Abstract
Antimony (Sb) and copper (Cu) are toxic heavy metals that are associated with a wide variety of minerals. Sb(III)-oxidizing bacteria that convert the toxic Sb(III) to the less toxic Sb(V) are potentially useful for environmental Sb bioremediation. A total of 125 culturable Sb(III)/Cu(II)-resistant bacteria from 11 different types of mining soils were isolated. Four strains identified as Arthrobacter, Acinetobacter and Janibacter exhibited notably high minimum inhibitory concentrations (MICs) for Sb(III) (>10 mM),making them the most highly Sb(III)-resistant bacteria to date. Thirty-six strains were able to oxidize Sb(III), including Pseudomonas-, Comamonas-, Acinetobacter-, Sphingopyxis-, Paracoccus- Aminobacter-, Arthrobacter-, Bacillus-, Janibacter- and Variovorax-like isolates. Canonical correspondence analysis (CCA) revealed that the soil concentrations of Sb and Cu were the most obvious environmental factors affecting the culturable bacterial population structures. Stepwise linear regression was used to create two predictive models for the correlation between soil characteristics and the bacterial Sb(III) or Cu(II) resistance. The concentrations of Sb and Cu in the soil was the significant factors affecting the bacterial Sb(III) resistance, whereas the concentrations of S and P in the soil greatly affected the bacterial Cu(II) resistance. The two stepwise linear regression models that we derived are as follows: 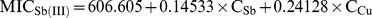 and
and 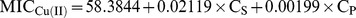 [where the MICSb(III) and MICCu(II) represent the average bacterial MIC for the metal of each soil (µM), and the CSb, CCu, CS and CP represent concentrations for Sb, Cu, S and P (mg/kg) in soil, respectively, p<0.01]. The stepwise linear regression models we developed suggest that metals as well as other soil physicochemical parameters can contribute to bacterial resistance to metals.
[where the MICSb(III) and MICCu(II) represent the average bacterial MIC for the metal of each soil (µM), and the CSb, CCu, CS and CP represent concentrations for Sb, Cu, S and P (mg/kg) in soil, respectively, p<0.01]. The stepwise linear regression models we developed suggest that metals as well as other soil physicochemical parameters can contribute to bacterial resistance to metals.
Introduction
Antimony (Sb) and Cu compounds are both considered as hazardous pollutants by the US Environmental Protection Agency (1979) and the Council of the European Communities (1976). The maximum concentrations permissible of Sb and Cu in drinking water have been set by the World Health Organization at 5 µg/L and 10 µg/L, respectively [1]. Sb is a chalcophile element that is commonly found in Cu, coal, Ag and Pd ores [2]. China is the largest producer of Sb and Cu. Over 80% of the world's supply of Sb comes from the Xikuangshan antimony mine located in Hunan province of southwestern China, and the Daye copper mines in the Hubei province produce large quantities of copper (Fig. S1). Sb and Cu contaminations of soil and water are serious concerns in these locations [3]–[4].
Copper (Cu) is an essential element and exists mainly as Cu(I) and Cu(II) in nature [5]. An excess of Cu is toxic and can cause a whole range of health concerns (http://www.ncbi.nlm.nih.gov/pubmed/17454552). In bacteria, Cu may undergo redox changes between Cu(I) and Cu(II), however, the redox change makes copper very dangerous for cells due to the involvement of Fenton-like reactions creating reactive oxygen species (ROS) [6], [7], [8]. Some bacteria have evaluated various Cu detoxification strategies that were mainly mediated by extrusion of Cu(I)/(II) out of the cells and by preventing cellular damage with Cu chaperones [8].
Sb has some chemical and toxicological properties in common with arsenic (As) [9], but Sb resistance in bacteria is much less studied compared to As resistance [10], [11]. The toxicity of Sb is dependent upon its chemical species: elemental Sb is more toxic than its salts, and inorganic species are more toxic than the organic species. Animal studies have shown that Sb trioxide is carcinogenic [2]. In soil and water systems, Sb exists mainly in the antimonite Sb(III) and the Sb(V) forms, and Sb(III) compounds are much more toxic than Sb(V), which is an important consideration for environmental Sb bioremediation [12], [13]. Biotransformation of metals by microorganisms is a useful strategy for bioremediation [14], [15]. Microorganisms can use Sb in oxidation and reduction reactions that play important roles in the biogeochemical cycle of antimony [16]. Heavy metal-resistant bacteria have also been used in bioremediation strategies to adsorb metals, or in combination with plants for phytoremediation of contaminated soil [17].
So far, the knowledge of microbial Sb mechanism is still very limited including the strategies of Sb uptake, efflux, methylation, oxidation and reduction [16]. In Escherichia coli, disruption of the glycerol transporter gene glpF reduced the uptake amount of Sb(III), clearly demonstrating the role of GlpF in Sb(III) uptake [18]. In Saccharomyces cerevisiae, a protein known as Fsp1 that is homologous to GlpF plays a similar role in the uptake of Sb(III). Sb(V) and Sb(III) enter cells by different routes, and the entrance mechanism for Sb(V) remains unknown [19]. Three families of transporters are associated with Sb(III) efflux: the ArsB protein, the Acr3p family and the ABC transporter superfamily [16]. In addition, biomethylation of Sb(III) has been documented for bacteria and fungi, in which the enzyme S-adenosylmethionine methyltransferase appeared to play a pivotal role [20], [21]. To the best of our knowledge, only a small number of Sb(III)-oxidizing bacteria have been reported to date, including Stibiobacter senarmontii [22], Agrobacterium tumefaciens 5A [23], six strains identified by our group (Acinetobacter sp. JL7, Comamonas spp. JL25, JL40, and S44, Stenotrophomonas sp. JL9 and Variovorax sp. JL23) [13], and two strains (Pseudomonas sp. S1 and Stenotrophomonas sp. A3) identified recently by Hamamura et al. [24]. Strain 5A can oxidize both As(III) and Sb(III); however, a strain harboring a mutated version of the As(III) oxidase gene aioAB, which is required for the oxidation of As(III), still exhibited Sb(III) oxidation ability [23]. An As(III)-oxidizing Sinorhizobium isolate possessing the aerobic arsenite oxidase gene (aioA) did not show any Sb(III) oxidation phynotype [24]. The results suggested that bacterial Sb(III) oxidation is catalyzed by a pathway different from the As(III) oxidation pathway catalyzed by Aio [23], [24].
Stepwise linear regression analysis is a statistical technique used to investigate and model the relationships between variables from multi-factor data. This analysis produces an equation that expresses the relationship between a variable of interest and a set of related predictor variables, following a conceptually logical process [25]. It is a method of regressing multiple variables while simultaneously removing unimportant variables. The stepwise linear regression essentially involves performing a standard regression analysis multiple times, each time removing the most weakly correlated variable. In the end, the only remaining variables are those that explain the distribution best [25]. The stepwise linear regression is widely used in plant and food sciences [26], but is less common in microbiological analyses [27].
Most mining areas are heavily polluted by different metals, including Sb and Cu. Therefore, the objectives of this study were to isolate Sb(III)-resistant, Cu(II)-resistant and Sb(III)-oxidizing bacterial strains from soils in different mining areas of China and to analyze the correlations among soil characteristics, bacterial resistance levels and environmental factors. Understanding the correlation between bacterial resistance and environmental factors is important for successful bioremediation of metal contamination.
Materials and Methods
Site description and soil sample collection
Eleven soil samples (LS, LH, JC, DF, DC, DN, DS, DA, TF, TM and TC) representing different types of mines (Sb, coal, Cu, Au, Fe and Sn) in China were collected from 2010–2011 (Fig. S1). No specific permissions were required for these location/activities. In addition, the field studies did not involve endangered or protected species.
For each soil sample, one portion was stored at 4°C for bacterial isolation, and another portion was dried and sieved through a 2 mm screen, to determine the main soil properties and total soil antimony content (Table 1). To measure the total Sb and As contents in the soil, we used an approach that combines HPLC with hydride-generation atomic fluorescence spectroscopy (HPLC-HG-AFS) (Beijing Titan Instruments Co., Ltd., China). The Sb and As contents of the soils were extracted as described in Okkenhaug et al. [28], and Johnston and Barnard [29], respectively. The physical and chemical features of the soil samples, including organic matter (O-M), S, N, P, NO3 −, Cu, Fe, and pH, were analyzed as described previously by Liao et al. [30].
Table 1. Soil characteristics of the 11 different mining soils.
| Soil | Soil texture/mine type | pH | O-M (g/kg) | S (g/kg) | N (g/kg) | P (g/kg) | Fe (g/kg) | Cu (mg/kg) | NO3 − (mg/kg) | As (mg/kg) | Sb (mg/kg) |
| LS | Sandy loam/Sb soil | 7.9±0.03 | 39.34±0.65 | 0.27±0.03 | 1.91±0.06 | 0.14±0.02 | 0.55±0.03 | 5.89±0.11 | 0.85±0.06 | 87.23±3.60 | 475.02±11.07 |
| LH | Sandy loam/Sb mine | 7.3±0.17 | 43.00±0.76 | 0.09±0.02 | 0.05±0.01 | 0.81±0.07 | 20.62±0.74 | 47.00±2.86 | 6.22±0.49 | 0.11±0.01 | 36425.25±141.1 |
| JC | Sandy loam/Coal mine | 7.2±0.11 | 303.04±4.49 | 0.18±0.01 | 3.82±0.69 | 0.63±0.01 | 17.96±0.58 | 91.67±2.90 | 48.18±0.27 | 7.45±1.06 | 11.84±0.93 |
| DF | Sandy loam/Fe mine | 7.4±0.21 | 14.62±0.19 | 0.43±0.02 | 0.39±0.03 | 1.57±0.02 | 282.46±0.85 | 5887.50±21.61 | 51.29±0.40 | 2.96±1.28 | 40.48±2.19 |
| DC | Sandy loam/Cu mine | 8.1±0.05 | 18.21±0.10 | 0.26±0.02 | 0.11±0.01 | 0.39±0.05 | 179.73±1.05 | 7455.98±25.46 | 18.65±2.01 | 4.96±0.09 | 21.94±3.30 |
| DN | Sandy loam/Sn soil | 8.2±0.11 | 36.98±3.95 | 0.79±0.03 | 0.53±0.03 | 29.27±0.23 | 79.47±9.67 | 5183.25±9.17 | 39.53±0.58 | 279.44±35.37 | 89.25±7.81 |
| DS | Sandy/Cu-Fe mine | 7.9±0.48 | 36.57±1.67 | 4.78±0.27 | 0.17±0.01 | 0.34±0.03 | 17.79±0.21 | 5072.85±2.73 | 4.55±0.47 | 0.42±0.02 | 4.47±1.85 |
| DA | Sandy/Gold mine | 8.4±0.10 | 12.82±0.55 | 0.14±0.02 | 0.30±0.01 | 1.03±0.03 | 18.06±3.95 | 2722.34±4.92 | 39.53±0.78 | 5.99±0.64 | 3.33±0.07 |
| TF | Sandy loam/Fe mine | 8.2±0.07 | 41.82±0.79 | 0.30±0.02 | 0.50±0.05 | 0.01±0.01 | 30.65±2.79 | 34.60±1.58 | 1.41±0.17 | 6.25±0.08 | 1.47±0.07 |
| TM | Sandy loam/Mn mine | 7.8±0.24 | 38.61±1.02 | 0.34±0.02 | 0.83±0.02 | 1.50±0.17 | 29.13±2.22 | 38.40±0.78 | 1.41±0.33 | 32.26±1.77 | 6.12±0.39 |
| TC | Sandy/Coal mine | 7.5±0.04 | 47.44±6.93 | 1.60±0.21 | 2.21±0.21 | 0.41±0.01 | 20.68±0.61 | 42.33±0.92 | 37.83±2.33 | 9.15±0.22 | 5.37±0.06 |
The LS etc. are the soil names shown in Figure S1;pH, 1:2.5 soil-H2O suspension;O–M, organic matter concentration;N: nitrogen concentration;±, Standard deviation (SD) calculated based on triplicates.
Isolation and identification of Sb(III)/Cu(II)-resistant and Sb(III)-oxidizing bacteria
One hundred gram of soil were supplemented with antimony potassium tartrate (C8H4K2O12Sb2·3H2O) to a final concentration of 10 mg/kg and incubated at 28°C. After one week, 1 g samples of the enriched soils (in triplicate) were added to 9 mL 0.85% sterilized NaCl solution and shaken at 180 r/min for 30 min. The mixture was then serially diluted and plated on chemically defined medium (CDM) plates (For 1 L: MgSO4·7H2O, 2.0 g; NH4Cl, 1.0 g; Na2SO4, 1.0 g; K2HPO4, 0.013 g; CaCl2·2H2O, 0.067 g; Na-lactate, 5.0 g; Fe2SO4·7H2O, 0.033 g; NaHCO3, 0.798 g and agar, 15.0 g; pH 7.2) [31] containing 25 µM C8H4K2O12Sb2·3H2O. The plates were incubated at 28°C for another week. Single colonies were obtained and each Sb(III)-resistant colony was purified repeatedly to obtain pure isolates.
For the Sb(III) oxidation test, each strain was inoculated into 5 mL liquid CDM medium containing 25 µM C8H4K2O12Sb2·3H2O and incubated at 28°C with 180 r/min shaking for 7 d, and the quantity of Sb(V) and Sb(III) was measured using HPLC-HG-AFS [32]. The parameters and conditions for the HPLC-HG-AFS analysis are shown in supplementary material (Table S1). A total of 36 Sb(III)-oxidizing bacteria were found, and 4 strains showing high Sb(III)-oxidizing efficiency were each inoculated into 100 mL liquid CDM medium each (for strains LH3, LH11, DS8 and DA6), incubated at 28°C and shaken at 180 r/min. When the OD600 value reached approximately 0.2, the CDM medium was supplemented with 10 µM C8H4K2O12Sb2·3H2O. Every 4 h, 2 mL samples were taken and analyzed for OD600 value and the growth of the strains. At the same time, 1 mL cultures were centrifuged, filtered through 0.2 mm filter membranes, and diluted 100 times with sterile ddH2O, and the concentration of Sb(III) or Sb(V) was measured using the HPLC-HG-AFS. The known Sb(III)-oxidizing bacterium A. tumefaciens 5A [23] was used as a positive control.
Total DNA from each strain was extracted using standard molecular methods. The nearly full-length 16S rDNA was amplified by PCR using the 16S rRNA gene universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [13]. Colony morphology and 16S rDNA PCR-RFLP were used to eliminate repeated isolates from each sample, as described previously [11].
DNA sequencing and phylogenetic analysis
DNA sequencing was performed using the ABI 3730XL DNA analyzer from Sunbiotech (Beijing, China). All sequences were compared with the sequences available in NCBI GenBank using a BLASTN search. Multiple alignments were performed using the CLUSTAL_X program [33]. Phylogenetic analysis was performed in PHYML online web server using the maximum-likelihood method [34] with bootstrap analyses based on 1,000 replications. Some reference sequences from the GenBank were used in the construction of the phylogenetic tree for the sake of clarity. The phylogenetic tree was viewed with MEGA 4.0 [35].
Determination of the bacterial minimal inhibitory concentrations (MICs) for Sb(III), Sb(V) and Cu(II)
The MIC, defined as the lowest concentration of Sb(III), Sb(V) or Cu(II) that inhibited bacterial growth was determined. A portion of each single colony was inoculated into CDM broth containing increasing concentrations of C8H4K2O12Sb2·3H2O, C12H19Na3O18Sb2·9H2O and CuSO4·5H2O for Sb(III), Sb(V) and Cu(II), respectively, and the cultures were incubated at 28°C for 7 d.
Statistical analyses
Stepwise linear regression was used to investigate the impact of physicochemical soil characteristics on the average MICs of the Sb(III)/Cu(II)-resistant bacteria from each soil sample, using the analytical software Statistix 8.0 [36]. Stepwise linear regression built a regression model by iteratively including or eliminating items from a list of independent variables. The average MIC of each soil was calculated as “average MIC of each soil = the total MICs of all bacteria of each soil/bacterial members of each soil”. All the environmental factors (Table 1) and the bacterial MIC values (Table S2) were used to generate the stepwise linear regression models. Variables whose p-values are bigger than 0.05 would be eliminated (not significant), while the variables whose p-values are less than 0.05 would be involved in construction of the models. The p-value ≤0.01 is considered as extremely significant. In statistics, the variance inflation factor (VIF) quantifies the severity of multicollinearity in an ordinary least squares regression analysis and provides an index that measures how much the variance of an estimated regression coefficient is increased because of collinearity [36]. Canonical correspondence analysis (CCA) was performed to analysis the correlation between the soil characters and the culturable microbial population structures using the Canoco program for Windows 4.5 (Biometris, Wageningen, Netherlands). CCA is an eigenvalue ordination technique designed for analysis of the relationships between multivariate ecological data which integrates regression and ordination techniques [37]. The 16S rDNA-based sequence similarity was selected to differentiate bacterial population structures [38], [39]
Nucleotide sequence accession numbers
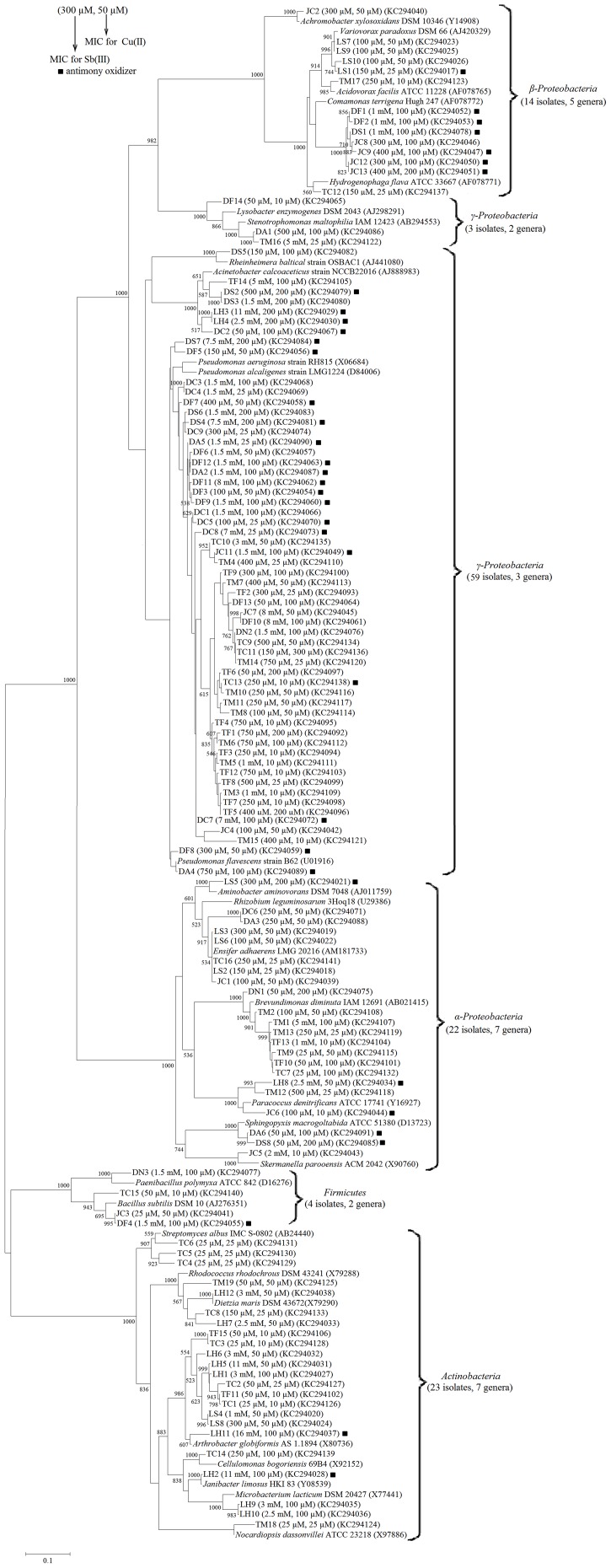
The NCBI GenBank accession numbers for the 16S rRNA gene sequences of the 125 metal-resistant bacteria are shown in Fig. 1.
Figure 1. A maximum-likelihood phylogenetic tree on the basis of 16S rRNA gene sequences.
The numbers at the nodes indicate bootstrap values (>500) based on 1000 replicates. Bar 0.1, 10 substitutions per 100 nucleotides. The Sb(III)/Cu(II)-resistant bacteria (including Sb(III)-oxidizing bacteria) were isolated using CDM medium as described in Material and Methods. ▪ represents the Sb(III)-oxidizing bacteria. The MIC values for Sb(III) and Cu(II), and the nucleotide accession numbers are shown after each strain's name.
Results
Characterization of soil samples
Eleven mining soil samples representing different types of mines in China were used in this study (supplementary materials, Fig. S1). Several physicochemical soil parameters that potentially influence Sb or Cu metabolism were analyzed. The total Sb concentration was significantly higher in the LH soil (from the Lengshuijiang high Sb content mine) than the other ten soils (LS-TC). The five soils from metal (gold, copper and iron) mines in Daye City (DF, DC, DN, DS and DA) showed significantly higher Cu concentrations compared to the other soil samples. The two coal mine soils (JC and TC) had high organic matter (O–M) and total nitrogen (N) concentrations (Table 1).
Distribution and diversity of Sb(III)/Cu(II)-resistant bacteria in soils from different mining areas
Analysis of phylogenetic diversity was performed for the bacterial species isolated from the 11 soil samples. Based on colony morphology and 16S rDNA-RFLP analysis, a total of 125 Sb(III)/Cu(II)-resistant bacterial strains were obtained. Overall, 10 (LS1–LS10), 12 (LH1–LH12), 13 (JC1–JC13), 14 (DF1–DF14), 9 (DC1–DC9), 3 (DN1–DN3), 8 (DS1–DS8), 6 (DA1–DA6), 15 (TF1–TF15), 19 (TM1–TM19) and 16 (TC1–TC16) strains were isolated from the LS, LH, JC, DF, DC, DN, DS, DA, TF, TM and TC soils, respectively.
The nearly full-length 16S rDNA sequences of the 125 strains were compared to the GenBank sequences. Fifteen strains showed 100% 16S rDNA sequence identity to at least one available 16S rDNA sequences, 103 strains showed 99%, 4 showed 98% and 3 showed 97% identities (Paracoccus spp. JC6, TH8 and TM12). Phylogenetic analysis identified the 125 strains into 27 genera belonging to five major bacterial lineages: α-Proteobacteria (22 strains, 7 genera), β-Proteobacteria (14 strains, 5 genera), γ-Proteobacteria (63 strains, 5 genera), Actinobacteria (23 strains, 7 genera) and Firmicutes (4 strains, 2 genera) (Fig. 1). Most of the Sb(III)/Cu(II)-resistant isolates were identified as Pseudomonas spp. (52/125 = 42%), which were found in all of the soils except for the LS and LH antimony mine soils (Fig. 1; Fig. S2A). Other major Sb(III)/Cu(II)-resistant bacteria were identified as Arthrobacter (9%), Comamonas (6%), Brevundimonas (6%), Acinetobacter (5%), Ensifer (4%) and Variovorax (3%). A number of identified isolates appeared to be specific for a given sampling site: isolates identified as Aminobacter and Variovorax were only found in LS soil; isolates identified as Dietzia, Janibacter, and Microbacterium were specific for LH soil; isolates identified as Acidovorax and Nocardiopsis were specific for TM soil; isolates identified as Achromobacter and Skermanella were specific for JC soil; isolates identified as Cellulomonas, Hydrogenophaga and Streptomyces were specific for TC soil; and isolates identified as Lysobacter, Paenibacillus and Rheinheimera were exclusively found in the Daye area (DF, DN and DS soils) (Fig. 1).
The majority of the Sb(III)/Cu(II)-resistant isolates belonged to the γ-Proteobacteria (mainly Pseudomonas), α-Proteobacteria (mainly Brevundimonas) and Actinobacteria (mainly Arthrobacter) classes. Bacteria from other phylogenetic groups, such as Firmicutes and β-Proteobacteria, were also found, but they were in the minority (Fig. 1; Fig. S2B). Actinobacteria was the dominant class in the high Sb-content soil from the LH site. α-Proteobacteria and β-Proteobacteria were the major classes in the LS soil. γ-Proteobacteria was the dominant class in the rest of the soils (JC-TC). γ-Proteobacteria and α-Proteobacteria were found in most of the soil samples. The TC soil sample showed the greatest bacterial diversity, yielding isolates from all five bacterial classes (Fig. S2B).
The bacterial Sb(III)/Cu(II) resistance levels
Using CDM medium, we determined the MIC for Sb(III) in each of the 125 isolated strains. The MICs ranged from 25 µM to 16 mM (Fig. 1, Table S2). Among the seven dominant genera of the Sb(III)-resistant bacteria, Acinetobacter showed the highest average MIC for Sb(III) (3.43 mM, SD = 4.12, n = 6). The other six genera showed different average MIC for Sb(III), in decreasing order from Arthrobacter (3.14 mM, SD = 5.37, n = 11) to Pseudomonas (1.65 mM, SD = 2.43, n = 52), Brevundimonas (0.81 mM, SD = 1.72, n = 8), Comamonas (0.63 mM, SD = 0.35, n = 7), Ensifer (0.18 mM, SD = 0.09, n = 5) and Variovorax (0.11 mM, SD = 0.03, n = 4). In addition, the MIC for Sb(V) was higher than 10 mM in all of the strains, indicating that Sb(V) is much less toxic than Sb(III) for these microorganisms (data not shown).
Certain correlations were found among the Sb(III) resistant levels, the bacterial species and the Sb concentrations of the soils: (1) Among the tested strains, Arthrobacter spp. LH11 and LH5, Acinetobacter sp. LH3 and Janibacter sp. LH2, whose MICs exceeded 10 mM, were all obtained from the LH soil, which was collected from the Lengshuijiang high Sb content mine; (2) The strains isolated from the LH soil had significantly higher average MIC (average MIC = 5.90 mM, SD = 4.86, n = 12) than the other ten soil samples (LS-TC, average MIC = 1.10 mM, SD = 1.95, n = 113); (3) The nine Sb(III)-resistant bacteria with the lowest MIC (25 µM) were all obtained from the three low Sb-content soil samples (TC, TM and JC) (Arthrobacter spp. TC1 and TC3, Streptomyces spp. TC4, TC5 and TC6, Brevundimonas spp. TM9 and TC7, Nocardiopsis sp. TM18, and Bacillus sp. JC3); (4) The MICs of different strains of the same genus varied greatly. For example, Arthrobacter sp. LH11 (16 mM) showed the highest resistance to Sb(III), but Arthrobacter spp. TC1 and TC3 (25 µM) showed the lowest MICs. In addition, the MIC of Brevundimonas sp. TM1 was 5 mM, but the MIC of Brevundimonas sp. TC7 was 25 µM.
The MIC for Cu(II) was also examined in all of the strains using the same method. The MICs for Cu(II) ranged from 10 to 300 µM, which were, in general, much lower than the MICs for Sb(III) (Fig. 1, Table S2).
Identification of Sb(III)-oxidizing bacteria
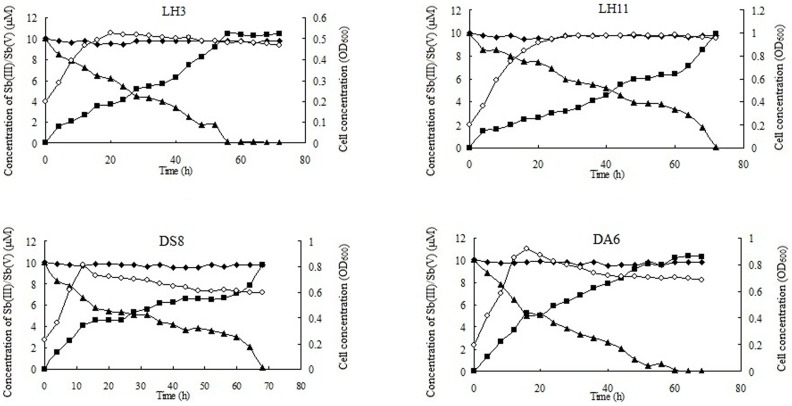
Out of the 125 Sb(III)/Cu(II)-resistant strains tested, a total of 36 strains showed Sb(III) oxidation ability, including strains identified as 17 Pseudomonas, 6 Comamonas, 4 Acinetobacter, 2 Sphingopyxis, and 2 Paracoccus strains, and one strain each of Variovorax, Aminobacter, Bacillus, Janibacter and Arthrobacter (Fig. 1). Pseudomonas (47%), Comamonas (17%) and Acinetobacter (11%) were the 3 major genera and Pseudomonas was the most dominant genus of the Sb(III)-oxidizing bacteria. Four of the strains (identified as Pseudomonas spp. DF3, DF9 and DF12 and Sphingopyxis sp. DA6) showed both Sb(III) and As(III) oxidation. Four strains (identified as Acinetobacter sp. LH3, Arthrobacter sp. LH11, and Sphingopyxis spp. DA6 and DS8) showing high Sb(III) oxidation efficiency were analyzed in detail (Fig. 2). Strain LH3 showed the highest Sb(III) oxidation efficiency (11.9 µM/h•g). The other three strains showed different Sb(III) oxidation rates, decreasing in order from DA6 to DS8 to LH11. No obvious Sb(III) oxidation was observed in the controls without bacterial inoculation (Fig. 2). Among the four strains, LH11 showed the highest resistance to Sb(III) (MIC = 16 mM), but the lowest Sb(III) oxidation rate. Strains DS8 and DA6 showed very low MICs for Sb(III) (both were 50 µM), but higher Sb(III) oxidation rates than LH11 (Fig. 2). There did not appear to be a positive correlation between the Sb(III) oxidation efficiency and Sb(III) resistance level.
Figure 2. The growth and Sb(III) oxidation curves of the Sb(III)-oxidizing strains identified as Acinetobacter sp. LH3, Arthrobacter sp. LH11, and Sphingopyxis spp. DA6 and DS8.
The concentrations of Sb(III) or Sb(V) were analyzed using HPLC-HG-AFS as described in the Material and Methods. ▪, concentration of Sb(V). ▴, concentration of Sb(III). ○, cell concentration (OD600). ⧫, concentration of Sb(III) in the controls without bacterial inoculation. The data shown are the representative of three independent experiments.
Correlations between the culturable bacterial population structure and the environmental factors
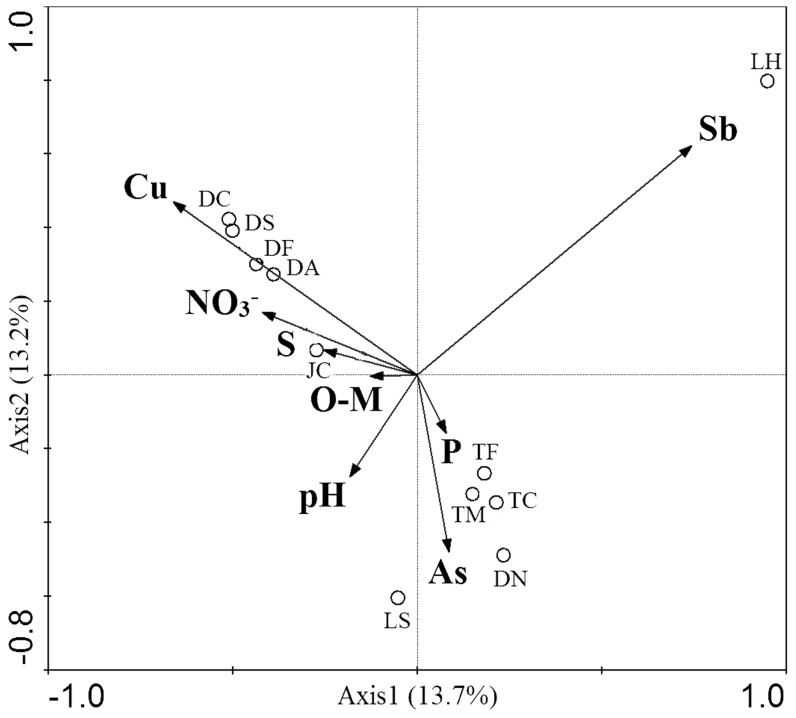
The ten environmental variables (Sb, Cu, As, Fe, N, NO3 −, S, P, pH and O-M, Table 1) were analyzed by canonical correspondence analysis (CCA) for correlation with the bacterial population structure. The CCA biplot revealed significant correlations between population structure and eight environmental factors including Sb, Cu, As, NO3 −, S, P, pH and O–M (p-value = 0.03, variable 8; F-ratio = 1.39; number of permutations = 999). The total canonical eigenvalue was 6.993. The first canonical axis represented 13.7% of the population structure detected, and the second axis showed 13.2% variance. A total variation of 26.9% was found (Fig. 3). The population structure of the bacteria from the high Sb content LH soil was quite distinct from the other soil samples, and correlated positively with Sb concentration and negatively with pH (Fig. 3). The DF, DC, DS and DA soils clustered together, showing that the four soil samples shared a similar culturable bacterial population structure, which correlated positively with Cu, NO3 − and S concentrations, demonstrating that the amount of Cu is an important factor affecting the microbial population structure in the four soils. The DN, TF, TM and TC sites clustered together and spread along the second axis, showing a positive correlation with soil As and P concentrations (Fig. 3). The concentrations of Sb and Cu were the two obvious factors affecting the population structure, and the Sb concentration was the most important factor for the microbial population structure.
Figure 3. Canonical correspondence analysis (CCA) showing correlations between the culturable microbial population structure and the environmental factors for the 11 soil samples (○, the soil names shown in Fig. S1).
The percentages of variation explained by each axis are shown.
Correlation between soil characteristics and the bacterial Sb(III)/Cu(II) resistance levels
In order to determine the impact of the soils' physicochemical characteristics on the bacterial resistances to Sb(III) and Cu(II), we compared the average MIC of the bacteria of every soil against the different environmental factors by stepwise linear regression analysis, since we believe that the average MIC could somehow represent the general bacterial metal resistance level in a sample. Based on the analysis of all the parameters of the 11 soil samples (Table 1), eight environmental factors (O–M, S, N, P, NO3 −, Fe, As and pH) did not reach significant correlation with the average MIC for Sb(III) (p>0.05), while the soil Sb and Cu concentrations were significantly correlated with the average MIC for Sb(III) in the bacteria from each soil sample (p<0.01) (Table 2). The stepwise linear regression model derived for the 11 soil samples is as follows:
| (1) |
Table 2. Stepwise linear regression models showing the correlations between the bacterial average MIC from each soil and the soil factors (based on the data of the 11 soil samples).
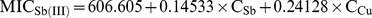 (R2 = 0.9298a, p<0.01)
(R2 = 0.9298a, p<0.01)
| ||||
| Variable | Coefficient | Std Error | P | VIFb |
| Constantc | 606.605 | 184.833 | 0.0112 | 1.1 |
| Cu | 0.24128 | 0.04759 | 0.0010 | 1.1 |
| Sb | 0.14533 | 0.01273 | 0.0000 | |
R2 = 0.9298 or 0.6467 shows that the average MIC was significantly correlated with the soil characters (R2>0.362, significant correlation, [32]); b variance inflation factor; c intercept. The MICSb(III) and MICCu(II) are the average bacterial MIC for the metal of each soil (µM), and the CSb, CCu, CS and CP are the soil concentrations for Sb, Cu, S and P (mg/kg), respectively.
Where the MICSb(III), CSb and CCu represent the average MIC for Sb(III) in the bacteria from each soil (µM), soil Sb concentration (mg/kg) and soil Cu concentration (mg/kg), respectively, p<0.01.
Eight environmental factors (O–M, Sb, Cu, N, P, NO3 −, Fe, As and pH) did not reach significant correlation with the average MIC for Cu(II) (p>0.05). The average MIC for Cu(II) in the bacteria from each soil was significantly correlated with the soil P and S concentrations (p<0.01) (Table 2). The stepwise linear regression model derived for this relationship is as follows:
| (2) |
Where the MICCu(II), CS and CP represent the average MIC for Cu(II) in the bacteria from each soil sample (µM), soil S concentration (mg/kg) and soil P concentration (mg/kg), respectively, p<0.01.
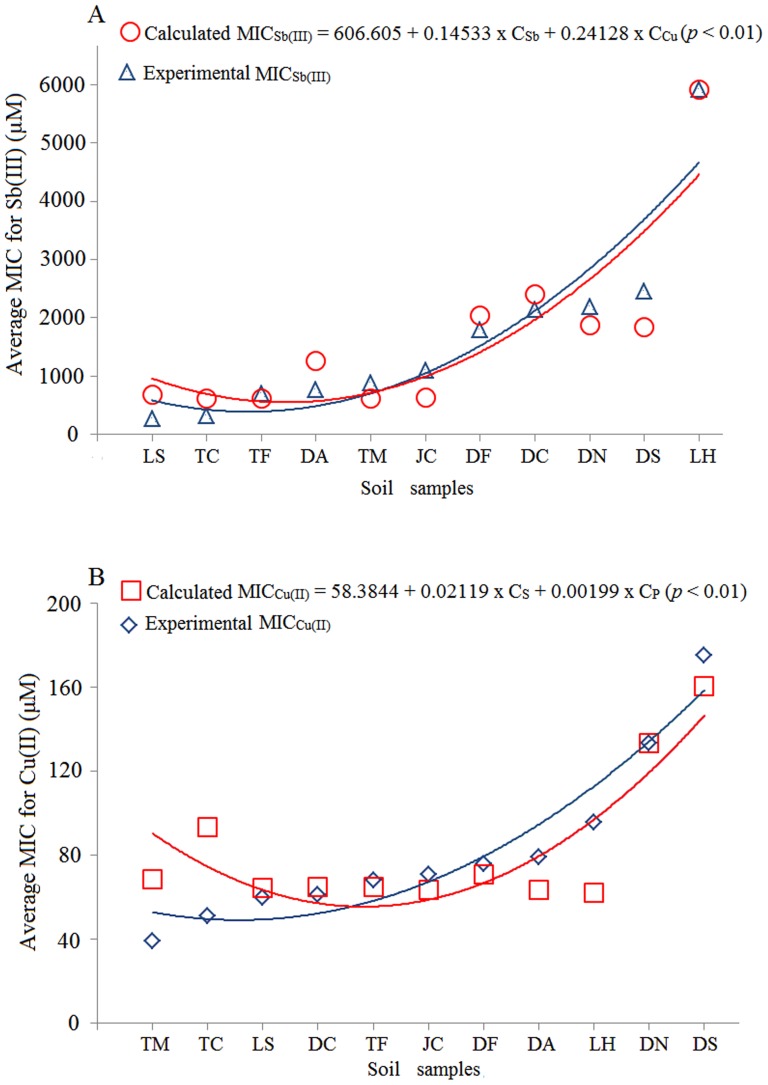
Fitting curves were used to determine the correlation of the observed average MICs with the back-tested average MICs using the stepwise linear regression models. Based on our analysis of these 11 soils, we showed good correlations and trends between the data using both the experimental values and the back-tested MIC values (Fig. 4, Table 3).
Figure 4. The fitting curves between the experimental MICs and the calculated MICs using the stepwise linear regression models for Sb(III) (A) and for Cu(II) (B).
Blue represents the experimental average MIC values tested in the CDM medium. Reds represents the model back-calculated MIC values using stepwise linear regression analysis. The soil names are shown in Fig. S1. The MICSb(III) and MICCu(II) are the average bacterial MIC for the metal of each soil (µM), and the CSb, CCu, CS and CP are the soil concentrations for Sb, Cu, S and P (mg/kg), respectively.
Table 3. Comparison between the experimental MIC and the calculated MIC for Sb(III) and Cu(II) in the respective stepwise linear regression models shown in Table 2.
| Soil | Range of MICs (mM) | Experimental average MIC for Sb(III) (mM) | Calculated average MIC for Sb(III) (mM) | Absolute value of error | Relative error*(%) | Fitting degree of accuracy ** (%) |
| LS | 0.1–1 | 0.26 | 0.68 | 0.42 | 61.76 | 38.24 |
| LH | 2.5–16 | 5.92 | 5.91 | 0.01 | 0.17 | 99.83 |
| JC | 0.025–8 | 1.10 | 0.63 | 0.47 | 74.60 | 25.40 |
| DF | 0.05–8 | 1.79 | 2.03 | 0.24 | 11.82 | 88.18 |
| DC | 0.05–7 | 2.13 | 2.41 | 0.28 | 11.62 | 88.38 |
| DN | 0.05–5 | 2.18 | 1.87 | 0.31 | 16.58 | 83.42 |
| DS | 0.05–7.5 | 2.46 | 1.83 | 0.63 | 34.43 | 65.57 |
| DA | 0.05–1.5 | 0.76 | 1.26 | 0.50 | 39.68 | 60.32 |
| TF | 0.05–5 | 0.70 | 0.62 | 0.08 | 12.90 | 87.10 |
| TM | 0.025–5 | 0.87 | 0.62 | 0.25 | 40.32 | 59.68 |
| TC | 0.025–3 | 0.31 | 0.62 | 0.31 | 50.00 | 50.00 |
| Average | / | / | / | / | 32.17 | 67.83 |
Relative error (%) = 100% x (Absolute value of error/ Experimental average);
Fitting degree of accuracy (%) = 100% - relative error. The experimental MIC for Sb(III) or Cu(II) of each isolate was tested using the CDM medium as described in the Material and Methods.
Correlations between the bacterial resistances to Sb(III) and Cu(II)
Since the average MIC for Sb(III) of the Sb(III)-resistant bacteria of each soil were most significantly correlated with the soil Sb and Cu concentrations in the stepwise regression model, we suspected that some correlation may exist between the bacterial resistant level to Sb(III) and Cu(II). Thus, we analyzed the MICs for Sb(III) and Cu(II) of the 125 strains. A unimodal scatter diagram showed that, in general, for each strain, the resistant levels to Sb(III) and to Cu(II) do not appear to be positively correlated, but certain correlation may exist: (1) The strains with low MICs for Sb(III) had a large range MICs for Cu(II) (10 – 300 µM); (2) The strains with high MICs for Sb(III) clustered together and showed MICs for Cu(II) of around 100 µM (Fig. S3).
Discussion
Microbial oxidation that converts the toxic Sb(III) to the less toxic Sb(V) provides a potential approach for environmental Sb bioremediation [13], [24]. To date, however, there are only a handful of known Sb(III)-oxidizing bacteria, which are strain identified within the genera Stibiobacter [22], Agrobacterium [23], Acinetobacter, Comamonas, Stenotrophomonas, Variovorax [13], Pseudomonas and Stenotrophomonas [24]. Furthermore, the genes or enzymes required for Sb(III) oxidation are still unknown. Previously, we identified 25 Sb(III)-resistant bacterial strains from Lengshuijiang Sb mined soil which was sampled in 2007 (Li et al., 2013). In this study, we obtained 125 Sb(III)-resistant bacteria including 36 Sb(III)-oxidizing bacteria from 11 soils representing different mining areas in China and performed a comprehensive analysis to understand the correlation among the soil characteristics, the microbial diversity and the bacterial Sb(III) resistance levels. Interestingly, at this time, we found four strains that show the highest level of Sb(III) resistance in bacteria reported to date (i.e., MICs greater than 10 mM). We also determined several novel Sb(III)-oxidizing bacteria identified as Aminobacter, Arthrobacter, Bacillus, Janibacter, Paracoccus and Sphingopyxis. Bacteria from the Agrobacterium, Pseudomonas, Variovorax and Bacillus genera have been reported to oxidize As(III) [10], [11], [23], [40]; however, members of the Comamonas genus appear to oxidize only Sb(III) and not As(III) (Li et al., 2013 and this study). We sequenced the whole genome of the Sb(III)-oxidizing bacterium Comamonas sp. S44 and did not find a putative As(III) oxidase gene [13], [41]. In addition, the mutational analysis indicated that another mechanism [i.e. other than the action of As(III) oxidase on Sb(III)] is responsible for Sb(III) oxidation [42]. Recently, we performed bacterial proteomics analysis of Sb(III) and As(III) oxidation and showed significant differences in the induced protein types (manuscript in preparation). Thus, the molecular basis for the oxidation of Sb(III) and As(III) appears to be quite distinct.
Although it is known that the bacteria-mediated oxidation of Sb(III) to Sb(V) is a detoxification process [23], [24], the toxicity of Sb(V) for bacteria has not been determined In this study, we found that the MICs of Sb(V) for the bacterial strains tested were all higher than 10 mM, indicating that Sb(V) is much less toxic than Sb(III). Due to the low solubility of C12H19Na3O18Sb2·9H2O and the precipitates that appeared in the CDM medium when Sb(V) concentration exceeded 10 mM, the upper limit of MICs of Sb(V) was difficult to determine. So far, the entrance mechanism for Sb(V) remains unknown. It is possible that bacterial cells do not take up Sb(V), resulting in Sb(V) 's low toxicity.
The 125 identified Sb(III)-resistant bacterial strains also showed resistance to Cu(II). Microbial resistance levels to different metals are not well characterized. Based on our experience working with different metal(loid)s, it appears that bacteria are more resistant to As(III) than to Sb(III) but much less resistant to Cu(II) compared with other metal(loid)s, The dominant genera within the Sb(III)/Cu(II)-resistant bacterial strains isolated during this study were identified as Pseudomonas, Arthrobacter, Comamonas, Brevundimonas, Acinetobacter, Ensifer and Variovorax. We previously described Sb(III)-resistant bacteria from three of these genera: Acinetobacter, Pseudomonas and Comamonas [13]. Other studies reported that Acinetobacter, Agrobacterium, Bacillus and Pseudomonas species are commonly found at As-contaminated sites [43]–[47], and indeed, we found three of these species in this study.
One limitation to our study is that the selective medium only isolates culturable bacteria; however, this method has previously been used successfully in microbial ecological research [11], [48], [49]. The major advantage of the culturable approach is the ability to determine the metal resistance and other physiological traits of the strains (e.g., Sb(III) oxidation level). Furthermore, these strains may be very useful for subsequent studies of Sb resistance mechanisms and are important applicable resources for bioremediation purposes. Using the culturable method, we found that the Sb(III) and Cu(II) resistance levels of different strains identified within the same genus differed greatly. Moreover, no positive correlation between the Sb(III) oxidation efficiency and Sb(III) resistance level was found, indicating that Sb(III) oxidation may contribute only partially to Sb(III) resistance; other mechanisms, such as Sb(III) efflux, may also play a role. We have observed that in many strains, the oxidation of As(III) is approximately 100-fold quicker than Sb(III) oxidation (data not shown).
CCA analysis revealed that the culturable bacterial population structure was greatly affected by soil concentrations of Sb and Cu. Our experiments did not show Fe to be a significant driver in population structure. Turpeinen et al. [50] reported that microbial community structure and microbial activity are affected by the amounts of As, Cr and Cu in soils near abandoned wood impregnation plants. Xiong et al. [51] reported that microbial community structure is affected by the amounts of As, O–M and P in As-contaminated soil. However, other environmental factors, such as spatial isolation, C/N ratio [41] and pH [52], [53], also contribute to community structure in metal-contaminated soils.
Stepwise linear regression models are used in many studies, but their application is less common in the field of microbiology [26]. In this study, for the first time, we created two equation models for the relationship between soil characteristics and the levels of Sb(III) and Cu(II) resistance in culturable bacteria. We also used fitting curves to show the accuracy of our stepwise linear regression models. In rare cases, the relative error was high (Table 3). This may be caused by the inclusion of a small subset of MICs that was much higher or lower than the others. For example, excluding the MIC for strain JC7 (8 mM) from the 13 MICs of the JC soil sample would reduce the relative error from 74.60% to 21.25%; In the LH soil sample, excluding the MICs for strains LH3 and LH4 (each of 200 µM) would reduce the relative error from 66.67% to 25%. In future studies of MICs, outliers may be eliminated to reduce the relative error.
The Stepwise linear regression model (1) indicated that the Sb and Cu concentrations in soil are significant factors affecting the Sb(III) resistance of bacterial strains. Sb is a chalcophile element and commonly found in ores of copper, silver, lead and coal [2]. Sb and Cu are often found in the same environments, which may result in the further evolution of bacterial species that were already well adapted to the high amounts of Sb- and Cu-rich soil. The model (2) showed that the most significant environmental factors affecting the bacterial Cu(II) resistance were the soil concentrations of S and P. Most of the Cu found in nature is in the form of Cu-sulfide, which could explain the importance of S [54]. However, how P concentration affects bacterial resistance to Cu is still not clear. To the best of our knowledge, there is no evidence that Cu and P are metabolized in a similar manner at the molecular level. Further analysis of bacterial resistance to Cu at different P concentrations or gene mutation experiments may shed more light to understand this relationship.
It has been reported that heavy metal concentration is the primary environmental factor favoring the presence of high numbers of heavy metal-resistant bacteria [11], [13]. In this study, we found that strains isolated from soils with high Sb content showed significantly higher Sb(III) resistance than those strains from soils with low Sb content. This is consistent with our previous report that bacterial strains with higher As resistance were isolated from highly As-contaminated soils [11]. However, bacterial resistance levels to Cu(II) did not correlate with soil Cu(II) content. The stepwise linear regression models we developed suggest that metals as well as other soil physicochemical parameters can contribute to bacterial resistance to metals. It is important to be aware of such an association as it may provide useful information on the genetics, physiology, and ecology of microbes in polluted environments and guide the design of in situ bioremediation schemes.
Supporting Information
A map showing the location of the 11 soil sampling sites in the P. R. of China. These sites include: the Lengshuijiang Sb mine (subsurface soil) (LS) (27°45′ N, 111°28′), the Lengshuijiang high Sb content mine (LH) (27°45′ N, 111°28′), the Jixi coal mine (JC) (45°18′ N, 130°57′ E), the Daye iron mine (DF) (30°12′ N, 114°56′ E), the Daye Tonglvshan copper mine (DC) (30°04′ N, 115°01′ E), Daye tin soil (DN) (30°00′ N, 115°01′ E), Daye delafossite with high sulfur content (DS) (29°59′ N, 114°57′ E), the Daye gold mine (DA) (30°03′ N, 114°59′ E), and the Tianjin iron mine (TF), manganese mine (TM) and coal mine (TC) (39°01′N, 117°11′ E).
(PDF)
(A). Distribution of the isolates of the 52 Pseudomonas strains among the 11 soil samples (Figure S1). (B). The percentage of the bacterial classes among the 11 soil samples.
(PDF)
The unimodal scatter diagram determined using Excel program showing the correlation between the MICs for Sb(III) and for Cu(II) of the 125 Sb(III)-resistant bacterial strains.
(PDF)
Experimental conditions for HPLC-HG-AFS analysis.
(PDF)
MIC for Sb(III) and Cu(II) (µM) of each strain in the 11 different mining soils. The soil names are the same shown in Figure S1.
(PDF)
Acknowledgments
We thank Dr. Timothy McDermott for providing strain A. tumefaciens 5A and for Dr. Christopher Rensing for his valuable comments and suggestions.
Funding Statement
This work was supported by a Major International Joint Research Project, National Natural Science Foundation of China (31010103903) and by the Chinese 863 project (2012AA101402-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liu F, Le XC, McKnight-Whitford A, Xia Y, Wu F, et al. (2010) Antimony speciation and contamination of waters in the Xikuangshan antimony mining and smelting area, China. Environ Geochem Health 32: 401–413. [DOI] [PubMed] [Google Scholar]
- 2. Zhang DY, Pan XL, Mu GJ, Wang JL (2010) Toxic effects of antimony on photosystem II of Synechocystis sp. as probed by in vivo chlorophyll fluorescence. J Appl Phycol 22: 479–488. [Google Scholar]
- 3. He M, Ji H, Zhao C, Xie J, Wu X, et al. (2002) Preliminary studies of heavy metal pollution in soil and plant near antimony mine area. J Beijing Norm Univ Nat Sci 38: 417–420. [Google Scholar]
- 4. Zhang X, Hu M, Zhao Y, Zhao B (2005) A survey of heavy metals pollution in Daye Tieshan Area. Environ Sci Technol (China) 28: 40–43. [Google Scholar]
- 5. Dupont CL, Grass G, Rensing C (2011) Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics 3: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 6. Rensing C, McDevitt SF (2013) The copper metallome in prokaryotic cells. Met Ions Life Sci 12: 417–450. [DOI] [PubMed] [Google Scholar]
- 7. Grass G, Rensing C (2001) CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli . Biochem Bioph Res Co 286: 902–908. [DOI] [PubMed] [Google Scholar]
- 8. Lucas BP, Fernando CS (2009) Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mole Microbiol 73: 212–225. [DOI] [PubMed] [Google Scholar]
- 9. Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51: 730–750. [DOI] [PubMed] [Google Scholar]
- 10. Fan H, Su C, Wang Y, Yao J, Zhao K, et al. (2008) Arsenite-oxidizing and arsenate-reducing bacteria associated with the geological arsenic groundwater contamination in Shanyin, Northwestern China. Journal of Applied Microbiology 105: 529–539. [DOI] [PubMed] [Google Scholar]
- 11. Cai L, Liu G, Rensing C, Wang G (2009) Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smichowski P (2007) Antimony in the environment as a global pollutant: a review on analytical methodologies for its determination in atmospheric aerosols. Talanta 75: 2–14. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Wang Q, Zhang S, Qin D, Wang G (2013) Phylogenetic and genome analyses of antimony-oxidizing bacteria isolated from antimony mined soil. Int Biodeterior Biodegrad 76: 76–80. [Google Scholar]
- 14. Christopher W, Simon CW, Geoffrey MG (1995) The role of microorganisms in biosorption of toxic metals and radionuclides. Int Biodeterior Biodegrad 35: 17–40. [Google Scholar]
- 15. Hong YG, Gu JD (2009) Bacterial anaerobic respiration and electron transfer relevant to the biotransformation of pollutants. Int Biodeterior Biodegrad 63: 973–980. [Google Scholar]
- 16. Filella M, Belzile N, Lett M (2007) Antimony in the environment: A review focused on natural waters. III. Microbiota relevant interactions. Earth-Science Reviews. 80: 195–217. [Google Scholar]
- 17. Wang Q, Xiong D, Zhao P, Yu X, Tu B, et al. (2011) Effect of applying an arsenic-resistant and plant growth-promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoids LH05-17. J Appl Microbiol 111: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 18. Meng Y, Liu Z, Rosen BP (2004) As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli . J Biol Chem 279: 18334–18341. [DOI] [PubMed] [Google Scholar]
- 19. Brochu C, Wang J, Roy G, Messier N, Wang XY, et al. (2003) Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob Agents Chemother 47: 3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentley R, Chasteen TG (2002) Microbial methylation of metalloids: arsenic, antimony and bismuth. Microbiol Mol Biol Rev 66: 250–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins RO (2002) Microbial biomethylation of antimony. Trends Organomet Chem 4: 109–122. [Google Scholar]
- 22. Lialikova NN (1974) Stibiobacter senarmontii: a new microorganism oxidizing antimony. Mikrobiologiia 43: 941–943. [PubMed] [Google Scholar]
- 23. Lehr CR, Kashyap DR, McDermott TR (2007) New insights into microbial oxidation of antimony and arsenic. Appl Environ Microbiol 73: 2386–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamamura N, Fukushima K, Itai T (2013) Identification of antimony- and arsenic-oxidizing bacteria associated with antimony mine tailing. Microbes Environ. 28: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery DC, Peck EA, Vining GG (2012) Introduction to linear regression analysis. Fifth Eidition. John Wiley & Sons, Inc.
- 26. Zhao L, Chen Y, Schaffner DW (2001) Comparison of logistic regression and linear regression in modeling percentage data. Appl Environ Microbiol 67 (5): 2129–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang QY, Peng SB, Buresh RJ, Zou YB, Castilla NP, et al. (2007) Rice varietal difference in sheath blight development and its association with yield loss at different levels of N fertilization. Field Crops Res 102: 219–227. [Google Scholar]
- 28. Okkenhaug G, Zhu YG, Luo L, Lei M, Li X, et al. (2011) Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ Pollut 159: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 29. Johnston SE, Barnard WM (1979) Comparative effectiveness of fourteen solutions for extracting arsenic from western New York soils. Soil Sci. Soc. Amer. J. 43: 304–308. [Google Scholar]
- 30. Liao XY, Chen TB, Xie H, Liu YR (2005) Soil as contamination and its risk assessment in areas near the industrial districts of Chenzhou City, Southern China. Environ Int 31: 791–798. [DOI] [PubMed] [Google Scholar]
- 31. Weeger W, Lievremont D, Perret M, Lagarde F, Hubert JC, et al. (1999) Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals 12: 141–149. [DOI] [PubMed] [Google Scholar]
- 32.Liao SJ, Zhou JX, Wang H, Chen X, Wang HF, et al. (2013) Arsenite oxidation using biogenic manganese oxides produced by a deep-sea manganese-oxidizing bacterium, Marinobacter sp. MnI7-9. Geomicrobiol J 30:22013 JAN 15.
- 33. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The clustal_x windows interface: flexible strategies for multiple alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guindon SEP, Dufayard JCCO, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 35. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 36.Statistix (2003) Statistix 8.0, User's Manual. Analytical Software, Tallahassee, FL.
- 37. Angers B, Magnan P, Plante M, Bernatchez L (1999) Canonical correspondence analysis for estimating spatial and environmental effects on microsatellite gene diversity in brook charr (Salvelinus fontinalis). Mol Ecol 8: 1043–1053. [Google Scholar]
- 38. Palacios C, Zettler E, Amils R, Amaral-Zettler L (2008) Contrasting Microbial Community Assembly Hypotheses: A Reconciling Tale from the Rı ´o Tinto. PLoS ONE 3(12): e3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koeppel A, Perry EB, Sikorski J, Krizanc D, Warner A, et al. (2008) Identifying the fundamental units of bacterial diversity: A paradigm shift to incorporate ecology into bacterial systematics. Proc Natl Acad Sci U S A 105: 2504–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green HH (1918) Description of a bacterium which oxidizes arsenite to arsenate, and of one which reduces arsenate to arsenite, isolated from a cattle-dipping tank. S Afr J Sci 14: 465–467. [Google Scholar]
- 41.Xiong J, He Z, Van Nostrand JD, Luo G, Tu S, et al. (2012) Assessing the microbial community and functional genes in a vertical soil profile with long-term arsenic contamination. PloS One http://dx.plos.org/10.1371/journal.pone.0050507 [DOI] [PMC free article] [PubMed]
- 42. Hao X, Lin Y, Johnstone L, Liu G, Wang G, et al. (2012) Genome sequence of the arsenite-oxidizing strain Agrobacterium tumefaciens 5A. J Bacteriol 194: 903–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol 48(5): 341–347. [DOI] [PubMed] [Google Scholar]
- 44. Pepi M, Volterrani M, Renzi M, Marvasi M, Gasperini S, et al. (2007) Arsenic-resistant bacteria isolated from contaminated sediments of the Orbetello Lagoon, Italy, and their characterization. J Appl Microbiol 103(6): 2299–2308. [DOI] [PubMed] [Google Scholar]
- 45. Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158(2): 128–137. [DOI] [PubMed] [Google Scholar]
- 46. Jackson CR, Dugas SL, Harrison KG (2005) Enumeration and characterization of arsenate-resistant bacteria in arsenic free soils. Soil Biol Biochem 37: 2319–2322. [Google Scholar]
- 47. Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60: 107–130. [DOI] [PubMed] [Google Scholar]
- 48. Quemeneur M, Heinrich-Salmeron A, Muller D, Lievremont D, Jauzein M, et al. (2008) Diversity surveys and evolutionary relationships of aoxB genes in aerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74: 4567–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Valverde A, Gonzalez-Tirante M, Medina-Sierra M, Santa-Regina I, Garcia-Sanchez A, et al. (2011) Diversity and community structure of culturable arsenic-resistant bacteria across a soil arsenic gradient at an abandoned tungsten–tin mining area. Chemosphere 85: 129–134. [DOI] [PubMed] [Google Scholar]
- 50. Turpeinen R, Kairesalo T, Haggblom MM (2004) Microbial community structure and activity in arsenic-, chromium- and copper-contaminated soils. FEMS Microbiol Ecol 47: 39–50. [DOI] [PubMed] [Google Scholar]
- 51. Xiong J, Wu L, Tu S, Van Nostrand JD, He Z, et al. (2010) Microbial communities and functional genes associated with soil arsenic contamination and the rhizosphere of the arsenic-hyperaccumulating plant Pteris vittata L. Appl Environ Microbiol. 76: 7277–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci 103: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88: 1354–1364. [DOI] [PubMed] [Google Scholar]
- 54. Whiteside LS, Goble RJ (1986) Structural and compositional changes in copper sulfide during leaching and dissolution. The Canadian Mineralogist 24: 247–258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A map showing the location of the 11 soil sampling sites in the P. R. of China. These sites include: the Lengshuijiang Sb mine (subsurface soil) (LS) (27°45′ N, 111°28′), the Lengshuijiang high Sb content mine (LH) (27°45′ N, 111°28′), the Jixi coal mine (JC) (45°18′ N, 130°57′ E), the Daye iron mine (DF) (30°12′ N, 114°56′ E), the Daye Tonglvshan copper mine (DC) (30°04′ N, 115°01′ E), Daye tin soil (DN) (30°00′ N, 115°01′ E), Daye delafossite with high sulfur content (DS) (29°59′ N, 114°57′ E), the Daye gold mine (DA) (30°03′ N, 114°59′ E), and the Tianjin iron mine (TF), manganese mine (TM) and coal mine (TC) (39°01′N, 117°11′ E).
(PDF)
(A). Distribution of the isolates of the 52 Pseudomonas strains among the 11 soil samples (Figure S1). (B). The percentage of the bacterial classes among the 11 soil samples.
(PDF)
The unimodal scatter diagram determined using Excel program showing the correlation between the MICs for Sb(III) and for Cu(II) of the 125 Sb(III)-resistant bacterial strains.
(PDF)
Experimental conditions for HPLC-HG-AFS analysis.
(PDF)
MIC for Sb(III) and Cu(II) (µM) of each strain in the 11 different mining soils. The soil names are the same shown in Figure S1.
(PDF)