Abstract
Objective:
To determine structural MRI and digital microscopic characteristics of REM sleep behavior disorder in individuals with low-, intermediate-, and high-likelihood dementia with Lewy bodies (DLB) at autopsy.
Methods:
Patients with autopsy-confirmed low-, intermediate-, and high-likelihood DLB, according to the probability statement recommended by the third report of the DLB Consortium, and antemortem MRI, were identified (n = 75). The clinical history was assessed for presence (n = 35) and absence (n = 40) of probable REM sleep behavior disorder (pRBD), and patients' antemortem MRIs were compared using voxel-based morphometry. Pathologic burdens of phospho-tau, β-amyloid, and α-synuclein were measured in regions associated with early neuropathologic involvement, the hippocampus and amygdala.
Results:
pRBD was present in 21 patients (60%) with high-likelihood, 12 patients (34%) with intermediate-likelihood, and 2 patients (6%) with low-likelihood DLB. Patients with pRBD were younger, more likely to be male (p ≤ 0.001), and had a more frequent neuropathologic diagnosis of diffuse (neocortical) Lewy body disease. In the hippocampus and amygdala, phospho-tau and β-amyloid burden were lower in patients with pRBD compared with those without pRBD (p < 0.01). α-Synuclein burden did not differ in the hippocampus, but trended in the amygdala. Patients without pRBD had greater atrophy of temporoparietal cortices, hippocampus, and amygdala (p < 0.001) than those with pRBD; atrophy of the hippocampus (p = 0.005) and amygdala (p = 0.02) were associated with greater phospho-tau burdens in these regions.
Conclusion:
Presence of pRBD is associated with a higher likelihood of DLB and less severe Alzheimer-related pathology in the medial temporal lobes, whereas absence of pRBD is characterized by Alzheimer-like atrophy patterns on MRI and increased phospho-tau burden.
In several autopsy series, more than half of patients with Lewy-related pathology, including Lewy bodies and Lewy neurites, have concomitant Alzheimer pathology.1–4 Thus, the third report of the dementia with Lewy bodies (DLB) Consortium criteria for neuropathologic diagnosis of DLB requires assessment of both presence and severity of Alzheimer-related pathology as well as density and distribution of Lewy-related pathology, with a final diagnosis expressed as a probability statement (low-, intermediate-, or high-likelihood) that the observed pathology would be associated with clinical features of DLB.5 Both Alzheimer- and Lewy-related cerebral pathologies are characterized by early involvement of the limbic structures,6,7 specifically the entorhinal cortex, amygdala, and hippocampus. Preserved hippocampal volumes measured on antemortem MRI have been shown to be associated with high-likelihood DLB.8,9 Conversely, hippocampal atrophy was associated with low- and intermediate-likelihood DLB. Furthermore, patients with probable REM sleep behavior disorder (pRBD) had lower Braak neurofibrillary tangle (NFT) stage and lower neuritic plaque scores compared with those without RBD.10 Because both structural MRI changes and RBD are associated with the pathologic classification of DLB, we sought to characterize the pathologic underpinnings of these associations.
Our objectives were 3-fold: first, to determine neuropathologic differences among patients with and without RBD in hippocampus and amygdala; second, to determine the structural MRI differences among patients with and without RBD; third, to determine the relationship between structural MRI abnormalities and pathologic hallmarks of Alzheimer- and Lewy-related pathologies—specifically, phospho-tau, β-amyloid, and α-synuclein burdens as assessed with state-of-the-art digital microscopic methods.
METHODS
Subjects.
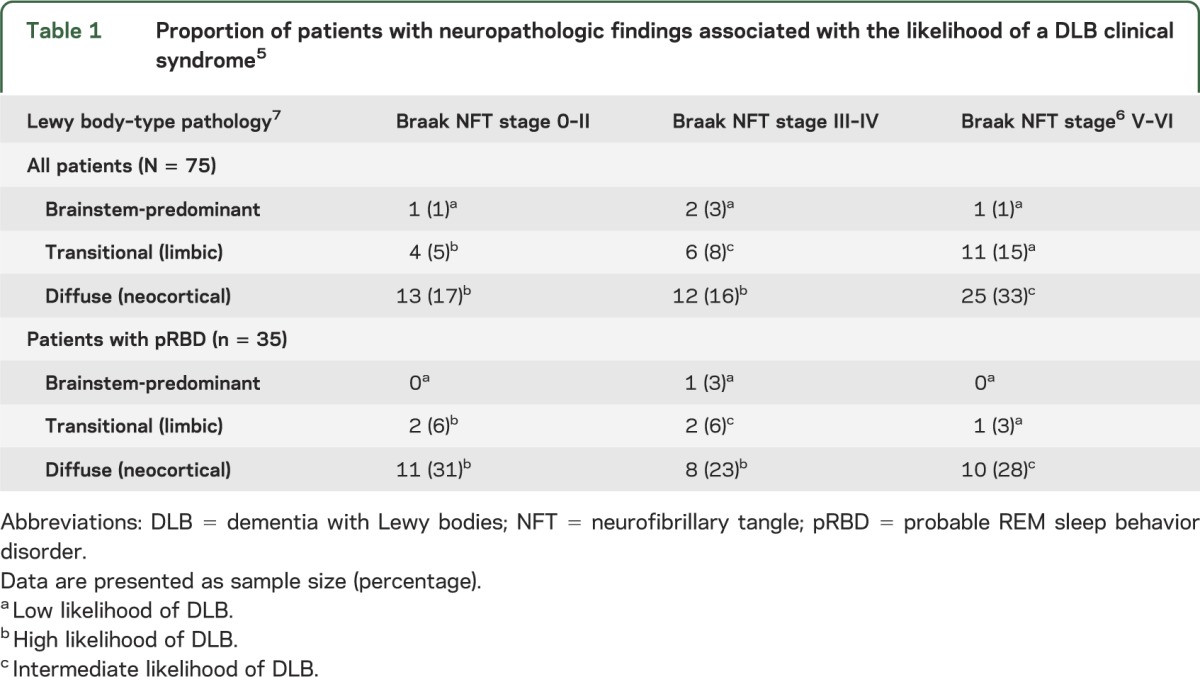
The initial study cohort included participants who had been prospectively followed at the Rochester site of the Mayo Clinic Alzheimer's Disease Research Center (dementia clinic referral–based sample) or Alzheimer's Disease Patient Registry (community-based sample). Participants who had a 1.5-tesla research MRI and had come to autopsy between August 1998 and January 2012 were considered (n = 636). Study subject's pathologic diagnosis had to include brainstem, transitional (limbic), or diffuse (neocortical) Lewy body disease,7 which was later used with Braak NFT stage6 to classify low-, intermediate-, and high-likelihood DLB according to the third report of the DLB Consortium (table 1).5 Note that subjects with Lewy-related pathology limited to the amygdala were not included, as this type of pathology does not conform to neuropathologic classification of DLB likelihood.11 Of the remaining subjects (n = 157), further exclusions were made for significant neuropathology, such as neurodegenerative tauopathies, frontotemporal lobar degeneration, and hippocampal sclerosis. Patients were also excluded if they had concurrent illness at the time of MRI, or had structural abnormalities sufficient to interfere with cognitive function (e.g., cortical infarct, tumor, and subdural hematoma). Subjects were not excluded for leukoaraiosis or lacunar infarcts. Of the remaining 78 subjects who met inclusion/exclusion criteria, clinical history was then reviewed for a history of RBD—3 subjects lacked sufficient details to determine a positive or negative history. The final study cohort included 75 autopsied individuals (45 men and 30 women; age at death 56–99 years) who met all inclusion and exclusion criteria.
Table 1.
Proportion of patients with neuropathologic findings associated with the likelihood of a DLB clinical syndrome5
Standard protocol approvals, registrations, and patient consents.
All patients or their informants/legal representatives signed consent to disclosure of clinical information, neuroimaging, and brain donation before time of death with appropriate ethical approval from the Mayo Clinic Institutional Review Board.
Neuropathologic measures.
Standardized methods for sampling and neuropathologic examination were performed according to the CERAD (Consortium to Establish a Registry for Alzheimer's Disease) and the third report of the DLB Consortium.5,12 Braak NFT stage was determined based on the distribution of NFTs assessed with Bielschowsky silver stain.6 Regional involvement of Lewy-related pathology was assessed with immunohistochemistry with a monoclonal α-synuclein antibody (appendix e-1 on the Neurology® Web site at www.neurology.org). Table 1 shows the distribution of patients according to DLB neuropathologic classification.
Serial 5-μm-thick sections of amygdala and hippocampus were analyzed using Aperio digital microscopy hardware and software (Aperio Technologies, Vista, CA). Alzheimer- and Lewy-related pathologies were immunostained and then analyzed using 3 custom-designed color deconvolution ImageScope algorithms.13 Antibody information and detailed methods on quantification are available in appendix e-1. All neuropathologic analyses were performed blinded to group status.
Neuroimaging procedures.
Antemortem MRIs were performed at 1.5 tesla (GE Healthcare, Waukesha, WI) using 3-dimensional spoiled gradient recalled acquisition. For each subject, the T1 MRI scan was spatially normalized to a custom template and segmented in SPM5 as previously described.14 RBD-positive and RBD-negative patients were compared with voxel-based morphometry (VBM) adjusting for age, sex, and time from MRI to death.15 An in-house modified Automated Anatomic Labeling atlas was used for measuring hippocampus and amygdala volumes, as previously described.14 The measured volume of amygdala and hippocampus ipsilateral to the pathologically assessed hemisphere in each individual was referenced to the total intracranial volume.
Clinical features and diagnoses.
A consensus clinical diagnosis made at the time of the MRI was assigned by a panel of neurologists, neuropsychologists, and research nurses who reviewed the patient information. The diagnosis of dementia was made based on DSM-III-R.16 The clinical diagnosis was made according to established criteria for DLB,5 but because RBD was assessed in this study, only the core features were considered and RBD was not considered as a supporting feature for the clinical diagnosis of DLB. Diagnostic criteria for Parkinson disease,17 Alzheimer disease (AD),18 and mild cognitive impairment (MCI) were also used.19
A Mayo Fluctuations Scale score ≥3 out of 4 was used to determine the presence/absence of fluctuations.19 Information regarding the presence or absence of fully formed visual hallucinations was obtained from the informant. The presence of parkinsonism was determined by neurologic examination and required 2 of the 4 cardinal features: tremor, bradykinesia, rigidity, and postural instability.
Thirty-five patients were considered to have a positive history of RBD based on informant report from the Mayo Sleep Questionnaire, a highly sensitive (98%) and specific (74%) assessment of RBD, as previously described.20 Overnight polysomnography was performed in 22 (63%) of these patients with a history of RBD with dream enactment behavior, and REM sleep without atonia was confirmed in all of them using established criteria by board-certified sleep specialists.21 Polysomnography was not routinely performed on those without a history of RBD. In this report, patients with polysomnography-confirmed or clinically pRBD are referred to as pRBD-positive, and those without a history of RBD are referred to as pRBD-negative.
Statistical analyses.
The median and interquartile ranges are reported- along with the unadjusted p values from a 2-sided, 2-sample Wilcoxon rank sum test for the continuous variables. For categorical variables, the number of subjects is reported as well as the percent and p values from a χ2 test. Pearson correlations are reported between imaging and pathology variables, adjusted for age, sex, and time from MRI scan to death. Results of multiple regression models across the entire cohort test the association between imaging and pathologic variables, again adjusted for age, sex, and time from MRI scan to death.
RESULTS
Characteristics of patients.
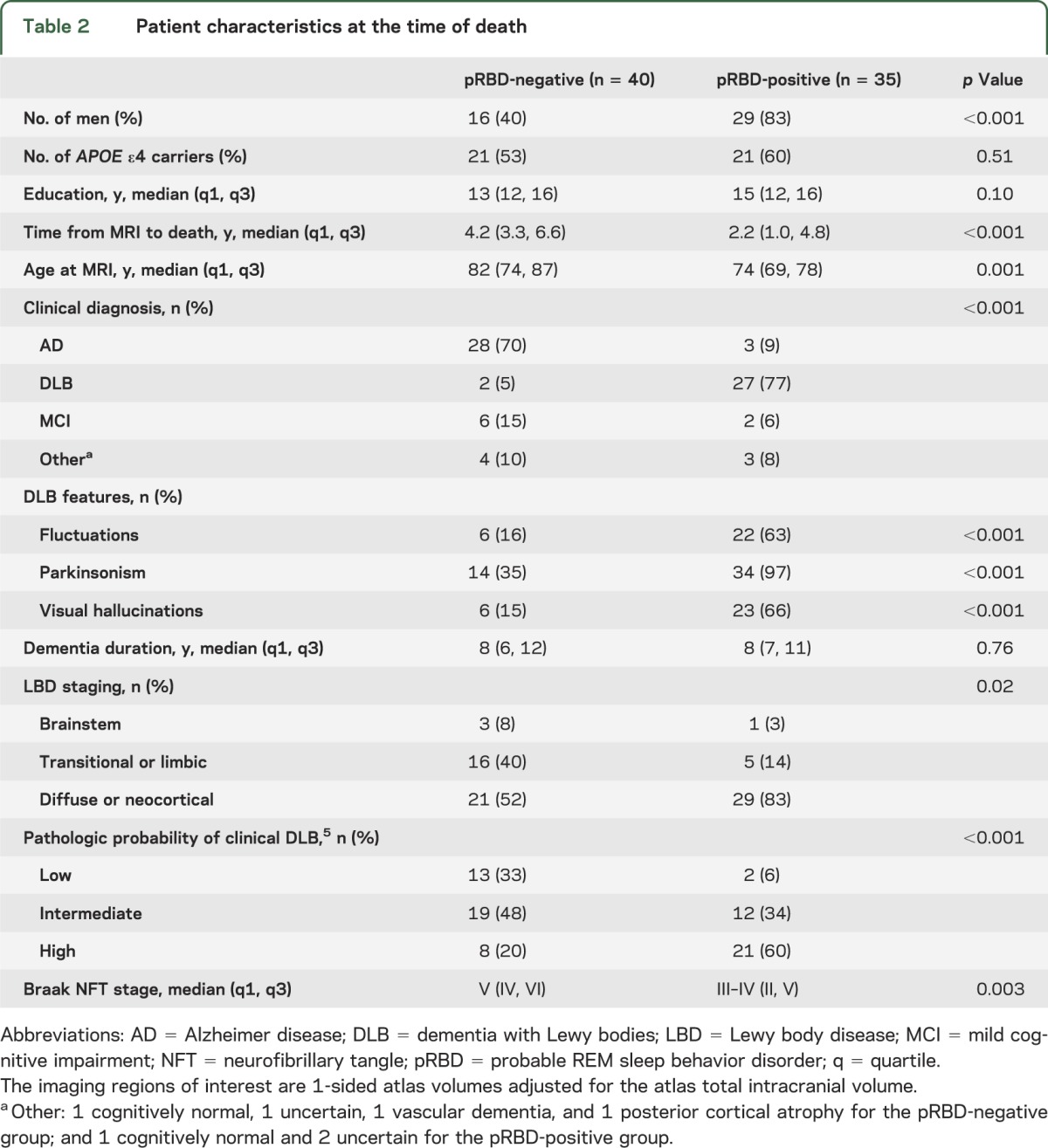
Patients were classified according to pathologic criteria from the third report of the Consortium for DLB as low-likelihood (n = 15; 20%), intermediate-likelihood (n = 31; 41%), or high-likelihood (n = 29; 39%) DLB (table 1). The demographic, clinical, and pathologic information of pRBD-negative and -positive patients are summarized in table 2. Of the 75 patients studied, 47% were pRBD-positive. Those classified as pRBD-positive were younger and were more likely to be male. Given the age and sex differences between the pRBD groups, as well as differences in the MRI-to-death interval, all further analyses were adjusted for these 3 covariates. Table 2 shows that 85% of pRBD-negative patients carried an antemortem clinical diagnosis of AD or MCI, and 80% had a low-likelihood or at most intermediate-likelihood of the clinical DLB syndrome based on neuropathology. In contrast, 89% of pRBD-positive patients carried antemortem clinical diagnoses of DLB or MCI, and 94% had intermediate- or high-likelihood DLB neuropathology. A greater proportion of pRBD-positive patients had diffuse cortical Lewy-related pathology (83%) compared with pRBD-negative patients (52%). A lower proportion of pRBD-positive patients had a neuropathologic diagnosis of AD (34%) compared with pRBD-negative patients (70%). Fluctuations, parkinsonism, and visual hallucinations were more frequent in pRBD-positive patients (table 2). Braak NFT stage was higher in pRBD-negative patients and indicative of NFT pathology in limbic regions as well as association cortices, whereas pRBD-positive patients had lower Braak NFT stage indicative of NFT pathology more restricted to limbic regions.
Table 2.
Patient characteristics at the time of death
Pathologic findings in the hippocampus and amygdala.
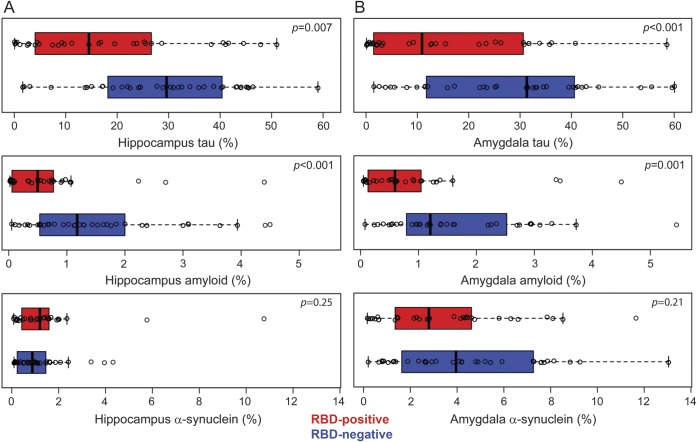
Figure e-1 shows an example of the phospho-tau, β-amyloid, and α-synuclein immunohistochemistry and their respective analyzed markup images from a hippocampal section of a pRBD-negative patient. In the hippocampus (figure 1A), median phospho-tau was higher in the pRBD-negative group than in the pRBD-positive group (29.6% vs 14.5%, p = 0.007). Similarly, β-amyloid burden was greater in the pRBD-negative compared with pRBD-positive group (1.2% vs 0.5%, p < 0.001). Hippocampal α-synuclein burden did not differentiate pRBD groups (0.9% vs 1.2%, p = 0.25). In the amygdala (figure 1B), median phospho-tau was higher in the pRBD-negative group than in the pRBD-positive group (31.3% vs 10.9%, p < 0.001), and a similar relationship, to a lesser degree, was seen with β-amyloid burden (1.2% vs 0.6%, p < 0.001). α-Synuclein burden in the amygdala did not differentiate between those with and without pRBD (4.0% vs 2.8%, p = 0.21).
Figure 1. Quantitative neuropathology differences in the hippocampus and amygdala of pRBD-negative and pRBD-positive patients.
Graphical depiction of quantitative neuropathology in the (A) hippocampus and (B) amygdala of patients with a spectrum of Alzheimer-related (hyperphosphorylated tau and β-amyloid) and Lewy-related (α-synuclein) pathology, neuropathologically classified as low- to high-likelihood dementia with Lewy bodies. A scatterplot overlays box plots that represent the interquartile range of pathology in pRBD-positive (red box) and pRBD-negative (blue box) patients. pRBD = probable REM sleep behavior disorder.
MRI findings and pathologic correlation.
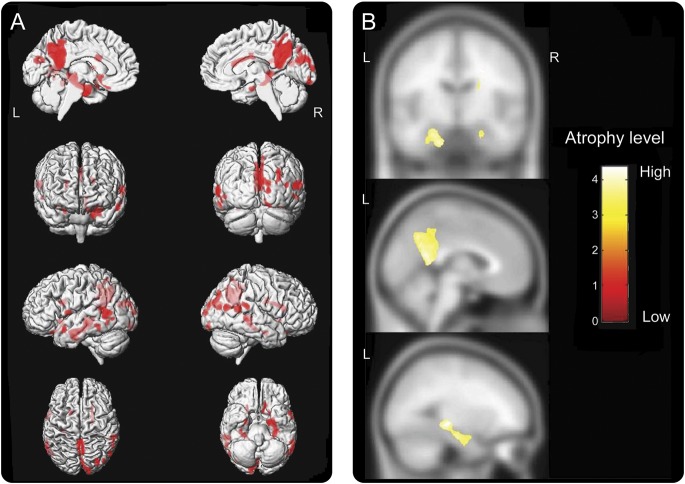
Group comparisons of structural MRI were performed using VBM analysis in patients negative and positive for pRBD using a threshold of p < 0.001 adjusting for age, sex, and time from MRI to death and correction at a cluster level of 200 voxels (figure 2). Results show greater atrophy in pRBD-negative than pRBD-positive patients in the posterior cingulate, precuneus, lateral temporoparietal, hippocampus, and amygdala. The findings disappeared when we used a threshold of 0.05 correcting for multiple comparisons using false discovery rate. We did not find greater atrophy in patients who were positive compared with negative for pRBD on VBM analysis (p < 0.001; uncorrected for multiple comparisons).
Figure 2. Neuroimaging differences between pRBD-negative and pRBD-positive patients.
The 3-dimensional surface render maps (A) and overlays from the custom template (B) demonstrated greater atrophy in the posterior cingulate, precuneus, lateral parietal and posterior temporal cortex, hippocampus, and amygdala in RBD-negative compared with RBD-positive patients (p < 0.001; corrected at cluster level of 200 voxels, adjusted for age, sex, and time from MRI to death). The posterior cingulate and precuneus atrophy appeared to be symmetric, but closer examination of the hippocampus and amygdala revealed atrophy in the midsagittal sections of the surface render maps, and the coronal section from the custom template demonstrated asymmetric left-greater-than-right atrophy. The inferior view from the maps demonstrated more pronounced uncal atrophy in the left, which is demonstrated in the sagittal view from the custom template. pRBD = probable REM sleep behavior disorder; RBD = REM sleep behavior disorder.
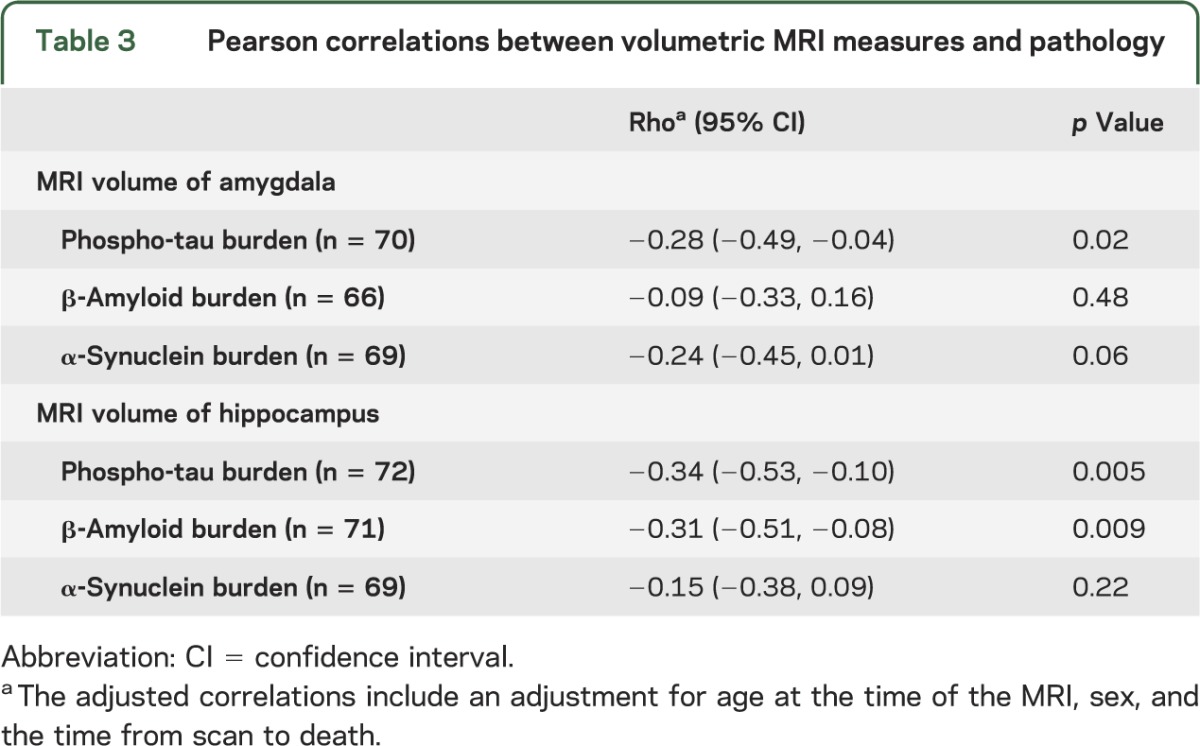
The association of MRI volumes with underlying Alzheimer- and Lewy-related pathologies was assessed in the hippocampus and amygdala by combining the cases for Pearson correlation tests in the entire group, after adjusting for age at the time of MRI, sex, and the scan-to-death interval for each correlation (table 3). There is an inverse relationship between the antemortem amygdala volume and phospho-tau burden, but not β-amyloid burden (table 3). The association between volumes of the amygdala with α-synuclein burden did not reach statistical significance (p = 0.056). Antemortem hippocampal volumes were inversely correlated with phospho-tau and β-amyloid, but not α-synuclein burdens (table 3). Because both phospho-tau and β-amyloid burdens correlated with hippocampal volume, we performed multiple regression modeling to determine the extent of this relationship, adjusting for age, sex, and scan-to-death interval. When both phospho-tau and β-amyloid burden were included in the same model, phospho-tau burden (p = 0.05) was negatively associated (regression coefficient −6 × 10−4, 95% confidence interval −1 × 10−3, −1 × 10−5) with hippocampal volumes, but β-amyloid burden was not (p = 0.11). The regression model with both phospho-tau and β-amyloid burdens, adjusting for age at the time of the MRI, sex, and scan-to-death interval, explained 25% of the variance in hippocampal volume.
Table 3.
Pearson correlations between volumetric MRI measures and pathology
DISCUSSION
We report on a subset of patients with Lewy body pathology who met pathologic criteria for low-, intermediate-, and high-likelihood DLB and who underwent detailed MRI scans during life. Consistent with the literature, pRBD-positive patients were more often male and had a younger age at death.22,23 Not surprisingly, despite the presence of Lewy body pathology, the majority of patients in the low-likelihood DLB group met criteria for a clinical diagnosis of dementia with AD, whereas those in the intermediate- and high-likelihood DLB group were mixed in whether they met clinical criteria for probable DLB according to core features. pRBD was represented in both intermediate- and high-likelihood DLB, but was not present in all patients in those classification stages. As such, this comparison of pRBD-negative and pRBD-positive individuals is more than just a comparison between DLB and AD, although these pathologies are intermixed and one goal of this study was to examine whether relationships are evident among Lewy body and AD pathologies, MRI characteristics, and pRBD. Patients who were pRBD-positive had less Alzheimer-related pathology, greater frequency of diffuse (neocortical) Lewy body disease, and lower degree of atrophy on MRI compared with those who were pRBD-negative.
Neuroimaging is recognized as a useful biomarker for the clinical diagnosis of DLB, and several neuroimaging findings have been included as suggestive and supportive features (e.g., preservation of medial temporal lobe on CT/MRI).5,9,24 Consistent MRI findings in DLB point to relative preservation of medial temporal lobe structures, especially compared with AD.8,9,25,26 Given the increased odds of autopsy-proven DLB when pRBD is considered,1 we retrospectively demonstrated that neuroimaging differences existed in an autopsy cohort with a range of Alzheimer- and Lewy-related pathologies. Previous antemortem studies investigating neuroimaging differences in incidental RBD have reported inconsistent results, describing increased hippocampal gray matter density27 and decreased parahippocampal gray matter volume compared with controls.28 Although neuronal reorganization is speculated to underlie an increase in gray matter density,27 our results support the concept of hippocampal preservation due to a lack of Alzheimer-related neuropathology. Specifically, we show that atrophy in the hippocampus and amygdala on antemortem MRI in this autopsy cohort with a range of Alzheimer- and Lewy-related pathology was associated with phospho-tau burden. Moreover, regional cortical volumes on VBM further demonstrate preservation of temporoparietal cortical volume in DLB that is typically atrophic in AD.9 While the investigations into the pathologic basis of medial temporal atrophy are limited,29 investigations into neuroimaging differences in autopsied patients with a spectrum of Lewy- and Alzheimer-related neuropathology dichotomized by their clinical history of pRBD are even less common.
Both Alzheimer-related and Lewy body–related pathologies are considered in the neuropathologic diagnosis of DLB,5 and this study evaluates imaging techniques as a marker of this underlying pathology. Regardless of Lewy body–related pathology, the Braak NFT stage correlated with the extent of antemortem hippocampal atrophy, and the pRBD-negative group demonstrated an atrophy pattern mirroring what has been described as an “AD signature” in autopsy-confirmed AD.30 Similarly, the posterior cingulate, precuneus, lateral temporoparietal cortex, hippocampus, and amygdala displayed greater atrophy in the pRBD-negative patients, a group that despite brainstem or limbic Lewy body pathology appears most dominated by Alzheimer-related pathology. This supports observations of high phospho-tau and β-amyloid burden in pRBD-negative patients, and highlights the need for assessment of both Alzheimer- and Lewy-related pathologies when predicting the DLB clinical syndrome from pathologic data.2,5 Evaluation of neuroimaging associations with intermediate-likelihood DLB patients are of interest; however, hemispheric atrophy on structural MRI is at a disadvantage in distinguishing Lewy body–related pathologies. Other imaging markers, such as striatal dopamine transporter binding on PET or SPECT should be considered in follow-up studies seeking to discriminate low-, intermediate-, and high-likelihood DLB patients in retrospective autopsy cohorts.
Our quantification methods included the area of burden occupied by the inclusions (i.e., NFT, senile plaques, and Lewy bodies), as well as the area occupied by neuritic pathology (i.e., neuropil threads and Lewy neurites). This distinguishes our methods from a previous study that excluded neuritic pathology on phospho-tau and α-synuclein immunohistochemistry in the qualitative assessment of medial temporal lobe atrophy in AD, DLB, and vascular cognitive impairment.25 That study reported higher Braak NFT stage, area of NFT, and area of senile plaques correlated with greater medial temporal lobe atrophy across the entire sample. A regression analysis of Braak NFT stage, NFTs, senile plaques, and Lewy bodies found that only Braak NFT stage significantly predicted medial temporal lobe atrophy across the entire sample. These findings point to the significant effects of NFT pathology on medial temporal lobe structures; however, inclusion of Braak NFT stage in the model could have biased the results given the high level of correlation between NFT severity in the hippocampus and Braak NFT stage.31 Regardless, the difference between this previous report and the current findings may point to the importance of considering both inclusions and neuritic pathology as the neuropathologic substrate underlying volume loss in hippocampus and amygdala. In a follow-up study utilizing quantitative neuroimaging methods to measure volumes of amygdala and hippocampus, the same methods for NFT area and plaque area were used, but both neuritic and Lewy body pathologies were quantified.29 This study found that the greater burden of Lewy-related pathology, but not phospho-tau or β-amyloid, significantly correlated with smaller volumes of amygdala. We found a trend for association between volume of amygdala and α-synuclein burden, supporting that α-synuclein burden may modify integrity of the amygdala; however, a much stronger and significant association32 was found between volume of amygdala and phospho-tau burden. Interestingly, hippocampal volume was not associated with α-synuclein burden in the current cohort. To determine the relative importance of β-amyloid and phospho-tau burdens for hippocampal volume, a multivariable regression model was used, which demonstrated that phospho-tau accumulation and not β-amyloid contributes to hippocampal volume loss.31
A limitation of this study is the time from MRI to death, averaging 4.0 years for the entire cohort. This was addressed by controlling for MRI-to-death interval as a covariate in regression models. Another limitation is the use of a lenient threshold for VBM analyses that was not corrected for multiple comparisons. An additional limitation is reliance on clinical judgment or bed-partner descriptions in patients in whom a polysomnography was not performed. Ideally, pRBD status would be confirmed in all patients (and controls) using overnight polysomnography. Unfortunately, sleep studies are costly and rarely performed in controls without abnormal sleep indications. Lastly, referral bias could be a factor for any clinical setting and the current findings need to be replicated in epidemiology samples.
The importance of Alzheimer-related pathology in DLB cannot go unappreciated, especially when imaging markers are being used for patient selection in clinical trials, and likely will be used for treatment decisions in the future.33 Patients with DLB who lack Alzheimer-like atrophy patterns on MRI and who have low cortical amyloid load on PET are more likely to cognitively respond to acetylcholinesterase inhibitor treatment compared with those with hippocampal atrophy and high cortical amyloid load on PET.33 Thus, pRBD status and medial temporal lobe atrophy on MRI may have implications for identifying patients who may benefit from treatments targeting disease-specific proteins and associated clinical syndromes.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and their families who have participated in these prospective clinical and imaging studies, and especially for the generous donation of their brain tissue to help further our knowledge in neurodegeneration. The authors acknowledge the continuous commitment and teamwork offered by Linda G. Rousseau, Virginia R. Phillips, and Monica Castanedes-Casey, and thank Kris Johnson and Jeremiah Aakre for assistance in collection of data and pathologic material.
GLOSSARY
- AD
Alzheimer disease
- DLB
dementia with Lewy bodies
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- MCI
mild cognitive impairment
- NFT
neurofibrillary tangle
- pRBD
probable REM sleep behavior disorder
- RBD
REM sleep behavior disorder
- VBM
voxel-based morphometry
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Murray and Dr. Ferman: study concept and design, acquisition of data, analysis and interpretation of the data, drafting the manuscript. Dr. Boeve: acquisition of data, critical revision of the manuscript for important intellectual content. Mr. Przybelski and Mr. Lesnick: study concept and design, analysis or interpretation of the data, critical revision of the manuscript for important intellectual content. Ms. Liesinger, Mr. Senjem, Dr. Gunter, Mr. Preboske, and Dr. Lowe: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Vemuri: acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content. Dr. Dugger: study concept and design, critical revision of the manuscript for important intellectual content. Dr. Knopman and Dr. Smith: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Parisi: acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content. Dr. Silber, Dr. Graff-Radford, and Dr. Petersen: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Jack: acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content. Dr. Dickson: study concept and design, acquisition of data, analysis and interpretation of the data, drafting the manuscript. Dr. Kantarci: study concept and design, acquisition of data, analysis and interpretation of the data, drafting the manuscript.
STUDY FUNDING
Supported by a Mayo Alzheimer Disease Research grant (P50 AG016574), Mayo Clinic Study on Aging (also known as the Alzheimer Disease Patient Registry; U01 AG006786), R01-AG040042, R01-AG015866, R01-AG011378, P50-NS72187-03, Mangurian Foundation, and the Robert H. and Clarice Smith and Abigail van Buren Alzheimer Disease Research Program.
DISCLOSURE
M. Murray is funded by P50-NS72187-03 (coinvestigator), and Robert H. and Clarice Smith and Abigail van Buren Alzheimer Disease Research Fellowship. T. Ferman is funded by R01-AG015866 (principal investigator), P50 AG016574 (coinvestigator), P50-NS72187 (coinvestigator), and Mangurian Foundation (coinvestigator). B. Boeve has served as an investigator for clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals, and GE Healthcare. He receives royalties from the publication of a book titled Behavioral Neurology of Dementia (Cambridge Medicine, 2009). He has received honoraria from the American Academy of Neurology. He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the National Institute on Aging (NIA) (P50 AG16574 [coinvestigator], U01 AG06786 [coinvestigator], RO1 AG15866 [coinvestigator], and U24 AG26395 [coinvestigator]), and the Alzheimer's Association (IIRG-05-14560 [principal investigator]). S. Przybelski, T. Lesnick, A. Liesinger, M. Senjem, J. Gunter, and G. Preboske report no disclosures. V. Lowe receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, NIA R01 DC010367-01 (coinvestigator), NIA P50 AG016574-12 Project 1 (co–principal investigator), and NIA R01 AG011378-16 (coinvestigator). P. Vemuri and B. Dugger report no disclosures. D. Knopman serves as an Associate Editor for Neurology®; serves on a data safety monitoring board for Lilly Pharmaceuticals; is an investigator in a clinical trial sponsored by Janssen Pharmaceuticals; and receives research support from the NIH (R01-AG11378 [coinvestigator], P50 AG16574 [coinvestigator], and U01 AG 006786 [coinvestigator]). G. Smith serves on the external advisory board of the University of Wisconsin Alzheimer's Disease Center (nonprofit) and is an editorial board member of The Clinical Neuropsychologist and Journal of International Neuropsychological Society. He receives research support from the National Center for Research Resources U54 RR 24150, Mayo Clinic Center for Clinical and Translational Research (coinvestigator, Chair, postdoctoral programs), Leader of Behavioral Sciences Core National Institute on Aging (co–principal investigator), AG16574 Information Transfer Core, Mayo Clinic Alzheimer's Disease Research Center (principal investigator), NIA AG15866 (coinvestigator), National Institute on Neurological Disease & Stroke Subcontract of NS6-2366-02 Tasks of Executive Function (site principal investigator). J. Parisi receives publishing royalties for Principles & Practice of Neuropathology, 2nd edition. M. Silber serves as Deputy Editor of Sleep and on the editorial board of the Journal of Clinical Sleep Medicine; receives publishing royalties for Sleep Medicine in Clinical Practice, 2nd edition (Informa Healthcare, 2010) and Atlas of Sleep Medicine (Informa Healthcare, 2010); and has received speaker honoraria from the American Academy of Neurology and the American Academy of Sleep Medicine. N. Graff-Radford serves on a scientific advisory board for Codman; serves on the editorial boards of The Neurologist and Alzheimer Disease and Therapy; has received publishing royalties from UpToDate, Inc.; and receives research support from Pfizer Inc., Janssen, Forest Laboratories, Inc., Medivation, Inc., Allon Therapeutics, Inc., and the NIH/NIA. R. Petersen serves on scientific advisory boards for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH (P50-AG16574 [principal investigator] and U01-AG06786 [principal investigator], and R01-AG11378 [coinvestigator]). C. Jack serves as a consultant for Janssen, Bristol-Myers Squibb, General Electric, and Johnson & Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from NIH grant R01-AG011378. D. Dickson is an editorial board member of American Journal of Pathology, Annals of Neurology, Parkinsonism & Related Disorders, Journal of Neuropathology and Experimental Neurology, and Brain Pathology. He is Editor-in-Chief of American Journal of Neurodegenerative Disease, and International Journal of Clinical and Experimental Pathology. He receives research support from the NIH (P50 AG016574 [coinvestigator], P50 NS072187 [principal investigator]) and CurePSP/Society for Progressive Supranuclear Palsy. K. Kantarci serves on the data safety monitoring board for Pfizer Inc., Janssen Alzheimer Immunotherapy, Takeda Global Research & Development Center, Inc.; and she is funded by the NIH (R01AG040042 [principal investigator]), Mayo Clinic Alzheimer's Disease Research Center/Project 1 P50 AG16574/P1 (principal investigator), P50 AG44170/Project 2 (principal investigator), and R01 AG11378 (coinvestigator). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011;77:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Isla T, Growdon WB, McNamara M, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology 1999;53:2003–2009 [DOI] [PubMed] [Google Scholar]
- 3.Galasko D, Hansen LA, Katzman R, et al. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol 1994;51:888–895 [DOI] [PubMed] [Google Scholar]
- 4.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204 [DOI] [PubMed] [Google Scholar]
- 5.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872 [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 7.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—a new disease? Clin Neuropathol 1984;3:185–192 [PubMed] [Google Scholar]
- 8.Kantarci K, Ferman TJ, Boeve BF, et al. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology 2012;79:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain 2007;130:708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dugger BN, Boeve BF, Murray ME, et al. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord 2012;27:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006;65:685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 13.Janocko NJ, Brodersen KA, Soto-Ortolaza AI, et al. Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 2012;124:681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarci K, Lowe VJ, Boeve BF, et al. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging 2012;33:2091–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association Work Group to Revise DSM-III. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R, 3rd ed. Washington, DC: American Psychiatric Association; 1987 [Google Scholar]
- 17.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39 [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 19.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–187 [DOI] [PubMed] [Google Scholar]
- 20.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine The International Classification of Sleep Disorders: Diagnostic and Coding Manual, 2nd ed Westchester, IL: American Academy of Sleep Medicine; 2005 [Google Scholar]
- 22.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 1998;51:363–370 [DOI] [PubMed] [Google Scholar]
- 23.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577 [DOI] [PubMed] [Google Scholar]
- 24.Berg D. Tracking of striatal degeneration in prediagnostic Parkinson's disease: first steps into a promising future. Lancet Neurol 2011;10:775–776 [DOI] [PubMed] [Google Scholar]
- 25.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 2009;132:195–203 [DOI] [PubMed] [Google Scholar]
- 26.Sabattoli F, Boccardi M, Galluzzi S, Treves A, Thompson PM, Frisoni GB. Hippocampal shape differences in dementia with Lewy bodies. Neuroimage 2008;41:699–705 [DOI] [PubMed] [Google Scholar]
- 27.Scherfler C, Frauscher B, Schocke M, et al. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol 2011;69:400–407 [DOI] [PubMed] [Google Scholar]
- 28.Hanyu H, Inoue Y, Sakurai H, et al. Voxel-based magnetic resonance imaging study of structural brain changes in patients with idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 2012;18:136–139 [DOI] [PubMed] [Google Scholar]
- 29.Burton EJ, Mukaetova-Ladinska EB, Perry RH, Jaros E, Barber R, O'Brien JT. Neuropathological correlates of volumetric MRI in autopsy-confirmed Lewy body dementia. Neurobiol Aging 2012;33:1228–1236 [DOI] [PubMed] [Google Scholar]
- 30.Vemuri P, Simon G, Kantarci K, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. Neuroimage 2011;55:522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 2008;71:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarci K, Avula R, Senjem ML, et al. Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology 2010;74:1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graff-Radford J, Boeve BF, Pedraza O, et al. Imaging and acetylcholinesterase inhibitor response in dementia with Lewy bodies. Brain 2012;135:2470–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.