SUMMARY
SETTING
Central Rio de Janeiro, Brazil.
OBJECTIVE
To compare the impact of routine DOTS vs. enhanced DOTS (DOTS-Ampliado or DOTS-A) on tuberculosis (TB) incidence.
DESIGN
Cluster-randomized trial in eight urban neighborhoods pair-matched by TB incidence and randomly assigned to receive either the DOTS-A or DOTS strategy. DOTS-A added intensive screening of household contacts of active TB cases and provision of treatment to secondary cases and preventive therapy to contacts with latent TB infection (LTBI) to the standard DOTS strategy. The primary endpoint was the TB incidence rates in communities after 5 years of intervention.
RESULTS
From November 2000 to December 2004, respectively 339 and 311 pulmonary TB cases were enrolled and 1003 and 960 household were identified in DOTS and DOTS-A communities. Among contacts from DOTS-A communities, 26 (4%) had active TB diagnosed and treated, 429 (61.3%) had LTBI detected and 258 (60.1%) started preventive therapy. TB incidence increased by 5% in DOTS communities and decreased by 10% in DOTS-A communities, for a difference of 15% after 5 years (P = 0.04).
CONCLUSION
DOTS-A was associated with a modest reduction in TB incidence and may be an important strategy for reducing the burden of TB.
Keywords: tuberculosis, DOTS, household contacts, latent TB infection treatment
Detection of cases and institution of effective chemotherapy is considered the most important strategy for controlling tuberculosis (TB), and is the cornerstone of global public health efforts.1 The World Health Organization’s (WHO’s) DOTS Strategy2 is widely followed, but has had variable success in reducing TB incidence rates in developing countries and in settings with a high prevalence of human immunodeficiency virus (HIV) infection.3,4 The TB epidemic in developing countries demands evaluation of broader approaches in addition to DOTS.
Evaluation of contacts of TB cases is integral to control efforts in industrialized countries, but is little used in high-burden settings. Routine contact evaluation usually yields a small proportion of active TB cases and varying proportions of contacts with latent TB infection (LTBI).5 Chemoprophylaxis of LTBI with isoniazid (INH) reduces the risk of subsequent TB disease in high-risk household contacts and HIV-infected people.6 Recent TB transmission contributes to a large proportion of active TB cases in urban settings.7–9 Mathematical models have suggested that preventive therapy, in combination with DOTS, may contribute substantially to TB control efforts.10,11 We undertook a cluster-randomized trial to assess the impact of a TB control strategy that included evaluation and treatment of contacts of newly diagnosed TB patients on the community incidence of TB in Rio de Janeiro City, Brazil. Enhanced DOTS (DOTS-Ampliado or DOTS-A) added intensive screening of household contacts of active TB cases and provision of TB treatment to secondary cases and preventive therapy to contacts with LTBI to the standard DOTS program.
METHODS
This community-randomized trial involved urban neighborhoods with high rates of TB in the downtown area of Rio de Janeiro City, Brazil. The study area has a population of 265 000 and the highest TB incidence rate in the city (240 cases per 100000 population).
Within the study area, a group of eight neighborhoods was selected based on access to health services and distinct geographic boundaries, and divided into four pairs matched by TB incidence over the previous 4 years. The matched communities were similar in socio-economic indicators such as life expectancy, adult literacy and monthly family income, as well as human development index ratings (Table 1). Within each pair, neighborhoods were randomly assigned to receive either the DOTS-A or the standard DOTS strategy within the public TB control services. We used a constrained randomization design to determine which communities would be allocated to DOTS or DOTS-A.
Table 1.
Randomized communities: population, TB incidence rates and socio-economic indicators
| Communities | TB incidence rate/100000 1998 | Population 2000 n | Monthly family income US$ | Literacy rate % | Human development index |
|---|---|---|---|---|---|
| DOTS | |||||
| Estácio | 542 | 20 667 | 45.89 | 94.0 | 0.829 |
| Rio Comprido | 225 | 35 526 | 65.85 | 97.0 | 0.849 |
| Santo Cristo | 54 | 9 920 | 35.62 | 94.2 | 0.792 |
| Benfica | 244 | 19 112 | 41.85 | 94.5 | 0.825 |
| DOTS-A | |||||
| São Cristóvão | 304 | 38 847 | 45.82 | 95.4 | 0.833 |
| Cidade Nova | 266 | 5 563 | 71.15 | 96.2 | 0.867 |
| Catumbi | 492 | 12 868 | 36.09 | 95.8 | 0.802 |
| Mangueira | 496 | 14 031 | 39.71 | 94.2 | 0.800 |
TB = tuberculosis; DOTS-A = DOTS ampliado (enhanced DOTS).
Subjects with newly diagnosed pulmonary TB living in the selected neighborhoods who were treated at one of the three municipal health centers serving the area and who provided written informed consent were enrolled in the study. Patients who refused to consent to the study or to receive directly observed therapy (DOT) were given self-administered treatment according to the Brazilian guidelines.12 Furthermore, residents of the communities who had TB diagnosed and treated at a hospital or health clinic outside the study area were not enrolled.
An index case was defined as the first member of a household diagnosed with active TB. Active TB diagnosis was defined according to Brazilian guidelines12 using acid-fast bacilli (AFB) sputum smear, mycobacterial sputum culture on Löwenstein-Jensen medium and chest radiographs. A household contact was defined as any individual who slept in the same dwelling as the index case for at least 2 nights per week during the period of time that the index case was symptomatic before diagnosis. Consenting index cases from both groups of communities were interviewed using a structured interview for clinical and socio-demographic information.
DOTS in the study areas incorporated all of the elements of the WHO policy, including supervision of the Brazilian standard three-drug treatment regimen (INH, rifampin, pyrazinamide) provided in the clinics.13,14
In the DOTS communities, contact evaluation followed Brazil’s National TB Program guidelines.12 Clinic staff informed TB index cases of the need for their household contacts to come to the clinic for evaluation. Those contacts who did attend were examined to rule out active TB. In keeping with practice in Brazilian clinics during the study period, information on contact evaluations was not formally collected and no official records were established. However, it is very unusual for contacts to undergo evaluation in routine practice.
The DOTS-A strategy enhanced routine DOTS with the addition of a program to intensively screen household contacts of TB cases for evidence of active TB or LTBI. Index cases living in the DOTS-A neighborhoods were asked to provide a list of names of their household contacts and to ask contacts to come to the health centers for evaluation. If contacts failed to come to the clinic, outreach workers from the health centers visited consenting patients’ homes. Contacts were informed of the nature of the study and asked to provide written informed consent for participation. Parental consent was obtained for minors, and assent was obtained from children between the ages of 5 and 18 years. Contact evaluation consisted of a structured interview, clinical examination, chest X-ray and a tuberculin skin test (TST) using 2 tuberculin units (TUs) of purified protein derivative RT23 given by the Mantoux method and read by a trained reader 2–3 days after application. TB in contacts was diagnosed and treated as in cases.
Contacts with a TST induration of ≥5 mm without evidence of active TB were considered to have LTBI and were offered prophylaxis if they had no history of previous TB or LTBI therapy and no evidence of active TB disease. Such individuals were offered INH preventive therapy twice weekly (800 mg), supervised, or daily, self-administered (300 mg), for 6 months, or were invited to participate in a clinical trial of preventive therapy if they were aged ≥18 years.15
Statistical analysis
We compared the change in incidence of TB in the two groups of communities over 5 years. Data were obtained from the city TB registry from 1999 to 2004. We determined the number of TB cases per year in each community based on reported cases with an address in one of the eight communities. We used the population enumerated for each neighborhood by the national census in 2000. Calculation of rates began with the 12-month period before study implementation. The standard paired t-test was used to compare the mean difference in incidence rates from 1999 to 2004 in the DOTS and DOTS-A communities.
The study protocol was approved by the institutional review boards of the Rio de Janeiro Municipal Health Secretariat and of the Johns Hopkins University School of Medicine. The trial was listed on a National Institutes of Health (NIH) web-based research registry, but preceded clinicaltrials.gov.
RESULTS
From November 2000 to December 2004, respectively 1292 and 923 TB cases were notified in DOTS and DOTS-A neighborhoods. In DOTS communities, 371 (29%) patients were enrolled and reported 1119 household contacts. In DOTS-A communities, 341 (37%) patients were enrolled and reported 1028 household members. Among the enrolled TB cases, 339 (91%) from DOTS communities and 311 (91%) from DOTS-A had pulmonary disease and identified respectively 1003 and 960 contacts. TB index cases from DOTS and DOTS-A communities did not differ significantly with regard to socio-demographic and clinical characteristics (Tables 2 and 3).
Table 2.
DOTS and DOTS-A communities: socio-demographic characteristics of tuberculosis index cases
| Variables | DOTS n (%) | DOTS-A n (%) |
|---|---|---|
| Age, years, mean | 36 | 36 |
| Sex | ||
| Male | 244 (66) | 236 (69) |
| Female | 127 (34) | 105 (31) |
| Race | ||
| White | 158 (42) | 116 (34) |
| Non-White | 213 (57) | 225 (66) |
| Number of people at home | ||
| <4 | 197 (53) | 166 (49) |
| 4–6 | 114 (31) | 93 (27) |
| >6 | 21 (6) | 27 (8) |
| Number of rooms | ||
| 1 | 65 (18) | 77 (23) |
| 2–3 | 239 (64) | 206 (60) |
| ≥4 | 64 (17) | 49 (14) |
| Education, years | ||
| None | 27 (7) | 28 (8) |
| <8 | 198 (53) | 197 (58) |
| ≥8 | 146 (40) | 116 (34) |
| Unemployed | 141 (38) | 135 (40) |
| Monthly family income | ||
| <1 minimum wage | 59 (16) | 55 (16) |
| 1 to <2 minimum wage | 114 (31) | 103 (30) |
| 2 to <4 minimum wage | 109 (29) | 88 (26) |
DOTS-A = DOTS ampliado (enhanced DOTS).
Table 3.
DOTS and DOTS-A communities: clinical characteristics of TB index cases
| Variables | DOTS n (%) | DOTS-A n (%) |
|---|---|---|
| Type of TB | ||
| Pulmonary | 339 (91) | 311 (91) |
| Extra-pulmonary | 32 (9) | 30 (9) |
| Prior TB treatment | ||
| Yes | 83 (22) | 86 (25) |
| No | 286 (77) | 254 (74) |
| Unknown | 2 (1) | 1 (1) |
| TST induration result, mm | ||
| 0–4 | 60 (16) | 63 (18) |
| ≥5 | 232 (62) | 217 (64) |
| Not done | 67 (18) | 61 (18) |
| Unknown | 12 (3) | 0 |
| HIV test | ||
| Prior HIV screening | 121 (33) | 109 (32) |
| Prior HIV-positive | 9 (7) | 16 (15) |
| Current HIV screening | 246 (66) | 193 (57) |
| Current HIV-positive | 26 (7) | 24 (12) |
| Chest X-ray | ||
| Abnormal, suspicious TB | 337 (91) | 316 (93) |
| Cavitation present | 199 (59) | 151 (48) |
| Bacteriological status | ||
| SS+, culture-negative or ND | 61 (16) | 55 (16) |
| SS+, culture-positive | 203 (55) | 183 (54) |
| SS−, culture-positive | 15 (4) | 15 (4) |
| SS−, culture-negative or ND | 92 (25) | 88 (26) |
| Treatment outcomes* | ||
| Completed treatment | 226 (79) | 195 (77) |
| Defaulter | 21 (7) | 28 (11) |
| Deaths | 7 (2.4) | 14 (5) |
New cases.
DOTS-A = DOTS ampliado (enhanced DOTS); TB = tuberculosis; TST = tuberculin skin testing; HIV = human immunodeficiency virus; SS+ = sputum smear-positive; SS− = sputum smear-negative; ND = not done.
Information on contacts is available only for DOTS-A communities (Table 4), as the DOTS program does not collect standardized information on contacts of TB cases. Of the 960 household contacts identified in the DOTS-A communities, 699 (73%) came to the clinic at least once and underwent evaluation for active TB and/or LTBI, while 261 (27%) never came to the clinic and were consequently not screened, despite attempts of outreach staff to facilitate participation. At screening, 26/699 (4%) contacts had active TB identified and were given therapy. Of the remaining 673 who started screening, 632 (94%) completed the interview, 583 (83%) returned for TST reading and 429 (73%) had an induration of ≥5 mm.
Table 4.
Socio-demographic and clinical characteristics of the household contacts in the DOTS-A communities
| Variables | Contacts (n = 632) n (%) |
|---|---|
| Age, years, mean ± SD | 23 ±18.7 |
| Sex | |
| Male | 268 (42) |
| Female | 363 (58) |
| Missing | 1 |
| Race | |
| White | 173 (27) |
| Non-white | 459 (73) |
| Education, years of schooling* | |
| None | 53 (8) |
| <8 | 355 (56) |
| ≥8 | 134 (21) |
| Employment status | |
| Currently employed | 160 (25) |
| Unemployed | 96 (15) |
| Student | 217 (34) |
| Retired | 21 (3) |
| Other, and preschool | 138 (22) |
| Monthly family income† | |
| <1 minimum wage | 29 (14) |
| 1 to <2 minimum wage | 70 (33) |
| 2 to <4 minimum wage | 68 (33) |
| ≥4 minimum wage | 29 (14) |
| Unknown | 13 (6) |
| BCG vaccination | |
| Yes | 504 (80) |
| No | 114 (18) |
| Unknown | 14 (2) |
| Initial TST result, mm | |
| 0–4 | 154 (24) |
| ≥5 mm | 429 (68) |
| Not done | 49 (8) |
| Chest X-ray | |
| Normal | 409 (65) |
| Abnormal, suspicious TB | 7 (1) |
| Abnormal, not suspicious for TB | 2 (4) |
| Abnormal, inactive TB | 5 (1) |
| Not done | 188 (29) |
| HIV status | |
| Negative | 67 (10) |
| Positive | 2 (1) |
| Not done | 394 (62) |
| Unknown | 169 (27) |
For those of school age or older (n = 542).
Brazilian minimum wage = US$145 (n = 209).
DOTS-A = DOTS ampliado (enhanced DOTS); SD = standard deviation; BCG = bacille Calmette-Guérin; TST = tuberculin skin testing; TB = tuberculosis; HIV = human immunodeficiency virus.
Of 429 contacts with a positive TST, 258 (60%) started preventive therapy (197 [76%] received INH and 61 [24%] were enrolled in the clinical trial), 34 (8%) refused chemoprophylaxis and 44 (10%) did not start chemoprophylaxis due to physician’s decision. The remaining 93 (22%) contacts did not complete the evaluation or were lost to follow-up. Of those 258 contacts who started preventive therapy, 178 (69%) completed it. The outcome of the contact evaluation is summarized in Figure 1.
Figure 1.
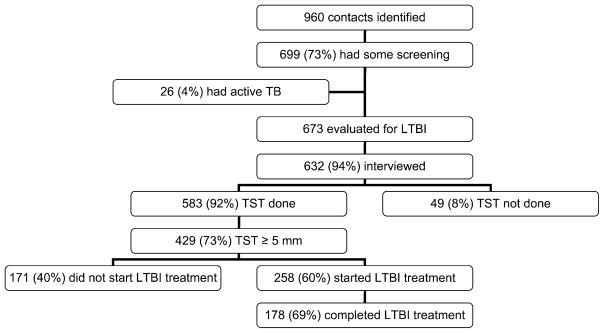
Outcome of contact investigation. TB = tuberculosis; LTBI = latent TB infection; TST = tuberculin skin test.
Incidence rates
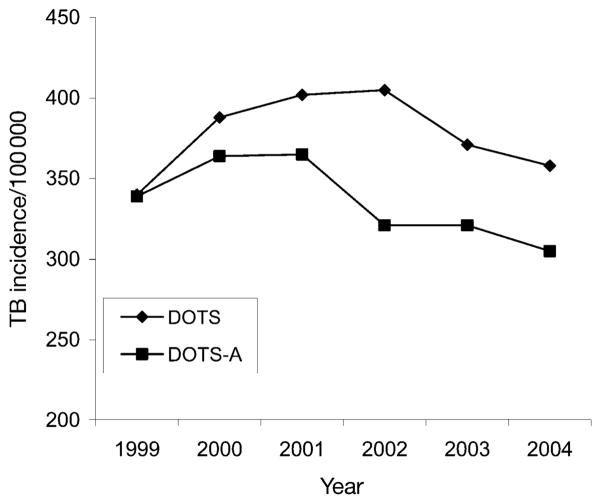
At baseline, TB incidence rates were the same in DOTS and DOTS-A communities, 340 and 339/100 000, respectively. The total incidence rate among the four DOTS communities increased during the study period by 5%, from 340 to 358 cases/100 000 residents (Table 5), while the total incidence in the DOTS-A communities decreased during the study period by 10%, from 339 to 305 cases/100 000. Thus, at the end of 5 years, the DOTS-A communities had an incidence rate that was 15% lower than in the DOTS communities, after beginning with equal incidence rates in 1999 (Table 5). The mean difference in incidence rates between the DOTS and DOTS-A communities was statistically significant (P = 0.04).
Table 5.
DOTS and DOTS-A communities: changes in incidence from 1999 to 2004
| Study arm | Incidence rate/100000
|
Change | ||
|---|---|---|---|---|
| 1999 | 2004 | |||
| Community | ||||
| Estácio | DOTS | 499 | 523 | +24 (+4.8%) |
| Santo Cristo | DOTS | 509 | 572 | +63 (+12.4%) |
| Benfica | DOTS | 210 | 237 | +27 (+12.9%) |
| Rio Comprido | DOTS | 270 | 267 | −3 (−1.1%) |
| All DOTS | 340 | 358 | +18 (+5.3%) | |
| São Cristóvão | DOTS-A | 256 | 242 | −14 (−5.5%) |
| Catumbi | DOTS-A | 372 | 379 | +7 (+1.9%) |
| Mangueira | DOTS-A | 434 | 324 | −110 (−25.3%) |
| Cidade Nova | DOTS-A | 625 | 530 | −95 (−15.2%) |
| All DOTS-A | 339 | 305 | −34 (−10%) | |
DOTS-A = DOTS ampliado (enhanced DOTS).
In the first 2 years of DOTS implementation (2000 and 2001), the incidence rates increased steadily in the DOTS and DOTS-A communities. In the following year (2002), there was a slight further increase (1%) in the incidence rate in DOTS communities and a 12% decrease on the incidence in DOTS-A communities. Thereafter, both groups of communities experienced declines in their TB incidences rates (Figure 2).
Figure 2.
Tuberculosis incidence in DOTS and DOTS-A communities. TB = tuberculosis; DOTS-A = DOTS ampliado (enhanced DOTS).
DISCUSSION
We have shown that the implementation of an expanded program of the WHO DOTS strategy where contact evaluation and treatment is provided, DOTS-A, was associated with a significantly lower TB incidence in urban communities in Rio de Janeiro City than standard DOTS. Compared to 1999, the year before implementation of the protocol, TB incidence in 2004 had declined in three of the four DOTS-A communities, whereas substantial increases in TB incidence were seen in three of the four control DOTS-only communities (Table 5). Given the similarity of the communities at baseline and the consistency of the findings in the intervention and control groups, our findings indicate that the addition of evaluation of household contacts for both active and latent TB, with appropriate treatment of each condition, resulted in a decline in TB incidence at the community level.
Treatment completion rates among new TB cases enrolled in the trial increased from 68% to 77% over the course of the study, but the completion rates were not significantly different in the two study arms. This finding suggests that the improvement in cure rates as a result of DOTS implementation was not the principal factor related to the decrease in incidence rates. Rather, we believe that the identification and treatment of secondary cases and treatment of LTBI among contacts was the likely reason for the effect seen. Early identification and treatment of secondary cases results in a shorter period of infectiousness and fewer secondary infections among contacts of these cases. Treatment of LTBI in contacts reduces the risk of subsequent TB by as much as 70–90%,16 depending on the duration of preventive therapy and patient adherence.
The impact of DOTS alone on TB incidence is unclear. In the United States, implementation of DOTS was associated with long-term decline in TB incidence in Baltimore,17 but no impact on incidence was seen in Tarrant County, TX, USA.18 Subsequent experience with DOTS in New York City suggests that multiple interventions, rather than DOTS alone, are likely to be associated with declines in TB incidence.19 In developing countries, some cluster randomized trials have assessed the impact of models of TB control on clinical outcomes, but neither measured the effects of TB control policies on TB incidence.20,21 Widespread application of DOTS in Peru in the 1990s was associated with marked and sustained decline in TB incidence.22
A recent study that modeled the impact of changing case detection rates (CDRs) on overall TB incidence suggested that DOTS and subsequent improved CDRs may have a dramatic effect on TB incidence in the short term, but that maintaining those CDRs may not reduce TB incidence by more than 1–2% per year in the long term.23 This supports the hypothesis that additional measures beyond DOTS are needed to reduce TB incidence.
Seventy three per cent (73%) of household contacts were infected, and 4% had active TB. The prevalence of LTBI among contacts found in our study was higher than the rates described in previous studies in Brazil,24–26 but similar to those described in some African countries.27,28 One potential explanation for this finding could be that we used a cut-off point of 5 mm for a positive TST, which might overestimate the infection rate. However, in a recent study in Rio de Janeiro of contacts of pulmonary smear-positive TB patients who did not receive chemoprophylaxis, no difference was found in the incidence of TB during follow-up between those with TST results of 5–9 mm and those with ≥10 mm.29 These findings suggest that the use of a cut-off point of 10 mm induration in household contacts may result in withholding TB preventive therapy from individuals who would benefit from it.
We found a reduction in TB incidence in DOTS-A communities over a period of 5 years. Overall, only 30% of TB patients participated in the intervention, largely as a result of patients being diagnosed elsewhere in the city and therefore being ineligible, or because of refusal to consent to the study procedures. Given the small proportion of the overall population that received TB preventive therapy, it is surprising that our intervention could have resulted in a 15% difference in TB incidence, and this finding could be due to chance, despite the randomized nature of our trial. Because the number of randomized neighborhoods in each arm was small, unmeasured events such as nosocomial outbreaks or several highly infectious cases could have resulted in the rates observed in the DOTS communities. The consistency of the results in three of four communities in each arm, however, is reassuring.
There is also a biologically plausible explanation for our results. Studies conducted in San Francisco7 and in South African gold mines30 have shown that over several years a single TB index case can account for a substantial proportion of subsequent cases in a community. In San Francisco, the proportion of cases attributed to a single index patient was 6% over several years, and in the gold mines a single index case was associated with 10% of all subsequent cases. An intervention such as ours that detected secondary, active TB cases in households and brought them into treatment, and that prevented subsequent cases in contacts by using preventive therapy, could conceivably reduce the community incidence through a stochastic process.
In summary, our trial showed that the addition of contact evaluation to identify secondary TB cases and to provide treatment of latent TB infection was associated with a lower incidence of TB than DOTS alone over a period of 5 years. Intensive contact evaluation and preventive therapy for contacts may be an important strategy for reducing the incidence of TB in a high-incidence community.
Acknowledgments
The authors thank J Coberly, L H Moulton, and R M Ferreira for assistance with the study. They also thank E Higgs and the National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases Protocol Review Group for invaluable advice. The contributions and cooperation of the staff of Municipal Health Department clinics is greatly appreciated. Supported by NIH Grants AI 45432, AI 01637 and TW05574, and United States Agency for International Development Cooperative Agreement HRNA-00-96-90006-00.
References
- 1.Enarson D, Rieder H, Arnadottir T, Trébucq A. A guide for low income countries. 5. Paris, France: International Union Against Tuberculosis and Lung Disease; 2000. Management of tuberculosis. [Google Scholar]
- 2.World Health Organization. WHO report 1994. Geneva, Switzerland: WHO; 1994. [Accessed November 2009]. Framework for effective tuberculosis control. WHO/TB/94.179. http://whqlibdoc.who.int/hq/1994/WHO_TB_94.179.pdf. [Google Scholar]
- 3.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–464. [PubMed] [Google Scholar]
- 4.Kenyon TA, Mwasekaga MJ, Huebner R, et al. Low levels of drug resistance amidst rapidly increasing tuberculosis and human immunodeficiency virus co-epidemics in Botswana. Int J Tuberc Lung Dis. 1999;3:4–11. [PubMed] [Google Scholar]
- 5.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 6.Comstock GW, O’Brien RJ. Tuberculosis. In: Evans AS, Brachman PS, editors. Bacterial infections of humans: epidemiology and control. 2. New York, NY, USA: Plenum; 1991. [Google Scholar]
- 7.Small PM, Hopewell PC, Singh SP, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 8.Alland D, Kalkut GE, Moss AR, et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1711–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 9.Cohn DL, O’Brien RJ. The use of restriction fragment length polymorphism (RFLP) analysis for epidemiological studies of tuberculosis in developing countries. Int J Tuberc Lung Dis. 1998;2:16–26. [PubMed] [Google Scholar]
- 10.Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 11.Chaulk CP, Friedman M, Dunning R. Modeling the epidemiology and economics of directly observed therapy in Baltimore. Int J Tuberc Lung Dis. 2000;4:201–207. [PubMed] [Google Scholar]
- 12.Ministério da Saúde, Fundação Nacional de Saúde. . Guia de vigilância epidemiológica. Brasília DF, Brazil: Ministério da Saúde; 2002. Portuguese. [Google Scholar]
- 13.Cavalcante SC, Soares ECC, Rocha MS, et al. A implantação da estratégia DOTS na cidade do Rio de Janeiro. Pulmão RJ. 2003;12:51–56. Portuguese. [Google Scholar]
- 14.Soares ECC, Pacheco AGF, Mello FCQ, et al. Improvements in treatment success rates with directly observed therapy in Rio de Janeiro City. Int J Tuberc Lung Dis. 2006;10:690–695. [PubMed] [Google Scholar]
- 15.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med. 2006;173:922–926. doi: 10.1164/rccm.200512-1953OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin skin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 17.Chaulk PC, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculois. JAMA. 1995;274:945–951. [PubMed] [Google Scholar]
- 18.Weiss SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 19.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333:229–333. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 20.Newell JN, Baral SC, Pande SB, Bam DS, Malla P. Family-member DOTS and community DOTS for tuberculosis control in Nepal: cluster-randomised controlled trial. Lancet. 2006;367:903–909. doi: 10.1016/S0140-6736(06)68380-3. [DOI] [PubMed] [Google Scholar]
- 21.Thiam S, LeFevre AM, Hane F, et al. Effectiveness of a strategy to improve adherence to tuberculosis treatment in a resource-poor setting. JAMA. 2007;297:380–386. doi: 10.1001/jama.297.4.380. [DOI] [PubMed] [Google Scholar]
- 22.Suárez PG, Watt CJ, Alarcón E, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–478. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 23.Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Organ. 2009;87:296–304. doi: 10.2471/BLT.08.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira L, Perkins MD, Johnson JL, et al. Infection and disease among household contacts of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:321–328. [PubMed] [Google Scholar]
- 25.Carvalho ACC, Kritski AL, De Riemer K. Tuberculin skin testing among BCG-vaccinated children who are household contacts. Int J Tuberc Lung Dis. 2001;5:297. [PubMed] [Google Scholar]
- 26.Carvalho ACC, De Riemer K, Nunes ZB, et al. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med. 2001;164:2166–2171. doi: 10.1164/ajrccm.164.12.2103078. [DOI] [PubMed] [Google Scholar]
- 27.Nunn P, Mungai M, Nyamwaya J, et al. The effect of human immunodeficiency virus type-1 on the infectiousness of tuberculosis. Tubercle Lung Dis. 1994;75:25–32. doi: 10.1016/0962-8479(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 28.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cailleaux-Cezar M, Melo AD, Xavier GM, et al. Tuberculosis incidence among contacts of active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13:190–195. [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey-Faussett P, Sonnenberg P, Shearer SC, et al. Tuberculosis control and molecular epidemiology in a South African gold-mining community. Lancet. 2000;356:1066–1071. doi: 10.1016/s0140-6736(00)02730-6. [DOI] [PubMed] [Google Scholar]