Abstract
Despite the promise of targeting γδ T cells for cancer immunotherapy, the mechanisms underpinning the recruitment of this T-cell subsets to neoplastic lesions remain poorly understood. We have recently identified the pro-inflammatory chemokine CCL2 and its receptor CCR2 as key molecular determinants of γδ T-cell migration and tumor infiltration.
Keywords: CCL2, CCR2, chemokines, tumor-infiltrating lymphocytes, γδ T cells
Inflammation is a doubled-edged hallmark of cancer that comprises both tumor-promoting and tumor-inhibitory components, including the infiltration of neoplastic lesions by various immune cell subsets with strikingly different functions.1 The recruitment of these immune cells to tumors is largely orchestrated by chemokines, among which chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP1), has been intensively studied.
CCL2 can be detected in the majority of solid tumors and has traditionally been linked to the recruitment of myeloid cells expressing the cognate receptor CCR2. In particular, CCL2 is a key signal for the infiltration of neoplastic lesions by tumor-associated macrophages (TAMs) that are biased toward a pro-tumor M2 phenotypic and functional profile. Moreover, CCL2 has been shown to recruit monocytes to pulmonary metastases in a murine model of breast carcinoma. In this setting, the neutralization of CCL2 with specific antibodies inhibited metastatic spread and prolonged the survival of tumor-bearing mice.2 Along similar lines, CCL2 has been implicated in tumor infiltration by myeloid-derived suppressor cells (MDSCs) in animal models of lung carcinoma, sarcoma, melanoma and lymphoma.3 Finally, CCR2-expressing MDSCs reportedly accumulated in melanoma lesions, where they hamper CD8+ T-cell recruitment and hence limit the efficacy of immunotherapy.4
In humans, the levels of CCL2 have been positively correlated with tumor infiltration by TAMs and are generally associated with poor prognosis among patients suffering from breast, prostate, and colon carcinoma.5 Collectively, these results have prompted the development of therapeutic protocols based on agents that would block either CCL2 or CCR2, which are nowadays under clinical evaluation.
Although the CCL2 and CCR2 have historically been implicated in tumor progression, a series of studies have identified a potential antitumor role for this signaling axis, which would reflect the recruitment of cytotoxic lymphocyte subsets including CD8+ T6-8and natural killer (NK) cells.8 Recently, we have added the recruitment of γδ T cells to the list of pleiotropic functions that CCL2 plays in the tumor microenvironment9 (Fig. 1). We demonstrated indeed that the CCL2/CCR2 signaling axis is required for γδ T-cell infiltration in a preclinical melanoma model based on the transplantation of murine B16 cells, which are a target for γδ T-cell cytotoxicity. In particular, we observed a significant reduction in the number of γδ T cells infiltrating B16 melanomas developing in Ccr2−/− as compared with wild-type (WT) mice. Interestingly, although myeloid cells (TAMs and MDSCs) were also less abundant in B16 tumors growing in Ccr2−/− animals, these lesions grew faster than those implanted in WT mice.9 These data suggest that, at least in some settings, the antitumor functions of CCL2—as mediated by γδ T cells, which were shown to inhibit tumor growth in vitro and in vivo—predominate over its pro-tumor activities (mainly mediated by myeloid cells). It should be noted, however, that these experiments focused on the early phases (first 2 wk) of tumor progression. It is therefore possible that a different scenario would have emerged at later stages of the disease, which could not be studied in this model owing to the rapid and eventually lethal growth of B16 cells.
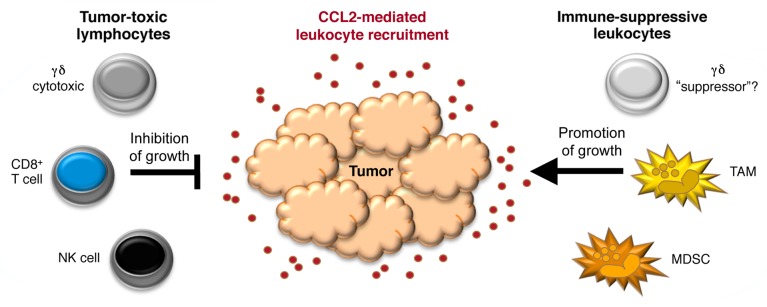
Figure 1. Pleiotropic roles of CCL2 in the recruitment of tumor-inhibitory (cytotoxic) vs. tumor-promoting (immunosuppressive) leukocyte subsets. MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage.
The complexity of CCL2 functions within the tumor microenvironment may be further enhanced by the heterogeneity of each leukocyte subset recruited via CCR2. For example, TAMs can display diametrically opposed phenotypes, often referred to as M1 and M2, which are associated with tumor inhibition vs. support, respectively.1 Heterogeneity may also be an important factor for human Vδ1 T cells, which abundantly infiltrate several tumor types,10 express CCR2 and migrate in response to CCL2.9 Thus, melanoma-infiltrating Vδ1 T cells have been shown to produce tumor necrosis factor α (TNFα) and interferon γ (IFNγ) and hence mediate potent cytotoxic functions. Conversely, Vδ1 T cells isolated from breast cancer lesions reportedly inhibit dendritic cell and CD4+ T-cell functions in vitro, and their abundance inversely correlated with CD8+ T-cell infiltration and overall survival in a cohort of advanced breast carcinoma patients.10 The latter results point to the existence of a (hitherto uncharacterized) “suppressor” subset of human Vδ1 T cells, which would contrast with its well-studied cytotoxic counterpart (Fig. 1).
Based on our data on the chemotactic response of Vδ1 T cells to CCL2, it will be interesting to correlate the abundance of tumor-infiltrating Vδ1 T cells with the intratumoral CCL2 levels. Transcriptomic studies performed in our laboratory revealed that the CCL2 mRNA is expressed to variable levels by a panel of distinct neoplasms. For example, squamous cell carcinoma, breast carcinoma, and primary prostate carcinoma overexpressed CCL2 in comparison with histologically matched healthy tissues. By contrast, liver, lung, and metastatic prostate carcinomas expressed lower levels of CCL2 than control tissues.9 We therefore propose that intratumoral CCL2 expression levels may constitute an important predictive factor for future immunotherapeutic strategies targeting Vδ1 T cells.
Another interesting aspect to consider in the context of the CCL2-dependent recruitment of tumor-infiltrating lymphocytes (TILs) are the potential post-translational modifications of this chemokine within tumors. Pioneering work from Viola, Bronte, and collaborators demonstrated that tumor-derived reactive nitrogen species result in CCL2 nitration, thereby hindering the recruitment of CD8+ T cells to the tumor bed.7 This team is currently developing chemical inhibitors of nitration, hoping to improve tumor infiltration by CD8+ T cells, which would likely extend to γδ T cells.
Notwithstanding our conviction that CCL2 plays a key role in tumor immunosurveillance (relying on γδ T cells and other cytotoxic lymphocytes) (Fig. 1), this pro-inflammatory chemokine can undoubtedly contribute to tumor progression and metastasis.2 Thus, the net effect of CCL2 on neoplastic lesions may well depend on multiple variable, including tumor stage and perhaps the concomitant presence of additional cytokines. Our recent study9 focused on the early stage of tumor progression, a setting in which the antineoplastic effects of CCL2 seemingly dominate over its tumor-supporting activity. Future studies will have to clarify if, at later stages of the disease, the CCL2-dependent accumulation of myeloid cells inevitably results in the stimulation of tumor growth. The resolution of this paradox will be critical to interpret previous results and potentially (re)design therapeutic strategies aimed at modulating CCL2 levels in cancer patients.
Glossary
Abbreviations:
- MDSC
myeloid-derived suppressor cell
- NK
natural killer
- TAM
tumor-associated macrophage
- TIL
tumor-infiltrating lymphocyte
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25461
References
- 1.Lança T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–25. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–86. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, et al. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485–93. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 6.Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–41. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 7.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakasone Y, Fujimoto M, Matsushita T, Hamaguchi Y, Huu DL, Yanaba M, et al. Host-derived MCP-1 and MIP-1α regulate protective anti-tumor immunity to localized and metastatic B16 melanoma. Am J Pathol. 2012;180:365–74. doi: 10.1016/j.ajpath.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Lança T, Costa MF, Gonçalves-Sousa N, Rei M, Grosso AR, Penido C, et al. Protective Role of the Inflammatory CCR2/CCL2 Chemokine Pathway through Recruitment of Type 1 Cytotoxic γδ T Lymphocytes to Tumor Beds. J Immunol. 2013;190:6673–80. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 10.Dieli F, Stassi G, Todaro M, Meraviglia S, Caccamo N, Cordova A. Distribution, function and predictive value of tumor-infiltrating γδ T lymphocytes. Oncoimmunology. 2013;2:e23434. doi: 10.4161/onci.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]


