Abstract
Improved understanding of the differential diagnosis of endemic treponematoses is needed to inform clinical practice and to ensure the best outcome for a new global initiative for the eradication of yaws, bejel, and pinta. Traditionally, the human treponematoses have been differentiated based upon their clinical manifestations and epidemiologic characteristics because the etiologic agents are indistinguishable in the laboratory. Serological tests are still considered standard laboratory methods for the diagnosis of endemic treponematoses and new rapid point-of-care treponemal tests have become available which are extremely useful in low-resource settings. In the past ten years, there has been an increasing effort to apply polymerase chain reaction to treponematoses and whole genome fingerprinting techniques have identified genetic signatures that can differentiate the existing treponemal strains; however, definitive diagnosis is also hampered by widespread unavailability of molecular diagnostics. We review the dilemmas in the diagnosis of endemic treponematoses, and advances in the discovery of new diagnostic tools.
Methods
References for this review were identified through searches of PubMed and WHO databases from January 1, 1905 to January 1, 2013, by use of terms “yaws,” “pian,” “bejel,” “pinta,” “carate,” “endemic Treponematoses,” and “Treponema pallidum.” Many articles were identified through searches in the authors' personal files. Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English, French, Spanish, and Portuguese were included.
Introduction
Treponematoses are infections caused by the spirochetal organisms of the Treponema species. These bacteria are the cause of both syphilis (Treponema pallidum ssp. pallidum) and the so-called nonvenereal or endemic treponematoses (ETs) consisting of yaws (T. pallidum spp. pertenue), bejel (or endemic syphilis) (T. pallidum spp. endemicum), and pinta (T. carateum) [1]. Dilemmas exist in the diagnosis of patients with ETs because clinical findings do not always accurately identify patients with the disease, and serologic methods are unable to differentiate these disease entities from venereal syphilis and from each other [2]. Furthermore, in resource-poor countries with high rates of syphilis and poor laboratory diagnostics, establishment of the diagnosis of yaws, bejel, and pinta can be even more difficult.
The WHO has now embraced yaws and dracunculiasis (Guinea worm disease) as the only two diseases targeted for eradication on its official list of 17 neglected tropical diseases (NTDs) [3]. As progress is made in yaws eradication in many countries, it becomes increasingly important to acquire more information on a number of aspects of the disease in order to ensure the effective and economical conduct of eradication campaigns. One such aspect is the accurate differential diagnosis with certain skin and other lesions not caused by ETs.
In this review, we focus on dilemmas in the diagnosis of endemic treponematoses in children and adults. We review the clinical presentation and differential diagnosis of the disease, use of epidemiological data, and interpretation of serological tests results, and draw attention to advances in specific diagnostic tools and the use of molecular biology to increase the sensitivity and differentiate the existing strains of pathogenic treponemes [4], [5].
Epidemiological and Geographical Differences
For differentiation of treponematoses, a point that might give some assistance would be residence in an area where one of these treponematoses was preponderant or present to the exclusion of the others. Transmission of ETs appears to be defined by climate and opportunity because T. pallidum is readily killed by drying; it can survive only briefly outside the body (one to two hours) [6]. Yaws and pinta are found in warm, moist climates, mainly in forested tropical regions, and are transmitted by direct skin-to-skin contact. Lesions are promoted by high atmospheric humidity, increasing their oozing and infectiousness [7]. Bejel is found in drier climates, but the bejel treponeme takes shelter in moist areas of the body, like the mouth, thus the infection spreads by direct contact (e.g., children kissing their siblings) or indirect contact through infected communal utensils [8], [9].
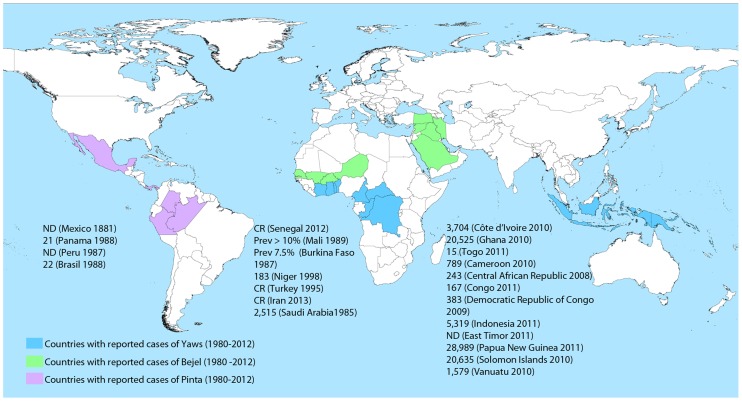
The upsurge in ETs observed in the period 1980–2012 (after they were almost eradicated by the mass penicillin treatment campaigns of the 1950s and 60s) is still poorly understood but is probably related to lack of follow-up care. Their current geographic extent remains uncertain, but there is growing evidence that the number of cases in some countries continues to increase. Figure 1 shows the countries where cases of yaws, bejel, and pinta have been reported in the last 30 years, though underreporting is likely to be common.
Figure 1. Countries with reported data on yaws, bejel, and pinta from 1980 to 2012 [10]–[29], [32].
Data are number of cases reported in a year, unless otherwise indicated. Prev: prevalence. CR: Case report. ND: No data. Shading indicates the countries where cases of endemic treponematoses have been reported within the last 30 years. In some countries the transmission may be restricted to small areas, rather than affecting the entire country. India interrupted transmission in 2004 and declared elimination in 2006. Since 2004, no new cases have been reported. The principal sources of data are WHO reports [10], [16], [24] and scientific articles identified through searches of PubMed about epidemiology of yaws [11]–[15], bejel [17]–[23], and pinta [25]–[27], [29].
Yaws is the most prevalent of the ETs [10]. Fourteen countries have reported cases of yaws to the WHO in the past three years, including Ghana, Papua New Guinea, and the Solomon Islands, which each reported over 20,000 cases in 2010 or 2011 [11]–[15]. Bejel is encountered in the arid areas of the Sahel (southern border of the Sahara desert), including Senegal, Burkina Faso, Mali, and Niger [16]–[19]. It has also been described in the Arabian Peninsula (Saudi Arabia, Iraq, and Syria) [20], [21]. There was a report of three cases of bejel in southeast Turkey in 1995 (where the disease was considered to be eliminated) [22] as well as a case report from southwest Iran in 2012 [23]. Data on the prevalence of pinta are limited, but it might remain endemic in remote regions of Mexico (states of Oaxaca, Guerrero, Michoacan, and Chiapas), where it was common in the 1980s [24], [25]. It has also been reported among Indian tribes in the Amazon region of Brazil, Colombia, and Peru [26]–[28]. A survey in Panama in the 1980s noted evidence of active or inactive pinta among 20% of the population [29].
Imported cases of yaws and bejel have been documented in children in Europe and the United States [30]–[32], and a case of local transmission of bejel was reported in 2012 in Canada among a family who had lived in a refugee camp in the Republic of Senegal and whose children were all infected [33]. Diagnosis of imported cases is hampered by the limited knowledge and lack of awareness among health care workers in western countries, who may need to include them in the differential diagnosis.
Distinct Clinical Features
The clinical symptoms and signs of ETs have been widely described and illustrated ( Table 1 ) [1], [2], [7]. ETs share clinical features with a number of other conditions common in the tropics and serologically negative suspected cases should be investigated for the aetiology of those lesions ( Table 2 ) [34], [35]. Broadly speaking, bejel can be considered to be a semimucosal disorder and yaws a cutaneous condition, which are both moderately invasive producing bone and cartilage involvement in the secondary stage; pinta is a noninvasive condition causing only local dermal lesions.
Table 1. Characteristics of the four treponemal diseases.
| T. p. pallidum | T. p. pertenue | T. p. endemicum | T. carateum | |
| Epidemiology | ||||
| Geographical distribution (climate) | Worldwide | Tropics (hot, humid areas) | Deserts of Africa and Saudi Arabia (hot, dry areas) | Central and South America (hot, humid areas) |
| Age group (peak incidence) | Adults (18–30) | Children (2–10) | Children (2–10) | Adults (15–50) |
| Transmission | Sexual and congenital | Skin-to-skin contact | Mouth-to-mouth or utensils | Skin-to-skin contact |
| Clinical characteristics | ||||
| Initial lesion (location) | Common (genitals) | Common (lower extremities) | Rare (oral mucosa) | Common (extremities) |
| Dissemination | Widespread and systemic | Widespread to skin and bone | Limited to intertriginous areas and facial bone | Limited only to skin |
| Late complications without treatment (%) | Gummas (10%), neurological (10%), cardiovascular (10–15%) | Destructive lesions of skin and bones (10%) | Destruction of nose/palate | Local skin hypo- achromia |
Table 2. Differential diagnosis for the mucocutaneous manifestations of endemic treponematoses.
| Yaws | Pinta | Bejel |
| Primary and secondary ulcers and papilloma | Primary and secondary lesions | Mucosal and perioral lesions |
| Syphilis | Eczema | Oral herpes simplex |
| Leishmaniasis | Classic or athrophic lichen planus | Aphtous ulcers |
| Paracoccidiomysosis | Tinea corporis | Angular cheilitis |
| Pyoderma | Syphilis | Syphilis |
| Ecthyma | Tuberculoid leprosy | |
| Tropical ulcer | Psoriasis | |
| Pellagra | ||
| Yaws | ||
| Papulosquamous secondary lesions | Dyschromic lesions | Cutaneous primary and secondary lesions |
| Syphilis | Pytiriasis alba | Syphilis |
| Psoriasis | Vitiligo | Condyloma acuminata |
| Eczema | Tinea versicolor | Molluscum contagiosum |
| Arthropod bites | Melasma | Seborrheic dermatitis |
| Scabies | Leprosy | Psoriasis |
| Dermatophytosis | Erythema dyschronicum perstans | Dermatophytosis |
| Tuberculoid leprosy | ||
| Tertiary gummatous lesions | Tertiary gummatous lesions | |
| Syphilis | Syphilis | |
| Lupus vulgaris | Lupus vulgaris | |
| Deep fundal infections | Deep fundal infections | |
| Mycobacterial infection | Mycobacteris infection | |
| Rhinosporidiosis | Rhinosporidiosis | |
| Rhinoscleroma | Rhinoscleroma |
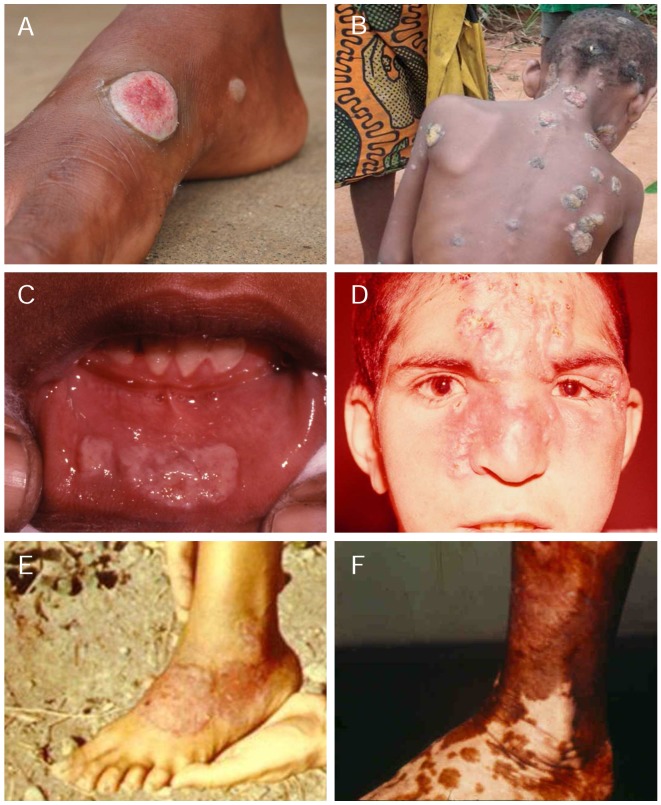
The clinical manifestations of ETs occur in three distinct stages ( Figure 2 ). In yaws, the primary lesion—the so-called mother yaw—is usually a localized papilloma or solitary ulcer 2–5 cm in diameter that may be mistaken for cutaneous leishmaniasis, tropical ulcer, or pyoderma [35]. Yaws skin ulcers are typically circular in shape, have central granulating tissue and elevated edges. Tropical ulcers, caused by anaerobic fusobacteria and Treponema vincentii, are painful and characteristically produce a disagreeable odour and blood-stained discharge, which are rarely met in yaws. H. ducreyi may be an emerging aetiological agent in chronic extragenital skin ulcers in patients from the Pacific Island region [36]. In contrast, the pinta primary lesion is an itchy, scaly papule or plaque that expands to greater than 10 cm but does not ulcerate [37]. In bejel, the primary lesion is seldom seen because of its small size and location within the oral and oropharyngeal mucosa [37]. Primary lesions in yaws and pinta are most commonly found on the exposed lower extremities, on the legs and ankles (65–85% of cases) [35], [38] but also on the buttocks, arms, hands, and face.
Figure 2. Common yaws, bejel, and pinta lesions in 2013.
Papillomatous primary yaws lesion (A); disseminated papilloma of secondary yaws (B); labial mucosal plaques of primary bejel (C); disfiguring infiltration of the nose, glabella, and forehead in a patient with secondary bejel (D); squamous plaque of primary pinta (E); late achromic pinta (F). Sources of photographs: O. Mitjà, Papua New Guinea (A, B); A. Abdolrasouli, Iran (C, D); F. Gómez, Mexico (E, F). The individuals photographed for this publication were informed of the purpose of the photograph and they agreed to have their photograph taken and potentially published.
Secondary skin lesions are very different in each of the ETs. Yaws is the most polymorphous condition, mainly affecting the tegumentum with papulosquamous lesions that must be distinguished from common disorders such as psoriasis, dermatophytosis, or crusted scabies [34], [35]. Secondary yaws lesions usually heal spontaneously after three to six months, though infectious relapses may occur for up to five years. The most common secondary-stage manifestations of bejel, according to the analysis of 3,507 cases in Iraq, include mucosal eroding lesions and plaques in the oropharynx and the lips, which can extend locally to the larynx causing hoarseness [39]. Syphilis should always be considered in the differential diagnosis of bejel; both bejel and syphilis may present with mucosal lesions, papules at the oral commissures, and intertriginous condyloma lata [40].
In pinta, lesions remain active and infectious for many years [41] leading to extensive depigmentation and acral dyschromia. Disorders associated with dyspigmentation, such as tinea versicolor, vitiligo, melasma, and leprosy, are often in the differential diagnosis of pinta [42], [43].
Osteoperiostitis is present in yaws, bejel, and syphilis; progressing slowly; it presents as diffuse cortical thickening followed by bone deformation. This mainly affects the long bones (i.e., forearm, tibia, or fibula) and it is generally bilateral in ETs and unilateral in syphilis. Dactylitis is more common with yaws and it may be difficult to distinguish from sickle-cell anaemia [44]. Gummatous lesions, which may be seen in tertiary yaws and bejel, may resemble manifestations of cutaneous tuberculosis, leishmaniasis, deep fungal infection, or mycobacterial infection. Gumma are neither clinically nor histologically specific; the presence of plasmocytes is nonetheless evocative [45], [46]. A dramatic reduction in the number of late-stage cases with destructive lesions has been noted in recent years [47].This is likely the consequence of more accessible health systems, resulting in earlier diagnosis, and the widespread use of antibiotics [47].
Patients with ETs generally do not present with organ involvement, whereas syphilis preferentially affects the central nervous and cardiovascular systems [48]. Neurologic and ophthalmologic abnormalities possibly caused by yaws and bejel have been reported, but without firm evidence of a causal relationship [49]–[51]. Also, it has been suggested that tertiary yaws can cause cardiovascular disease on basis of a large analysis of autopsies in Ghana [52].
Direct Diagnostic Methods
Direct diagnostic methods are limited by the fact that the T. pallidum treponemes cannot be cultured on synthetic media. The rabbit infectivity test (RIT) is the gold standard for demonstrating T. pallidum infection, but is impractical for clinical use because of high costs and delayed test results. If direct methods are pursued, treponemes may be identified in a wet preparation of material obtained from early lesions by dark-field microscopy. Direct fluorescent antibody tests using anti–T. pallidum antibodies can distinguish pathogenic treponemal infections from saprophyte treponemes [53], [54]. Microscopy, however, is impractical in the field and its sensitivity may be severely decreased if the bacterial load is low or viability of the treponemes is reduced by oral antibiotics or topical antiseptics [55].
The skin pathology using the silver impregnation technique in ETs is largely similar to that of venereal syphilis and despite the existence of relative differences, these cannot be used to differentiate them. The early lesions of yaws show epidermal hyperplasia with collections of neutrophils, and a typical plasmocytic dermal infiltrate [56], [57]. The histopathologic picture of bejel closely resembles that of yaws; though in early bejel, granulomas consisting of epithelioid cells and multinuclear giant cells may be present [58]. In pinta, there is no ulcer formation comparable to that in yaws [59]. In the early lesion, there is loss of melanin in basal cells and liquefaction degeneration. Epidermal atrophy and the presence of many melanophages in the dermis are typical findings of late-stage pinta [60].
Serological Tests
The serological tests used to diagnose ETs are the same as those used to diagnose syphilis [61]. Serologic testing traditionally involves a nonspecific nontreponemal antibody test followed by a more specific treponemal test for diagnostic confirmation [62]. Nontreponemal agglutination tests, RPR or VDRL, are positive in untreated cases; treponemal tests TPHA, TPPA, and FTA-Abs are more specific, but remain positive for life [63], [64]. The equipment and personnel requirements, such as refrigeration for storing reagents, centrifugation for separating serum, an electric rotator for operating flocculation tests, and experienced laboratory staff for conducting and interpreting the tests, are often barriers to conducting these laboratory-based tests in low-resource settings in developing countries or during yaws community screening campaigns.
Rapid point-of-care (PoC) treponemal tests have become available in the form of immunochromatographic strips; these can be used with whole blood and do not require refrigeration [65]–[67]. However, the treponemal rapid test is not able to differentiate between active and treated infection and unfortunately its result correlates poorly with that of active infection, especially in areas where prevalence of yaws is low or after one or more rounds of mass treatment. Therefore, diagnosis based on the result of a treponemal test alone is likely to result in unnecessary treatment and increases probability of selection for antibiotic-resistant pathogens.
A combined point-of-care test which detects both treponemal and nontreponemal antibodies has been evaluated for the diagnosis of syphilis [68], [69]. The test has been designed for field use, not requiring any kind of laboratory support and it could help to detect yaws cases in isolated rural settings. It could also assist in the surveillance that will be needed for years after endemic communities are treated with antibiotics during eradication programs. In addition, measurement with an automatic reader has the potential to monitor the changes in density of nontreponemal antibodies (corresponding to the titer on nontreponemal test) and allow for quantitative serological follow-up. However, as of this year, there was limited published data in this regard to allow for exploration of their accuracy in syphilis or ETs. More research on these combination tests is highly warranted.
Caution should be exercised in interpretation of the any of the above-mentioned serologic tests results for yaws: i) the antibody response to treponemal infections is often not detectable during the first one to three weeks of infection [70], ii) diagnosis of congenital syphilis can be confused by transferred antibodies from the mother, and most importantly iii) serological tests cannot distinguish between endemic treponematoses infections and syphilis [71], [72]. The whole genome analysis of treponemes is an important avenue of current research, in an attempt to identify new targets for strain- and subspecies-specific molecular and serological diagnosis.
Whole Genome Sequencing: New Diagnostic Targets
Historically, syphilis and yaws treponemes were considered to be separate species (based on differences in clinical manifestations of the corresponding diseases), but since 1984 they have been classified as subspecies [73], based on DNA hybridization experiments [74]. The whole genome fingerprinting technique [5], [75] revealed high sequence relatedness among all investigated Treponema genomes, with the most divergent genomic sequence found in the rabbit pathogen (T. paraluiscuniculi). However, T. paraluiscuniculi differed from syphilis-causing strains in less than 2% of the genome sequence [76]. These data indicated that complete, high-quality sequences were required for treponeme genome comparisons. T. carateum (pinta agent), unlike T. p. pertenue or T. p. endemicum, is not currently classified as a T. pallidum subspecies since our molecular knowledge at this time is insufficient to warrant such a classification.
The whole genome analyses of a number of pathogenic treponemes have been performed, including five T. p. pallidum strains (Nichols, SS14, Chicago, DAL-1, and Mexico A) [77]–[80], three T. p. pertenue strains (Samoa D, CDC-2, and Gauthier) [81], and other genomes have been sequenced but not published yet (the Fribourg-Blanc simian isolate, T. p. endemicum strain Bosnia A) [82]. Genome size, percentage of sequence identity with T. p. pallidum, and selected diverse genetic regions are shown in Table 3 . In all strains, no major rearrangements and a nearly identical gene order was found, further establishing the close genetic relationship between these Treponema pathogens. The observed differences, in the presence of restriction target sites, grouped T. p. pallidum strains into a separate cluster compared to T. p. pertenue strains. Analyses revealed a closer relationship between the Fribourg-Blanc simian treponemes and T. p. pertenue strains, and showed that T. p. endemicum (Bosnia A strain) also clustered with T. p. pertenue strains, though more distantly than that of the Fribourg-Blanc isolate. The genomic data have provided new opportunities to study pathogenic treponemes and have revealed chromosomal regions with accumulated genetic diversity between the subspecies [82].
Table 3. Genomic features and historic overview of selected genetic differences found in nonvenereal treponemes.
| T. p. pallidum | T. p. pertenue | Fribourg-Blanc | T. p. endemicuma | Year (Ref.) | ||
| Genome features | ||||||
| Genome size (kbp) | 1138.0–1140.0 | 1139.3–1139.7 | 1140.5 | 1137.7 | ||
| Genome sequence identity with sequences of T. p. pallidum (%) | N/Ab | 99.8 | 99.8 | ∼99.7 | 1998 [77], 2008 [78], 2012 [79]–[81], [96] | |
| GenBank acc. no. | AE000520.1 | CP002374.1 | CP003902.1 | N/A | ||
| CP000805.1 | CP002375.1 | |||||
| CP001752.1 | CP002376.1 | |||||
| CP003115.1 | ||||||
| CP003064.1 | ||||||
| Selected genetic differences c | ||||||
| Differential genetic locus (type of change) | Position in gene/genome (according to CP002374): | |||||
| tpf1, TP1038 (nt change) | 122d/1136540 | A | G | G | G | 1989 [91] |
| tpp15, 5′-flanking region, TP0170 (nt change/Eco47III digestion pattern) | 759/191605 | C/cut | T/no cut | T/no cut | T/no cut | 1998 [92] |
| gpd, TP0257 (nt change/PleI digestion pattern) | 579/269535 | A/no cut | G/cut | G/cut | G/cut | 1999 [93] |
| tprC, TP0117(nt change/BsrDI digestion pattern) | 1726–1733/134662–134669 | CATTG/cute | TATTA/no cut | CATTG/cut | CATTG/cut | 2006 [4] |
| tprI, TP0620 (nt change/BsrDI digestion pattern) | 1759–1766/672713–672720 | T/no cut | T/no cut | T/no cut | C/cut | 2006 [4] |
| arp, TP0433 (no. of repeats) | 547–1266/462430–463149 | 4–16 | 3–12 | 15 | 8, 10f | 2007, 2008 [94], [95] |
| IGR19, intergenic spacer/hlyB (TP0027)g | 20–25/34079–34084 | 9xC, 12xC | CCCTCC | CCCTCC | CCCTCC | 2011 [96] |
Genome positions are shown in the T. p. pertenue Samoa D genomic sequence (GenBank acc. no. CP002374.1).
For T. p. endemicum, the genome size and the genome sequence identity with T. pallidum were estimated based on preliminary data.
N/A, not applicable.
Genetic differences between T. p. pallidum and T. p. pertenue were also determined in tprJ (TP0621), in tp92 (TP0326), and in six genomic regions as described by Stamm et al. (1998) [89], Cameron et al. (2000) [90], and Mikalová et al. (2010) [5], respectively.
Position 123 published by Noordhoek et al. (1989) (tpf1 vs. tyf1); this position is in fact position 122.
No cut for T. p. pallidum Mexico A, SS14, and Sea81-3 strains.
8 and 10 repeats found in the T. p. endemicum Bosnia A and Iraq B, respectively [95].
IGR19 is an intergenic spacer between fliG (TP0026) and putative hemolysin gene hlyB (TP0027) in the Nichols genome (AE000520.1); this region is a part of the hlyB gene (TP0027) in the Samoa D genome (CP002374.1).
Polymerase Chain Reaction Analyses of Clinical Samples
During the past 20 years, there has been an increasing effort to apply polymerase chain reaction (PCR) techniques for direct diagnosis of treponematoses. PCR detection of treponemal DNA is a direct method with detection thesholds as low as a few copies of the treponemal chromosome per PCR reaction (sensitivity of 10−2–10−3 organism equivalents).
Non-subspecies–specific T. pallidum PCR is a developing technology, available as an in-house assay at a few laboratories. Several target sequences have been evaluated with some, such as tpf-1, bmp, tpp47, tmpA, and 47-kDa [83]–[85], encoding subsurface lipoproteins and others, such as polA [86], being involved in genome duplication; most recently, polA assay has been adapted to real-time TaqMan PCR. [87]. This could be applied to swab specimens for rapid detection of nonvenereal treponemes; however, the low numbers of treponemes in blood put limitations on PCR diagnosis of ETs from blood samples [88].
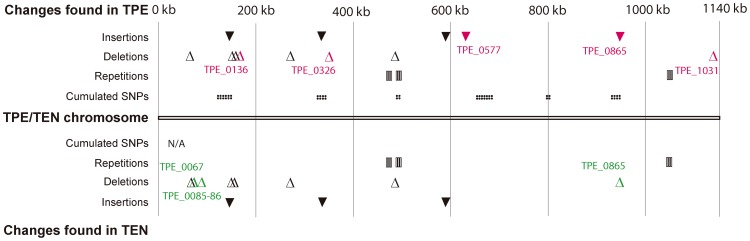
Investigational PCR assays to distinguish nonvenereal T. pallidum subspecies have also been evaluated. T. p. pertenue and T. p. endemicum genetic signatures have been identified ( Table 3 ). The regions of sequence divergence could be used for the molecular detection and discrimination of syphilis, yaws, and bejel strains; this option is not possible with currently available diagnostic tests [4], [89]–[96]. Moreover, recent accumulation of genomic data revealed many genetic regions potentially suitable for the detection of T. p. pertenue and T. p. endemicum strains ( Figure 3 ). However, these regions need to be tested in other strains before selecting the most suitable target for a molecular diagnosis of the yaws- or bejel-causing strains.
Figure 3. Identified genetic loci showing sequence differences in T. p. pertenue and T. p. endemicum .
[1] , [81] . These regions are potentially suitable for detection of TPE or TEN strains; however, further testing of these regions in additional strains is needed. Indels specific to tested TPE and TEN strains are shown in red and green, respectively. Only genetic differences consistently present between all completely sequenced TPE and TPA strains are shown. In the case of TEN, only one strain (Bosnia A) has been analyzed on a genome level. Coordinates of insertions in the TPE genomes are taken from the Samoa D genome (GenBank acc. no. CP002374.1): 148701–148781, 331996–332630, 594549–594600, 629981–629992, 945694–945717; coordinates of deletions (CP002374.1): 72694–72695, 150386–150387, 153904–154035, 158201–158202, 279101–279102, 348027–348028, 492496–492501, 1125682–1125683; coordinates of repetitions (CP002374.1): 462430–463149, 498895–499200, 1051990–1052003. Coordinates of cumulated SNPs (defined as ten or more nucleotide changes present in the 100-bp genome window, CP002374.1): 135500–135599, 148700–148799, 153800–153899, 154000–154099, 331200–331399, 331700–332899, 493000–493099, 672000–672199, 672500–673699, 675300–675899, 800500–800599, 936700–936799, 938000–938099. For TEN Bosnia A, only coordinates of insertions, deletions, and repetitions are shown; coordinates of insertions (according to CP002374.1): 148701–148781, 331996–332630, 594549–594600; coordinates of deletions: 72694–72695, 72719–72734, 94986–94998, 150386–150387, 153904–154035, 279101–279102, 492496–492501, 945694–945717; coordinates of repetitions: 462430–463149, 498895–499200,1051990–1052003. N/A, not applicable.
Experience with DNA sequencing to confirm a clinical suspicion of ETs is limited. A ten-year-old boy with active skin lesions, who migrated from the Republic of the Congo, was confirmed to suffer from yaws [30], and a one-year-old girl with an oral ulcer was diagnosed with bejel during an outbreak of local transmission in Canada [33]. Real-time PCR is useful for identifying the organism and would be a good test to differentiate between the T. pallidum subspecies in a single assay, although such techniques are expensive, and unlikely to be available outside reference laboratories [97].
Laboratory Support and Approach to Evaluation of the Programme to Eradicate Yaws
New WHO yaws eradication strategy and treatment policies for yaws with azithromycin were issued in 2012. The recommended treatment for eradication is one dose of oral azithromycin to be given to entire populations in areas known to harbour yaws. The mass treatment should be followed by immediate mop-ups and resurveys every 6 months to detect and treat remaining cases until zero prevalence is reached. During all stages of the newly embraced yaws eradication programmes, regular surveillance and laboratory support is essential to assess the progress and identify lacunae and gaps. During the mass-treatment phase, active case-finding and treatment campaigns will be organized and diagnosis will be mainly on clinical findings with serological confirmation [62], [65]. During the verification phase after no new active cases have been reported, a combination of treponemal and nontreponemal serological tests will be required for confirmation of all new clinically suspected cases. Interruption of transmission of yaws disease is measured by zero reporting of cases consistently for three consecutive years and verified by no sero-reactors among a randomly selected sample of children one to five years old [10], [98]. If the new rapid dual test accuracy is determined, this could be easier to perform in the field during the verification phase instead of the traditional tests.
Discussion
The nonvenereal treponematoses continue to be transmitted among rural communities in developing countries and a risk remains for importation of the disease into areas where it is not endemic, and subsequently for local transmission of the etiologic agent. Clinical identification of yaws, bejel, and pinta has important implications regarding diagnostic approach, case management and prevention strategies during implementation of eradication campaigns. Therefore, the clinical assessment skills among health workers and community agents in endemic areas need to be developed through training courses.
In addition, the diagnosis of ETs is difficult and usually requires serological confirmation; a combination of a treponemal and a nontreponemal test is advisable to exclude past-treated infection, but this is challenging in limited-resource settings. Well-designed studies of diagnostic accuracy are needed for the new rapid and PoC combination assay that add potential value to the standard nontreponemal test because it can be easily performed in the field.
In recent years, much effort has been devoted to the development of molecular techniques that enable health care workers to distinguish between the T. pallidum subspecies. However, at present the use of clinically useful molecular biological techniques that affect turnaround time, diagnosis accuracy, and patient outcome, and reduce overall costs, is not in sight. Further support is needed to facilitate and expedite the practical application of these scientific discoveries to clinical medicine.
Learning Points
The clinical diagnosis for endemic treponematoses may be difficult because these infections produce lesions which can resemble several other diseases in the tropics; thus, support of laboratory techniques and epidemiologic characteristics is necessary.
The serological tests are the same as those used to diagnose syphilis. The nontreponemal tests are a better indication of active infection and ongoing transmission in an area.
Available T. pallidum PCR assays can be applied to the diagnosis of endemic treponematoses as a direct method with high sensitivity; new molecular diagnostic techniques to differentiate the existing subspecies of pathogenic treponemes are under development.
Key Articles
Hackett CJ (1953) Extent and nature of the yaws problem in Africa. Bull World Health Organ 8: 129–182.
Mitjà O, Hays R, Lelngei F, Laban N, Ipai A, et al. (2011) Challenges in recognition and diagnosis of yaws in children in Papua New Guinea. Am J Trop Med Hyg 85: 113–116.
Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, et al. (2010) Novel point-of-care test for simultaneous detection of non-Treponemal and Treponemal antibodies in patients with syphilis. J Clin Microbiol 48: 4615–4619.
Čejková D, Zobaníková M, Chen L,Pospíšilová P, Strouhal M, et al. (2012) Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl Trop Dis 6: e1471. doi:10.1371/journal.pntd.0001471.
Acknowledgments
We thank A. Abdolrasouli for his kind permission to use clinical photographs in Figure 2.
Funding Statement
The authors have indicated that no funding was received for this work.
References
- 1.Perine PL, Hopkins DR, Niemel PLA, St. John RK, Causse G, et al.. (1984) Handbook of endemic Treponematoses: yaws, endemic syphilis, and pinta. Geneva: World Health Organization.
- 2. Antal GM, Lukehart SA, Meheus AZ (2002) The endemic Treponematoses. Microbes Infect 4: 83–94. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2012) Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation – executive summary. Geneva: World Health Organization.
- 4. Centurion-Lara A, Molini B, Godornes C, Sun E, Hevner K, et al. (2006) Molecular differentiation of Treponema pallidum subspecies. J Clin Microbiol 44: 3377–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mikalová L, Strouhal M, Čejková D, Zobaníková M, Pospíšilová P, et al. (2010) Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: most of the genetic differences are localized in six regions. PLoS ONE 5: e15713 doi:10.1371/journal.pone.0015713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner TB, Hollander DH (1957) Biology of the treponematoses. Geneva: World Health Organization.
- 7. Hackett CJ (1953) Extent and nature of the yaws problem in Africa. Bull World Health Organ 8: 129–182. [PMC free article] [PubMed] [Google Scholar]
- 8. Hudson H (1938) Bejel: syphilis as a contagious disease of children. Am J Trop Med Hyg 18: 675–687. [Google Scholar]
- 9. Arslanagic N, Bokonjic M, Macanovic K (1989) Eradication of endemic syphilis in Bosnia. Genitourin Med 65: 4–7. [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization (2012) Eradication of yaws - the Morges Strategy. Wkly Epidemiol Rec 87: 189–194. [PubMed] [Google Scholar]
- 11. Wirski WR (1985) Surveillance and control of resurgent yaws in the African region. Rev Infect Dis 7: s227–232. [DOI] [PubMed] [Google Scholar]
- 12. Touré B, Koffi NM, Assi KP, Ake O, Konan DJ (2007) Yaws in Côte d'lvoire: health problem forgotten and neglected. Bull Soc Pathol Exot 100: 130–132 [Article in French]. [PubMed] [Google Scholar]
- 13. Manning LA, Ogle GD (2002) Yaws in the periurban settlements of Port Moresby, Papua New Guinea. P N G Med J 45: 206–212. [PubMed] [Google Scholar]
- 14. Capuano C, Ozaki M (2011) Yaws in the Western pacific region: a review of the literature. J Trop Med 642832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health, Division of Planning and Policy National Health Statistics Office, Solomon Islands (2008) Annual health report 2007. Tech Rep, Solomon Islands.
- 16. World Health Organization (1985) International Symposium on Yaws and Other Endemic Treponematoses. Washington DC, April 16–18, 1984. Rev Infect Dis 7: 217–351. [PubMed] [Google Scholar]
- 17. Gazin P, Meynard D (1988) A clinical and serologic survey of bejel in north Burkina Faso. Bull Soc Pathol Exot Filiales 81: 827–831 [Article in French]. [PubMed] [Google Scholar]
- 18. Autier P, Delcambe JF, Sangaré D, Lamine D, Kessier W, et al. (1989) Serological and clinical studies of endemic Treponematosis in the Republic of Mali. Ann Soc Belg Med Trop 69: 319–329 [Article in French]. [PubMed] [Google Scholar]
- 19. Julvez J, Michault A, Kerdelhue V (1998) Serologic studies of non-venereal Treponematoses in infants in Naimey, Niger. Med Trop (Mars) 58: 38–40 [Article in French]. [PubMed] [Google Scholar]
- 20. Csonka G, Pace J (1985) Endemic nonvenereal Treponematosis (bejel) in Saudi Arabia. Rev Infect Dis 7: 260–265. [DOI] [PubMed] [Google Scholar]
- 21. Pace Jl, Csonka GW (1984) Endemic non-venereal syphiis (bejel) in Saudi Arabia. Br J Vener Dis 60: 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yakinci C, Ozcan A, Aslan T, Demirhan B (1995) Bejel in Malatya, Turkey. J Trop Pediatr 41: 117–20. [DOI] [PubMed] [Google Scholar]
- 23. Abdolrasouli A, Croucher A, Hemmati Y, Mabey D (2013) A case of endemic syphilis, Iran [letter]. Emerg Infect Dis 19: 162–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization (1981) Endemic Treponematoses. Wkly Epidemiol Rec 56: 241–244. [Google Scholar]
- 25. Giuliani M, Latini A, Palamara G, Maini A, Di Carlo A (2005) The clinical appearance of pinta mimics secondary syphilis: another trap of Treponematosis [letter]. Clinical Inf Dis 40: 1548. [DOI] [PubMed] [Google Scholar]
- 26. Pecher SA, Croce J (1988) Immunology of tertiary pinta. Med Cutan Ibero Lat Am 16: 111–114 [Article in Portuguese]. [PubMed] [Google Scholar]
- 27. Hopkins DR, Flórez D (1977) Pinta, yaws, and venereal syphilis in Colombia. Int J Epidemiol 6: 349–355. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (1987) Programme for the Control of the Endemic Treponematoses. Geneva: World Health Organization, VDT/EXBUD/87.1.
- 29. Fohn MJ, Wignall S, Baker-Zander SA, Lukehart SA (1988) Specificity of antibodies from patients with pinta for antigens of Treponema pallidum subspecies pallidum . J Infect Dis 157: 32–37. [DOI] [PubMed] [Google Scholar]
- 30. Pillay A, Chen CY, Reynolds MG, Mombouli JV, Castro AC, et al. (2011) Laboratory-confirmed case of yaws in a 10-year-old boy from the Republic of the Congo. J Clin Microbiol 49: 4013–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Engelkens HJ, Oranje AP, Stolz E (1989) Early yaws, imported in the Netherlands. Genitourin Med 65: 316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vabres P, Roose B, Berdah S, Fraitag S, Prost YD (1999) Le bejel: une cause inhabituelle de stomatite de l'enfant. Ann Dermatol Venereol 126: 49–50 [Article in French]. [PubMed] [Google Scholar]
- 33. Fanella S, Kadkhoda K, Shuel M, Tsang R (2012) Local transmission of imported endemic syphilis. Emerg Infect Dis 18: 1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrouche N, Ghosn SH (2012) Endemic (nonvenereal) treponematoses. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller SA, Lefell DJ, et al.., editors. Fitzpatrick's dermatology in general medicine. 8th edition. McGraw Hill, p. 2493.
- 35. Mitjà O, Hays R, Lelngei F, Laban N, Ipai A, et al. (2011) Challenges in recognition and diagnosis of yaws in children in Papua New Guinea. Am J Trop Med Hyg 85: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW (2010) Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med J Aust 192: 348–350. [DOI] [PubMed] [Google Scholar]
- 37. Leon y Blanco F (1940) La lesion inicial del mal de pinta. Rev Med Trop Parastiol Bacteriol Clin Lab 6: 21 [Article in Spanish]. [Google Scholar]
- 38. Guderian RH, Guzman JR, Calvopiña M, Cooper P (1991) Studies on a focus of yaws in the Santiago Basin, province of Esmeraldas, Ecuador. Trop Geogr Med 43: 142–147. [PubMed] [Google Scholar]
- 39. Csonka GW (1953) Clinical aspects of Bejel. Br J Vener Dis 29: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basset A, Maleville J, Basset M (1969) Aspects de la syphilis endemique des Touareg du Niger. Bull Soc Pathol Exot 62: 80–92. [PubMed] [Google Scholar]
- 41. Martinez SA, Mouney DF (1982) Treponemal infections of the head and neck. Otolaryngol Clin North Am 27: 220–223. [PubMed] [Google Scholar]
- 42. Marquez F, Rein CR, Arias O (1955) Mal de pinto in Mexico. Bull World Health Organ 13: 299–322. [PMC free article] [PubMed] [Google Scholar]
- 43. Fuchs J, Milbradt R, Pecher SA (1993) Tertiary pinta: case reports and overview. Cutis 51: 425–430. [PubMed] [Google Scholar]
- 44. Mitjà O, Hays R, Ipai A, Wau B, Bassat Q (2011) Osteoperiostitis in early yaws: case series and literature review. Clin Infect Dis 52: 771–774. [DOI] [PubMed] [Google Scholar]
- 45. Kanan MW, Abbas M, Girgis HY (1971) Late mutilating bejel in the nomadic Bedouins of Kuwait. Dermatologica 143: 277–286. [DOI] [PubMed] [Google Scholar]
- 46. Jones LGG (1953) Mutilating bejel. Br J Vener Dis 29: 104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fegan D, Glennon MJ, Thami Y, Pakoa G (2010) Resurgence of yaws in Tanna, Vanuatu: time for a new approach? Trop Doct 40: 68–69. [DOI] [PubMed] [Google Scholar]
- 48.Merrit HH, Adams RD, Solomon HC (1946) Neurosyphilis. New York: Oxford University Press.
- 49. Roman GC, Roman LN (1986) Occurrence of congenital, cardiovascular, visceral, neurologic, and neuro-ophthalmologic complications in late yaws: a theme for future research. Rev Infect Dis 8: 760–770. [PubMed] [Google Scholar]
- 50. Tabbara KF, Al Kaff AS, Fadel T (1989) Ocular manifestations of endemic syphilis (bejel). Ophtalmology 96: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 51. Hoff H, Shaby JA (1940) Nervous manifestations of Bejel. Trans Royal Soc Trop Med Hyg 33: 549–551. [Google Scholar]
- 52. Edington GM (1954) Cardiovascular disease as a cause of death in the Gold Coast African. Trans R Soc Trop Med Hyg 48: 419–425. [DOI] [PubMed] [Google Scholar]
- 53. Satter EK, Tokarz VA (2010) Secondary yaws: an endemic Treponemal infection. Pediatr Dermatol 27: 364–367. [DOI] [PubMed] [Google Scholar]
- 54. Hoang MP, High WA, Molberg KH (2004) Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol 31: 595–599. [DOI] [PubMed] [Google Scholar]
- 55. Heymans R, van der Helm JJ, de Vries H, Fennema HS, Coutinho RA, et al. (2010) Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J Clin Microbiol 48: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Engelkens HJ, ten Kate FJ, Judanarso J, Vuzevski VD, van Lier JB, et al. (1993) The localisation of treponemes and characterization of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis or early infectious yaws. Genitourin Med 69: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Engelkens HJ, Vuzevski VD, ten Kate FJ, van der Heul P, van der Sluis JJ, et al. (1991) Ultraestructural aspects of infection with Treponema pallidum subspecies pertenue (Pariaman strain). Genitourin Med 67: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lever WF, Schaumburg-Lever G (1992) Treponemal diseases. In: Lever WF, Schaumburg-Lever G, editors. Histopathology of the skin. 7th edition. Philadelphia, PA: J.B. Lippincott. pp. 352–359.
- 59. Hasselmann CM (1957) Comparative studies on the histopathology of syphilis, yaws, and pinta. Br J Venereal Dis 33: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pecher SA, Azevedo EB (1987) Aspectos histopatologicos da pinta terciaria. Med Cutan Ibero Lat Am 15: 239–242 [Article in Portuguese]. [PubMed] [Google Scholar]
- 61. Menke HE, Veldkamp J, Brunings EA, Niemel PLA, Notowicz A, et al. (1979) Comparison of cardiolipin and Treponemaltests in the serodiagnosis of yaws. Br J Vener Dis 55: 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Association of public health laboratories (2009) Laboratory diagnostic testing for Treponema pallidum . Atlanta January 13–15. [Google Scholar]
- 63. Garner MF, Backhouse JF, Daskolopolous G, Walsh JL (1972) Treponema pallidum haemagglutination test for yaw; comparison with the TPI and FTA-ABS tests. Br J Vener Dis 48: 479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Backhouse JL, Hudson BJ (1995) Evaluation of immunoglobulin G enzyme immunoassay for serodiagnosis of Yaws. J Clin Microbiol 33: 1875–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jafari Y, Peeling RW, Shivkumar S, Claessens C, Joseph L, et al. (2013) Are Treponema pallidum specific rapid and point-of-care tests for syphilis accurate enough for screening in resource limited settings? Evidence from a meta-analysis. PLoS ONE 8: e54695 doi:10.1371/journal.pone.0054695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Herring AJ, Ballard RC, Pope V, Adegbola RA, Changalucha J, et al. (2006) A multi-centre evaluation of nine rapid, point-of-care syphilis tests using archived sera. Sex Transm Infect 82: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mabey D, Peeling RW, Ballard R, Benzaken AS, Galbán E, et al. (2006) Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect 82: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, et al. (2010) Novel point-of-care test for simultaneous detection of non-Treponemal and Treponemal antibodies in patients with syphilis. J Clin Microbiol 48: 4615–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yin YP, Chen XS, Wei W, Gong KL, Cao WL, et al. (2013) A dual point-of-care test shows good performance in simultaneously detecting non-Treponemal and Treponemal antibodies in patients with syphilis - a multi-site evaluation study in China. Clin Infect Dis 56: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li HY (1955) Serological study of yaws in Java. Bull World Health Organ 12: 905–943. [PMC free article] [PubMed] [Google Scholar]
- 71. Noordhoek GT, Cockayne A, Schouls LM, Meloen RH, Stolz E, et al. (1990) A new attempt to distinguish serologically the subspecies of Treponema pallidum causing syphilis and yaws. J Clin Microbiol 28: 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baker-Zander SA, Lukehart SA (1983) Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue . Infect Immun 42: 634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smibert RM (1984) Genus III Treponema. In: Krieg NR, Holt JG, editors. Bergey's manual of systematic bacteriology, vol. 1. Baltimore: Williams and Wilkins, pp. 49–57.
- 74. Miao RM, Fieldsteel AH (1980) Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol 141: 427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Strouhal M, Šmajs D, Matějková P (2007) Genome differences between Treponema pallidum subsp. pallidum strain Nichols and T. paraluiscuniculi strain Cuniculi A. Infect Immun 75: 5859–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Šmajs D, Zobaníková M, Strouhal M, Čejková D, Dugan-Rocha S, et al. (2011) Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS ONE 6: e20415 doi:10.1371/journal.pone.0020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fraser CM, Norris SJ, Weinstock GM (1998) Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281: 375–388. [DOI] [PubMed] [Google Scholar]
- 78. Matějková P, Strouhal M, Šmajs D, Norris SJ, Palzkill T, et al. (2008) Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Giacani L, Chattopadhyay S, Centurion-Lara A, Jeffrey BM, Le HT, et al. (2012) Footprint of positive selection in Treponema pallidum subsp. pallidum genome sequences suggests adaptive microevolution of the syphilis pathogen. PLoS Negl Trop Dis 6: e1698 doi:10.1371/journal.pntd.0001698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zobaníková M, Mikolka P, Čejková D, Pospíšilová P, Chen L, et al. (2012) Complete genome sequence of Treponema pallidum ssp. pallidum strain DAL-1. Stand Genomic Sci 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Čejková D, Zobaníková M, Chen L, Pospíšilová P, Strouhal M, et al. (2012) Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl Trop Dis 6: e1471 doi:10.1371/journal.pntd.0001471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Šmajs D, Norris SJ, Weinstock GM (2012) Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect Genet Evol 12: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Noordhoek G T, Woltes T, De Jonge M, Van Embden J (1991) Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol 29: 1976–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hay PE, Clarke J, Strugnell R, Taylor-Robinson D, Goldmeier D (1990) Use of polymerase chain reaction to detect DNA sequences specific to pathogenic treponemes in cerebrospinal fluid. FEMS Microbiol 68: 428–432. [DOI] [PubMed] [Google Scholar]
- 85. Grimprel E, Sanchez P, Wendel G, Burstain JM, McCracken GH Jr, et al. (1991) Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid. J Clin Microbiol 29: 1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu H, Rodes B, Chen CY, Steiner B (2001) New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol 39: 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leslie DE, Azzato F, Karapanagiotidis T, Leydon J, Fyfe J (2007) Development of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay's performance by comparison with serological testing. J Clin Microbiol 45: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grange PA, Gressier L, Dion PL (2012) Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J Clin Microbiol 50: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stamm LV, Greene SR, Bergen HL, Hardham JM, Barnes NY (1998) Identification and sequenceanalysis of Treponema pallidum tprJ, a member of a polymorphic multigenefamily. FEMS Microbiol 169: 155–163. [DOI] [PubMed] [Google Scholar]
- 90. Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, et al. (2000) Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 181: 1401–1413. [DOI] [PubMed] [Google Scholar]
- 91. Noordhoek GT, Hermans PWM, Paul AN, Schouls LM, van der Sluis JJ, et al. (1989) Treponema pallidum subspecies pallidum and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: comparison of two homologous antigens. Microb Pathogenesis 6: 29–42. [DOI] [PubMed] [Google Scholar]
- 92. Centurion-Lara A, Castro C, Castillo R, Shaffer JM, Van Voorhis WC, et al. (1998) The flanking regions sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J Infect Dis 177: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 93. Cameron CE, Castro C, Lukehart SA, Van Voorhis WC (1999) Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect Immun 67: 3168–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu H, Rodes B, George R, Steiner B (2007) Molecular characterization and analysis of a gene encoding the acidic repeat protein (Arp) of Treponema pallidum . J Med Microbiol 56: 715–721. [DOI] [PubMed] [Google Scholar]
- 95. Harper KN, Liu H, Ocampo PS, Steiner BM, Martin A, et al. (2008) The sequence of the acidic repeat protein (arp) gene differentiates venereal from nonvenereal Treponema pallidum subspecies, and the gene has evolved under strong positive selection in the subspecies that causes syphilis. FEMS Immunol Med Microbiol 53: 322–332. [DOI] [PubMed] [Google Scholar]
- 96. Pětrošová H, Zobaníková M, Čejková, Mikalová L, Pospíšilová P, et al. (2012) Whole genome sequence of Treponema pallidum ssp. pallidum, strain Mexico A, suggests recombination between yaws and syphilis strains. PLoS Negl Trop Dis 6: e1832 doi:10.1371/journal.pntd.0001832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Noordhoek G, Wieles B, Sluis J, Embden J (1990) Polymerase chain reaction and synthetic DNA probes: a means of distinguishing the causative agents of syphilis and yaws? Infect Immun 58: 2011–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. World Health Organization (2008) Elimination of yaws in India. Wkly Epidemiol Rec 15: 125–132. [PubMed] [Google Scholar]