid concentration, and type of juice polyphenols. Sugars and organic acids fractions were antagonistic or synergistic to TAC of intrinsic polyphenols, and played a determinant role in the interaction of ascorbic acid fortification in juices. The addition of ascorbic acid to juices or their polyphenol fractions was also synergistic for total phenol values. Thus, using the total phenol assay to assess juices with ascorbic acid may result in overestimation of total phenol values because of synergy. More work is warranted to elucidate the mechanisms of these synergistic and antagonistic interactions.
Supplementary Material
Abbreviations
- AAPH
2-2′-azobis (2-amidinopropionamidine) dihydrochloride
- DPPH
di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium
- DV
daily value
- FRAP
ferric reducing antioxidant power
- GAE
gallic acid equivalents
- ORAC
oxygen radical absorbance capacity
- ORAC,FRAP
ORAC to FRAP ratio
- RSD
relative standard deviations
- TAC
total antioxidant capacity
- TEAC
Trolox-equivalent antioxidant capacity
Footnotes
Supported by POM Wonderful, LLC, and U.S. Department of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. BWB was supported by NIGMS K12GM074869. The contents of this publication do not necessarily reflect the views or policies of the NIH or USDA nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. government. Parts of this study were presented at the Experimental Biology 2010 conference.
References
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bolling BW, Chen YY, Kamil AG, Chen CY. Assay dilution factors confound measures of total antioxidant capacity in polyphenol-rich juices Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. Journal of Food Science. 2012;77:H69–75. doi: 10.1111/j.1750-3841.2011.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Blumberg JB. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. Journal of Agricultural and Food Chemistry. 2008;56:4427–4434. doi: 10.1021/jf800061z. [DOI] [PubMed] [Google Scholar]
- Choueiri L, Chedea VS, Calokerinos A, Kefalas P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC-MS. Food Chemistry. 2012;133:1039–1044. [Google Scholar]
- Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. Journal of Agricultural and Food Chemistry. 2010;58:8139–8144. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BL, Eggett DL, Parker TL. Synergistic and Antagonistic Interactions of Phenolic Compounds Found in Navel Oranges. Journal of Food Science. 2010;75:C570–C576. doi: 10.1111/j.1750-3841.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- Gardner PT, White TAC, McPhail DB, Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chemistry. 2000;68:471–474. [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. Journal of Agricultural and Food Chemistry. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization of Red Radish Anthocyanins. Journal of Food Science. 1996;61:322–326. [Google Scholar]
- Gökmen V, Kahraman N, Demir N, Acar J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. Journal of Chromatography A. 2000;881:309–316. doi: 10.1016/s0021-9673(00)00080-7. [DOI] [PubMed] [Google Scholar]
- Hollman PCH, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The Biological Relevance of Direct Antioxidant Effects of Polyphenols for Cardiovascular Health in Humans Is Not Established. The Journal of Nutrition. 2011;141:989S–1009S. doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Prior RL. The Chemistry behind Antioxidant Capacity Assays. Journal of Agricultural and Food Chemistry. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Kirakosyan A, Mitchell Seymour E, Noon KR, Urcuyo Llanes DE, Kaufman PB, Warber SL, Bolling SF. Interactions of antioxidants isolated from tart cherry (Prunus cerasus) fruits. Food Chemistry. 2010;122:78–83. [Google Scholar]
- Lutz M, Jorquera K, Cancino B, Ruby R, Henriquez C. Phenolics and antioxidant capacity of table grape (Vitis vinifera L.) cultivars grown in Chile. Journal of Food Science. 2011;76:C1088–C1093. doi: 10.1111/j.1750-3841.2011.02298.x. [DOI] [PubMed] [Google Scholar]
- Mermelstein N. Dealing with antioxidant assay issues. Food Technology. 2010;62:72–75. [Google Scholar]
- Miller NJ, Rice-Evans CA. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chemistry. 1997;60:331–337. [Google Scholar]
- Ou B, Hampsch-Woodill M, Prior RL. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. Journal of Agricultural and Food Chemistry. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Parker TL, Miller SA, Myers LE, Miguez FE, Engeseth NJ. Evaluation of Synergistic Antioxidant Potential of Complex Mixtures Using Oxygen Radical Absorbance Capacity (ORAC) and Electron Paramagnetic Resonance (EPR) Journal of Agricultural and Food Chemistry. 2010;58:209–217. doi: 10.1021/jf903080f. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Ichiyanagi T, Komiyama T, Hatano Y, Konishi T. Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radical Research. 2006;40:993–1002. doi: 10.1080/10715760600815322. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed Polyphenol-Rich beverages in the united states. Journal of Agricultural and Food Chemistry. 2008;56:1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Ravent RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods of Enzymology. 1999;299:152–178. [Google Scholar]
- Stalmach A, Edwards CA, Wightman JD, Crozier A. Identification of (Poly)phenolic Compounds in Concord Grape Juice and Their Metabolites in Human Plasma and Urine after Juice Consumption. Journal of Agricultural and Food Chemistry. 2011;59:9512–9522. doi: 10.1021/jf2015039. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–1674. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. Oxygen Radical Absorbing Capacity of Anthocyanins. Journal of Agricultural and Food Chemistry. 1997;45:304–309. [Google Scholar]
- Xu BJ, Yuan SH, Chang SKC. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. Journal of Food Science. 2007;72:S167–S177. doi: 10.1111/j.1750-3841.2006.00261.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.