Abstract
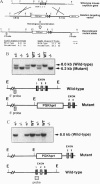
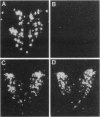
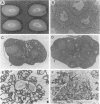
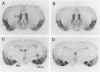
Oxytocin, a neurohypophyseal hormone, has been traditionally considered essential for mammalian reproduction. In addition to uterine contractions during labor and milk ejection during nursing, oxytocin has been implicated in anterior pituitary function, paracrine effects in the testis and ovary and the neural control of maternal and sexual behaviors. To determine the essential role(s) of oxytocin in mammalian reproductive function, mice deficient in oxytocin have been generated using embryonic stem cell technology. A deletion of exon 1 encoding the oxytocin peptide was generated in embryonic stem cells at a high frequency and was successfully transmitted in the germ line. Southern blot analysis of genomic DNA from homozygote offspring and in situ hybridization with an exonic probe 3' of the deletion failed to detect any oxytocin or neurophysin sequences, respectively, confirming that the mutation was a null mutation. Mice lacking oxytocin are both viable and fertile. Males do not have any reproductive behavioral or functional defects in the absence of oxytocin. Similarly, females lacking oxytocin have no obvious deficits in fertility or reproduction, including gestation and parturition. However, although oxytocin-deficient females demonstrate normal maternal behavior, all offspring die shortly after birth because of the dam's inability to nurse. Postpartum injections of oxytocin to the oxytocin deficient mothers restore milk ejection and rescue the offspring. Thus, despite the multiple reproductive activities that have been attributed to oxytocin, oxytocin plays an essential role only in milk ejection in the mouse.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang H. L., Ungefroren H., De Bree F., Foo N. C., Carter D., Burbach J. P., Ivell R., Murphy D. Testicular oxytocin gene expression in seminiferous tubules of cattle and transgenic mice. Endocrinology. 1991 Apr;128(4):2110–2117. doi: 10.1210/endo-128-4-2110. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Collu M., Gessa G. L., Melis M. R., Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol. 1988 May 10;149(3):389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Barberis C., Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10(1):119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Chibbar R., Miller F. D., Mitchell B. F. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J Clin Invest. 1993 Jan;91(1):185–192. doi: 10.1172/JCI116169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo N. C., Carter D., Murphy D., Ivell R. Vasopressin and oxytocin gene expression in rat testis. Endocrinology. 1991 Apr;128(4):2118–2128. doi: 10.1210/endo-128-4-2118. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Dietl M. M., Stoeckel M. E., Palacios J. M., Richard P. Quantitative autoradiographic mapping of neurohypophysial hormone binding sites in the rat forebrain and pituitary gland--II. Comparative study on the Long-Evans and Brattleboro strains. Neuroscience. 1988 Jul;26(1):273–281. doi: 10.1016/0306-4522(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Hara Y., Battey J., Gainer H. Structure of mouse vasopressin and oxytocin genes. Brain Res Mol Brain Res. 1990 Oct;8(4):319–324. doi: 10.1016/0169-328x(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Harbaugh C. R. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989 May;45(5):1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Insel T. R. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Shapiro L. E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. R., Winslow J. T., Witt D. M. Homologous regulation of brain oxytocin receptors. Endocrinology. 1992 May;130(5):2602–2608. doi: 10.1210/endo.130.5.1315251. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Young L., Witt D. M., Crews D. Gonadal steroids have paradoxical effects on brain oxytocin receptors. J Neuroendocrinol. 1993 Dec;5(6):619–628. doi: 10.1111/j.1365-2826.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Russell J. A. Oxytocin: cellular and molecular approaches in medicine and research. Rev Reprod. 1996 Jan;1(1):13–18. doi: 10.1530/ror.0.0010013. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992 Apr 9;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Ruppert S., Schmale H., Rehbein M., Richter D., Schütz G. Deduced amino acid sequence from the bovine oxytocin-neurophysin I precursor cDNA. Nature. 1983 Mar 24;302(5906):342–344. doi: 10.1038/302342a0. [DOI] [PubMed] [Google Scholar]
- Larcher A., Neculcea J., Breton C., Arslan A., Rozen F., Russo C., Zingg H. H. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology. 1995 Dec;136(12):5350–5356. doi: 10.1210/endo.136.12.7588281. [DOI] [PubMed] [Google Scholar]
- Laurent F. M., Hindelang C., Klein M. J., Stoeckel M. E., Felix J. M. Expression of the oxytocin and vasopressin genes in the rat hypothalamus during development: an in situ hybridization study. Brain Res Dev Brain Res. 1989 Mar 1;46(1):145–154. doi: 10.1016/0165-3806(89)90152-1. [DOI] [PubMed] [Google Scholar]
- Lefebvre D. L., Farookhi R., Larcher A., Neculcea J., Zingg H. H. Uterine oxytocin gene expression. I. Induction during pseudopregnancy and the estrous cycle. Endocrinology. 1994 Jun;134(6):2556–2561. doi: 10.1210/endo.134.6.8194482. [DOI] [PubMed] [Google Scholar]
- Lefebvre D. L., Giaid A., Bennett H., Larivière R., Zingg H. H. Oxytocin gene expression in rat uterus. Science. 1992 Jun 12;256(5063):1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- Lefebvre D. L., Giaid A., Zingg H. H. Expression of the oxytocin gene in rat placenta. Endocrinology. 1992 Mar;130(3):1185–1192. doi: 10.1210/endo.130.3.1537285. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Finegold M. J., Su J. G., Hsueh A. J., Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992 Nov 26;360(6402):313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- McCarthy M. M. Oxytocin inhibits infanticide in female house mice (Mus domesticus). Horm Behav. 1990 Sep;24(3):365–375. doi: 10.1016/0018-506x(90)90015-p. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Ascher J. A., Monroe Y. L., Prange A. J., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982 May 7;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Prange A. J., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehbein M., Hillers M., Mohr E., Ivell R., Morley S., Schmale H., Richter D. The neurohypophyseal hormones vasopressin and oxytocin. Precursor structure, synthesis and regulation. Biol Chem Hoppe Seyler. 1986 Aug;367(8):695–704. doi: 10.1515/bchm3.1986.367.2.695. [DOI] [PubMed] [Google Scholar]
- Robinson G., Evans J. J. Oxytocin has a role in gonadotrophin regulation in rats. J Endocrinol. 1990 Jun;125(3):425–432. doi: 10.1677/joe.0.1250425. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Scherer G., Schütz G. Recent gene conversion involving bovine vasopressin and oxytocin precursor genes suggested by nucleotide sequence. Nature. 1984 Apr 5;308(5959):554–557. doi: 10.1038/308554a0. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Coirini H., Frankfurt M., McEwen B. S. Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6798–6801. doi: 10.1073/pnas.86.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M., Coirini H., Pfaff D. W., McEwen B. S. Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science. 1990 Nov 2;250(4981):691–694. doi: 10.1126/science.2173139. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bolwerk E. L., Liu B., Burbach J. P. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988 Mar;122(3):945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Wathes D. C. Possible actions of gonadal oxytocin and vasopressin. J Reprod Fertil. 1984 May;71(1):315–345. doi: 10.1530/jrf.0.0710315. [DOI] [PubMed] [Google Scholar]
- Wathes D. C., Swann R. W. Is oxytocin an ovarian hormone? Nature. 1982 May 20;297(5863):225–227. doi: 10.1038/297225a0. [DOI] [PubMed] [Google Scholar]
- Witt D. M., Insel T. R. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991 Jun;128(6):3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986 Dec;387(3):231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L. Oxytocin and vasopressin gene expression during gestation and lactation. Brain Res. 1988 Aug;464(1):1–6. doi: 10.1016/0169-328x(88)90011-3. [DOI] [PubMed] [Google Scholar]
- van Leengoed E., Kerker E., Swanson H. H. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987 Feb;112(2):275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]