Stroke is the clinical syndrome of rapid onset of focal, or sometimes global, cerebral deficit with a vascular cause, lasting more than 24 hours or leading to death.1 Eighty per cent of strokes are ischaemic, 15% are due to intracerebral haemorrhage, and 5% to subarachnoid haemorrhage. Correct diagnosis is important because treatments for ischaemic stroke2 may be contraindicated in intracerebral haemorrhage.3 The diagnosis requires imaging of the brain.4 But which imaging—computed tomography or magnetic resonance—how quickly should it be done, should this include imaging cerebral blood flow, and what is the most cost effective approach?
The average general hospital (catchment population 250 000-500 000) will see two to three patients with stroke per day. Many patients have poor airway control, are confused, or are unable to communicate. Routine imaging for most patients must therefore be quick (speed is of the essence for patients, salvaging their brain, and for the radiology department), practical, readily available, and yield the key diagnostic information. There is, however, no imaging technique that does all of these perfectly.
Computed tomography scanning is practical, quick (a few minutes to scan a brain), widely available, and easy to use in ill patients. It accurately identifies intracerebral haemorrhage as soon as it has occurred, but the technique has limitations. Intracerebral haemorrhage will be misinterpreted as ischaemic stroke if computed tomography is not done within 10-14 days after stroke.5 Delays in seeking medical attention or poor access to computed tomography for stroke will result in failure to identify up to three quarters of intracerebral haemorrhage5 and may lead to inappropriate treatment (for example, aspirin or carotid endarterectomy) for many.
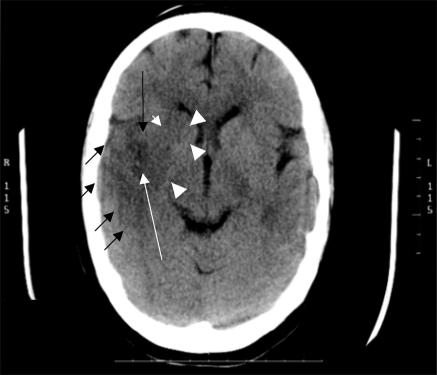
Computed tomography scanning shows positive features of ischaemic stroke in many patients with severe and moderate strokes scanned two to seven days after the event, but early signs of ischaemia (within three to six hours) are difficult to recognise.6 Many patients with mild stroke never develop a visible infarct on computed tomography, no matter when they are scanned.7 The clinical diagnosis of stroke can be difficult in the first few hours after onset, so many doctors would value a diagnostic test not only to exclude intracerebral haemorrhage or tumour (found in 4-20% initially diagnosed as “stroke”), but also positively to confirm ischaemic stroke. The subtle early signs of infarction on computed tomography include grey matter becoming isodense with white matter, loss of the basal ganglia and insular ribbon outlines, a little swelling reducing the visibility of sulci or ventricles, and a hyperdense artery (figure). If any of these signs are important for making decisions regarding treatment—for example, deciding whether or not to give thrombolysis8—then improving their recognition is vital. The acute cerebral CT evaluation of stroke study (ACCESS), in which as many doctors and radiologists as possible worldwide interpret typical computed tomography scans of stroke over the internet, aims to improve recognition of early signs of infarction (please try your hand at www.neuroimage.co.uk—completion carries five category 1 credits for continuing medical education).
Figure 1.
Computed tomography scan showing typical subtle signs of early infarct in the right temporoparietal and basal ganglia regions. Note the loss of the insular grey matter (long black arrow), sulcal effacement, and loss of cortical grey-white matter junction (short black arrows), loss of outline of the caudate and lentiform nuclei in the basal ganglia (arrowheads—compare with the left side of the brain) and the hyperdense artery in the Sylvian fissure (long white arrow)
The quest for a positive diagnosis of ischaemic stroke fuelled the use of magnetic resonance imaging—for example, diffusion weighted imaging, which shows early ischaemic changes as a bright white lesion (“lightbulb”). More ischaemic strokes show up on diffusion weighted imaging than on computed tomography or conventional magnetic resonance imaging in the first few hours9 and weeks later, which makes this technique especially useful for positive identification of an ischaemic stroke in patients presenting up to eight weeks after stroke.10 Previous intracerebral haemorrhage is visible indefinitely as a low signal (black) ring or dot on gradient echo magnetic resonance sequences, which makes this technique useful in patients presenting too late for computed tomography.5 However, magnetic resonance imaging may not identify hyperacute intracerebral haemorrhage correctly; is difficult to use routinely in acute, particularly severe stroke; is less often available than computed tomography; requires more cooperation by the patient for a longer time; is very noisy and upsets confused patients; and about a fifth of patients cannot undergo magnetic resonance imaging (because they are too ill or confused or have an intraocular or intracerebral metallic foreign body or pacemaker).
Whether lesions seen on diffusion weighted imaging indicate permanent neuronal damage or include some tissue that could recover is not clear. Magnetic resonance perfusion imaging shows under-perfused brain, often over a larger area of brain than the lesion seen on diffusion weighted imaging. The difference between the lesions seen on diffusion weighted imaging and perfusion scans may indicate potentially salvageable brain (“ischaemic penumbra”), although many issues remain—for example, defining the imaging boundaries of recoverable tissue, the “point of no return,” and the relation with the duration of ischaemia.
Computed tomographic perfusion imaging, similar in concept to magnetic resonance perfusion,11 is now available. Although this procedure is still in development, combined with plain computed tomography it could define the ischaemic penumbra without the problems of accessibility and patient compatibility that magnetic resonance imaging entails.
How should imaging be used most cost effectively? In our NHS health technology assessment study, on average each hospital admission for stroke cost about £11 000 ($19 800; €16 000).12 A few patients per day per hospital quickly accumulate high costs. A computed tomography scan costs between £44 and £130.12 The average length of stay after stroke is shorter for patients who are independent after stroke (14 days) than patients who are dependent (51 days) or who die (33 days).12 The imaging strategy for computed tomography producing the highest number of quality adjusted life years at the lowest cost was “scan all immediately.”12 Strategies that delay scanning reduce quality adjusted life years and increase cost. Thus even a marginal shift from dependent to independent survival after stroke through correct early diagnosis with computed tomography and appropriate treatment (aspirin for most patients with ischaemic stroke and its avoidance in intracerebral haemorrhage,2) is the most cost effective strategy, despite the marginal benefits of treatment. Although some gaps remain in knowledge on how best to use complex brain imaging, timely and judicious use of computed tomography improves outcomes and reduces the cost of caring for patients with stroke.
ACCESS is funded by Chest, Heart, and Stroke Scotland, the Universities of Edinburgh and Mannheim, DesAcc, and The Health Foundation. The study of cost effectiveness of imaging in stroke was funded by the NHS HTA programme (96/08/01)—the views expressed are those of the authors and not the funders.
Competing interests: JMW has received a contribution towards the cost of attending a symposium from Boehringer Ingelheim, a fee for speaking on one occasion in the past five years at a symposium organised by Boehringer Ingelheim, and is on the CT reading panel for the third European cooperative acute stroke study, for which she received a consulting fee from Boehringer Ingelheim. She also received consulting fees from Boehringer Ingelheim during the licensing application of alteplase in acute stroke.
References
- 1.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull WHO 1976;54: 541-53. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40 000 randomized patients from the Chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke 2000;31: 1240-9. [DOI] [PubMed] [Google Scholar]
- 3.Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovasc Dis 2002;14: 197-206. [DOI] [PubMed] [Google Scholar]
- 4.Weir CJ, Murray GD, Adams FG, Muir KW, Grosset DG, Lees KR. Poor accuracy of stroke scoring systems for differential clinical diagnosis of intracranial haemorrhage and infarction. Lancet 1994;344: 999. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Keir SL, Dennis MS. The impact of delays in computed tomography of the brain on the accuracy of diagnosis and subsequent management in patients with minor stroke. J Neurol Neurosurg Psychiatry 2003;74: 77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalafut MA, Schriger DL, Saver JL, Starkman S. Detection of early CT signs of > 1/3 middle cerebral artery infarctions: interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke 2000;31: 1667-71. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Lewis SC, Dennis MS, Counsell C, McDowall M. Is visible infarction on computed tomography associated with an adverse prognosis in acute ischemic stroke? Stroke 1998;29: 1315-9. [DOI] [PubMed] [Google Scholar]
- 8.Patel SC, Levine SR, Tilley BC, Grotta JC, Lu M, Frankel M, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 2001;286: 2830-8. [DOI] [PubMed] [Google Scholar]
- 9.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002;33: 2206-10. [DOI] [PubMed] [Google Scholar]
- 10.Schulz UGR, Briley D, Meagher T, Molyneux A, Rothwell PM. Abnormalities on diffusion weighted magnetic resonance imaging performed several weeks after a minor stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 2003;74: 734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastwood JD, Lev MH, Provenzale JM. Perfusion CT with iodinated contrast material. Am J Roentgenol 2003;180: 3-12. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Seymour J, Cairns J, Sandercock PAG, Keir S, Lewis SC, et al. What is the best imaging strategy for acute stroke? NHS Health Technology Assessment Project No 96/08/01. Southampton: HTA Monographs, 2003 (in press). www.ncchta.org (accessed 30 Dec 2003). [DOI] [PubMed]