Abstract
Objectives:
The objective of this study was to evaluate whether patients with surgically refractory medial temporal lobe epilepsy (MTLE) exhibit a distinct pattern of structural network organization involving the temporal lobes and extratemporal regions.
Methods:
We retrospectively studied 18 healthy controls and 20 patients with medication refractory unilateral MTLE who underwent anterior temporal lobectomy for treatment of seizures. Patients were classified as seizure-free or not seizure-free at least 1 year after surgery. The presurgical brain connectome was calculated through probabilistic connectivity from MRI–diffusion tensor imaging from 83 anatomically defined regions of interest encompassing the whole brain. The connectivity patterns were analyzed regarding group differences in regional connectivity and network graph properties.
Results:
Compared with controls, patients exhibited a decrease in connectivity involving ipsilateral thalamocortical regions, with a pathologic increase in ipsilateral medial temporal lobe, insular, and frontal connectivity. Among patients, those not seizure-free exhibited a higher connectivity between structures in 1) the ipsilateral medial and lateral temporal lobe, 2) the ipsilateral medial temporal and parietal lobe, and 3) the contralateral temporal pole and parietal lobe. Patients not seizure-free also exhibited lower small-worldness in the subnetwork within the ipsilateral temporal lobe, with higher subnetwork integration at the expense of segregation.
Conclusions:
MTLE is associated with network rearrangement within, but not restricted to, the temporal lobe ipsilateral to the onset of seizures. Networks involving key components of the medial temporal lobe and structures traditionally not removed during surgery may be associated with seizure control after surgical treatment of MTLE.
In patients with medial temporal lobe epilepsy (MTLE), the hippocampus is traditionally considered to be the site of seizure onset.1,2 Surgical removal of the hippocampus is usually associated with a high probability of complete seizure control.1,3 Nonetheless, histopathologic4–6 and imaging studies7–10 have consistently demonstrated extrahippocampal abnormalities in MTLE.
The clinical significance of extrahippocampal pathology in patients with MTLE remains unclear. For patients who achieve seizure control after surgery, it may represent a remote pathologic effect of seizures, without influence on epileptogenesis. For patients with surgically refractory MTLE, however, extrahippocampal pathology may be crucial to initiate and sustain seizures. Unfortunately, before surgery, patients who do not achieve seizure freedom are indistinguishable from patients who will become seizure-free. They are expected to have a good outcome, but continue to experience seizures despite technically successful surgery.
A promising theory suggests that a distinctly organized network supporting epileptogenesis involves the temporal lobe of patients with surgically refractory MTLE.11 In this study, we tested this hypothesis by comparing the structural brain connectome constructed from probabilistic white matter tractography obtained from diffusion tensor MRI of seizure-free vs not-seizure-free surgically treated patients with MTLE. We hypothesized that patients with suboptimal postsurgical results would exhibit more intricate presurgical temporal lobe connectivity.
METHODS
Subjects.
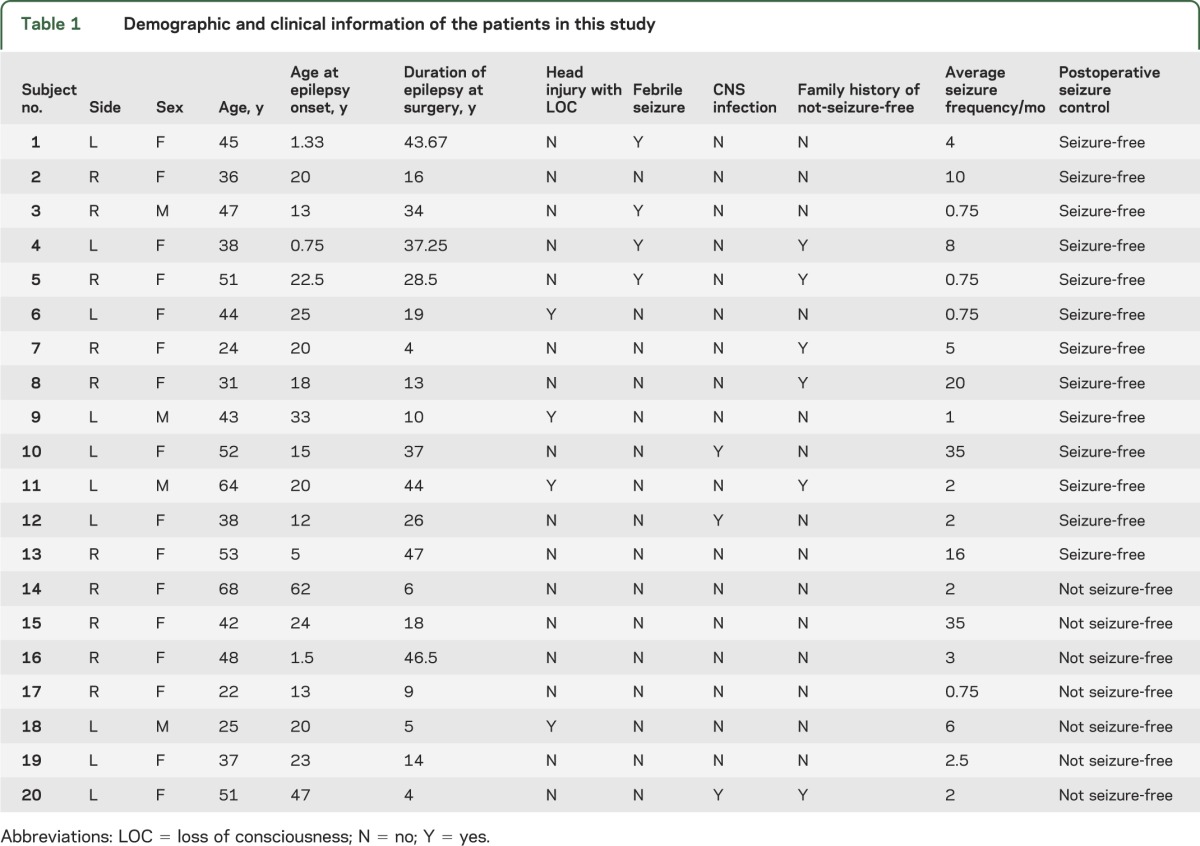
We retrospectively studied 20 consecutive patients with medication refractory MTLE treated at the Medical University of South Carolina (MUSC) (mean age 42.9 ± 12.2 years, 4 males). All patients were diagnosed with MTLE according to the criteria defined by the International League Against Epilepsy,12 after a comprehensive neurologic evaluation, ictal EEG recording of unilateral seizures localized to the temporal region, and high-resolution diagnostic MRI demonstrating unilateral hippocampal atrophy concordant with the side of ictal EEG seizure onset. All patients were refractory to antiepileptic pharmacologic treatment, and the indication for surgical treatment was achieved by consensus at the Refractory Epilepsy Conference at the MUSC. None of the patients enrolled in this study had evidence of structural abnormalities other than hippocampal atrophy on high-resolution presurgical MRI. All patients underwent anterior temporal lobectomy. None of the patients had intra- or perioperative complications, and the preoperative antiepileptic regimen was continued for all patients in the postoperative period.
Surgical outcome was defined with at least 1 year of postsurgical follow-up according to the Engel Surgical Outcome Scale.1 Patients were placed into 1 of 2 groups: 1) free of disabling seizures (i.e., seizure-free), equivalent to Engel class I; or 2) not seizure-free (Engel classes II, III, or IV). We collected presurgical information regarding 1) epilepsy risk factors (family history of epilepsy, history of head trauma with loss of consciousness, history of meningitis or encephalitis, and history of febrile seizures); 2) age at epilepsy onset; 3) duration of epilepsy; and 4) seizure frequency. The clinical and demographic information of all patients in this study is summarized in table 1. Two-sample independent-measures t test was used to compare the groups. We also studied a control group composed of 18 healthy individuals (mean age 40.5 ± 5.33 years, 8 males) recruited from the local community, with no significant medical history of neurologic or psychiatric problems.
Table 1.
Demographic and clinical information of the patients in this study
Standard protocol approvals, registrations, and patient consents.
This study was approved by the MUSC institutional review board. Written informed consent was obtained from all control subjects. Data from patients was obtained retrospectively through chart review and MRI analyses. Patient data were obtained as standard of care for medication refractory epilepsy and were reviewed under the “waived of consent” category.
Image acquisition.
All subjects underwent the same imaging protocol, performed on a Siemens 3T Verio MRI scanner (Siemens AG, Erlangen, Germany) equipped with a 12-channel head coil. The imaging protocol yielded a high-resolution T1-weighted image, with an isotropic voxel size of 1 mm (repetition time = 2,250 milliseconds, echo time = 41 milliseconds, field of view = 256 × 256 mm). Diffusion-weighted images (DWIs) were obtained using 2 diffusion weightings (b = 0 and 1,000 s/mm2) along 60 diffusion-encoding directions (repetition time = 10,600 milliseconds, echo time = 100 milliseconds, field of view = 224 × 224 mm2, parallel imaging factor of 2, slice thickness = 2 mm, and 60 axial slices).
Image processing.
DICOM-to-NIfTI format conversion and extraction of diffusion gradient directions were performed with dcm2nii (http://www.mccauslandcenter.sc.edu/mricro/mricron/dcm2nii.html). The package FMRIB Software Library's Diffusion Toolbox (FDT) (www.fmrib.ox.ac.uk/fsl) was used for preprocessing of DWIs and for diffusion tensor estimation. The DWIs underwent eddy current correction through affine transformation of each DWI to the base b = 0 T2-weighted image. FDT's DTIFIT was then used to perform voxel-wise calculation of the diffusion tensor within the brain.
Probabilistic tractography.
For each subject, we estimated the brain connectome, i.e., the whole brain structural connectivity, by performing the quantification of the number of white matter fibers connecting each possible pair of brain regions. Brain regions were individually defined according to an anatomical atlas, and white matter fibers were quantified through probabilistic distribution of fiber tractography streamlines obtained from diffusion tensor MRI. Structural connectivity was obtained by applying FDT's probabilistic method using FDT's probtrackx for fiber tracking on diffusion data after voxel-wise calculation of the diffusion tensor. We chose probabilistic tractography because it can, to some extent, resolve intravoxel fiber crossings,13,14 thus increasing the authenticity of fiber tracking. Of note, fiber tractography is dependent on MRI signal variation, subject motion, and physiologic noise, and it is also increased by a higher number of encoding directions.15
Seed regions for tractography were obtained from an automatic cortical and subcortical segmentation of T1-weighted images using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) with cortical parcellation into regions of interest (ROIs) according to the Lausanne anatomical atlas (http://www.connectome.ch), yielding 83 ROIs (42 regions in each hemisphere and one ROI corresponding to the brainstem) (table e-1 on the Neurology® Web site at www.neurology.org). The regions were then transformed into each subject's DTI space using an affine transformation obtained with FMRIB Software Library's FLIRT. Probabilistic tractography was performed using each of the 83 ROIs as the seed region.
Connectivity matrix.
The weighted connectivity between regions i and j was defined as the number of probabilistic streamlines arriving at j when i was seeded, averaged with the number of probabilistic streamlines arriving at i when j was seeded. The raw connectivity was normalized to the total volume of the originating and terminating regions. For each subject, the structural connectivity matrix “A” had 83 × 83 entries, with Aij corresponding to the weighted connectivity between structures i and j, also referred to as the link between nodes i and j.
Connectivity-based statistics.
Permutation analysis was used to evaluate regional network differences in link weight between patients vs controls, and seizure-free vs not-seizure-free patients. For every entry Aij, group labels (i.e., patients vs controls, or seizure-free vs not-seizure-free) were randomly permuted 5,000 times. For each permutation, the 2-sample t test statistic T was computed comparing one group vs another. The maximal T and minimal T values were recorded across the entire matrix A for each permutation, and a distribution of maximal and minimal T values was obtained. To account for multiple comparisons, the thresholds for statistical significance were defined as the T value in the lowest 2.5 percentiles and the T value in the highest 97.5 percentile of the minimal and maximal T value distributions, respectively.16 The T values from the original patient labels were computed and the links with a T score higher than the highest cutoff T score, or lower than the lowest cutoff T score, were considered statistically significantly.
Graph-theoretic measures.
Graph theory enables the quantification of network properties, assessing quantifiable features such as topological organization, theoretical efficiency of information flow, and node segregation.17 Graph measures for each individual connectivity matrix were calculated with the Brain Connectivity Toolbox (https://sites.google.com/a/brain-connectivity-toolbox.net/bct/). We evaluated the following global network measures: 1) average degree, 2) average clustering coefficient, 3) global efficiency, 4) average betweenness centrality, and 5) small-worldness (defined as the ratio between the average clustering coefficient and the characteristic path length of the network18). Regional network measures were also calculated for each node, corresponding to 1) degree, 2) clustering coefficient, 3) local efficiency, and 4) betweenness centrality. The interpretation of these metrics is described in detail in table e-2. Differences between groups were calculated through a 2-sample t test comparing the node metric across groups.
Subnetwork analyses.
Given that seizures in MTLE are traditionally considered to arise from the medial temporal lobe, we hypothesized that structural differences in subnetworks within the ipsilateral temporal lobe would be most likely associated with epileptogenicity and clinical outcome. We performed a subnetwork analysis evaluating differences between seizure-free vs not-seizure-free patients focusing on the ipsilateral temporal lobe subnetwork and its nodes (the regions contained in this analysis are marked with an asterisk in table e-1). Graph properties of the temporal lobe subnetwork evaluate the relationship between nodes within this subnetwork. We used 2-sample t tests to compare nodal properties between groups with p value adjustment to account for multiple comparisons.
RESULTS
Patient demographics.
Patients and controls were not different in age (p = 0.42) or sex distribution (p = 0.18). There were no differences in clinical variables between the not-seizure-free vs seizure-free patients (age: p = 0.8; age at onset: p = 0.21; duration of epilepsy: p = 0.09; seizure frequency: p = 0.45; history of head trauma: p = 0.4; history of febrile seizures: p = 0.16; history of meningitis or encephalitis: p = 0.25; family history of epilepsy: p = 0.25).
Image analysis (patients vs controls): Connectivity statistics.
Patients with MTLE exhibited a significant reduction in connectivity between the ipsilateral thalamus and the ipsilateral precentral gyrus (T = 4.99, p = 0.0001). Patients also exhibited a higher connectivity between the ipsilateral inferior parietal lobule and the ipsilateral supramarginal gyrus (T = 4.18, p = 0.0001). An exploratory analysis using a less stringent statistical threshold (p < 0.001) demonstrated an increase in connectivity in patients with MTLE involving the ipsilateral hippocampus, amygdala, insula, putamen, and middle frontal areas, with decrement in connectivity in the orbitofrontal, accumbens, and inferior parietal lobule. These results are summarized in figure e-1 and table e-3.
Image analysis (patients seizure-free vs patients not seizure-free).
Connectivity statistics.
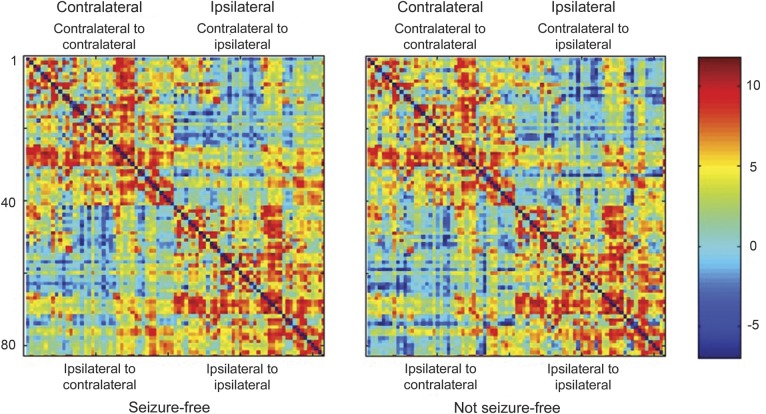
The average connectivity matrix for each patient group is displayed in figure 1. Qualitative visual inspection of the connectivity matrices suggested that not-seizure-free patients demonstrated a subtle pattern of higher connectivity within each hemisphere compared with seizure-free patients, with reduced magnitude of connectivity between hemispheres. Within hemisphere connectivity is observed in the upper left and lower right quadrants of each connectivity matrix (respectively representing contralateral-contralateral connections, and ipsilateral-ipsilateral connections). To facilitate the visualization of network patterns in each group, circular connectivity diagrams are illustrated in figure e-2, where it is possible to appreciate a subtle decrease in the number of connections between the contralateral frontal and temporal lobes, with relative sparing of connectivity in the ipsilateral temporal and frontal regions.
Figure 1. Connectivity matrices for seizure-free and not-seizure-free patients.
Average connectivity matrices for seizure-free and not-seizure-free patients. The brain structures are numbered from 1 to 83 in accordance with the labels in table e-2. Regions 1 to 42 represent the hemisphere contralateral to seizure onset, 43 to 82 represent the hemisphere ipsilateral to seizure onset, and 83 represents the brainstem. Within each hemisphere, the regions are grouped in the following order: frontal lobe, temporal lobe, basal nuclei, parietal lobe, and occipital lobe. Each entry in the matrix represents the log of the number of probabilistic streamlines between the regions in the corresponding row and column.
The T-score distribution from the comparison of the data between the not-seizure-free and seizure-free patients is displayed in figure 2. The following links exhibited a higher number of probabilistic streamlines in not-seizure-free patients in the following reciprocal connections: 1) ipsilateral entorhinal cortex and ipsilateral superior temporal gyrus (T = −4.83, p = 0.0001); 2) contralateral temporal pole and contralateral supramarginal gyrus (T = −3.92, p = 0.0010); and 3) ipsilateral parahippocampal gyrus and ipsilateral superior parietal gyrus (T = −3.89, p = 0.0011). These results are demonstrated in figure 3.
Figure 2. T-score distribution (seizure-free vs not-seizure-free patients).
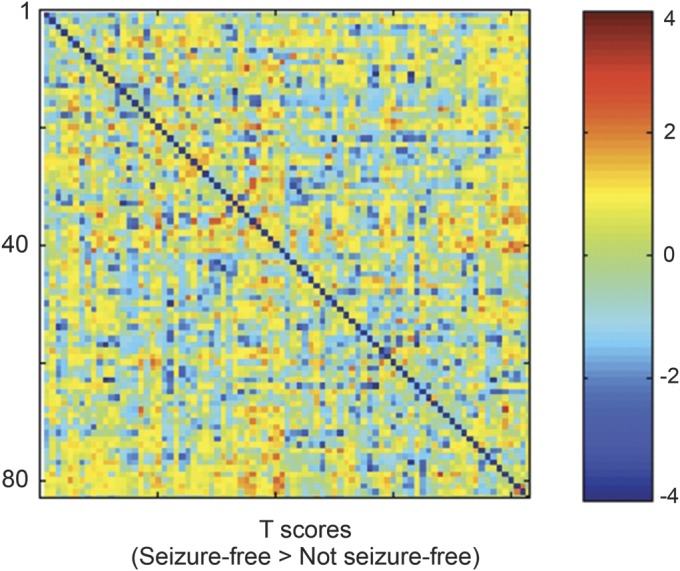
The distribution of T scores from the comparison of the weighted connectivity matrices between the seizure-free and not-seizure-free patients. Each matrix entry represents the T score from the t test comparison evaluating differences in link weight between seizure-free and not-seizure-free patients.
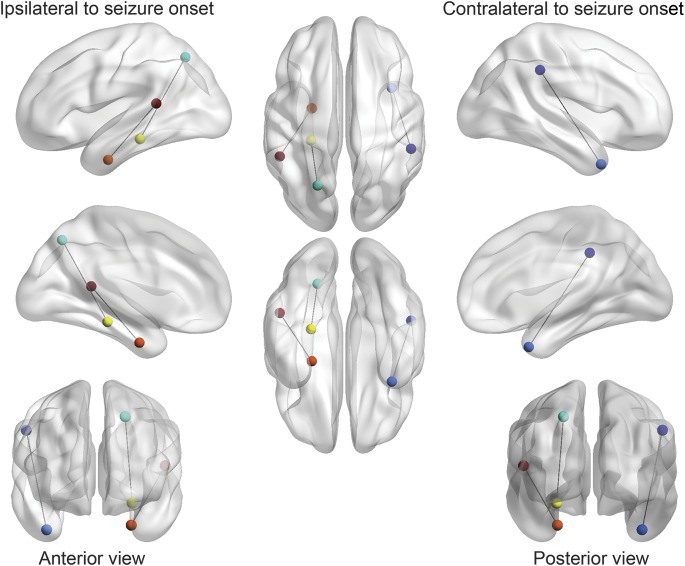
Figure 3. Connectivity differences in patients with surgically refractory medial temporal lobe epilepsy.
Patients who did not achieve seizure freedom with surgery exhibited a higher degree of connectivity between the 1) entorhinal cortex (orange) and superior temporal gyrus (red) (T = −4.83, p = 0.0001); 2) contralateral temporal pole (light blue) and contralateral supramarginal gyrus (dark blue) (T = −3.92, p = 0.0010); and 3) ipsilateral parahippocampal gyrus (yellow) and ipsilateral superior parietal gyrus (green) (T = −3.89, p = 0.0011). This figure was generated with the visualization software BrainNet Viewer.29
We did not observe a significant correlation between the 3 links described above and clinical variables such as age at onset, duration of epilepsy, or seizure frequency (when all patients were evaluated, or when only seizure-free or not-seizure-free patients were evaluated separately).
Graph-theory measures.
Despite the observed regional abnormalities, whole network graph properties were not different between the 2 patient groups.
Nodal graph properties were not significantly different between groups using the correction for multiple comparisons defined by the distribution of T scores based on permuted data. We applied an exploratory threshold of p < 0.01 and this method revealed that the ipsilateral transverse temporal region demonstrated an increase in clustering coefficient (p = 0.003, T = −3.39) and local efficiency (p = 0.009, T = −2.93) in patients who were not seizure-free. No other nodes exhibited group differences at a threshold of p < 0.01. There was no difference in global network small-worldness between seizure-free and not-seizure-free patients.
Subnetwork analyses.
We investigated a subnetwork consisting of 11 nodes within the temporal lobe ipsilateral to the side of seizure onset (highlighted in table e-1). Not-seizure-free patients exhibited a significant increase in weighted degree within the superior temporal gyrus (p = 0.012, T = 2.79) and a significant decrease in degree within the lingual gyrus (p = 0.006, T = 3.13). The subnetwork average degree, clustering coefficient, local efficiency, and betweenness centrality were not different between groups. We observed a significant decrease in small-worldness within the temporal lobe subnetwork in the not-seizure-free patients (p = 0.015). These results are shown in figure e-3.
DISCUSSION
In this study, we investigated whether patients who do not achieve seizure freedom after temporal lobectomy exhibit a different pattern of brain structural connectivity compared with patients who become free of seizures after surgery.
By evaluating the whole brain and the temporal subnetwork connectomes, we observed 4 main findings. First, patients with MTLE exhibited loss of connectivity involving the hemisphere ipsilateral to the side of seizure onset, associated with the pathologic increase in connectivity within the ipsilateral medial temporal and limbic regions. Second, comparing seizure-free vs not-seizure-free patients, there were no differences in global properties related to network integration and segregation, namely, global efficiency and clustering coefficient. Third, even though there were no global differences between patient groups, there were regional network differences mostly involving the ipsilateral and contralateral temporal lobes. Not-seizure-free patients exhibited higher connectivity between regions within the ipsilateral medial and lateral temporal lobe regions, between regions within the ipsilateral medial temporal lobe and the ipsilateral parietal lobe, and between regions within the contralateral temporal pole and parietal lobe. Fourth, not-seizure-free patients demonstrated a disruption of small-worldness in the subnetwork within the ipsilateral temporal lobe, which reflects an increase in integration at the expense of segregation within the ipsilateral temporal lobe.
These results corroborate previous findings suggesting an abnormal pattern of connectivity in patients with MTLE.19 They also indicate a distinct pattern of structural connectivity in patients with MTLE who do not achieve seizure freedom with surgery. The structural network rearrangement in patients with surgically refractory MTLE was observed more notably in, but not restricted to, the temporal lobe ipsilateral to the onset of seizures. These findings can have implications for the understanding of the mechanisms underlying seizure control and epileptogenesis in patients with partial epilepsy and MTLE. This is particularly relevant as the concept of partial epilepsy is evolving to include the role of supporting networks in epileptogenesis.11,20 The growing evidence of extrahippocampal pathology in MTLE suggests that a larger network of abnormal structures may be involved in the generation of seizures. It is not clear whether the extensive network damage in MTLE may have an impact on epileptogenesis, or may be only a consequence of the excitotoxic effect of seizures with no direct impact on seizure generation. However, the lack of seizure control in patients with MTLE who appear to be optimal candidates for surgery suggests that seizures may be originating from extrahippocampal structures. This observation is at times confirmed by intracranial EEG studies suggesting that seizures may arise from a temporal structure nearby the hippocampus in patients with failed surgery.21 A study using resting-state functional MRI also observed that the pattern of activation in patients with suboptimal surgical outcome is less localized.22 This converging evidence suggests broader networks in patients with refractory MTLE obtained by methods with different spatial and temporal resolutions.
The results from this study should be interpreted in the context of its limitations. Namely, this was a small sample cross-sectional study. Also, this study evaluated a relatively large number of female patients. As noted by previous studies, male subjects may experience a marginally better surgical outcome.23 Furthermore, some studies suggest that males experience a more “extended” seizure propagation,24 while other studies suggest that female patients more often exhibit PET hypometabolism and an ictal spread to the contralateral temporal lobe.25 Another limitation of our study is the grouping of left and right MTLE into a single group. Previous studies suggest that the side of seizure onset in relationship with the dominant hemisphere may influence connectivity and structural abnormalities in MTLE.26,27 Interpretation of the results of the study should take this factor in consideration, and further studies may address whether a presurgical pattern is dependent on the side of seizure onset.
It is possible that patients may have different subtypes of MTLE, which are yet invisible to the routine presurgical workup. The different subtypes of MTLE may be determined by the extent and location of the epileptogenic network arrangement.28 Our findings suggest that there are specific regional reconfigurations in the temporal lobe and in extratemporal regions in not-seizure-free patients.
Importantly, our observations do not prove causality between abnormal connections and surgical outcome. It is possible that abnormal connectivity may represent a consequence of surgical refractoriness and not directly support epileptogenesis. It is also noteworthy that some abnormal connections, namely, between the ipsilateral entorhinal cortex and ipsilateral superior temporal gyrus, may be partially removed during anterior temporal lobectomy. Even though these connections may be removed incompletely during surgery, the formation of an abnormal network may be of relevance to epileptogenesis. These findings warrant further investigation. In particular, this study aimed to identify a common pattern among various patients with MTLE. The causes of surgical failure in MTLE may be diverse and individually determined. The disease process may be different across various patients, and a clinically useful tool should enable the identification of individual markers pertaining to prognosis. This study suggests that abnormal networks can be identified, and it remains to be defined whether there is a typical pattern that can be recognized on an individual basis, and whether this pattern can be used for personalized surgical prediction in MTLE.
Supplementary Material
GLOSSARY
- DWI
diffusion-weighted image
- FDT
FMRIB's Diffusion Toolbox
- MTLE
medial temporal lobe epilepsy
- MUSC
Medical University of South Carolina
- ROI
region of interest
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Leonardo Bonilha: study design, data analyses and interpretation, manuscript drafting, critical revision of the manuscript for important intellectual content. Joseph A. Helpern: study design and supervision, critical revision of the manuscript for important intellectual content. Rup Sainju: data collection and preliminary analyses of clinical data. Travis Nesland: imaging data analyses and interpretation of data. Jonathan C. Edwards and Steven S. Glazier: critical revision of the manuscript for important intellectual content. Ali Tabesh: study design, manuscript drafting, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, through NIH grants UL1 RR029882 and UL1 TR000062.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–547 [DOI] [PubMed] [Google Scholar]
- 2.Engel J., Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 2001;7:340–352 [DOI] [PubMed] [Google Scholar]
- 3.Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol 1996;40:446–450 [DOI] [PubMed] [Google Scholar]
- 4.Eriksson SH, Free SL, Thom M, et al. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods 2009;181:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson SH, Thom M, Symms MR, et al. Cortical neuronal loss and hippocampal sclerosis are not detected by voxel-based morphometry in individual epilepsy surgery patients. Hum Brain Mapp 2009;30:3351–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain 2005;128:1344–1357 [DOI] [PubMed] [Google Scholar]
- 7.McDonald CR, Hagler DJ, Jr, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 2008;49:794–803 [DOI] [PubMed] [Google Scholar]
- 8.Bonilha L, Rorden C, Castellano G, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 2004;61:1379–1384 [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776–1784 [DOI] [PubMed] [Google Scholar]
- 10.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 2008;49:741–757 [DOI] [PubMed] [Google Scholar]
- 11.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43:219–227 [DOI] [PubMed] [Google Scholar]
- 12.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for Revised Classification of Epilepsies and Epileptic Syndromes. Epilepsia 1989;30:389–399 [DOI] [PubMed] [Google Scholar]
- 13.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 2007;245:367–384 [DOI] [PubMed] [Google Scholar]
- 14.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JY, Abdi H, Bakhadirov K, Diaz-Arrastia R, Devous MD., Sr A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage 2012;60:1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JA, Terashima KH, Burggren AC, et al. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc Natl Acad Sci USA 2011;108:20760–20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilha L, Nesland T, Martz GU, et al. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 2012;83:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 2012;83:1238–1248 [DOI] [PubMed] [Google Scholar]
- 21.Wennberg R, Arruda F, Quesney LF, Olivier A. Preeminence of extrahippocampal structures in the generation of mesial temporal seizures: evidence from human depth electrode recordings. Epilepsia 2002;43:716–726 [DOI] [PubMed] [Google Scholar]
- 22.Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia 2011;52:1733–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2008;49:1308–1316 [DOI] [PubMed] [Google Scholar]
- 24.Janszky J, Schulz R, Janszky I, Ebner A. Medial temporal lobe epilepsy: gender differences. J Neurol Neurosurg Psychiatry 2004;75:773–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savic I, Engel J., Jr Sex differences in patients with mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 1998;65:910–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemmotsu N, Girard HM, Bernhardt BC, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011;52:2257–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonilha L, Rorden C, Halford JJ, et al. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2007;78:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonilha L, Martz GU, Glazier SS, Edwards JC. Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 2012;53:1–6 [DOI] [PubMed] [Google Scholar]
- 29.Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS One 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.