Abstract
The human pathogen Listeria monocytogenes is susceptible to the β-lactam antibiotics penicillin G and ampicillin, and these are the drugs of choice for the treatment of listerial infections. However, these antibiotics exert only a bacteriostatic effect on this bacterium and consequently, L. monocytogenes is regarded as β-lactam tolerant. It is widely accepted that the phenomenon of bacterial tolerance to β-lactams is due to the lack of adequate autolysin activity, but the mechanisms of L. monocytogenes tolerance to this class of antibiotics are poorly characterized. A ferritin-like protein (Fri) was recently identified as a mediator of β-lactam tolerance in L. monocytogenes, but its function in this process remains unknown. The present study was undertaken to improve our understanding of L. monocytogenes tolerance to β-lactams and to characterize the role of Fri in this phenomenon. A comparative physiological analysis of wild-type L. monocytogenes and a fri deletion mutant provided evidence of a multilevel mechanism controlling autolysin activity in cells grown under β-lactam pressure, which leads to a reduction in the level and/or activity of cell wall-associated autolysins. This is accompanied by increases in the amount of teichoic acids, cell wall thickness and cell envelope integrity of L. monocytogenes grown in the presence of penicillin G, and provides the basis for the innate β-lactam tolerance of this bacterium. Furthermore, this study revealed the inability of the L. monocytogenes Δ fri mutant to deplete autolysins from the cell wall, to adjust the content of teichoic acids and to maintain their D-alanylation at the correct level when treated with penicillin G, thus providing further evidence that Fri is involved in the control of L. monocytogenes cell envelope structure and stability under β-lactam pressure.
Introduction
Listeria monocytogenes is a ubiquitous Gram-positive opportunistic pathogen that causes rare but severe disease in humans and animals. While listeriosis may occur in otherwise healthy individuals, those primarily at risk are immunocompromised patients, pregnant women, the very young and the elderly. Septicemia, meningitis and other infections of the central nervous system are commonly seen in patients with listeriosis. In at-risk groups, the mortality rate is 20–30%, even with antibiotic treatment, making listeriosis one of the most deadly bacterial infections [1], [2].
The treatment of choice for L. monocytogenes infections is a β-lactam antibiotic (penicillin G or ampicillin), alone or in combination with an aminoglycoside [3]. The general susceptibility of L. monocytogenes isolates to β-lactams is reflected by the low MIC (minimal inhibitory concentration) of these antibiotics. However, the MIC and MBC (minimal bactericidal concentration) values of β-lactam antibiotics against most isolates of L. monocytogenes are markedly different, and consequently this bacterium is regarded as tolerant to β-lactams [3], [4]. The tolerance of L. monocytogenes to β-lactams is one of the most important factors contributing to the not infrequent failure of antibiotic therapy against listeriosis. The mechanism underlying L. monocytogenes tolerance to β-lactams is largely unknown.
In other bacterial species, β-lactam tolerance is caused by reduced activity of murein (peptidoglycan) hydrolases, also referred to as autolysins. Peptidoglycan hydrolases are present in all bacteria synthesizing murein cell walls, and due to their ability to catalyze selective hydrolysis of covalent bonds in murein, they are involved in numerous cellular processes including cell growth, cell wall turnover, murein maturation, cell division, separation, differentiation and pathogenicity [5], [6]. These enzymes are also believed to promote bacterial cell lysis in response to reduced penicillin binding protein activity following treatment with β-lactams [7], [8], [9]. Five L. monocytogenes autolysins have been identified so far: Iap (Invasion associated protein), also known as CwhA (cell-wall hydrolase A) or p60 (60 kDa protein); MurA (muramidase A) also called NamA (N-acetylmuramidase A); Spl (p45); Ami and Auto [10], [11], [12], [13], [14], [15].
Since murein hydrolases play a crucial role in bacterial cell lysis, their activity is subject to tight control. In the case of Gram-positive bacteria, a complex control mechanism regulating autolysin activity was proposed in which the main role is played by teichoic acids (TA), which inhibit murein hydrolase activity depending on the presence or absence of protonated D-alanines [16]. TA are polyanionic polymers of Gram-positive bacteria, covalently bound to peptidoglycan in the cell wall. Different species have diverse requirements for TA, suggesting that this polymer may perform various functions. As major constituents of the surfaces of Gram-positive bacteria, TA influence a number of important biological processes, such as autolysis, the binding of cations and surface-associated proteins, cell adhesion, biofilm formation, coaggregation, resistance to antimicrobial agents such as cationic peptides, protein secretion, acid tolerance, virulence and stimulation of the host immune response [17], [18]. The modification of TA with D-alanine is highly conserved and it occurs in the cell wall compartment after TA biosynthesis is completed [17]. The dltABCD operon encodes the enzymes catalyzing this process, and is therefore responsible for modulating the net charge of teichoic acid polymers [19].
It was recently shown that Fri, a ferritin-like protein, plays a crucial role in the tolerance of L. monocytogenes to β-lactam antibiotics [20]. Fri of L. monocytogenes belongs to the Dps (DNA-binding proteins from starved cells) family of proteins, which play an important role in counteracting the adverse effects of iron under aerobic conditions. Dps family proteins resemble ferritins and heme-containing ferritins (bacterioferritins) in their structure and function [21]. Thus, these proteins form cage-like polymers with iron oxidation/storage properties that serve two purposes. One is the removal of Fe(II) from the cytoplasm to prevent the potential generation of the highly toxic hydroxyl radical through the Fenton reaction: Fe(II) + H2O2→Fe(III) + OH− + OH•. The other is to overcome the low solubility of Fe(III) by storing it in the protein internal cavity as ferric hydroxide micelles. Dps proteins use H2O2 as the physiological iron oxidant [22], [23], whereas ferritins employ molecular oxygen [24]. This characteristic enables Dps proteins to protect microorganisms from both Fe(II) and H2O2. There is significant variability in the type and number of ferritin-like proteins expressed in different bacterial species. Escherichia coli and Salmonella enterica possess two ferritins, one bacterioferritin and a Dps protein [25], [26], Porphyromonas gingivalis and Campylobacter jejuni contain one ferritin and a Dps protein [27], [28], while Bacillus subtilis and Bacillus anthracis contain two Dps proteins [29], [30]. This coexistence of multiple ferritin-like proteins within a single bacterium suggests that each protein plays a distinct physiological role, as exemplified in the case of S. enterica [26]. L. monocytogenes is unusual in that it possesses only a single Dps protein encoded by the fri gene [31].
The expression of fri in L. monocytogenes is subject to complex control. Transcription of the fri gene originates from three distinct promoters, one σB-dependent and two σA-dependent [32]. It is also known to be repressed by Fur and derepressed in a perR mutant background [33], [34], and to be upregulated under conditions of iron limitation, heat and cold shock [35], [36]. The ferritin-like protein of L. monocytogenes contributes to virulence and plays a role in protection against multiple stresses including oxidative, iron-starvation, cold- and heat-shock [32], [37], [38]. In addition, the deletion of L. monocytogenes fri was shown to abolish the innate tolerance of this bacterium to β-lactam antibiotics, which was accompanied by visible lysis of the cells [20], [39]. These observations led us to hypothesize that the role of Fri protein in the tolerance of L. monocytogenes to β-lactams might be associated with the regulation of bacterial cell envelope integrity.
This study was undertaken to pinpoint the role of Fri in the tolerance of L. monocytogenes to β-lactams. Fri was found to participate in the regulation of the components that are vital to the stability and function of the Gram-positive cell envelope. Under adverse conditions, such as β-lactam pressure, the activity of this protein is indispensable for the maintenance of L. monocytogenes cell envelope integrity. These findings also provide insight into the basis of the innate tolerance of this pathogen to β-lactams.
Materials and Methods
Bacterial strains, media and DNA techniques
Escherichia coli strain DH5α used in cloning experiments was grown in Luria-Bertani medium. The L. monocytogenes EGD wild-type strain and isogenic EGDΔfri deletion mutant were grown in brain heart infusion (BHI, Oxoid) medium. When required, the media were supplemented with kanamycin (50 µg/ml). The EGDΔfri deletion mutant [32] was a generous gift from Hanne Ingmer, Royal Veterinary and Agricultural University, Denmark. Plasmid pTCV-lac was a kind gift from Birgitte H. Kallipolitis, University of Southern Denmark, Denmark. The isolation of chromosomal and plasmid DNA, digestion of DNA with restriction enzymes, and PCR were performed according to standard protocols [40].
Growth conditions
The L. monocytogenes strains used in all experiments were cultured as described previously [20]. Briefly, the strains were grown overnight in BHI medium at 37°C with shaking. Each culture was then diluted 1∶100 into fresh BHI broth with no additions or supplemented with 0.09 µg/ml penicillin G (Sigma-Aldrich), which equates to 0.75 of the MIC value. The prepared cultures were then incubated as described above until they attained an OD600 of ∼0.35, which corresponds to the mid-log phase of growth.
Analysis of cell wall integrity
L. monocytogenes cultures were harvested by centrifugation and the cells resuspended in lysis buffer (25 mM phosphate buffer, pH 6.4) to an OD600 of 1.0. Hen egg lysozyme (Sigma-Aldrich) was then added to a final concentration of 10 µg/ml and the cell suspensions were incubated at 37°C. Lysis was monitored by following the decrease in OD600 of the samples at 15-min intervals for 90 min, as previously described [41]. The presented results are the averages from three independent experiments, each performed in triplicate.
HPLC analysis of the muropeptide composition of peptidoglycan
The peptidoglycan of the studied strains was isolated, purified and then digested with the muramidase mutanolysin M1 (Sigma-Aldrich) as described previously [42], except that the purified murein was not N-acetylated before digestion. Soluble muropeptides were reduced by treatment with sodium borohydride and then analyzed by HPLC on a Hypersil octadecylsilane (ODS) reversed-phase column (Teknochroma) according to the method of Glauner [43]. Eluted compounds were detected by monitoring the A205. HPLC analysis was performed twice using samples prepared in two independent peptidoglycan isolations.
Estimation of teichoic acids content
The TA content in the cell walls of the studied strains was determined on the basis of the cell wall phosphate concentration, essentially as described previously [41]. Briefly, L. monocytogenes cultures were harvested by centrifugation and each cell pellet resuspended in 50 mM Tris-HCl (pH 7.5), then sonicated to disrupt the cells. Any unbroken cells were pelleted by centrifugation (7,000 × g, 10 min, 4°C). The supernatant was removed and mixed with an equal volume of hot 8% sodium dodecyl sulfate (SDS), then boiled for 30 min. The insoluble cell wall preparation was collected by centrifugation (150,000 × g, 30 min) and washed five times with distilled water. The SDS-free cell walls were then mineralized and the phosphate concentration determined according to the method of Chen [44]. The presented results are the averages from three independent experiments, i.e. performed using three separate cell wall isolations, each carried out in triplicate.
Construction of lacZ transcriptional fusions and β-galactosidase assay
DNA fragments containing the promoter regions of genes of interest were amplified by PCR using the primer pairs listed in Table 1. The fragments were digested with EcoRI and BamHI (except that representing the dlt operon promoter, which was digested with SmaI and BamHI) and cloned into plasmid pTCV-lac [45], a shuttle vector for the construction of transcriptional fusions with the lacZ gene, digested with the corresponding restriction enzymes. Correct insertion of the promoter fragments into pTCV-lac was verified by DNA sequencing and the resulting plasmid constructs were introduced into the wild-type and Δfri mutant strains by electroporation [46]. For measurement of lacZ expression, cells from the L. monocytogenes cultures were collected by centrifugation and assayed for β-galactosidase activity [47]. Briefly, the cells were permeabilized by treatment with 0.5% toluene/4.5% ethanol, and the enzyme activity was determined according to a standard protocol [48]. The specific activity of β-galactosidase was calculated using the formula 103 × (OD420 of the reaction mixture - OD550 of the reaction mixture)/(reaction time in minutes × OD600 of the cells used in the reaction mixture). The specific β-galactosidase activities presented are the averages from three independent experiments, each performed in triplicate.
Table 1. Oligonucleotide primers used in this study.
| Primer | Sequence (5′ → 3′) |
| auto Fa | GAGAATTCAGTAGATAAACGTACCGATTGGA |
| auto R b | GAGGATCCGCTGCTTTAGCATGGAATAGAG |
| ami Fa | CTGAATTCCGCTGCCGGATTGTATATTA |
| ami R b | CTGGATCCTAATCATAGTAGCGGAGGTGC |
| iap Fa | TTGAATTCGCGGGCTATTTCTCGATACTG |
| iap R b | TTGGATCCTGTAGCCGCGATAGTTGCT |
| murA Fa | TAGAATTCGCCATCATACAAAGTGTTGC |
| murA R b | TAGGATCCACCCCAGCAATTGTTGCACC |
| spl Fa | TCGAATTCGAAGTTAGTGACAAGCTCGC |
| spl R b | TCGGATCCTGCTGCGAGTGAGATCGC |
| ftsEX-spl Fa | GTGAATTCGTGCAAGTGAAGTAATGGGTGA |
| ftsEX-spl R b | GTGGATCCGGCAGCTGTTATGCCGTTAG |
| dltABCD Fc | GCGCCCGGGACAAGCCTTTTCTCCTGCA |
| dltABCD Rb | CGGGATCCAGGGAAATCCGGTGTCTTCT |
| secA2 Fa | TCGAATTCTGTAGCCGCGATAGTTGCT |
| secA2 R b | TAGGATCCGCGGGCTATTTCTCGATACTG |
The sequence in boldface type is the EcoRI restriction enzyme site.
The sequence in boldface type is the BamHI restriction enzyme site.
The sequence in boldface type is the SmaI restriction enzyme site.
Protein isolation
Protein isolation was performed according to a previously described cell fractionation method [49] with modifications regarding the isolation of supernatant and cell wall proteins. Briefly, L. monocytogenes cultures were harvested by centrifugation and secreted proteins were isolated from the culture supernatants by precipitation with trichloroacetic acid (at a final concentration of 10%) as described previously [50]. The cell pellets were resuspended in phosphate-buffered saline (PBS) and cells disrupted by sonication. Following the removal of unbroken cells by centrifugation (7,000 × g, 10 min, 4°C), the cytoplasm was separated from the cell envelope by centrifugation (100,000 × g, 45 min). The supernatants containing the cytoplasmic proteins were collected and the pellets were resuspended in 0.5% sodium sarcosinate in PBS. The mixtures were then incubated at 25°C for 30 min with shaking. After centrifugation (100,000 × g, 45 min), the supernatants containing the cytoplasmic membrane proteins were collected. The pellets containing the peptidoglycan layer of the cell wall with noncovalently associated murein hydrolases [51] were resuspended in sample buffer (1% SDS, 5% 2-mercaptoethanol) and the cell wall-associated proteins were released by boiling the samples. The isolated proteins from various cell fractions were adjusted to a final volume that was commensurate with the cell density (0.20 ml/1010 cells).
Protein analysis by SDS-PAGE and Zymography
Isolated protein fractions were analyzed by sodium dodecyl sulfate (SDS) - 10% polyacrylamide gel electrophoresis (PAGE). After electrophoresis the gels were stained with Coomassie brilliant blue to visualize protein bands. Murein hydrolases were analyzed by renaturing gel electrophoresis as described previously [52], using 8–12% SDS-polyacrylamide gradient gels containing 0.1% lyophilized Micrococcus luteus cells (Sigma-Aldrich). After electrophoresis the gels were washed with 25 mM Tris-HCl (pH 7.5) containing 1% Triton X-100 and incubated with shaking at 37°C for 24–48 h. Bands of murolytic activity were detected by staining with 1% methylene blue (Sigma) in 0.01% KOH and subsequent destaining with distilled water. Murein hydrolase activity was visualized as zones of clearing in the blue-stained cell wall background, which were analyzed by densitometry using an ImageQuant™ 300 and ImageQuant™ TL software (GE Healthcare, United Kingdom). SDS-PAGE and zymography analysis were performed three times using proteins from three independent isolations.
Preparation of samples for electron microscopy
Bacterial cells were prepared for observation under a scanning electron microscope (SEM) as described previously [53]. Briefly, L. monocytogenes cultures were harvested by centrifugation and the cells were fixed by incubating for 30 min in 4% paraformaldehyde. After washing three times in PBS (pH 7.4), the cells were dehydrated using a graded ethanol series (25%, 50%, 75%, 96% ethanol; 15 min for each step). The cell suspension was then spread on a microcover, coated with gold and examined using a LEO 1430VP scanning electron microscope. For observation under a transmission electron microscope (TEM), the cells were prepared by thin sectioning as described previously [54]. Briefly, the cells were fixed (12 h, RT) in 1.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2). After washing three times in 0.1 M cacodylate buffer the cells were resuspended in cacodylate buffer and incubated for 30 min at RT. This step was repeated 4 times prior to fixing the cells in OsO4 solution for 2 h. The cell suspension was then dehydrated using a graded ethanol series (70%, 80%, 96%, absolute ethanol) followed by acetone. The dehydrated material was embedded in Epon 812 and thin sections were prepared using Tesla microtome. The samples were examined using a Leo 1912AB transmission electron microscope. Samples for electron microscopy were prepared twice for each strain using two independent bacterial cultures.
Statistical analysis
Statistical analysis was performed using STATISTICA® v10.0. Data were analyzed by Student's t-test, with differences considered significant when P values were □ 0.05.
Results and Discussion
Cell wall integrity of L. monocytogenes EGD and Δfri mutant strains
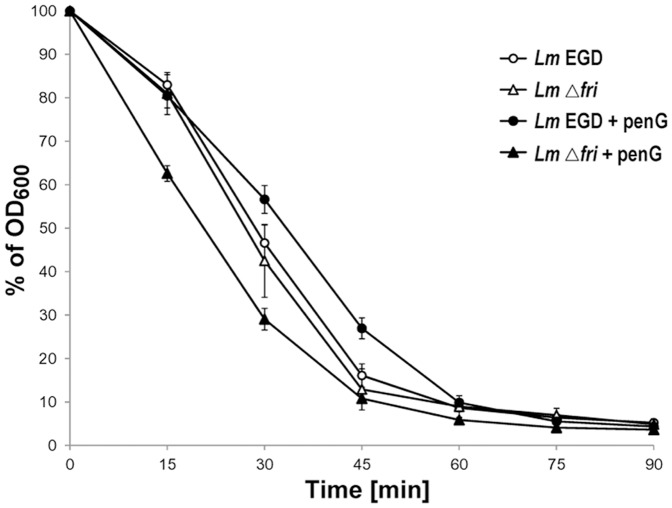
It has recently been shown that the Fri protein of L. monocytogenes plays a crucial role in cell death caused by β-lactam antibiotics [20]. Detailed studies were undertaken to define the involvement of Fri in the maintenance of L. monocytogenes cell envelope structure and stability under β-lactam pressure. In this study, we compared the properties of wild-type strain EGD and the Δ fri mutant grown in both the absence and presence of 0.09 µg/ml of penicillin G (corresponds to 0.75 of the MIC value). These β-lactam stress conditions were previously shown to permit efficient growth of both strains, but caused slight growth retardation of strain Δ fri compared to the wild type [20]. To examine the role of Fri in maintaining the integrity of the L. monocytogenes cell envelope under β-lactam pressure, the susceptibility of the studied strains to lysis induced by incubation with the cell wall hydrolase lysozyme was assessed (Figure 1). When strains grown without antibiotic pressure were used in this experiment, the rate of cell lysis of the Δ fri mutant was not significantly different to that observed for the wild-type strain. However, when the strains grown under penicillin G pressure were compared, the rate of cell lysis of the mutant strain was significantly increased. In contrast, wild-type strain EGD was less susceptible to the action of lysozyme than both the mutant strain grown under the same conditions and EGD grown without the antibiotic (Figure 1). This result demonstrates that in response to penicillin G pressure, cells of L. monocytogenes become less susceptible to lysis and that the lack of fri expression in these adverse conditions leads to impaired cell wall integrity. Subsequently, we examined whether the observed increase in the susceptibility of Δ fri cells to lysozyme was an effect of changes in the level of N-deacetylation of peptidoglycan, as has been observed in the case of the pdgA mutant of L. monocytogenes [55], [56]. However, HPLC analysis did not reveal any significant differences in the muropeptide composition of peptidoglycan of either strain, grown with or without penicillin G (Figure S1). Thus, the observed decrease in the integrity of the cell envelope of the Δ fri mutant was not due to alteration of the structure of the main component of the cell wall, i.e. peptidoglycan.
Figure 1. Integrity of the cell wall of L. monocytogenes strains.
Cells of the wild-type (Lm EGD) and Δ fri mutant (Lm Δ fri) strains grown in the absence or presence of penicillin G (+penG) were incubated with lysozyme (10 U/ml) at 37°C for 90 min. At selected intervals, the OD600 of the cell suspensions was determined. Error bars represent the standard deviation from three independent experiments, each performed in triplicate.
Role of Fri in teichoic acids synthesis
In a further attempt to explain the reduced integrity of the cell envelope of the Δ fri strain grown under β-lactam pressure, we examined the role of Fri in teichoic acid synthesis. Teichoic acids, along with peptidoglycan, are the main components of the cell wall of Gram-positive bacteria and are thought to control the overall surface charge and affect murein hydrolase activity [17]. L. monocytogenes mutants in genes involved in the synthesis of teichoic acids exhibit decreased integrity of the cell envelope [41], [42]. Furthermore, teichoic acids have been shown to contribute to lysozyme resistance in staphylococci, by preventing the binding of this cell wall hydrolase to its peptidoglycan target [57]. Examination of the TA content in the cell wall of the Δ fri mutant and wild-type strains during growth without antibiotic pressure revealed similar levels of cell wall phosphate (Table 2). In wild-type cells grown in the presence of penicillin G, the amount of cell wall phosphate increased significantly, but no such increase was observed in the case of the mutant strain (Table 2). This result indicates that in response to β-lactam pressure, the content of teichoic acids in the cell wall of L. monocytogenes increases in a process that depends on the presence of functional Fri protein.
Table 2. Cell wall TA content in L. monocytogenes strains.
| Strain | Mean cell wall phosphate (µmol/mg of cell wall) ± SDa | |
| BHI medium | BHI medium + PenG | |
| EGD | 1.47 ± 0.04 | 1.79 ± 0.03 b |
| Δ fri | 1.54 ± 0.05 | 1.41 ± 0.04c |
The results are the average of three independent experiments, each performed with separate cell wall preparations of the strains grown in the presence (+ PenG) or absence of 0.09 µg/ml penicillin G ± the standard deviation (SD).
Significant differences following growth in the presence and absence of penicillin G (Student's t-test; P<0.05).
Significant differences between the studied strains (Student's t-test; P<0.05).
Morphology of L. monocytogenes EGD and Δfri mutant strains
Abnormal cell wall structure and cell morphology have been observed in L. monocytogenes when the content of teichoic acids in the cell wall is reduced [41], [42]. In daptomycin-resistant isolates of S. aureus an increase in the TA content was accompanied by an increase in the thickness of the cell wall [58]. Thus, it may be concluded that changes in teichoic acids content often result in alterations in the structure of the Gram-positive cell wall, which can in turn influence cell morphology. Given the observed involvement of Fri in the control of cell envelope integrity as well as its influence on the content of teichoic acids in the cell wall of L. monocytogenes under β-lactam pressure, the effect of the fri mutation on cell morphology and the structure of the cell wall was examined.
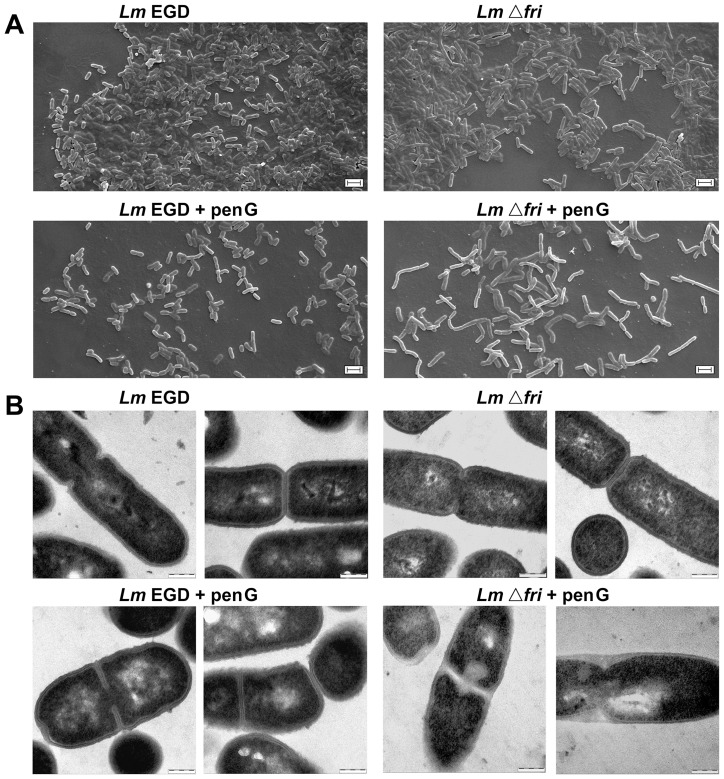
Scanning electron microscopy (SEM) revealed that cells of the L. monocytogenes strain lacking fri were significantly longer than those of the wild type, in both the absence and presence of penicillin G (Figure 2A). However, this effect was much greater in the latter conditions (Table 3) and there was also an increased percentage of Δ fri cells with irregular morphology in the presence of β-lactam pressure. In contrast, cells of the wild-type EGD strain were significantly shorter following growth in the presence of penicillin G with an increased percentage of cells linked in chains.
Figure 2. Cell morphology of L. monocytogenes strains.
Scanning electron micrographs (A) and transmission electron micrographs (B) of wild-type (Lm EGD) and Δ fri mutant (Lm Δ fri) cells grown in the absence or presence of penicillin G (+penG). Bars indicate 2 µm in the scanning electron micrographs and 200 nm in the transmission electron micrographs. The presented micrographs are representative of two independent sample preparations.
Table 3. Cell length and cell wall thickness of L. monocytogenes strains.
| Strain | Average cell length (µm) ± SDa | Average cell wall thickness (nm) ± SDb | ||
| BHI medium | BHI medium + PenG | BHI medium | BHI medium + PenG | |
| EGD | 1.52 ± 0.25 | 1.35 ± 0.22c | 26.52 ± 1.82 | 30.78 ± 2.86c |
| Δ fri | 2.02 ± 0.39d | 2.37 ± 0.76c,d | 25.59 ± 1.67 | 18.81 ± 2.49c,d |
Mean cell lengths were determined by measuring 100 cells of each strain grown in the presence (+ PenG) or absence of 0.09 µg/ml penicillin G. The analysis was performed using two independently prepared samples. From each independent sample, equal numbers of cells were analyzed ± standard deviation (SD).
Mean cell wall thickness was determined by measurement of 20 cells of each strain grown in the presence (+ PenG) or absence of 0.09 µg/ml penicillin G. The analysis was performed using two independently prepared samples. From each independent sample, equal numbers of cells were analyzed ± standard deviation (SD).
Significant differences following growth in the presence and absence of penicillin G (Student's t-test; P<0.05).
Significant differences between the studied strains (Student's t-test; P<0.05).
A detailed insight into the structure of the cell wall of the studied strains was obtained using transmission electron microscopy (TEM) (Figure 2B). In thin sections, both wild-type and Δ fri cells grown in the absence of the antibiotic presented the typical morphology of dividing bacteria, with the formation of a regular septum and normal cell wall thickness, which did not vary in the studied strains. In the presence of penicillin G, strain EGD had a normal morphology and no disorders in septum formation were observed, but the cell wall was significantly thicker (Table 3). In contrast, the Δ fri mutant grown in the presence of this β-lactam, exhibited a clearly altered morphology with reduced cell wall thickness, frequently accompanied by deformities. Furthermore, cell division was apparently arrested in many cells, with irregularities observed in septum structure. Together, these data indicate that fri expression is required to maintain both the shape of the bacterial envelope and normal cell division during the growth of L. monocytogenes under penicillin G pressure.
Participation of Fri in regulating the transcription of murein hydrolase genes and genes engaged in controlling the activity of murein hydrolases
Morphological changes, like the inability of daughter cells to separate following cell division, are the most frequently observed effect of murein hydrolase gene mutations in both Gram-positive and Gram-negative bacteria [16]. Likewise, cell division defects are clearly visible in L. monocytogenes strains deficient in two cell-wall hydrolases, Iap and MurA [59], [60], [61], [62]. Taking into consideration the major influence of murein hydrolases on the integrity of the cell envelope and bacterial cell lysis, we decided to investigate the possible role of Fri in regulating the transcription of genes encoding the known murein hydrolases of L. monocytogenes, i.e. Auto, Ami, MurA, Iap and Spl (p45). The activity of murein hydrolases is often regulated at the posttranslational level, e.g. by controlling the secretion of these enzymes. It is noteworthy that in L. monocytogenes, the auxiliary secretion protein SecA2 was shown to be specifically engaged in the secretion of the murein hydrolases Iap and MurA [61], [62]. Therefore, we also investigated the involvement of Fri in the transcriptional regulation of the secA2 gene. In many Gram-positive bacteria the dlt genes required for D-alanylation of teichoic acids play a vital role in modulating surface charge and controlling murein hydrolase activity [63], [64], [65]. Thus, the influence of Fri on the expression of the dltABCD operon was also examined.
To test whether some of the aforementioned genes are under the control of Fri, DNA fragments containing their putative promoter regions were amplified by PCR and fused to lacZ in the transcriptional fusion vector pTCV-lac. The resulting plasmids were introduced into the L. monocytogenes wild-type and Δ fri mutant strains, and the levels of β-galactosidase activity were determined. In the presence of penicillin G, the expression of the iap-lacZ, murA-lacZ and secA2-lacZ fusions was over 1.5-, 2.5- and 2-fold lower, respectively, than the expression under non-stress conditions in both strains (Table 4). This result demonstrates that the expression of these three genes is repressed by penicillin G only, and Fri is not involved in this regulation. Since secA2 is engaged in the secretion of Iap and MurA [61], [62], diminished membrane translocation of these two autolysins would be anticipated under β-lactam pressure. Interestingly, the expression of dltABCD-lacZwas reduced 2-fold during growth with penicillin G, but only in the Δ fri strain. This indicates that expression of the dlt operon under β-lactam pressure is controlled by Fri.
Table 4. Expression of promoter-lacZ fusions in L. monocytogenes strains.
| Promoter of gene or operonb | Expressiona | |||
| BHI medium | BHI medium + PenG | |||
| EGD strain | Δfri strain | EGD strain | Δfri strain | |
| auto | 108.1 ± 8.4 | 97.4 ± 7.1 | 99.8 ± 19.3 | 102.9 ± 16.7 |
| ami | 85.1 ± 4.0 | 71.9 ± 3.6 | 80.2 ± 8.0 | 64.0 ± 10.2 |
| iap | 132.3 ± 9.0 | 128.2 ± 7.4 | 77.1 ± 8.4c | 84.3 ± 6.3c |
| murA | 145.7 ± 13.3 | 121.5 ± 11.8 | 52.6 ± 6.7c | 45.8 ± 5.8c |
| spl | 103.7 ± 5.3 | 96.9 ± 4.9 | 97.0 ± 7.1 | 103.7 ± 8.4 |
| ftsEX-spl b | 124.4 ± 12.4 | 115.4 ± 7.7 | 97.4 ± 5.9 | 95.2 ± 10.3 |
| dltABCD b | 114.4 ± 9.1 | 109.4 ± 6.2 | 101.2 ± 5.2 | 46.6 ± 4.3c, d |
| secA2 | 76.3 ± 9.5 | 67.5 ± 7.9 | 33.3 ± 6.8c | 27.5 ± 8.5c |
The expression of promoter-lacZ fusions in response to the addition of 0.09 µg/ml penicillin G (+ PenG) or in the absence of the antibiotic was determined by β-galactosidase assays. Specific β-galactosidase activity was measured for wild-type (EGD) or Δfri mutant cells containing promoter-lacZ fusions, grown in the presence or absence of the antibiotic. The results are the average of three independent experiments, each performed in triplicate ± standard deviation.
Genes that are organized in an operon (according to Toledo-Arana et al. [66]).
Significant differences following growth in the presence and absence of penicillin G (Student's t-test; P<0.05).
Significant differences between the studied strains (Student's t-test; P<0.05).
Influence of Fri on murein hydrolase content in various cellular compartments
Simple transcriptional analysis of murein hydrolase genes is not sufficient to estimate the activity of the encoded enzymes, since the regulation of murein hydrolase activity is highly complex and is often controlled at the posttranslational level [16]. As mentioned above, the activity of murein hydrolases can be controlled at the level of secretion. Furthermore, following membrane translocation, murein hydrolase activity can also be regulated by their stability and/or localization. There is only very limited information on the control of stability of L. monocytogenes autolysins. However, the proteolytic processing of autolysins seems to be common among Gram-positive bacteria, and has been observed in the case of MurA and Ami of L. monocytogenes [61]. The control of autolysin localization is also poorly understood. The expected final destination of murein hydrolases of L. monocytogenes is their site of action in the cell wall. These enzymes are noncovalently bound to the cell wall via their LysM domain or GW modules [51], [67]. However, autolysins with LysM and GW regions have been detected not only in the cell wall, but also in the supernatant fraction. Furthermore, Iap and Spl are also found in the cell membrane fraction [61], [68], [69], [70].
To determine the posttranslational fate of L. monocytogenes murein hydrolases and thus their potential activity, and to examine the effect of β-lactam pressure and/or Fri on the posttranslational regulation of these enzymes, we compared their abundance in different cell compartments of EGD and Δ fri strains grown with and without penicillin G. Proteins were isolated from each cellular fraction, i.e. cytoplasm, cytoplasmic membrane, cell wall and culture supernatant. SDS-PAGE analysis revealed that the protein patterns of each cellular fraction were similar in the mutant and the wild-type strains, irrespective of the presence of penicillin G (Figure S2). Therefore, the lack of Fri does not result in global alterations of protein composition or in the non-specific release of proteins into the culture supernatant.
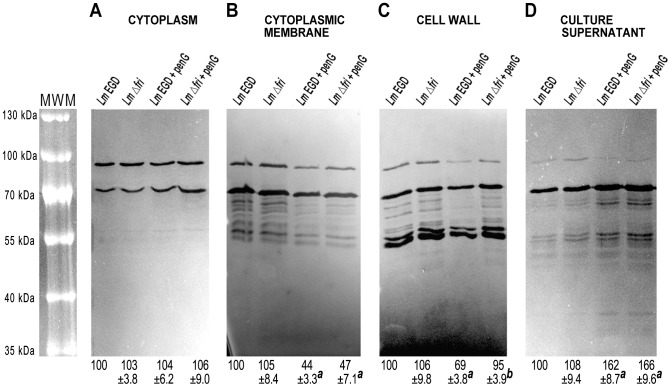
After this preliminary assay, equivalent quantities from each fraction were subjected to zymography analysis (Figure 3A-3D). Densitometry of zymograms revealed that in the presence of penicillin G, the total amount of murein hydrolases recovered in the cell membrane fraction was approximately 55% lower than under non-stress conditions in the two strains (Figure 3B). Furthermore, in the presence of penicillin G, the total amount of murein hydrolases released into both culture supernatants was approximately 60% higher than under non-stress conditions (Figure 3D).
Figure 3. Content of murein hydrolases in various cellular compartments of L. monocytogenes strains.
Zymography analysis of proteins from the cytoplasm (A), cytoplasmic membrane (B), cell wall fraction (C) and culture supernatant (D) were performed for the wild-type EGD strain grown without (Lm EGD) and with (Lm EGD + penG) penicillin G, and the Δ fri mutant strain grown without (Lm Δfri) and with (Lm Δfri + penG) the same antibiotic. Equivalent quantities of proteins isolated from each fraction were subjected to zymographic analysis in SDS-polyacrylamide gels. MWM – prestained Protein Molecular Weight Marker. The relative amounts of murein hydrolases estimated on the basis of the densitometric analysis are shown in the lower panel. Values in each analyzed compartment were normalized to the total intensity of murein hydrolases from the wild-type EGD strain grown without the antibiotic, which was assigned the value of 100%. The presented results are mean values from the analysis of three independent protein isolations ± the standard deviation. a Significant differences following growth in the presence and absence of penicillin G (Student's t-test; P<0.05). b Significant differences between the studied strains (Student's t-test; P<0.05).
These results indicate that the diminished amount of cell membrane-associated murein hydrolases as well as the increased release of these enzymes into the culture supernatant are an effect of the presence of penicillin G alone. Fri is not involved in regulating the translocation of these enzymes across the membrane, nor in their release to the supernatant. Interestingly, the total amount of murein hydrolases recovered in the cell wall fraction of strain Δ fri was about 35% higher than in the wild-type strain during growth under penicillin G pressure, whereas during growth without the antibiotic there was no significant difference between the two strains in the level of these enzymes (Figure 3C). Furthermore, the amount of cell wall-associated murein hydrolases isolated from the wild-type strain was approximately 30% lower in cells grown under penicillin G pressure compared to those grown without this antibiotic. This reduction in the level of cell wall-associated murein hydrolases caused by penicillin G was not observed in the case of the Δ fri mutant strain (Figure 3C). The reduction in the amount of cell membrane- and cell wall-associated murein hydrolases in response to β-lactam pressure suggests that the content of these enzymes in these compartments is subject to tight regulation. The lack of downregulation of cell wall-associated murein hydrolases in the Δ fri strain clearly indicates that under these adverse conditions, Fri is indispensible in controlling the abundance of these enzymes in their site of activity.
The results of the cell wall integrity assay demonstrate that Fri is indispensable for the observed increase in the integrity of the L. monocytogenes cell wall in response to penicillin G pressure. The higher cell wall integrity is correlated with an increased content of teichoic acids in response to penicillin G pressure, which depends on the presence of the functional Fri protein. Furthermore, the results of transcriptional analysis revealed that expression of the dlt operon under penicillin G pressure is controlled by Fri, indicating that this protein is also involved in modulating the net charge of the teichoic acid polymers.
Interestingly, one of the most well characterized and widespread functions of teichoic acids among bacterial species is the control of autolysins [17], [18]. It has been proposed that teichoic acids might control autolysins directly since these enzymes have high affinities for these polyanionic polymers [71], [72]. It is also possible that the ability of teichoic acids to provide cations may be more important in controlling autolysins than direct interaction, since the activity of the major autolysins depends on bivalent cations [18], [73]. Alternatively, TA may play an indirect role in autolysin control by providing a proton-binding mechanism that regulates the enzyme activity by influencing acidification of the environment [74]. Furthermore, D-alanylation of teichoic acids is considered to be crucial in modulating surface charge and controlling murein hydrolase activity [63], [64], [65]. For example, dlt mutations in B. subtilis resulted in enhanced autolysis and the cell walls of the mutants were apparently more negatively charged [63], [75]. Thus, the observed decrease in the cell wall integrity of the L. monocytogenes fri mutant could result from insufficient control of autolysin activity, which might be the effect of a reduced content of TA that are poorly D-alanylated. Insufficient control of autolysin activity could lead to the reduced cell wall thickness and septum irregularities observed in the fri mutant under penicillin G pressure. With regard to the latter, it is noteworthy that in addition to being regulated by teichoic acids, autolysins may be positioned at cell wall septa by these polymers [71], [72].
As mentioned above, it has been proposed that D-alanylation determines the number of anionic sites on TA for autolysin binding. According to this hypothesis, strains with reduced dlt expression should bind more autolysin, resulting in an increased rate of autolysis [63]. In the light of this hypothesis, it seems likely that the observed higher content of autolysins in the fri mutant compared to the EGD wild type is the direct result of reduced D-alanylation in the mutant strain and the consequent higher binding capacity of the teichoic acids. Furthermore, the observed decrease in the cell wall integrity of the fri mutant might result both from its inability to regulate the autolysin content of the cell wall compartment as well as insufficient control of the activity of these enzymes. However, it should be stressed that the final amount of autolysins in the cell wall depends on processes such as their secretion, stability in the cell envelope and transport across the cell wall into the extracellular milieu. In this respect, D-alanylation could play another important role in modulating the rate of protein transport across the cell wall, since the net negative charge of the wall influences protein folding [76], [77].
The results of the present study show that a ferritin-like protein plays an important role in maintaining the stability and structure of the cell envelope of L. monocytogenes under β-lactam pressure. Previously, Fri was shown to be involved in protection against multiple stresses and the expression of the fri gene was found to be subject to a complex control mechanism involving Fur, Per and SigB [32], [33], [34], [37], [38]. Alternative sigma factor SigB was shown to determine the tolerance of L. monocytogenes to cell envelope-acting antimicrobial agents and is thought to control the transcription of genes contributing to this tolerance, including the dlt operon [78]. Interestingly, overexpression of an anti-sigma B factor, RsbW, was observed in a fri mutant strain, [37], which strongly suggests negative modulation of SigB activity by the Fri protein. This might explain the important role of Fri in maintaining the stability of the L. monocytogenes cell envelope observed in the current study. However, further studies are required to examine possible interactions between Fri and SigB under β-lactam pressure.
It is assumed that the important role of Fri in protecting the bacterial cells against multiple stresses relies on the prevention of oxidative damage by removing excess ferrous ions from the cytosol, making them unavailable for participation in the Fenton reaction. Interestingly, the study of Kohanski and co-workers revealed that bactericidal antibiotics increase cellular production of H2O2, the end product of an oxidative damage-cellular death pathway involving stimulation of the Fenton reaction [79]. This suggests that Dps proteins play a crucial role in the control of bacterial cell death caused by antibiotics. In line with this hypothesis is growing evidence of the role of Dps proteins in bacterial cell death in human pathogens including Salmonella enterica, Mycobacterium tuberculosis and Listeria monocytogenes [20], [80], [81]. Here, we describe for the first time, the effect of Dps protein on the stability and structure of the L. monocytogenes cell envelope under β-lactam antibiotic pressure. Under these adverse conditions the Dps protein plays an important role in regulating components that are critical for Gram-positive cell envelope function, i.e. the amount/activity of cell wall-associated murein hydrolases, the content of teichoic acids in the cell wall and the level of D-alanylation of teichoic acids.
Conclusions
Serious illnesses and fatalities that occur in susceptible individuals highlight the importance of understanding the mechanisms underlying the innate tolerance of L. monocytogenes to β-lactam antibiotics. The findings of the present study demonstrate that Fri protein, which has recently been identified as a mediator of β-lactam tolerance, is involved in regulating L. monocytogenes cell envelope structure and integrity under β-lactam pressure. Our detailed investigation revealed the inability of an L. monocytogenes strain lacking Fri to control the amount and/or activity of cell wall-associated murein hydrolases. This deficiency is caused by the inability of the Δ fri strain to (i) reduce the content of these enzymes associated with the cell wall, (ii) adjust the content of teichoic acids bound within the cell wall and (iii) maintain the D-alanylation of teichoic acids at the proper level under β-lactam pressure. We provide evidence that under β-lactam pressure, the lack of a functional Fri protein leads to the loss of control of both the amount and activity of cell wall-associated murein hydrolases, which has serious implications for L. monocytogenes cell envelope structure and stability in these conditions.
Furthermore, the results of the present study demonstrate that the content of cell wall-associated murein hydrolases in L. monocytogenes is subject to tight control in response to β-lactam pressure, which provides insight into the mechanism of L. monocytogenes tolerance to this class of antibiotics. The observed fate of murein hydrolases in L. monocytogenes cells grown under β-lactam pressure indicates the presence of a multilevel mechanism controlling murein hydrolase activity. This mechanism includes transcriptional downregulation of genes encoding the two main murein hydrolases and SecA2 protein involved in their secretion. Moreover, an analysis of the abundance of murein hydrolases in different cell compartments showed that penicillin G treatment causes a decrease in the relative content of these enzymes associated with the cell membrane and cell wall, and an increase in the relative content of these enzymes released into the supernatant. In light of these observations, we propose that the alterations in the level and/or activity of cell wall-associated murein hydrolases, accompanied by an increase in the amount of TA in this compartment, led to the observed increase in cell wall thickness and cell envelope integrity of L. monocytogenes grown in the presence of penicillin G. Undoubtedly, these changes help to protect the cells from lysis and provide the basis for the innate tolerance of L. monocytogenes to β-lactams.
Supporting Information
HPLC analysis of the muropeptide composition of the peptidoglycan of L. monocytogenes strains. The analyzed peptidoglycan was purified from the wild-type EGD strain (Lm EGD) and the Δ fri mutant strain (Lm Δfri) grown without the antibiotic (A), and the wild-type EGD strain (Lm EGD + penG) and the Δfri mutant strain (Lm Δfri + penG) grown in the presence of penicillin G (B). Muropeptides produced by the enzymatic hydrolysis of peptidoglycan were reduced and separated by reversed-phase HPLC and the A205 of the eluate was monitored. The presented results are representative of HPLC analysis of two independent peptidoglycan preparations.
(TIF)
Analysis of proteins isolated from different cellular compartments of L. monocytogenes strains. Equivalent quantities of protein from the cytoplasm (A), cytoplasmic membrane (B), cell wall fraction (C) and culture supernatant (D) were subjected to SDS-PAGE analysis and stained with Coomassie brilliant blue. This analysis was performed for proteins isolated from cells of the wild-type EGD strain grown without (Lm EGD) and with (Lm EGD + penG) penicillin G, and the Δfri mutant strain grown without (Lm Δfri) and with (Lm Δfri + penG) this antibiotic. MWM – prestained Protein Molecular Weight Marker. The presented results are representative of the analysis of three independent protein preparations.
(TIF)
Acknowledgments
We are grateful to Birgitte H. Kallipolitis for providing plasmid pTCV-lac and Hanne Ingmer for providing mutant EGDΔfri. We also thank Magdalena Popowska for expert technical assistance in HPLC analysis.
Funding Statement
This work was funded by the grant of Polish Ministry of Science and Higher Education N N302 229738. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McLaughlin J (1997) The pathogenicity of Listeria monocytogenes: a public health perspective. Rev Med Bacteriol 8: 1–14. [Google Scholar]
- 2. Schuchat A, Swaminathan B, Broome CV (1991) Epidemiology of human listeriosis. Clin Microbiol Rev 4: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hof H, Nichterlein T, Kretschmar M (1997) Management of listeriosis. Clin Microbiol Rev 10: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hof H (2003) Listeriosis: therapeutic options. FEMS Immunol Med Microbiol 35: 203–205. [DOI] [PubMed] [Google Scholar]
- 5. Blackman SA, Smith TJ, Foster SJ (1998) The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144: 73–82. [DOI] [PubMed] [Google Scholar]
- 6.Shockman GD, Höltje J-V (1994) Microbial murein (peptidoglycan) hydrolases. In: Ghuysen J-M, Hakenbeck R (eds) Bacterial Cell Wall pp. 131–144.
- 7. Tomasz A, Albino A, Zanati E (1970) Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227: 138–140. [DOI] [PubMed] [Google Scholar]
- 8. Tomasz A (1979) The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol 33: 113–137. [DOI] [PubMed] [Google Scholar]
- 9. Koch AL (2001) Autolysis control hypotheses for tolerance to wall antibiotics. Antimicrob Agents Chemother 45: 2671–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bubert A, Kuhn M, Goebel W, Kohler S (1992) Structural and functional properties of the p60 proteins from different Listeria species. J Bacteriol 174: 8166–8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLaughlan AM, Foster SJ (1998) Molecular characterization of an autolytic amidase of Listeria monocytogenes EGD. Microbiol 144: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 12. Schubert K, Bichlmaier AM, Mager E, Wolff K, Ruhland G, et al. (2000) P45, an extracellular 45 kDa protein of Listeria monocytogenes with similarity to protein p60 and exhibiting murein lytic activity. Arch Microbiol 173: 21–28. [DOI] [PubMed] [Google Scholar]
- 13. Carroll SA, Hain T, Technow U, Darji A, Pashalidis P, et al. (2003) Identification and characterization of a murein hydrolase, MurA, of Listeria monocytogenes, a muramidase needed for cell separation. J Bacteriol 185: 6801–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabanes D, Dehoux P, Dussurget O, Frangeul L, Cossar P (2004) Auto, a surface associated autolysin of Listeria monocytogenes required for entry into eukaryotic cells and virulence. Mol Microbiol 51: 1601–1614. [DOI] [PubMed] [Google Scholar]
- 15. Pilgrim V, Kolb-Maurer A, Gentschev L, Goebel W, Kuhn M (2003) Deletion of the gene encoding p60 in Listeria monocytogenes leads to abnormal cell division and loss of actin-based motility. Infect Immun 71: 3473–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rice KC, Bayles KW (2008) Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72: 85–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neuhaus FC, Baddiley J (2003) A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67: 686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weidenmaier C, Peschel A (2008) Teichoic acids and related cell wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 6: 276–287. [DOI] [PubMed] [Google Scholar]
- 19. Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, et al. (2002) Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes . Mol Microbiol 43: 1–14. [DOI] [PubMed] [Google Scholar]
- 20. Krawczyk-Balska A, Marchlewicz J, Dudek D, Wasiak K, Samluk A (2012) Identification of a ferritin-like protein of Listeria monocytogenes as a mediator of β-lactam tolerance and innate resistance to cephalosporins. BMC Microbiol 12: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haikarainen T, Papageorgiou AC (2010) Dps-like proteins: structural and functional insights into a versatile protein family. Cell Mol Life Sci 67: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su M, Cavallo S, Stefanini S, Chiancone E, Chasteen ND (2005) The socalled Listeria innocua ferritin is a Dps protein. Iron incorporation, detoxification, and DNA protection properties. Biochemistry 44: 5572–5578. [DOI] [PubMed] [Google Scholar]
- 23. Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, et al. (2002) Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells: A ferritin-like DNA-binding protein of Escherichia coli . J Biol Chemistry 277: 27689–27696. [DOI] [PubMed] [Google Scholar]
- 24. Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275: 161–203. [DOI] [PubMed] [Google Scholar]
- 25. Andrews SC (1998) Iron storage in bacteria. Adv Microb Physiol 40: 281–351. [DOI] [PubMed] [Google Scholar]
- 26. Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC (2007) The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron–sulphur cluster repair and virulence. Mol Microbiol 63: 1495–1507. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa T, Mizunoe Y, Kawabata S, Takade A, Harada M, et al. (2003) The iron-binding protein Dps confers hydrogen peroxide stress resistance to Campylobacter jejuni . J Bacteriol 185: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ueshima J, Shoji M, Ratnayake DB, Abe K, Yoshida S, et al. (2003) Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis . Infect Immun 71: 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, James LP, Helmann JD (1993) Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol 175: 5428–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X, Kim K, Leighton T, Theil EC (2006) Paired Bacillus anthracis Dps (Mini-ferritin) have different reactivities with peroxide. J Biol Chem 281: 27827–27835. [DOI] [PubMed] [Google Scholar]
- 31. Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, et al. (2001) Comparative genomics of Listeria species. Science 294: 849–852. [DOI] [PubMed] [Google Scholar]
- 32. Olsen KN, Larsen MH, Gahan CG, Kallipolitis B, Wolf XA, et al. (2005) The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology 151: 925–933. [DOI] [PubMed] [Google Scholar]
- 33. Fiorini F, Stefanini S, Valenti P, Chiancone E, De Biase D (2008) Transcription of the Listeria monocytogenes fri gene is growth-phase dependent and is repressed directly by Fur, the ferric uptake regulator. Gene 410: 113–121. [DOI] [PubMed] [Google Scholar]
- 34. Rea RB, Hill C, Gahan CGM (2005) Listeria monocytogenes PerR mutants display a small-colony phenotype, increased sensitivity to hydrogen peroxide, and significantly reduced murine virulence. Appl Environ Microbiol 71: 8314–8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hebraud M, Guzzo J (2000) The main cold shock protein of Listeria monocytogenes belongs to the family of ferritin-like proteins. FEMS Microbiol Lett 190: 29–34. [DOI] [PubMed] [Google Scholar]
- 36. Polidoro M, De Biase D, Montagnini B, Guarrera L, Cavallo S, et al. (2002) The expression of the dodecameric ferritin in Listeria spp. is induced by iron limitation and stationary growth phase. Gene 296: 121–128. [DOI] [PubMed] [Google Scholar]
- 37. Dussurget O, Dumas E, Archambaud C, Chafsey I, Chambon C, et al. (2005) Listeria monocytogenes ferritin protects against multiple stresses and is required for virulence. FEMS Microbiology Letters 250: 253–261. [DOI] [PubMed] [Google Scholar]
- 38. Mohamed W, Darji A, Domann A, Chiancone E, Chakraborty T (2006) The ferritin-like protein Frm is a target for the humoral immune response to Listeria monocytogenes genes and is required for efficient bacterial survival. Mol Genet Genomics 275: 344–353. [DOI] [PubMed] [Google Scholar]
- 39. Popowska M, Kloszewska M, Gorecka S, Markiewicz Z (1999) Autolysis of Listeria monocytogenes . Acta Microbiol Pol 48: 141–152. [PubMed] [Google Scholar]
- 40.Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring HarborN.Y.: Cold Spring Harbor Laboratory Press.
- 41. Dubail I, Bigot A, Lazarevic V, Soldo B, Euphrasie D, et al. (2006) Identification of an essential gene of Listeria monocytogenes involved in teichoic acid biogenesis. J Bacteriol 188: 6580–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Popowska M, Osińska M, Rzeczkowska M (2012) N-acetylglucosamine-6-phosphate deacetylase (NagA) of Listeria monocytogenes EGD, an essential enzyme for the metabolism and recycling of amino sugars. Arch Microbiol 194: 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glauner B (1988) Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem 172: 451–464. [DOI] [PubMed] [Google Scholar]
- 44. Chen PS (1956) Microdetermination of phosphorus. Anal Chem 18: 1756–1758. [Google Scholar]
- 45. Poyart C, Trieu-Cuot P (1997) A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in Gram-positive bacteria. FEMS Microbiol Lett 156: 193–198. [DOI] [PubMed] [Google Scholar]
- 46. Park SF, Stewart GS (1990) High efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94: 129–132. [DOI] [PubMed] [Google Scholar]
- 47. Kallipolitis BH, Ingmer H, Gahan CG, Hill C, Søgaard-Andersen L (2003) CesRK, a two-component signal transduction system in Listeria monocytogenes, responds to the presence of cell wall-acting antibiotics and affects β-lactam resistance. Antimicrob Agents Chemother 47: 3421–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JH (1972) Experiments in molecular genetics. Cold Spring HarborN.Y.: Cold Spring Harbor Laboratory Press.
- 49. Newton SM, Klebba PE, Raynaud C, Shao Y, Jiang X, et al. (2005) The svpA-srtB locus of Listeria monocytogenes: fur-mediated iron regulation and effect on virulence. Mol Microbiol 55: 927–940. [DOI] [PubMed] [Google Scholar]
- 50. Wiśniewski J, Krawczyk-Balska A, Bielecki J (2006) Associated roles of hemolysin and p60 protein for the intracellular growth of Bacillus subtilis . FEMS Immunol Med Microbiol 46: 330–339. [DOI] [PubMed] [Google Scholar]
- 51. Bierne H, Cossart P (2007) Listeria monocytogenes surface proteins: From genome predictions to function. Microbiol Mol Biol Rev 71: 377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Foster SJ (1992) Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol 174: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krawczyk-Balska A, Popowska M, Markiewicz Z (2012) Re-evaluation of the significance of penicillin binding protein 3 in the susceptibility of Listeria monocytogenes to β-lactam antibiotics. BMC Microbiol 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Korsak D, Vollmer W, Markiewicz Z (2005) Listeria monocytogenes EGD lacking penicillin-binding protein 5 (PBP5) produces a thicker cell wall. FEMS Microbiol Lett 251: 281–288. [DOI] [PubMed] [Google Scholar]
- 55. Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, et al. (2007) A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci USA 104: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Popowska M, Kusio M, Szymanska P, Markiewicz Z (2009) Inactivation of the wall-associated de-N-acetylase (PgdA) of Listeria monocytogenes results in greater susceptibility of the cells to induced autolysis. J Microbiol Biotechnol 19: 932–945. [DOI] [PubMed] [Google Scholar]
- 57. Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, et al. (2007) Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189: 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bertsche U, Yang S, Kuehner D, Wanner S, Mishra NN, et al. (2013) Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS ONE 8(6): e67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Machata S, Hain T, Rohde M, Chakraborty T (2005) Simultaneous deficiency of both MurA and p60 proteins generates a rough phenotype in Listeria monocytogenes . J Bacteriol 187: 8385–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lenz LL, Portnoy DA (2002) Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol Microbiol 45: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 61. Lenz LL, Mohammadi S, Geissler A, Portnoy DA (2003) SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci USA 100: 12432–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Renier S, Chambon C, Viala D, Chagnot C, Hébraud M, et al. (2013) Exoproteomic analysis of the SecA2-dependent secretion in Listeria monocytogenes EGD-e. J Proteomics 80: 183–195. [DOI] [PubMed] [Google Scholar]
- 63. Wecke J, Madela K, Fischer W (1997) The absence of D-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis . Microbiology 143: 2953–2960. [DOI] [PubMed] [Google Scholar]
- 64. Steen A, Palumbo E, Deghorain M, Cocconcelli PS, Delcour J, et al. (2005) Autolysis of Lactococcus lactis is increased upon D-alanine depletion of peptidoglycan and lipoteichoic acids. J Bacteriol 187: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hartung T, Morath S, Hols P (2006) D-Alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. J Bacteriol 188: 3709–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, et al. (2009) The Listeria transcriptional landscape from saprophytism to virulence. Nature 459: 950–956. [DOI] [PubMed] [Google Scholar]
- 67. Renier S, Micheau P, Talon R, Hébraud M, Desvaux M (2012) Subcellular localization of extracytoplasmic proteins in monoderm bacteria: rational secretomics-based strategy for genomic and proteomic analyses. PLoS One 7: e42982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Desvaux M, Dumas E, Chafsey I, Chambon C, Hebraud M (2010) Comprehensive appraisal of the extracellular proteins from a monoderm bacterium: theoretical and empirical exoproteomes of Listeria monocytogenes EGD-e by secretomics. J Proteome Res 9: 5076–5092. [DOI] [PubMed] [Google Scholar]
- 69. Wehmhoner D, Dieterich G, Fischer E, Baumgartner M, Wehland J, et al. (2005) "LaneSpector". a tool for membrane proteome profiling based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis/liquid chromatography-tandem mass spectrometry analysis: application to Listeria monocytogenes membrane proteins. Electrophoresis 26: 2450–2460. [DOI] [PubMed] [Google Scholar]
- 70. Kuhn M, Goebel W (1989) Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun 57: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giudicelli S, Tomasz A (1984) Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol 158: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bierbaum G, Sahl HG (1987) Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J Bacteriol 169: 5452–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heptinstall S, Archibald AR, Baddiley J (1970) Teichoic acids and membrane function in bacteria. Nature 225: 519–52. [DOI] [PubMed] [Google Scholar]
- 74. Biswas R, Martinez RE, Göhring N, Schlag M, Josten M, et al. (2012) Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS ONE 7: e41415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wecke J, Perego M, Fischer W (1996) D-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb Drug Resist 2: 123–129. [DOI] [PubMed] [Google Scholar]
- 76. Hyyrylainen HL, Vitikainen M, Thwaite J, Wu H, Sarvas M, et al. (2000) D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis . J Biol Chem 275: 26696–26703. [DOI] [PubMed] [Google Scholar]
- 77. Forster BM, Marquis H (2012) Protein transport across the cell wall of monoderm Gram-positive bacteria. Mol Microbiol 84: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Begley M, Hill C, Ross RP (2006) Tolerance of Listeria monocytogenes to cell envelope-acting antimicrobial agents is dependent on SigB. Appl Environ Microbiol 72: 2231–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130: 797–810. [DOI] [PubMed] [Google Scholar]
- 80. Calhoun LN, Kwon YM (2011) The ferritin-like protein Dps protects Salmonella enterica serotype Enteritidis from the Fenton-mediated killing mechanism of bactericidal antibiotics. Int J Antimicrob Agents 37: 261–265. [DOI] [PubMed] [Google Scholar]
- 81. Pandey R, Rodriguez GM (2012) A ferritin mutant of Mycobacterium tuberculosis is highly susceptible to killing by antibiotics and is unable to establish a chronic infection in mice. Infect Immun 80: 3650–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC analysis of the muropeptide composition of the peptidoglycan of L. monocytogenes strains. The analyzed peptidoglycan was purified from the wild-type EGD strain (Lm EGD) and the Δ fri mutant strain (Lm Δfri) grown without the antibiotic (A), and the wild-type EGD strain (Lm EGD + penG) and the Δfri mutant strain (Lm Δfri + penG) grown in the presence of penicillin G (B). Muropeptides produced by the enzymatic hydrolysis of peptidoglycan were reduced and separated by reversed-phase HPLC and the A205 of the eluate was monitored. The presented results are representative of HPLC analysis of two independent peptidoglycan preparations.
(TIF)
Analysis of proteins isolated from different cellular compartments of L. monocytogenes strains. Equivalent quantities of protein from the cytoplasm (A), cytoplasmic membrane (B), cell wall fraction (C) and culture supernatant (D) were subjected to SDS-PAGE analysis and stained with Coomassie brilliant blue. This analysis was performed for proteins isolated from cells of the wild-type EGD strain grown without (Lm EGD) and with (Lm EGD + penG) penicillin G, and the Δfri mutant strain grown without (Lm Δfri) and with (Lm Δfri + penG) this antibiotic. MWM – prestained Protein Molecular Weight Marker. The presented results are representative of the analysis of three independent protein preparations.
(TIF)