Abstract
Our laboratory investigates the immune tolerance mechanisms promoted by acute myeloid leukemia (AML). In a murine AML model, we have observed that leukemia antigen-specific T cells are specifically deleted from the host, presumably following interactions with immature host antigen-presenting cells (APCs). Ongoing work focuses on identifying APC subsets that induce T-cell tolerance in AML as well as the precise mechanisms that underlie this phenomenon.
Keywords: peripheral tolerance, AML, anergy, deletion, APC
Although T cells can recognize tumor-derived peptide antigens, the immune system is rarely able to control the growth of established malignancies, in part because the tumor environment employs a number of immune evasion mechanisms which prevent either the generation or execution of a productive antitumor immune response. A growing number of pathways whereby neoplastic cells escape the immune system have been uncovered, largely in the setting of solid tumors, and include (1) loss of MHC expression on malignant cells, (2) lack of T-cell co-stimulation by cancer cells, promoting T-cell anergy, (3) expression of inhibitory receptors on tumor-infiltrating lymphocytes, (4) expansion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) within the tumor environment, and (5) tumor-mediated production of immunosuppressive cytokines and enzymes.1-3 While such immunosuppressive pathways have been well-characterized in the context of solid tumors, the mechanisms that modulate immune responses against hematological malignancies, such as acute myeloid leukemia (AML), have been less well explored and are a primary focus of investigation in our laboratory.
To characterize leukemia antigen-specific CD8+ T-cell responses in hosts with AML, our laboratory utilizes a transplantable AML cell line (C1498 cells) which has been engineered to express the model SIY peptide antigen (C1498.SIY cells).4 Several years ago, a straightforward experiment in which C1498.SIY cells were inoculated either intravenously (i.v.) or subcutaneously (s.c.) into syngeneic C57BL/6 mice led to a critical observation.5 Vigorous SIY-specific CD8+ T-cell responses were generated in mice following the inoculation of C1498.SIY cells s.c. However, intravenous C1498.SIY-cell inoculation resulted in very poor CD8+ T-cell activation. To determine whether diminished T-cell responses following a systemic AML cell challenge resulted from passive immunological ignorance or active peripheral tolerance, CD8+ T-cell responses against the SIY antigen were analyzed in mice receiving an intravenous C1498.SIY cell challenge, followed several days later by the inoculation of C1498.SIY cells s.c. (referred to as IV/SC challenge). If immunological ignorance were occurring, the inoculation of AML cells i.v. should not impair the generation of a functional T-cell response against a subsequent subcutaneous challenge in the same animal. In fact, significantly reduced SIY-specific T-cell responses were observed in IV/SC AML cell-challenged animals, indicating that the systemic inoculation of AML cells induces T-cell tolerance.
T-cell tolerance in animals subjected to an intravenous C1498.SIY cell challenge was antigen-specific and did not depend on Tregs or MDSCs.5 T-cell receptor transgenic CD8+ T cells specific for the SIY antigen (2C T cells) adoptively transferred into mice harboring C1498.SIY cells i.v. failed to accumulate and demonstrated defects in cytokine secretion, suggesting that they were undergoing deletion. To formally investigate T-cell deletion as a peripheral tolerance mechanism in AML, 2C T cells were engineered to overexpress the anti-apoptotic protein Bcl-XL,6 which restored their ability to accumulate and to produce effector cytokines in AML-bearing hosts. These results confirmed that T-cell deletion is a critical mechanism of T-cell tolerance in this AML model.
Antigen presentation by immature antigen-presenting cells (APC) can lead to T-cell tolerance.7 To investigate whether host APCs were regulating T-cell dysfunction in mice bearing AML, we administered an agonistic anti-CD40 antibody to systemically active APCs in vivo. This maneuver significantly enhanced leukemia-specific T-cell responses in mice with systemic AML and, more impressively, prolonged the survival of animals with established disease. These results indicated that not only were host APCs mediating T-cell tolerance but also their activation could restore functional immune responses in AML-bearing hosts.
Initial evidence suggesting that hematological cancers induce T-cell tolerance came from experiments in the A20 lymphoma model.8 Following the systemic inoculation of A20 cells, antigen-specific CD4+ T cells failed to expand and produced low levels of effector cytokines upon re-stimulation, consistent with an anergic phenotype. The anergy of CD4+ T cells in this system was mediated by host hematopoietic cells that presumably cross-presented tumor-associated antigens. To model T-cell responses against a leukemia-associated antigen also expressed by normal peripheral tissues, the Greenberg lab utilized a transplantable AML cell line (FBL cells) that expresses an immunogenic peptide derived from the retroviral Gag protein.9 In this setting, Gag-specific CD8+ T cells were found to be tolerized in AML-bearing hosts in which the Gag antigen was also conditionally expressed in the liver. However, CD8+ T-cell responses elicited by a leukemia-specific antigen have not previously been explored. In the C1498 model, deletional tolerance appeared to be a dominant mechanism of immune evasion, which has not been observed in other hematological cancer models.
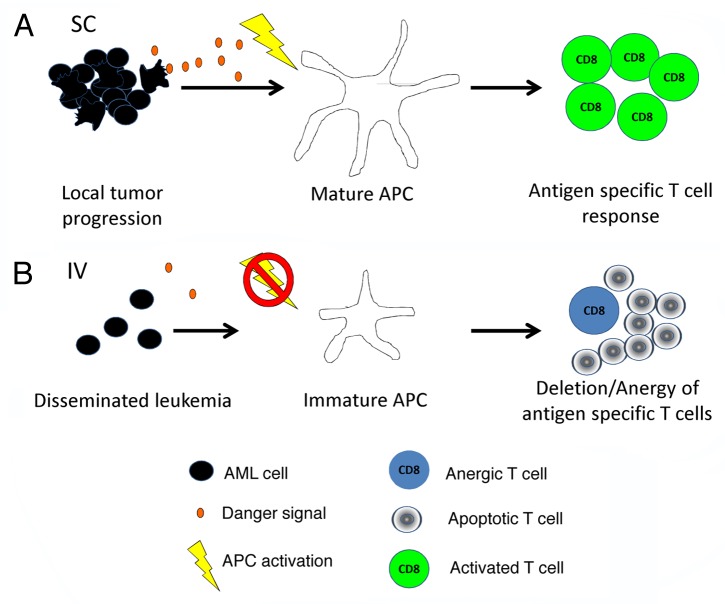
The death of malignant cells can cause local inflammation and deliver “danger signals” that promote APC maturation. Mature APCs can then cross-present tumor-associated antigens to initiate the productive priming of tumor-specific T cells.10 However, it is interesting to speculate that immunogenic cell death may not occur to a similar degree in disseminated leukemias as compared with solid tumors, in which nutrients and oxygen may be more limited. Also, because of its natural growth pattern, danger signals produced by dying AML cells may be less concentrated. Therefore, dying AML cells may not facilitate the maturation of APCs and hence prevent the proper priming of adaptive immune responses (Fig. 1).
Figure 1. Model of peripheral T-cell tolerance induction in hosts with acute myeloid leukemia. (A) Acute myeloid leukemia (AML) cells introduced locally into the host release danger signals as they die, resulting in the maturation of local antigen-presenting cells (APCs). Mature APCs can then cross-present leukemia-derived antigens to T cells along with the proper co-stimulatory ligands necessary for complete T-cell activation. (B) However, when AML cells are inoculated systemically, the relative degree of leukemia cell death at a given site is reduced. In this setting, APCs do not receive appropriate maturation signals and though they are able to cross-present leukemia antigens, they do so in a context that results in T-cell deletion and/or anergy.
In conclusion, we have uncovered a distinct mechanism through which AML induces T-cell tolerance by promoting the deletion of leukemia-specific CD8+ T cells. This phenomenon appears to be mediated by APCs that cross-present tumor-associated antigens in a context that is unfavorable for T-cell activation. Ongoing research in our laboratory is focused on identifying the APC population(s) that actually mediate immune tolerance to leukemia. We speculate that either a particular type of APC or the overall activation state of APCs in general may govern this phenomenon. Further advances in our understanding of how AML negatively regulates immune responses may ultimately identify targets for the development of efficient immunotherapies for leukemia patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25445
References
- 1.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 2.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–20. [PubMed] [Google Scholar]
- 3.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–52. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest. 2013;123:1999–2010. doi: 10.1172/JCI63980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–8. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 8.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–41. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 10.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8alpha+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]