Abstract
Multicellular tissues and organs often show planar cell polarity (PCP) where the constituent cells align along an axis to form coordinated patterns. Mammalian eye lenses are mainly comprised of epithelial-derived fiber cells which exhibit highly ordered alignment that is regulated by PCP signaling. Each fiber cell has an apically situated primary cilium and in most cases this is polarized towards the lens anterior pole. Here we describe how to visualize the global cellular alignment of lens fiber cells by examining the suture pattern that is formed by the tips of fibers meeting at the anterior pole. We also describe a method for whole mount preparation which allows observation of the polarized distribution of primary cilia at the apical surface of lens fibers. Given its relative simplicity, at least in cellular terms, and its requirement for a high degree of precision in cellular alignment and orientation, we predict that the lens will be an excellent model system to help elucidate the role of cilia and PCP components in development of three-dimensional organization in tissues and organs.
Keywords: planar cell polarity (PCP), mammalian eye lens, lens fiber cell, lens suture, primary cilium, whole mount
1. Introduction
Planar cell polarity (PCP) signaling provides a mechanism for aligning cells in a coordinated pattern along a particular axis to form functional tissues or organs. PCP was first identified in invertebrates and is now well established in several vertebrate systems. However, growing recognition of its importance in regulating many vital biological processes has led to an increased focus on PCP and its role in a wide range of tissues and organs (Lawrence et al., 2007; Seifert and Mlodzik, 2007; Strutt, 2008; Wang and Nathans, 2007). From studies of invertebrate model systems, several essential genes have been identified and most of these genes are now recognized as common players in vertebrate systems. Whilst there are many similarities between invertebrate and vertebrate systems that exhibit PCP, there are some differences. One such difference is the emerging recognition of the key role that primary cilia play in PCP in vertebrates (Axelrod, 2008; Park et al., 2006; Ross et al., 2005; Simons and Walz, 2006; Singla and Reiter, 2006). How cilia and PCP proteins cooperate to coordinate precise cell alignment and orientation has become a major focus of research.
Recently, we reported that the eye lens exhibits PCP, with each lens fiber cell having an apically situated cilium and in most cases this is polarized towards the anterior pole (Figure 1; Sugiyama et al., 2010). Concentrically arranged fibers are precisely aligned as they elongate along the anterior–posterior axis and orientate towards lens poles where they meet equivalent fibers from other segments to form characteristic sutures. This global alignment is regulated by the PCP pathway. Because of its lack of cellular complexity and its distinctive polarity, the lens has many advantages for PCP studies. Moreover, the ease with which lens can be isolated from other eye tissues as well as the ability to prepare pure populations of the two forms of lens cells make it a very convenient tissue for study. Thus, we predict the lens will be a valuable model system to help elucidate the role of cilia and PCP components in development of three-dimensional organization in tissues and organs.
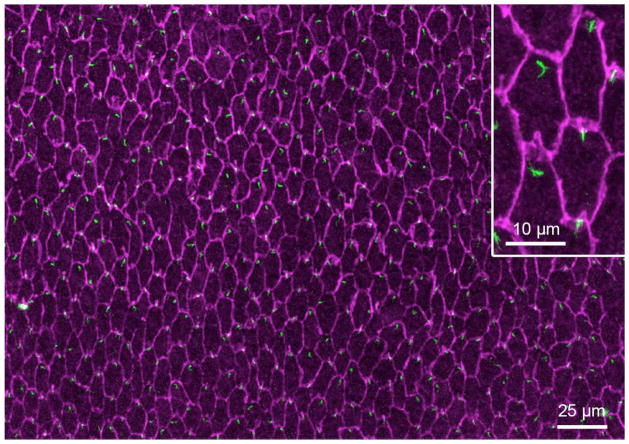
Figure 1.
Hexagonally aligned lens fiber apical tips with polarized centrosomes/primary cilia. A whole mount was prepared from a P34 rat lens and immuno-stained with anti-pericentrin (green) and anti-β-catenin (purple) antibodies. Pericentrin-reactivity localizes the centrosome (and associated primary cilium; see Sugiyama et al., 2010) and β-catenin-reactivity demarcates the cell borders and shows the hexagonal shape of the fiber cells in cross section. In virtually all cells, the centrosome/primary cilium is polarized to the side of the cell that faces the anterior pole (top of page).
We have developed several new techniques to observe lens PCP in detail. However, the standard histological approach of using paraffin embedded lenses or whole eyes to study lens cell alignment and orientation also gives valuable information. We mostly use rats and mice as experimental material is readily available and, particularly in the case of mice, transgenic models and mutants can be generated or accessed from other researchers. In PCP mutants like Loop-tail (Lp) or Sfrp2 overexpressing mice, lenses do not develop the spheroidal shape typical of their wild-type littermates but tend towards a flatter overall appearance (in Lp lenses) or become flatter anteriorly and more pointed posteriorly (in Sfrp2 mice) (Chen et. al., 2008; Sugiyama et. al. 2010). Cross sections that are horizontal to the lens equator also give a lot of information about cellular organization. From this perspective it is clearly evident that the normal lens consists of layers of well organized concentrically arranged fibers with similar global alignment and orientation. Any disturbance of this can be readily assessed in experimental PCP models. For example, in Sfrp2 overexpressing mice, although fibers show similar alignment within groups, different groups usually exhibit different orientations and this results in the formation of a distinct boundary where two groups of fibers meet. Because paraffin embedding and subsequent sectioning methodologies involve standard histological procedures we will not deal with these here, rather we will concentrate on the new techniques that we have developed to study PCP in the lens.
Here we describe two advanced methods for examining lens PCP in detail. First we introduce a method to observe the anterior suture pattern by using whole lens staining. This analysis provides information on whether global PCP is maintained in the particular lens under investigation. Second, we describe a method to visualize the primary cilium at the fiber apical tip to determine if it shows characteristic polarized distribution as well as to assess integrity of local PCP.
2. Materials
2.1. Samples
Mice: Transgenic mice (e.g. Sfrp2 overexpressing mice) or mutant or knockout mice (e.g. Lp, Crash) aged between embryonic day (E) 16.5 to about 1-month postnatal.
Rats: Wistar rats (about 30 days old). Cellular alignments are similar in the lenses of mice and rats, but the latter provide larger sized lenses that can often facilitate assessment of normal cellular patterns.
2.2. Dissection tools
Standard forceps
Scissors with curved tips
35 mm cell culture dishes (Nunc)
10 mL tubes with screw caps
Dissection microscope and light source (Leica)
Two pairs of fine forceps (Dumont Jeweller’s Forceps #5)
Filter papers (3M, Wattman)
2.3. Solutions
Sterile essential medium (M199) with Earle’s salts (Invitrogen) supplemented with 0.1% bovine serum albumin (Sigma-Aldrich), 2 mM L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin and 2.5 μg/mL of fungizone.
PBS: Prepared from tablets (Roche)
100% Methanol
4% (w/v) paraformaldehyde in PBS
Dye dilution buffer: 1% NP-40 in PBS
Fluorescent dyes: TRITC-lectin (Sigma), Alexa 488-phalloidin (Invitrogen)
2% (w/v) agarose in PBS; prepare in 100 mL screw capped glass bottle and melt by microwave oven
Washing buffer: 0.1% (w/v) BSA in PBS
Blocking solution: 10% normal donkey serum (Millipore) in washing buffer
Primary antibody solution: rabbit anti-pericentrin antibody (Abcam) and mouse anti-β-catenin antibody (BD) with 1.5% normal donkey serum in washing buffer
Secondary antibody solution: Alexa 488-conjugated donkey anti-rabbit IgG and Alexa 594-conjugated donkey anti-mouse IgG (Invitrogen) in washing buffer
2.4. Other materials
Water repelling marker: Dako Pen (Dako)
Humid chamber (Plastic airtight container with damp papers and supports for slides)
Fine pliers
Water-based mounting medium: Aqua-Poly/Mount (Polysciences), Pristine Mount (Invitrogen)
22 mm diameter coverslips
Glass slides
Plastic transfer pipette
2.5. Microscope
Upright microscrope (Axioskop2, Zeiss) with laser confocal system (LSM5 Pascal, Zeiss)
3. Methods
In mice and rats, PCP becomes progressively more prominent in lens fiber cells during postnatal development; consequently their highly organized alignment and orientation can be best visualized in adults rather than in neonates. Lenses also continue to grow throughout life, so for ease of manipulation it may be beneficial to use adult lenses. If the gene of interest causes embryonic lethality and it is not possible to get postnatal samples, aim to get the oldest stage samples as possible, ideally at E18.5, since by this time, suture alignment has been established to some extent and it is possible to determine if suture formation is defective. However, visualizing the positioning of cilia at the apical tips of fibers may be not possible even at postnatal day 3 because of the inherent technical difficulties of preparing whole mounts from small lenses. Conditional knockouts overcome such developmental problems as well as size issues. The availability of lens-specific Cre recombinase transgenic mice provides a practical solution to these problems. So far, several lines of lens-specific Cre mice which have variable Cre expression timings and patterns are available (Ashery-Padan et. al., 2000, Zhao et. al., 2004). Usually lens defects do not affect viability of mice and thus these models permit access to older stage lenses. The other major advantage of conditional knockouts is that the phenotype in the tissue of interest is unlikely to be due to secondary effects resulting from damage to an adjacent tissue.
3.1 Preparation of lens samples
Eyeballs are removed, by external approach, from euthanised animals and transferred to CO2 equilibrated, pre-warmed M199 medium in a 35 mm culture dish. For adult animals, use curved scissors and press down alongside two edges of the eye with the tips of scissors so that the eyeball protrudes; then pinch it off by sliding the scissor tips underneath eyeball. In younger animals, before about postnatal day 13, eyes are still closed and eyelids need to be removed. For this, lift eyelid with forceps and cut it off with scissors to expose eyeball underneath, then proceed as described for older animals.
To dissect out lenses from eyeballs, tear the posterior part of the eyeball by inserting the tips of fine forceps (use a pair of forceps in each hand) into the hole left by the cut optic nerve. Carefully open eyeball by tearing the retina layer and removing surrounding ocular tissue from lens. Filter paper can be used to wipe the tips of the forceps as these get sticky with tissue debris. Discard removed tissues from culture medium.
3.2 Anterior suture observation
Transfer dissected eyeballs in a 10 mL tube and fix with 4% PFA/PBS overnight at room temperature. After 1 hour wash with PBS, isolate lenses by following step 3.1.2. Tissues may become more elastic and opaque after fixation, but it is still possible to isolate lenses from eyeballs (see Note 1, Note 2).
Transfer isolated lenses to TRITC-lectin or Alexa 488-phalloidin (or both) in dye dilution buffer and incubate for a minimum of two days at room temperature with gentle agitation. To transfer small lenses without damaging them, use a plastic transfer pipette and take lenses up into the tip of the pipette with a small amount of solution.
Wash lenses in PBS for 1 hour at room temperature.
Transfer a lens to a coverslip with anterior pole side down (i.e. epithelium side facing the coverslip). Remove as much excess PBS as possible. Drop melted 2% agarose/PBS onto the lens and leave until agarose sets and lens is fixed in place (see Note 3).
To observe under the microscope, invert the coverslip over a glass slide and rest on 2 platforms, 3 mms tall and separated by about 15 mms, so that the lens is suspended in the space between the platforms. The platforms can be made by breaking a glass slide into 6 strips, each 1 mm thick, and forming 2 separate platforms each made up of 3 glass strips glued onto the slide.
Under the microscope, the lens capsule and lens epithelial layer will be visualized first (Figure 2A). Apical tips of lens fibers are visible just beneath lens epithelial cells (Figure 2B). Collect a confocal slice image that contains a suture image (Figure 2C, D) or reconstitute a 3D projection image to show suture structure in detail (Figure 2F). See Note 4.
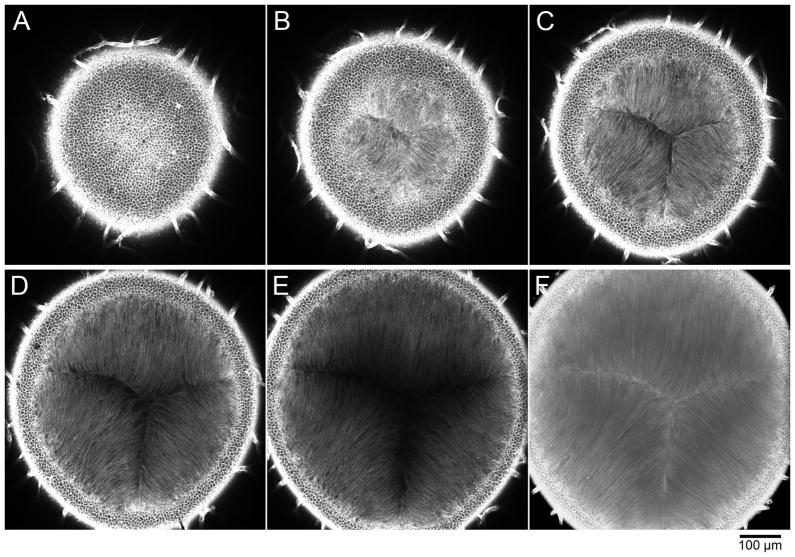
Figure 2.
Postnatal 5-day mouse lens stained with TRICT-lectin for 2 days and sequentially imaged by confocal microscopy starting from the anterior pole (x20 objective lens, 2 μm optical sections). A. In the first slice, cobblestone-like lens epithelial cells are in focus. Blood vessels that are associated with the lens surface are also evident at the periphery. B. The focal plane has now passed through the central lens epithelium region and apical tips of fibers are visible. C, D. In these planes, the typical Y-shape suture is clearly recognized. E. Deeper within the lens, fibers along the suture are devoid of staining because of limited dye penetration. F. Three-dimensional projection image reconstituted from a series of sliced images using the projection tool of Zeiss LSM software.
3.3 Whole mount preparation
In this method, apical tips of fibers are preserved as an attachment on the epithelial layer when lens capsule is peeled off from the fiber mass (Figure 3A). The extent of fiber remnants maintaining attachment to the epithelium may vary between samples; sometimes almost the whole epithelial sheet is covered by fiber tips so that the complete suture structure may be visible (Figure 3B). In other cases, only a few small patches of fiber tips may be retained on the epithelial layer. When this happens, try changing the peeling speed and/or the angle at which the capsule is pulled away from the fiber mass. To distinguish epithelial cells and fiber tips, E-cadherin staining may be helpful since E-cadherin is detected only in epithelial cells (Figure 3B). Apical tips of fibers have a characteristic hexagonal shape whereas the epithelial cells have random cobblestone-like appearance, so this morphological difference may also help to distinguish these two types of cells. This is a modified method originally developed to prepare lens epithelial explant cultures (Lovicu and McAvoy, 2008).
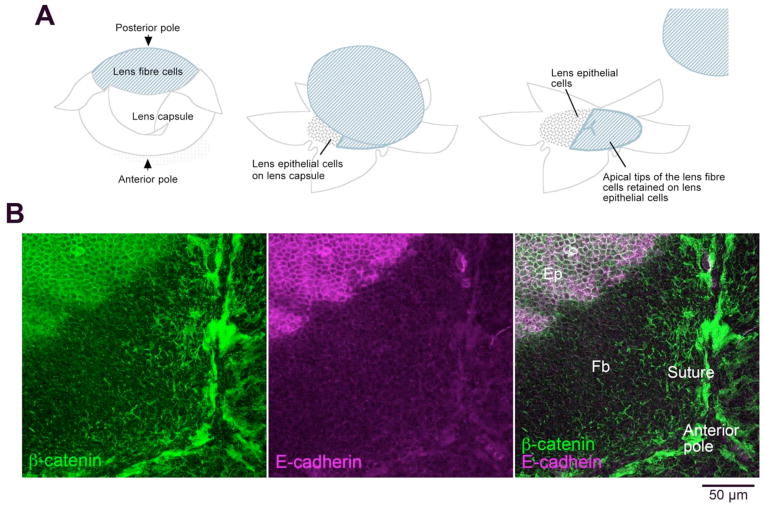
Figure 3.
Preparation of lens whole mounts. A. Diagram showing how the lens capsule is peeled away from the lens fiber cells. Lens epithelial cells remain firmly attached to the lens capsule; however, when the fibers are removed, in some regions, remnants of their apical ends are retained on the apical surface of the epithelial cells. B. β-catenin (green) is present at the margins of both epithelial and fiber cells but E-cadherin (purple) is specific for epithelial cells. In this whole mount, a large region near the anterior pole of the lens retains remnants of the apical ends of lens fiber cells. Abbreviations: Ep, epithelial cells; Fb, fiber cells. Adapted from Sugiyama et al, 2010.
Fix isolated lenses with 100% methanol for 45 seconds and wash twice with PBS for 5 minutes each. Keep lens-containing dishes on ice. Fixed lenses can be stored in PBS at 4°C for up to one week.
Transfer one or two lenses into a new cell culture dish filled with PBS. Place the lens anterior pole side down (facing the culture dish surface). By using two fine forceps, make a small tear on the capsule at the posterior pole.
From the small tear, make 5 or 6 tears toward anterior surface until just passing the equatorial region.
Peel off the capsule along the tears and expose posterior part of lens fibers. Remove lens fiber mass.
Gently press a tip of one flap of the capsule onto the plastic culture dish until the capsule is immobilised and sticks to the culture dish surface. Make one more attachment to fix the capsule onto the dish.
Stretch the lens capsule slightly to make it as flat as possible. If the capsule is rolled up when the fiber mass is removed try to unravel it before fixing it to the dish (see Note 5).
Pin all of the tips of the flaps onto the culture dish.
3.4 Immunostaining of whole mount
Decant PBS and remove remaining moisture around whole mounts with tissue paper. Circle whole mounts with Dako pen to make water-repellent barrier. Add PBS immediately into the circle to prevent any tissue dehydration.
Replace PBS with blocking medium containing 10% normal donkey serum. Keep whole mount-containing dishes in a humid chamber to prevent evaporation. Incubate for 30 minutes at room temperature or overnight at 4 °C (see Note 6).
Remove blocking solution and apply primary antibody solution. Incubate samples for 1 hour at 37°C or overnight at 4 °C in humid chamber.
Remove primary antibody solution and wash samples with washing buffer 3 times for 5, 10 and 15 minutes. Add secondary antibody solution and incubate for 1 hour at room temperature in the dark (place in box or some other container that blocks out the light (see Note 7).
Remove secondary antibody solution and wash samples with washing buffer 3 times for 5, 10 and 15 minutes. Keep samples in dark.
Break culture dish wall using pliers and remove surrounding wall completely to convert the dish into a disc. Add a drop of water-based mounting medium onto the whole mount and seal with a round coverslip. The disc of culture dish can then be placed in its original lid and labelled for future identification.
Place the disc containing the whole mount on a glass slide with a drop of water or glycerol to act as a glue to hold the specimen in place. Observe fiber apical tips with cilia using a 40 times objective lens or higher.
Acknowledgments
This work was supported by NHMRC (Australia), NIH (USA, R01 EY03177), ORIA, Australia and The Sydney Foundation for Medical Research. Y.S. was supported by an Endeavour Fellowship, Australia and The Sydney Eye Hospital Foundation. Some research illustrated here was undertaken as part of the Vision CRC, New South Wales, Sydney, Australia.
Footnotes
For whole lens staining, an alternative method that can be applied to large adult lenses is to dissect lenses prior to fixation. In this case treat dissected lenses with 4% PFA/PBS for 1 hour before washes. 100% methanol can be used for fixation by treating lenses for 45 seconds. Note phalloidin does not work with alcohol-based fixatives, so that only lectin staining is applicable if the lenses are fixed with methanol.
10% Neutralized formalin usually contains methanol to promote stability; however, be aware that this may affect phalloidin staining.
The anterior pole should be centrally positioned but sometimes this can be difficult to ascertain. If you can see the posterior suture, this helps to centre the lens, but otherwise try to use iris remnants on the lens as an indicator of lens orientation.
Efficiency of dye penetration is enough to observe the surface suture but usually, dye penetration is limited to the cortical region of lenses (see Figure 2E. In this sample, lectin still stains epithelial cells and outer regions of fibers, but the central portion of fibers is devoid of staining). Longer incubation times may enhance intensity of signal and depth of dye penetration, but it appears that after 2 days incubation no further penetration is achieved.
Whole mounts should be prepared so that they are flat, otherwise wrinkles/folds interfere with microscopy.
We recommend normal donkey serum as blocking reagent of immunostaining. Normal goat serum tends to give high background signal especially at the centrosome/cilium. Use freshly thawed serum and keep it at 4°C as repeated freeze-thaw causes higher background signals.
Nucleus detecting dyes (Hoechst, DAPI etc.) can be included in secondary antibody solution.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD. Basal bodies, kinocilia and planar cell polarity. Nat Genet. 2008;40:10–11. doi: 10.1038/ng0108-10. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways. Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Epithelial explants and their application to study developmental processes in the lens. In: Tsonis Panagiotis A., editor. Animal Models in Eye Research. Vol. 10. Academic Press; 2008. pp. 134–147. [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dolfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signaling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Strutt D. The planar polarity pathway. Curr Biol. 2008;18:R898–902. doi: 10.1016/j.cub.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, Murdoch JN, Lovicu FJ, McAvoy JW. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarized primary cilia. Dev Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:1930–1939. doi: 10.1167/iovs.03-0856. [DOI] [PubMed] [Google Scholar]