Abstract
Xylitol has been demonstrated to be a safe and effective tooth decay preventive agent when used habitually. Nevertheless, its application has been limited by absence of formulations that demand minimal adherence and are acceptable and safe in settings where chewing gum may not be allowed. A substantial literature suggests that a minimum of five to six grams and three exposures per day from chewing gum or candies are needed for a clinical effect. At the same time there is conflicting evidence in the literature from toothpaste studies suggesting that lower-doses and less frequent exposures might be effective. The growing use of xylitol as a sweetener in low amounts in foods and other consumables is, simultaneously, increasing the overall exposure of the public to xylitol and may have additive benefits.
In this paper the authors address the questions: (1) What is the minimum dose and frequency for use of xylitol containing chewing gum for significantly lowering mutans streptococci levels? And (2) can delivery vehicles be produced that are applicable in settings where chewing gum or similar confections might be permitted?
Chewing Gum
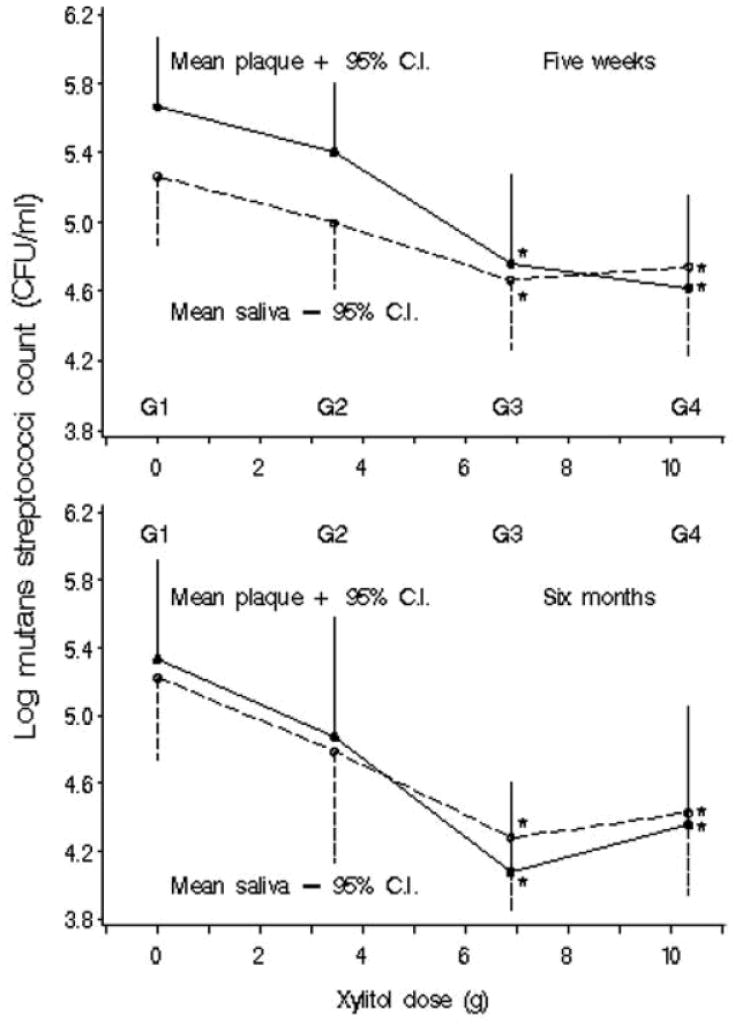
A randomized controlled trial was carried out to determine the dose-response effects of S. mutans in plaque and unstimulated saliva to xylitol gum (Milgrom et al., 2006). Participants (N=132) were randomized into four groups: controls of 9.83 g sorbitol/0.702 g maltitol/day (G1), 3.44 g xylitol/day (G2), 6.88 g xylitol/day (G3), and 10.32 g xylitol/day (G4) in the form of 12 pellets (3 pellets/4 times/day). Plaque was collected in a standardized manner from specific sites but was not weighed. Baseline, 5-week, and 6-month samples of plaque and unstimulated saliva showed decreasing levels of S. mutans across treatment groups of increasing dose. Xylitol at 6.88 g/day and 10.32 g/day reduced S. mutans in plaque at 5 weeks, and in plaque and saliva at 6 months (Figure 1). Results suggested a plateau effect for both plaque and saliva, indicating that exceeding the daily dose of xylitol 10.32 g/day is not likely to increase effectiveness. Alternatively, a dose of 3.44 g/day is not likely to show reductions in S. mutans levels.
Figure 1.
Mean log10 CFU mutans streptococci/mL in plaque and unstimulated saliva by xylitol dose at 5 wks and at 6 mos (N=33 in each group). *Significant-difference group compared with placebo (G1) in least-significant-difference multiple comparisons. (Reprinted from Milgrom P, et al. 2006 with permission)
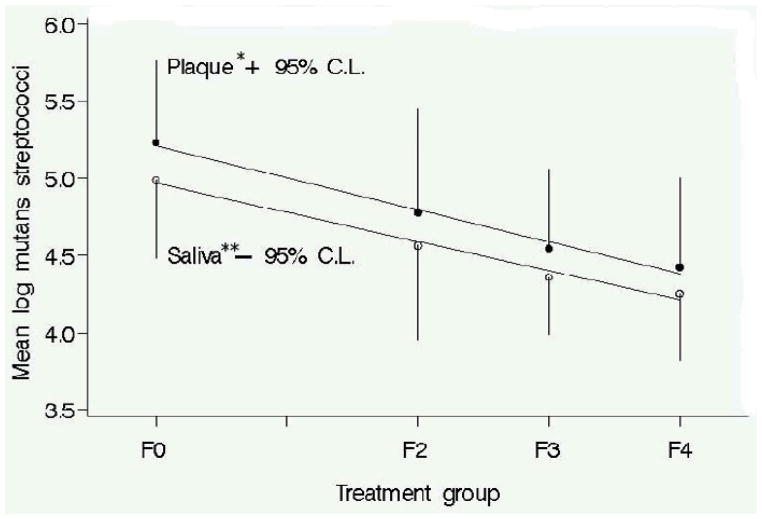
A five week randomized controlled trial was conducted in order to determine the reduction in S. mutans levels in plaque and unstimulated saliva to increasing frequency of xylitol gum use at a fixed daily dose of 10.32 g (Ly et al., 2006.) Participants (N=132) received either 10.32 g xylitol/day in the active group or 9.83 g sorbitol/0.7 g maltitol/day in the control group. The 10.32 g dose was used because it clearly would allow testing of the hypothesis even though a smaller dose (e.g. 6.88 g) might also have been possible. The number of pieces of gum did not change, and frequency of chewing (times per day) varied from 2 to 4 times/day within the active group; the control group chewed gum 4 times/day. There were no significant differences in S. mutans level among the groups at baseline. At five weeks, there was a linear reduction in S. mutans in plaque and unstimulated saliva to increasing frequency of xylitol gum use at a constant daily dose of 10.32 g (Figure 2). Although the difference observed for the xylitol two times/day group was consistent with the model, the difference was not statistically significant.
Figure 2.
Mutans streptococci counts in plaque and unstimulated saliva at five weeks and best fit linear line. Linear reduction of mutans streptococci levels of xylitol chewing gum use at constant daily dose (10.32 g/day). Linear line equations: plaque -*log mutans streptococci = −.21(Frequency)+5.21; unstimulated saliva-**log mutans streptococci =−.19(frequency)+5.07. Group F0 = Sorbitol Control; F2 = xylitol 2x/d; F3 = xylitol 3x/d; F4 = xylitol 4x/d. N=33 subjects per group. (reprinted from Ly KA, et al., 2006)
Alternative Vehicles
Study one of a recent experiment compared the potential of pediatric topical syrup to deliver xylitol versus chewing gum. The basic rationale was that if the salivary xylitol concentrations were similar to chewing gum over a similar period, the effect on the oral flora should be the same and a xylitol delivery system for the very young is desirable. Others also have considered syrup or child’s dummy (pacifier) as a delivery vehicle (Uhari, 1996, 1998; Taipale et al., 2006)
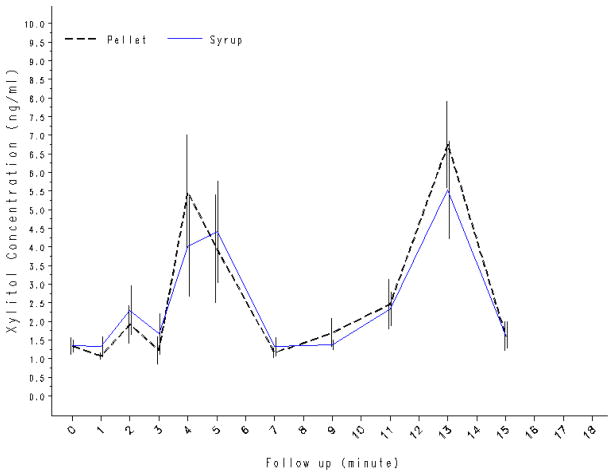
A within-subjects study design was employed to compare the presence and time course of xylitol concentrations in saliva from different delivery methods. Xylitol-containing pellet chewing gum (2.6 g) and 33% xylitol syrup (2.67 g) are presented here (Riedy et al., 2008). Adult subjects (N=15) consumed one product per visit with a 7-day washout period between products. Saliva samples were collected according to a standardized protocol at baseline and at ten regular intervals following exposure. HPLC was used to quantify xylitol concentrations. Mean salivary xylitol concentrations and bimodal time curves were similar for the two delivery methods (Figure 3); the correlation coefficient (r2) between the mean xylitol concentrations at each time point for xylitol pellet chewing gum and the syrup was 0.96. Total AUC for the two products did not differ significantly (pellet gum – 63.0 ng.min/mL, syrup – 59.0 ng.min/mL).
Figure 3.
Comparison of salivary xylitol concentrations (ng/mL) after using xylitol-containing gum and syrup (N=15). (Adapted from Riedy CA et al., 2008)
A randomized control trial of xylitol syrup on early childhood caries has been conducted (Milgrom et al., 2008 (under peer-review). Children at 9 to 15 months of age were randomized to three conditions in which all were given syrup orally three times per day by their mother/caretaker. The groups were: 3 doses of 2.67 g xylitol each (8 g/day); 2 doses of 4.0 g xylitol per day plus a single dose of a sorbitol placebo (8 g/day xylitol); or a single dose of 2.67 g xylitol plus two sorbitol placebo doses. Results show the pediatric topical syrup was highly effective in preventing early childhood caries in a population with very high rates of disease by 24 months of age.
In the second study of the xylitol salivary level experiment above (Riedy et al., 2008), bear shaped xylitol confections (2.6 g) were compared to xylitol pellet gum (2.6 g) at similar concentration. Another set of subjects (N=15) served as their own control. The study method and saliva sampling were as described for study one, the pellet gum compared to syrup study above. Mean salivary xylitol concentrations and bimodal time curves were similar for the two delivery methods; the correlation coefficient (r2) between the mean xylitol concentrations at each time point for xylitol pellet chewing gum and the gummy bears was 0.99. Total AUC for the two products did not differ significantly (pellet gum – 63.0 ng.min/mL, gummy bears – 55.9 ng.min/mL).
A randomized trial of the same bear shaped confection is now being conducted in which the target is prevention of tooth decay in first permanent molars. About 30 percent of first molars are decayed by first grade. This current study is designed to address the targeted use of xylitol when the first permanent molars are erupting (Hujoel et al., 1999). The study is a two group, 30-month randomized controlled clinical trial designed to assess the use of xylitol gummy bears as snack food during school hours to reduce dental caries among kindergarten children. Nearly all the children have untreated tooth decay in their primary teeth. Three hundred children are being randomized over two years into one of two treatment groups, receiving either six xylitol (1.3 g/piece, 2.6 g/dose—7.8 g/day) or six placebo gummy bears, distributed in the classroom evenly three times a day, for nine months.
Discussion
The work presented confirms the interpretation of data from clinical studies regarding frequency and dose (Isokangas, 1987; Rekola, 1989; Mäkinen et al., 1995). One caution is that the effectiveness of the lowest dose in the Milgrom and colleagues study (2006) may have been masked because the subjects had background levels of xylitol exposure, apparently from the diet. The bacterial reductions are a surrogate for reductions in tooth decay but this is permissible because the mechanism of action of xylitol is specifically antibacterial and a number of studies have demonstrated parallel reductions of S. mutans and tooth decay. Thus, the correspondence between the findings in the Milgrom series and the clinical studies already in the literature means that chewing gum can be used as a vehicle in institutional programs. However, there will still be adherence issues related to those who must administer or supervise use. Gum has been shown to be less effective in individual treatment programs because of lack of adherence (Isotupa et al., 1995; Stecksén-Blicks, 2004).
A controlled study of complex design of xylitol containing candies and gum was conducted in children about 10 years old (Alanen et al., 2000). This age group was targeted because of the potential to protect erupting second permanent molars. Three xylitol test groups received either candies (xylitol-maltitol or xylitol-polydextrose) or gum at 5 grams per day divided into three doses over several years depending on the group. The results showed 35 to 60 percent reductions in caries incidence in the test groups relative to the controls and no difference between xylitol delivery vehicles. This study is important both because of its result in the same dosage/frequency range as the previous studies and because the trial was intentionally sized to have adequate statistical power even with anticipated attrition.
In contrast there have been at least two studies attempting to demonstrate an effect of lower dosages. A non-randomized trial (Honkala et al., 2006) compared one xylitol candy three times per day (assumed to be 1.9 g total/day; the paper is unclear as to dose) to an untreated control in children and young adults in a school for the disabled. The control group consisted of students whose parents did not consent to the study. Baseline caries scores were fairly high and similar yet the test group showed a significant reduction in caries incidence relative to the untreated controls. This may indeed have been because the test candy, according to the manufacturer’s website, was actually a 1:1 mixture of xylitol and maltitol. Other studies have shown that confections sweetened with maltitol alone reduced S. mutans levels in daily use with children (Ly et al., 2008). Thus, it is likely inaccurate to assert that 1.9 g xylitol per day alone is effective.
Oscarson and colleagues (2006) attempted to prevent caries in preschool children using 0.5 to 1.0 grams of xylitol in lozenges beginning around age 2. This study failed to show any effect largely because the underlying caries rate was extremely low (less than 1 dmfs per child at 4 years old) and the study had not been designed to detect such small, perhaps clinically insignificant, differences in the first place. Neither of these publications gives any rationale for the low dosages.
Xylitol-containing dentifrice
Several studies have evaluated sodium fluoride toothpaste formulations with xylitol. In all they raise questions, in view of the previous data presented, as to how an exposure of as little as 0.1 to 0.2 g per day xylitol (assuming a 1 g dose of toothpaste that is 10% xylitol and given no more than twice per day) could result in significant reductions of S. mutans and dental caries. Unpublished work by Söderling and colleagues has shown that low-dose xylitol decreases the growth of specific mutans strains in culture during the growth phase but this is hardly the same situation as in the mouth. Early short–term study of a xylitol-glycerol dentifrice showed reductions in salivary mutans (Svanberg & Birkhed, 1991).
In a study of 155 university students with high S. mutans levels comparing three fluoridated dentifrices (toothpaste with or without triclosan, or triclosan plus 10% xylitol), only the toothpaste with triclosan and xylitol showed significant reductions in plaque and saliva mutans levels from the placebo at 6 months although the levels dropped in all the groups (Janneson et al. 2002). In this study the students were instructed to use about 1.5 cm of the dentifrice (about 1 g) and to refrain from rinsing. The authors argue that the proprietary toothpaste was formulated to optimize the bioavailability of the xylitol and that the dose used was larger than in other studies (for example, see Twetman & Peterson, 1995). No data were presented on how long the xylitol was present in the mouth after the exposures nor were there data on adherence. It is possible that the effects of triclosan and xylitol are synergistic. The time-response effect seen in this study is consistent with the Milgrom and colleagues studies of xylitol-containing chewing gum.
A prospective study of 2,630 Costa Rican children, initially eight to 10 years of age, brushing twice daily with fluoride toothpaste with 10% xylitol or fluoride toothpaste alone reported a 12% reduction in decayed/filled surfaces (DFS) and 11% reduction in decayed/filled buccal and lingual surfaces (DFS-BL) among those children brushing with fluoride toothpaste and xylitol after three years (Sintes et al., 1995). This study should to be interpreted cautiously as there was nearly 40 percent attrition in the subject population and the analysis did not employ intent-to-treat analytical methods. Another 30-month study by the same investigators of 3,394 seven to 12 year old children who used either fluoride toothpaste with and without 10% xylitol showed DFS and DFT increments of 1.30 and 0.69, respectively, for the 10% xylitol group when compared with the fluoride toothpaste only group (Sintes et al., 2002). Again, there were limitations in the study design and synergy between fluoride and xylitol cannot be ruled out. An additional concern is that these toothpastes contained sodium lauryl sulfate as a detergent, which may decrease the effectiveness of the xylitol (Assev et al., 1997).
Low-Dose Non-Intentional Exposure to Xylitol
In the U.S., for example, xylitol is being added in small non-clinical amounts as a sweetener or advertising gimmick to various foods and children’s vitamins. Tables 1 and 2 give examples of many of the products containing xylitol in the US. It is possible that frequent lower dose exposure to xylitol is beneficial without the effort to maintain special programs. It is not possible to answer this question from the existing literature; however, two-thirds of the subjects in the Milgrom and colleagues study (2006) had been exposed to low levels of xylitol in their diets (Roberts et al., 2002).
Table 1.
Xylitol Containing Gums and Mints Available in U.S. Markets, Their Xylitol Content, Preventive Potential, and Availability
| PRODUCTS† | XYLITOL grams (g) per piece | TOTAL POLYOLS per piece (g) | PIECES FOR 6 (10) g/day | PREVENTIVE Potential†† | AVAILABILITY |
|---|---|---|---|---|---|
| GUMS: | |||||
| B-Fresh Gums | 0.90 | 0.90 | 7 (11) | Yes | online |
| BioGenesis “Xylitol Fruit Gum” | 0.72 | 0.72 | 8 (14) | Yes | online |
| Clen-Dent Xylitol Gum | 0.67 | 0.67 | 9 (15) | Yes | online |
| ElimiTaste “Zapp” & “Smoke Screen” gums | 1.01 | 1.01 | 6 (10) | Yes | online |
| Emerald Forest “Ricochet Xylitol Gum” | 0.72 | 0.72 | 8 (14) | Yes | online |
| Epic Xylitol Gum | 1.06 | 1.06 | 6 (10) | Yes | online |
| Global Sweet Polyols “Xponent Gum” | 0.70 | 0.70 | 9 (14) | Yes | online |
| Lumi Line (Fennobon Oy) “XyliMax Maximum Gum” | 1.0 | 1.0 | 6 (10) | Yes | online |
| Lumi Line (Fennobon Oy) “XyliMax Gum” | 0.70 | 0.70 | 9 (14) | Yes | online |
| Nature’s Provision “Smart Smile Gum” (Xylipro) | 0.70 | 0.70 | 9 (14) | Yes | online |
| Omnii “Theragum” | 0.70 | 0.70 | 9 (14) | Yes | online |
| Peelu “Xylitol Gum” | 1.0 | 1.0 | 6 (10) | Yes | online |
| Spry Xylitol Gum | 0.72 | 0.72 | 8 (14) | Yes | retail & online |
| Tundra Trading “XyliChew Gum” | 0.80 | 0.80 | 8 (13) | Yes | retail |
| Vitamin Research “Unique Sweet Gum” | 0.72 | 0.72 | 8 (14) | Yes | online |
| XyloBurst Gum | 1.0 | 1.0 | 6 (10) | Yes | online |
| Zellies “Xylitol Gum” | 0.70 | 0.70 | 9 (14) | Yes | online |
| Altoids Sugar-Free Gum (Cinnamon/Peppermint) | 2nd of 4 polyols | 1.0 | NC* | Maybe | retail |
| BreathRx “Halispheres Sugar-Free Gum” | 1st of 3 polyols | < 1g/piece | NC | Maybe | online |
| Elma “MASTICgum” | 0.53g (1st of 3 polyols) | NC | 11 (19) | Maybe | online |
| Hershey “Ice Breakers Ice Cube” Gum | 1st of 5 polyols | 2.0 | NC | Maybe | retail |
| Starbucks “After Coffee” Peppermint/Tangerine | 1st of 2 polyols | 1.0 | NC | Maybe | retail |
| Zellies “Kids Gum” | 0.50 | 0.0 | 12 (20) | Maybe | online |
| Arm & Hammer “Dental Care Baking Soda Gum” | 2nd of 3 polyols | 1.0 | NC | No | retail |
| Arm & Hammer “Advance White Icy Mint Gum” | 2nd of 3 polyols | 1.0 | NC | No | retail |
| Biotene “Dry Mouth Gum” | 3rd of 3 polyols | 1.0 | NC | No | retail |
| Cadbury Adams “S Stride” Gum | 3rd of 3 polyols | 1.0 | NC | No | retail |
| Dentyne “Tango Gum” | 4th of 4 polyols | 1.0 | NC | No | online |
| Eco-Dent “Between Dental Gum” (various flavors) | 0.35 | 1.0 | 17 (29) | No | online |
| Ferndale “glean whitening gum” | 0.05 | 0.7 | 120 (200) | No | online |
| Tully’s “vanilla mint gum” | 5th of 5 polyols | 1.0 | NC | No | retail |
| Cadbury Adams “Trident Gum with Xylitol” (stick) | 2nd of 3 polyols | 1.0 | NC | No | retail |
| Cadbury Adams “Trident Gum with Xylitol” (pellet) | 3rd of 3 polyols | 1.0 | NC | No | retail |
| Cadbury Adams “Trident for Kids Gum” | 3rd of 3 polyols | 1.0 | NC | No | retail |
| Wrigley “Orbit Sugar-Free Gum” | 3rd of 3 polyols | 1.0 | NC | No | retail |
| MINTS: | |||||
| B-Fresh “Mints” | 0.70 | 0.70 | 9 (14) | Yes | online |
| Clen-Dent “Mints” | 0.67 | 0.67 | 9 (15) | Yes | online |
| Lumi Line “Sugar Free Chewy Mints” | 1.0 | 1.0 | 6 (10) | Yes | online |
| Biogenesis “Xylitol Peppermint Mints” | 0.55 | 0.55 | 11 (18) | Maybe | online |
| Emerald Forest “Ricochet Xylitol Mints/Sours” | 0.40 | 0.40 | 15 (25) | Maybe | online |
| Epic “Xylitol Mints” | 0.50 | 0.50 | 12 (20) | Maybe | online |
| Global Sweet Polyols “Xponent “Mints” | 0.55 | 0.55 | 11(18) | Maybe | online |
| Nature’s Provision “Smart Smile Mints” (Xylipro) | 0.55 | 0.55 | 11 (18) | Maybe | online |
| Omnii “Theramints” | 0.50 | 0.50 | 12 (20) | Maybe | online |
| OraHealth “Xylimelts” (time release formula) | 0.50 | 0.50 | 12 (20) | Maybe | online |
| Spry “Mints” | 0.50 | 0.50 | 12 (20) | Maybe | online |
| Tundra Trading “XyliChew Mints” | 0.55 | 0.55 | 11 (18) | Maybe | retail |
| Vitamin Research “Unique Sweet Mints” | 0.50 | 0.50 | 12 (20) | Maybe | online |
| XyloBurst Mints | 0.50 | 0.50 | 12 (20) | Maybe | online |
| Zellies “Xylitol Mints” | 0.50 | 0.50 | 12 (20) | Maybe | online |
| Mint Asure breath capsules | 0.063 | 0.063 | 95 (160) | No | retail |
| SMINT “Mints” (Regular & White) | < 0.20 | <0.20 | 30 (50) | No | retail |
| Xlear “SparX” (candy – various flavors) | 0.23 | 0.23 | 25 (40) | No | online |
| Brown & Haley “Zingos Caffeinated Peppermints” | 2nd of 2 polyols | 2.0 | NC | No | retail |
| Hershey “Ice Breakers Center Ice” mints | 1st of 2 polyols | 2.0 | NC | No | retail |
| Oxyfresh “Breath Mints” | 2nd of 2 polyols | NC | NC | No | online |
| Starbucks “After Coffee Mints” | 2nd of 2 polyols | < 0.14 | NC | No | retail |
Product list is not exhaustive. Xylitol market is rapidly changing and new xylitol containing products appear frequently.
The products in Table 1 (Gums and Mints) are sorted initially by their preventive potential. Products within the preventive potential groupings of “Yes”, “Maybe”, and “No” are listed alphabetically. “Yes”, “No”, or “Maybe” is based on the potential a person is willing to consume 2–3 pieces, 3 to 5 times per day to meet the effective dose range of 6 to 10 grams per day. Products with potential for effectiveness but xylitol dose is either unknown or required consumption is ≥ 10 pieces per day to provide 6 g of xylitol are assigned “Maybe”.
NC = Not Certain. Information can not be derived from internet vendor, or market packaging, or not successful in obtaining information from vendors’ information representatives.
Table 2.
Xylitol Containing Oral Hygiene, Healthcare, and Diet Products Available in U.S. Markets, and Their Xylitol Content
| PRODUCTS* | XYLITOL Content | AVAILABILITY |
|---|---|---|
| ORAL HYGIENE: | ||
| Biotene “Dry Mouth Toothpaste/Gel” (+/− Calcium) | 10% (0.14% sodium fluoride) | retail & online |
| Crest “Multicare Cool Mint Toothpaste” | 10% (0.24% sodium fluoride) | retail & online |
| Epic “Fluoride-Free Xylitol Toothpaste” (no F) | 25% | online |
| Epic “Xylitol & Fluoride Toothpaste” | 31% (0.24% sodium fluoride) | online |
| NOW “XyliWhite Toothpaste” Gel (no F) | 25% | retail & online |
| Spry Toothpaste (no F) | 25% | retail & online |
| Spry Toothpaste with Fluoride | 25% (0.24% sodium fluoride) | retail & online |
| Squigle “Enamel Saver Toothpaste” | 36% (0.24% sodium fluoride) | online |
| Tanner’s “Tasty Paste” Toothpaste | 15% (0.24% sodium fluoride) | online |
| ToothZone “Xylokid Toothpaste” (reduced F) | 30% (0.55% sodium fluoride) | online |
| Topex Toothpaste “Take Home Care”, “White Care” | 10% (1.1% sodium fluoride) | dental office & online |
| Orajel “Dry Mouth Toothpaste” | NC†† 2nd of 2 polyols (last ingre.) | retail & online |
| Oxyfresh “Super Relief Gel” & Fluoride Dental Gel” | NC only sweetener (2nd ingre.) | online |
| Oxyfresh “Power Paste” Toothpaste | NC 2nd of 2 polyols (7th ingre.) | online |
| Rembrandt Toothpaste “For Canker Sore” | NC only sweetener (5th ingre.) | retail & online |
| Tom’s of Maine Toothpaste/Gel lines except Anticavity Cinnamint & “for children” Toothpaste contain NO xylitol | 1%–7% (varies in ingredient list) | retail & online |
| Tom’s of Maine Anticavity Liquid Gel “for children” | NC 2nd of 2 polyols (6th ingre.) | retail & online |
| Tundra Trading “XyliBrush Toothpaste” | NC only sweetener (3rd ingre.) | online |
| Spry Infant “Tooth Gel” (no F or flavoring) | 35% | online |
| Gerber Infant “Tooth & Gum Cleanser” (no F) | NC 1st of 2 polyols (3rd ingre.) | retail & online |
| Gerber Toddler “Tooth & Gum Cleanser” (no F) | NC 2nd of 2 polyols (6th ingre.) | retail & online |
| Laclede “First Teeth” Baby Toothpaste (no F) | NC 1st of 2 polyols (3rd ingre.) | retail & online |
| Oral-B “Stages Baby Tooth & Gum Cleanser” (no F) | NC 2nd of 2 polyols (6th ingre.) | retail & online |
| Dr. Ray’s Products “Spiffies Dental Wipes” | 0.65 g/wipe (only sweetener) | online |
| Epic “Oral Rinse” (no F) | 25% | online |
| Parnell “MouthKote Oral Moisturizer” | 15–30% | online |
| Spry “Oral Rinse” (no F) | 22% | retail & online |
| Biotene “Oral Balance” Dry mouth gel or liquid | NC 2nd of 2 polyols | retail & online |
| Biotene “Mouthwash” | NC 1st of 2 polyols | retail & online |
| NOW “XyliWhite Mouthwash” | NC only sweetener (2nd ingre.) | retail & online |
| Oasis “Mouth Spray” | NC only sweetener (last ingre.) | retail & online |
| Orajel “Dry Mouth Moisturizing Spray” | NC 2nd sweetener (7th ingredient) | retail & online |
| Oxyfresh “Mouthrinses” (except Unflavored) | NC only polyol (2nd ingredient) | online |
| Spry “Rain” Dry mouth spray | NC 30% by volume | online |
| Tom’s of Maine “Anticavity (fluoride) Mouthwash” | NC (varies in ingredient list) | retail & online |
| ToothZone Sponge Floss (with xylitol) | NC | online |
| Xylifloss “Pocket Dental Flosser” | NC | retail & online |
| HEALTHCARE: | ||
| Omni “Cavity Shield” 5% Sodium Fluoride Varnish | NC (only sweetener) | Rx – Dental Office |
| Omni “Vanish” 5% Sodium Fluoride Varnish | NC (only sweetener) | Rx – Dental Office |
| Sultan “DuraShield” 5% Sodium Fluoride Varnish | NC (only sweetener) | Rx – Dental office |
| “VanishAmerica” 5% Sodium Fluoride Varnish | NC (has sucralose) | Rx – Dental office |
| Bayer “Flintstone Vitamins - Complete” | NC | retail & online |
| Bayer “One a Day Kids Vitamins - Complete” | NC | retail & online |
| Jarrow Formulas “Kid Bear Kids Multi” | NC | retail & online |
| Micro Spray “Vitamin Sprays” | NC (2nd ingredient) | online |
| NatureSmart “Disney Complete Multivitamin” | NC | retail & online |
| NOW Foods “Kid Cal Orange Dream” | NC | retail & online |
| Solgar “Kangavites Multivitamin/ Vitamin C” | NC | retail & online |
| Sundown “Spiderman Complete Vitamins” | NC | retail & online |
| B&T “Echina Spray” | NC | online |
| Nicorette “Gum” –Mint | NC (last ingredient) | retail & online |
| NOW “Activated Nasal Mist” with Xylitol | NC (2nd ingredient) | retail & online |
| Xlear “Nasal Wash” | NC (2nd ingredient) | retail & online |
| ENERGY BARS & FOOD: | ||
| Barry Farm Sugar Free Jam (various flavors) | 3.6 g/20 g serving (18%) | online |
| Barry Farm Sugar Free Pie Filling (various flavors) | 45 g/serving (36%) | online |
| Bayho “Ultra Low Carb Bar – Chocolate Coconut | 12 g/bar | online |
| Buddha Bars / Vitality Bar | 4 – 5 g/bar | online |
| Chocolate Passion “Gourmet Chocolate Sauces” | 7 g/1 tbsp serving | online |
| Emerald Forest “Lena’s Jam” (various flavors) | 12 g/1 tbsp serving | online |
| Emerald Forest “Chocolate Nut Bar” | 7 g/bar | online |
| Emerald Forest “Ricochet Coffee Shots” | 2 g/5 piece serving | online |
| Fran Gare’s “Decadent Desserts” Mix (various types) | 15 – 25 g/30 g serving | online |
| Garden of Life “Perfect Meal” shakes | 1st “other” ingredient | retail |
| Glycemic Level Solutions “Glycemic MRP Shakes” | 10 g/serving | online |
| Jarrow Formulas “Muscle Optimeal” shakes | 7th ingredient | retail & online |
| Jay Robb Enterprises “Egg White Protein” shakes | 2 g/33 g serving | retail & online |
| Jay Robb Enterprises “Jaybar” | 5 g/bar | online |
| Kraft Jell-O Pudding Sugar Free Chocolate | 4–7 g/serving | retail |
| Nature’s Hollow (Probst) – Sugar Free Jam Preserves | 4.5 g/20 g serving | online |
| Nature’s Hollow – Sugar Free Syrup (various flavors) | 2.8 g/40 ml serving (7%) | online |
| Nature’s Hollow – Sugar Free Ketchup | 0.8 g/20 g serving (4%) | online |
| Nature’s Hollow – Sugar Free Honey | 1.6 g/20 g serving (8%) | online |
| Nature’s Way “Metabolic Reset” shakes | 5 g/serving | retail & online |
| Renew Life Fiber35 Diet “Fit Smart” shakes | 8 g/46 g serving | retail & online |
| Smart Sweet Xylitol Jam (various flavors) | 8 g/20 g serving | online |
| Smart Sweet “Xylitol Maple Syrup” | 9 g/40 ml (1/6 cup) serving | online |
| Smart Sweet “Xylitol Honey” | 8 g/1 tbsp serving | online |
| Zipfizz energy drink (citrus & pink lemonade) | 2 g/serving | retail & online |
Product list is not exhaustive. Xylitol market is rapidly changing and new xylitol containing product appears frequently. Aside from toothpaste, most products have not been studied or published in peer-review journals thus the potential impact on caries reduction is not known.
NC = Not Certain. Information can not be derived from market packaging and not successful in obtaining information from company information representative.
Conclusions
In spite of the considerable evidence that xylitol is an effective caries preventive and cariostatic agent; an effective delivery system for xylitol, especially for children, demanding minimal adherence yet safe has not been developed. A substantial body of work suggests that a minimum of five to six grams and three exposures per day are needed for a clinical effect. At the same time there is conflicting evidence in the literature from the xylitol toothpaste studies suggesting that lower-doses and less frequent exposures might be effective but the synergistic effects of xylitol and fluoride or triclosan cannot be ruled out. Studies of new vehicles for xylitol such as a xylitol releasing dummy and a pediatric syrup have been conducted.
Acknowledgments
Supported by Grants U54 DE14254 from the NIDCR/NIH, R40MC03622 from the Maternal and Child Health Research Bureau, HRSA, and a Head Start Innovation and Improvement Project Grant No. 90YD0188 from the Office of Head Start, Agency for Children and Families.
References
- Alanen P, Isokangas P, Gutmann K. Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidemiol. 2000;28(3):218–24. doi: 10.1034/j.1600-0528.2000.280308.x. [DOI] [PubMed] [Google Scholar]
- Assev S, Wåler SM, Rolla G. Are sodium lauryl sulfate-containing toothpastes suitable vehicles for xylitol? Eur J Oral Sci. 1997;105(2):178–82. doi: 10.1111/j.1600-0722.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Honkala E, Honkala S, Shyama M, Al-Mutawa SA. Field trial on caries prevention with xylitol candies among disabled school students. Caries Res. 2006;40:508–13. doi: 10.1159/000095650. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Mäkinen KK, Bennett CA, Isotupa KP, Isokangas PJ, Allen P, Mäkinen P-L. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J Dent Res. 1999;778(3):797–803. doi: 10.1177/00220345990780031301. [DOI] [PubMed] [Google Scholar]
- Isokangas P. Xylitol chewing gum in caries prevention. A longitudinal study on Finnish school children. Proc Finn Dent Soc. 1987;83(Suppl 1):1–117. [PubMed] [Google Scholar]
- Isotupa KP, Gunn S, Chen CY, Lopatin D, Mäkinen KK. Effect of polyol gums on dental plaque in orthodontic patients. Am J Orthod Dentofacial Orthop. 1995;107:497–504. doi: 10.1016/s0889-5406(95)70117-6. [DOI] [PubMed] [Google Scholar]
- Jannesson L, Renvert S, Kjellsdotter P, Gaffar A, Nabi N, Birkhed D. Effect of a triclosan-containing toothpaste supplemented with 10% xylitol on mutans streptococci in saliva and dental plaque. A 6-month clinical study. Caries Res. 2002;36:36–39. doi: 10.1159/000057588. [DOI] [PubMed] [Google Scholar]
- Ly KA, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use: a randomized controlled trial [ISRCTN43479664] BMC Oral Health. 2006;6:6. doi: 10.1186/1472-6831-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, et al. Xylitol chewing gums and caries rates: A 40-month study. J Dent Res. 1995;74:1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85:117–181. doi: 10.1177/154405910608500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Tut OK, Ly KA, Gancio MJ, Roberts M, Mancl L, Langidrik JR, Briand K. Xylitol topical oral syrup prevents early childhood caries: A RCT. Abstract No. 3331, International Association of Dental Research Annual Meeting; Toronto. 2008. [Google Scholar]
- Oscarson P, Lif Holgerson P, Sjöström I, Twetman S, Stecksén-Blicks C. Influence of a low xylitol-dose on mutans streptococci colonisation and caries development in preschool children. Eur Arch Paediatric Dent. 2006;7(3):142–7. doi: 10.1007/BF03262555. [DOI] [PubMed] [Google Scholar]
- Rekola M. Correlation between caries incidence and frequency of chewing gum sweetened with sucrose or xylitol. Proc Finn Dent Soc. 1989;85:21–4. [PubMed] [Google Scholar]
- Riedy CA, Milgrom P, Ly KA, Rothen M, Mueller G, Hagstrom MK, Tolentino E, Zhou L, Roberts MC. A surrogate method for comparison analysis of salivary concentrations of Xylitol-containing products. BMC Oral Health. 2008 Feb 11;:8–5. doi: 10.1186/1472-6831-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC, Riedy CA, Coldwell SE, Nagahama S, Judge K, Lam M, et al. How xylitol-containing products affect cariogenic bacteria. J Am Dent Assoc. 2002;133:435–441. doi: 10.14219/jada.archive.2002.0201. [DOI] [PubMed] [Google Scholar]
- Sintes JL, Elias-Boneta A, Stewart B, Volpe AR, Lovett J. Anticaries efficacy of a sodium monofluorophosphate dentifrice containing xylitol in a dicalcium phosphate dihydrate base. A 30-month caries clinical study in Costa Rica. Am J Dent. 2002;15:215–219. [PubMed] [Google Scholar]
- Sintes JL, Escalante C, Stewart B, McCool JJ, Garcia L, Volpe AR, et al. Enhanced anticaries efficacy of a 0.243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent. 1995;8:231–235. [PubMed] [Google Scholar]
- Stecksén-Blicks C, Holgerson PL, Olsson M, Bylund B, Sjöström I, Sköld-Larsson K, et al. Effect of xylitol on mutans streptococci and lactic acid formation in saliva and plaque from adolescents and young adults with fixed orthodontic appliances. Eur J Oral Sci. 2004;112:244–248. doi: 10.1111/j.1600-0722.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Svanberg M, Birkhed D. Effect of dentifrices containing either xylitol and glycerol or sorbitol on mutans streptococci in saliva. Caries Res. 1991;25:449–53. doi: 10.1159/000261409. [DOI] [PubMed] [Google Scholar]
- Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Dissolution of xylitol from a food supplement administered with a novel slow-release pacifier: preliminary results. Eur Arch Paediatr Dent. 2007;8:123–125. doi: 10.1007/BF03262581. [DOI] [PubMed] [Google Scholar]
- Twetman S, Petersson LG. Influence of xylitol in dentifrice on salivary microflora of preschool children at caries risk. Swed Dent J. 1995;19:103–8. [PubMed] [Google Scholar]
- Uhari M, Kontiokari T, Koskela M, Niemelä M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ. 1996;313(7066):1180–1184. doi: 10.1136/bmj.313.7066.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998;102:879–884. doi: 10.1542/peds.102.4.879. [DOI] [PubMed] [Google Scholar]