Abstract
Background
Irritable bowel syndrome and other gastrointestinal (GI) and non-GI disorders such as functional dyspepsia, fibromyalgia, temporomandibular joint disorder, interstitial cystitis/painful bladder syndrome, and chronic fatigue syndrome are known as functional pain syndromes. They commonly coexist within the same individual. The pathophysiologic mechanisms of these disorders are not well understood, but it has been hypothesized that they share a common pathogenesis.
Purpose
The objective of this review is to discuss the proposed pathophysiologic mechanisms, which have been similarly studied in these conditions. These mechanisms include enhanced pain perception, altered regional brain activation, infectious etiologies, dysregulations in immune and neuroendocrine function, and genetic susceptibility. Studies suggest that these functional disorders are multifactorial, but factors which increase the vulnerability of developing these conditions are shared.
Keywords: chronic fatigue syndrome, fibromyalgia, functional dyspepsia, interstitial cystitis/painful bladder syndrome, irritable bowel syndrome, temporomandibular joint disorder
INTRODUCTION
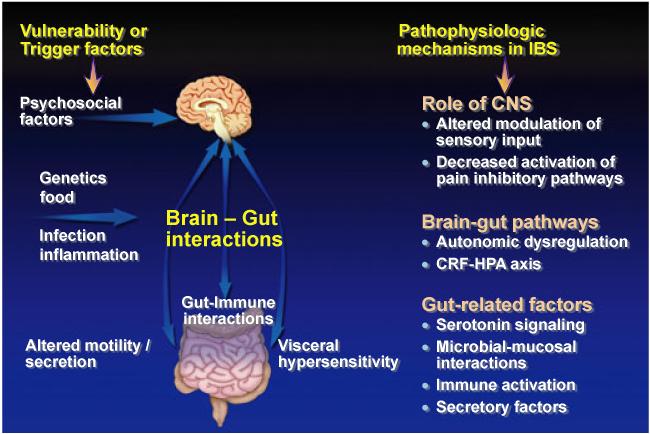
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that has a worldwide prevalence. The pathophysiology of this disorder is complex and not fully understood. Proposed pathophysiologic mechanisms of IBS include, but are not limited to, brain-gut axis dysregulation,1 enhanced visceral perception,2 altered intestinal microbiota,3 post infectious changes in gastrointestinal (GI) function,4 and enhanced immunologic reactivity (Fig. 1).5 Currently, there is no reliable diagnostic biomarker for IBS and therefore, IBS has been generally considered “functional” in etiology due to the lack of consistent anatomic or biochemical abnormalities to explain the symptoms.6 Similarly, there are other symptom-based disorders, which have been labeled “functional,” as their pathophysiologic mechanisms are yet to be clearly defined (see Table 1). These conditions include other GI disorders such as functional dyspepsia (FD) and non-GI disorders including fibromyalgia (FM), chronic fatigue syndrome (CFS), interstitial cystitis/painful bladder syndrome (IC/PBS), and temporomandibular joint disorder (TMD).
Figure 1.
Factors that increase the vulnerability of developing irritable bowel syndrome (IBS) or trigger symptom onset include psychosocial symptoms, genetic factors, and infection. The physiological effects of psychological and physical stressors on gut function and brain–gut interactions are mediated by outputs of the autonomic, neuroendocrine, and pain modulatory responses. Patients show an enhanced responsiveness of this system manifesting in altered modulation of gastrointestinal motility and secretion and in alterations in the perception of visceral events. Pathophysiologic mechanisms reported in IBS include alterations in central processing and modulation of sensory input, autonomic and neuroendocrine responses, and gut-related factors. Functional brain imaging techniques are beginning to identify brain circuits involved in the perceptual alterations. Adapted from229. This figure has been reproduced with permission of the International Association for the Study of Pain ® (IASP ®). The figure may not be reproduced for any other purpose without permission.
Table 1.
Selected functional pain disorders
| IBS | FD | FM | CFS | TMD | IC/PBS | |
|---|---|---|---|---|---|---|
| Definition | A functional bowel disorder in which abdominal pain or discomfort is associated with defecation or a change in bowel habit, and with features of disordered defecation30 |
Presence of symptoms thought to originate in the gastroduodenal region, in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms211 |
Chronic widespread somatic pain and is typically associated with fatigue, anxiety, sleep disturbances, and/or cognitive dysfunction37 |
Intense fatigue of an unknown cause, which is permanent and limits the patient’s functional capacity producing various degrees of disability47 |
Pain of the masticatory musculature and/or the temporomandibular joint and associated structures40 |
Suprapubic pain related to bladder filling, with other symptoms such as increased daytime and nighttime frequency in the absence of proven infection or other pathology45 |
| Prevalence (%) |
10–156 | 11–2915 | 2–524,25 | 0.1–140 | 6–1230 | 0.002–0.146 |
| Female : male ratio |
1.5–2 : 130,212 | 1–1.5 : 134,213 | 7–9 : 1214 | 3 : 1215 | 4 : 1216 | 5–10:146 |
| Usual age of onset or at time of diagnosis (years) |
29–33212 | 15 and up34 | 25–60214 | 40–56217 | ~14218 | 42–46219 |
Several observations support that these conditions share a common pathophysiology. For example, there is significant overlap between IBS and these other functional disorders (Table 2).7-10 This overlap may be seen with two syndromes, or even three with various combinations of comorbidities such as IBS, CFS, and IC/PBS11 or IBS, FM, and IC/PBS.12 For example, one study noted health-care provider diagnoses of comorbid conditions of TMD, FM, and CFS within an IBS group from a tertiary care outpatient clinic to be 16%, 59%, and 36%, respectively. When examining symptoms, it is commonly found that IBS is associated with non-GI symptoms that are commonly seen in other somatic syndromes such as FM, CFS, IC/PBS, and TMD. For example, sleep disturbance was observed in 28–74% of IBS patients,13-15 whereas urinary symptoms (i.e., frequency, urgency, nocturia, incomplete bladder emptying sensation) were found approximately in 50% of IBS patients.14
Table 2.
Overlap between irritable bowel syndrome (IBS) and other functional pain disorders
| Disorder | Prevalence of IBS in patients with the disorder |
Prevalence of the disorder in patients with IBS |
|---|---|---|
| Gastrointestinal | ||
| Functional dyspepsia |
11–37%8 | 32%220 |
| Non-gastrointestinal or somatic |
||
| Fibromyalgia | 30–70%221,222 | 32%223 |
| Chronic fatigue syndrome | 35–92%224,225 | 14%10 |
| Temporomandibular joint disorder |
64%*9 | 16%*9 |
| Interstitial cystitis | 30.2–40%226,227 | 35%†228 |
Based on one study.
Includes all pelvic pain disorders.
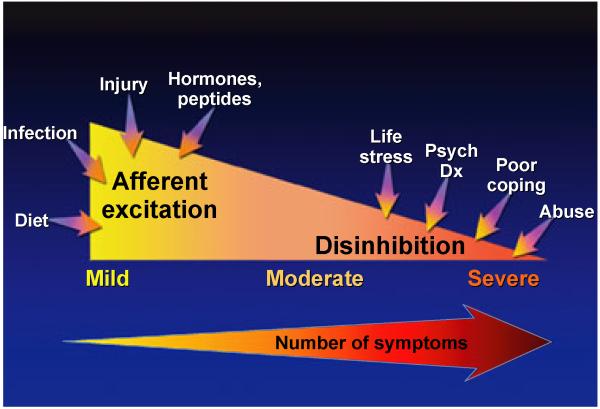
Furthermore, stress and other psychosocial factors appear to play a role in the development or the symptom exacerbations of these conditions (Fig. 2). Depression, anxiety, and other psychiatric comorbidities can frequently coexist with these functional syndromes particularly in more severe cases, although the prevalence can vary among studies (Table 2).16,17 A study conducted in the Netherlands noted that 30% of Rome II positive IBS patients had anxiety symptoms and 22% had depression symptoms based on the Hospital Anxiety and Depression (HAD) Scale.18 A study conducted in FD patients found that a coexistent anxiety disorder existed in 28.5% patients,16 while another study evaluating FD patients at a tertiary outpatient clinic found that 30.4% had a comorbid depression disorder.17 In FM, the prevalence of an anxiety disorder ranged between 20% and 80% and the prevalence of depression ranged between 13% and 63.8%.19 Furthermore, one survey study found that 57% of FM patients reported symptoms of posttraumatic stress disorder (PTSD).20 The prevalence of depression and anxiety based on DSM-IV criteria within a CFS population in the United Kingdom were each 14%, with coexistent depression and anxiety in 18%.21 However, a large United States survey study noted the lifetime prevalence of having a diagnosis of depression in a CFS population was as high as 57%.22 With respect to TMD patients, a US study demonstrated that the lifetime prevalence of a depressive disorder, which was diagnosed by a psychiatrist or a clinical psychologist, was 41%.23 In a study of IC/PBS female patients at a tertiary urology clinic setting, 5% had a diagnosis of depression, 11% had positive depression symptoms, and 14% had a panic disorder.24 However, this study noted that a “masking” effect was a confounder because patients who were taking an antidepressants or anxiolytics were unaccounted for. In contrast, based on a large community-based sample of IC/PBS patients, the probable diagnosis of depression was 34.8% and panic attacks were 52%.25 In summary, there are many studies conducted in these functional pain syndromes that demonstrate a significant comorbidity with psychological symptoms or psychiatric disorders with depression and anxiety being the more common diagnoses. However, studies varied in the type of patient population (tertiary vs community sample), geographic region, diagnostic criteria, and medication use.
Figure 2.
Peripheral and central influences on severity of functional pain syndromes. This figure depicts multiple factors which may contribute to pain symptoms in functional pain syndromes. Afferent excitation and thus upregulation of afferent pathways may be observed in patients with mild to moderate symptoms with contributory factors being diet, infection, injury, hormones, and peptides. Moderate to severe symptoms may be due to disinhibition at the level of central modulation of pain leading to a lack of inhibitory effects at the peripheral afferent level. Contributory factors associated with increasing severity include life stressors, greater psychosocial disturbances, poor coping skills, and abuse. Concurrently, there is an increase in symptom reporting. Adapted from229. This figure has been reproduced with permission of the International Association for the Study of Pain ® (IASP ®). The figure may not be reproduced for any other purpose without permission.
Another common observation in these functional somatic syndromes is a female predominance that is likely multifactorial. These factors include estrogen-related enhancement in pain sensitivity,26 increased pain amplification of sensory stimuli in women compared to men,27 gender differences in stress reactivity, and sociocultural differences in reaction to pain/coping mechanisms,28,29 and increased health-care seeking behavior in women.
Lastly, these functional syndromes are often treated similarly, such as with antidepressants and cognitive behavioral therapy. Thus, it can be hypothesized that these conditions have a unifying pathophysiology. The objective of this review is to discuss the overlap of IBS with these other functional syndromes and explore shared pathophysiologic mechanisms.
FUNCTIONAL GASTROINTESTINAL DISORDERS (FGIDS)
Irritable bowel syndrome
Irritable bowel syndrome is one of the most common FGIDs and affects between 10% and 15% of the US and European populations.30 Irritable bowel syndrome is associated with a female predominance although this is more evident in the clinic populations than in the general community.31 Currently, IBS is diagnosed using symptom-based criteria, such as the Rome III criteria,30 which is defined as the presence of abdominal pain or discomfort for at least 3 days per month in the last 3 months with two or more of the following features: (i) onset in relation to a change in frequency of stool, (ii) onset associated with a change in form of stool, and/or (iii) improvement with defecation.30
Functional dyspepsia
Dyspepsia is defined as “persistent or recurrent upper gastrointestinal symptoms, predominantly pain or discomfort localized in the epigastric region.”32,33 FD is a very common disorder, with a worldwide prevalence ranging from 11.5% to 29.2%.34 The Rome III diagnostic criteria for FD are at least 3 months or more of: (i) discomforting postprandial fullness, (ii) early satiation, (iii) epigastric pain, and/or (iv) epigastric burning for at least 3 months with the onset at least 6 months ago and no evidence of structural disease to explain all symptoms. In addition, FD is further subcategorized into epigastric pain syndrome and postprandial distress syndrome, which can also coexist. This subcategory is mainly to distinguish differences between dyspeptic symptoms induced by ingestion vs those that are not.35 Usually a negative endoscopy in the presence of dyspeptic symptoms confirms the diagnosis of FD.36
NON-GI FUNCTIONAL DISORDERS
Fibromyalgia
Fibromyalgia is defined by chronic widespread somatic pain and is typically associated with fatigue, anxiety, sleep disturbances, and/or cognitive dysfunction.37 It is prevalent in about 2–5% of the population.38,39 There is a higher prevalence in women that increases with age: 2% in women between the ages of 30 and 39 years and 7% in women between the ages of 60 and 69 years.39 Fibromyalgia is currently diagnosed using the modified 2010 American College of Rheumatology that utilizes two scales, the Widespread Pain Index (WPI) and the Symptom Severity (SS) scale. The diagnosis is based on meeting three criteria: (i) WPI ≥7 (0–19) and the SS score ≥5 (0–12) or a WPI of 3–6 and a SS is ≥9; (ii) a persistent similar level of symptoms for at least 3 months; and (iii) absence of another disorder to explain the pain.37
Temporomandibular joint disorder
Temporomandibular joint disorder is comprised of clinical symptoms related to pain of the masticatory musculature and/or the temporomandibular joint and associated structures.40 Temporomandibular joint symptoms are seen in 6-12% of the US population.41 Although imaging studies of temporomandibular joint can be done, TMD remains mainly a clinical diagnosis.40 The Research Diagnostic Criteria for temporomandibular disorders has a two-axis method of diagnosing TMD.42,43 Axis I focuses on the physical diagnosis of TMD, whereas Axis II assesses behavioral, social, and psychological factors associated with TMD.42,43
Interstitial cystitis/painful bladder syndrome
While some disagree, IC and PBS have been commonly used together, although they are sometimes used interchangeably. Interstitial cystitis was traditionally diagnosed using the National Institute for Diabetes and Diseases of the Kidney (NIDDK) criteria, which included bladder pain or urinary urgency, cystoscopic findings of either glomerulations or Hunner’s ulcers, and the lack of exclusion criteria.44 In 2002, the International Continence Society preferred the term PBS over IC and defined PBS in less restrictive terms as “suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and nighttime frequency in the absence of proven urinary infection or other obvious pathology.”45 The diagnosis of IC requires typical cytoscopic and histological features. Prevalence varies depending on the diagnostic criteria used, but it is between 0.002% and 0.1%.46
Chronic fatigue syndrome
Chronic fatigue syndrome is characterized by “intense fatigue of an unknown cause, which is permanent and limits the patient’s functional capacity producing various degrees of disability.”47 Chronic fatigue syndrome has a prevalence ranging from 0.1% to 1.0%.48 Diagnostic criteria have been established by The Centers for Disease Control and the CFS International Study Group where patients must have persistent chronic fatigue for at least 6 months, or unexplained relapsing intermittent fatigue that is not secondary to exertion, does not improve with rest, and results in remarkable reduction from previous normal activity.49 Other medical and psychiatric disorders that can explain CFS symptoms must be excluded.49
PROPOSED PATHOPHYSIOLOGIC MECHANISMS
There are multiple factors that may contribute to the development of functional somatic syndromes and their symptom expression (Fig. 2). Relatively greater contribution from peripheral factors, such as afferent excitation and upregulation of afferent pathways, may be observed in patients with mild to moderate symptoms. Moderate to severe symptoms may be due to disinhibition at the level of central modulation of pain leading to lack of pain inhibition at the peripheral afferent level. Contributory factors may be life stressors, psychiatric diagnoses, poor coping skills, and abuse. Common pathophysiologic mechanisms that have been proposed in functional pain syndromes will be discussed in this review.
Enhanced pain perception
Enhanced visceral perception, also referred to as visceral hypersensitivity, has been demonstrated in IBS, and has been proposed as a biomarker for the diagnosis or treatment response in a subset of IBS patients.50 Studies utilizing an electronic computerized barostat distention device report that enhanced visceral hypersensitivity can be observed in 30–40% of IBS patients.51,52 Proposed pathophysiologic mechanisms underlying enhanced visceral perception in IBS patients are as follows: (i) altered central processing of visceral afferent sensory input,53 (ii) peripheral sensitization of sensory afferents, (iii) increased intestinal membrane permeability associated with altered expression of colonic microRNAs,50 and (iv) psychological tendency to report pain and urgency.54 In a study where rectal perceptual thresholds were compared in IBS, IBS with coexistent FM (IBS+FM), and healthy controls, both the IBS and IBS+FM groups had significantly lower discomfort thresholds to rectal distention (increased visceral sensitivity) compared to controls.55
Somatic pain perception has also been measured in IBS and IBS with comorbid FM (IBS+FM). Originally, studies showed that IBS patients had somatic hyposensitivity.56 In one study, pressure stimuli were applied to the skin with increasing tension in IBS, IBS+FM, and healthy controls.57 Irritable bowel syndrome patients demonstrated somatic hypoalgesia and IBS+FM patients had somatic hyperalgesia compared to controls.56 In contrast, subsequent studies have shown somatic hypersensitivity in IBS.55,58 In a study done by Calderella et al.,55 IBS patients had normal skin sensitivity to electrical stimuli, but lowered pain thresholds at the subcutis and muscle when compared to healthy controls. However, patients with IBS+FM and FM alone had significantly lower pain thresholds to controls at all three sites.55 In another study by Moshiree et al.,59 IBS+FM patients had enhanced thermal sensitivity compared to IBS only patients during foot immersion in hot water.59 These studies support that IBS patients have visceral hyperalgesia and may also have somatic hypersensitivity depending on the presence of comorbid FM or greater illness severity.
Enhanced pain perception has also been demonstrated in FD.60,61 Balloon distention studies have demonstrated visceral hypersensitivity involving the stomach, but not the rectum.60,62 In a study by Tack et al.,62 gastric hypersensitivity was found in a subset of FD patients and was associated with symptoms of belching, postprandial epigastric pain, and weight loss.62 Similarly, in another study by Mertz et al.,60 reduced thresholds to discomfort, fullness, or pain was observed in FD patients compared to healthy controls and organic dyspeptic patients (dyspepsia associated with tissue injury/irritation).60 Studies have evaluated visceral perception in patients with coexistent FD and IBS (FD+IBS). In a study by Corsetti et al.,61 gastric perception to barostat-administered balloon distension was assessed during a meal in FD and FD+IBS patients.61 FD+IBS patients showed lower gastric thresholds (increased sensitivity) for first perception and discomfort compared to patients with FD only.61 In another study by Holtmann et al.,63 patients with FD and/or IBS collectively demonstrated lower thresholds for first perception and maximum tolerated pressure to small intestinal distension compared to controls.63 There were no differences between the FD only, IBS only, and FD+IBS groups. Similar to IBS, enhanced pain perception in FD is thought to be due to several factors including central and peripheral sensitization.60
Enhanced pain perception and persistent pain are the main defining features of FM.64 A number of studies have demonstrated enhanced pain perception in FM. A study employing self-reported surveys of daily lives of FM patients, rheumatoid arthritis patients, and healthy controls revealed increased generalized sensory hypersensitivity to somatic (tactile) and non-somatic (olfactory, taste, auditory) stimuli in FM patients compared to the other two groups.65 Other studies have demonstrated that FM patients have lowered thresholds to painful electrical stimuli of the hand and arm,66 and to thermal stimuli given via a CO2 laser stimulator to the dorsum of hand, and tender and nontender points compared to healthy controls.67 In a study by Chun et al.,68 patients with FM perceived pain at rectal phasic distension pressures that were intermediate between patients with IBS and healthy controls. Rectal pain thresholds in FM patients were no different from those in IBS patients or controls.68 However, the number of subjects within each group was small. The mechanisms of increased multisensory perception are postulated to be due to central sensitization and increased sensory processing of painful stimuli.67 However, peripheral involvement in the maintenance of central sensitization has been observed as well.69
Increased pain perception has also been demonstrated in patients with TMD.70,71 Lowered thresholds to various painful stimuli, such as thermal, mechanical, or ischemic pain were found in TMD patients compared to patients without TMD.70,71 One of these studies examined pain sensitivity at a symptomatic (i.e., oropharynx) and an asymptomatic site (i.e., upper extremity), and both sites showed increased pain sensitivity in TMD patients compared to controls.70 This finding suggests a generalized upregulation of central processing to sensory stimuli,70 similar to what has been postulated in FM.
In IC/PBS, somatic and visceral pain perception has been studied. In a small study, Ness et al.72 demonstrated enhanced hypersensitivity in IC/PBS patients, using multisensory stimuli. Thermal stimuli was delivered through a contact heat probe applied to the forearm, deep tissue stimuli was applied to the upper trapezius, masseter, and ulna using a handheld algometer, and ischemic stimuli was induced using a modified tourniquet on the arm. Furthermore, bladder distension pain was measured by intravesicle infusion of normal saline. These multisensory stimuli induced higher sensory ratings in IC/PBS patients compared to healthy subjects.72 In another study, IC/PBS patients reported increased pain compared to baseline during intravesicle instillation of ice water compared to patients with neurogenic detrusor overactivity and stress urinary incontinence.73 Increased pain sensitivity was thought to be due to an increased expression of immunoreactive nerve fibers, known as TRPM8.73 Central factors have been shown to play a role as well. Ness et al.72 found that IC patients had greater catastrophizing and vigilance to sensory stimuli than controls.
Persistent and widespread pain is notable in CFS patients74 and thus, the theory of enhanced pain perception has been postulated.75 Past studies showed enhanced pain perception or lowered pain thresholds to multiple types of sensory stimuli such as electrical, mechanical pressure, and heat to various regions of the body, including the skin and muscle tissue in CFS patients compared to healthy controls.75,76 The etiology of enhanced pain perception in CFS is not clear, but dysfunction in pain inhibitory control (“anti-nociceptive”) pathways within the CNS,75,77 dysfunction in afferent sensory input,75 decreased antioxidants with increased oxidative stress,76 and altered brain activation patterns78-80 have been proposed as mechanisms of this phenomenon.
In summary, patients with these functional pain syndromes demonstrate evidence of enhanced perception at sites associated with their predominant symptoms. However, when patients have coexistent conditions, they may have more widespread hypersensitivity.
Altered brain activation patterns
Irritable bowel syndrome has been commonly associated with altered brain-gut interactions. The brain-gut axis is comprised of bidirectional neural pathways linking the enteric, autonomic, neuroendocrine, and central nervous systems.81,82 It is still not completely clear which areas of the brain-gut axis are consistently dysregulated in IBS, as there could be sensitization at the level of the afferent enteric neurons,83 dysregulated processing of sensory information at the spinal or supraspinal level, abnormal brain modulation of sensory or nociceptive input from the gut,82,84 and/or failure to sufficiently activate descending pain inhibitory pathways or activation of pain facilitatory pathways.85 To further delineate the brain-gut axis and its role in IBS, neuroimaging studies have been performed. Although there are brain regions, which were similarly activated in response to rectal distension, there were also areas, which were differentially activated in IBS patients compared to controls86,87 and patients with inflammatory bowel disease.85 These differentially activated areas included somatosensory processing regions (e.g., thalamus, insula) and cognitive and affective processing regions (e.g., anterior cingulate cortex [ACC]), and limbic and paralimbic regions (e.g., amygdala).82,87 Interestingly, altered central activation patterns were similar in response to actual and expected but undelivered rectal distensions in IBS patients compared to healthy controls.87 The ACC has been a region of interest in IBS. Studies demonstrated greater activation of ACC in IBS patients compared to healthy controls.58,86,88 In a recent study, Larsson et al.88 found that IBS patients with visceral hypersensitivity (based on perceptual thresholds to rectal distension) had greater activation of the insula and reduced deactivation of the perigenual ACC during noxious rectal distensions compared to normosensitive patients and healthy controls.88
Neuroimaging studies conducted in FD patients support the presence of altered brain-gut interactions.89,90 Brain imaging during the resting state as well as during gastric distension has been performed in FD patients.91,92 A positron emission tomography (PET) study measuring resting state brain activity in FD patients showed increased activation of the ACC, insula, thalamus, cerebellum, and middle cingulate cortex compared to healthy controls.93 Significant positive correlations between dyspepsia symptom severity and activation of the ACC, insula, thalamus, cerebellum, and middle cingulate were seen, although significantly negative correlations between dyspepsia-specific quality of life and these regions were noted.93 Another neuroimaging study was performed during proximal stomach distension in FD patients and healthy controls.92 This study revealed that hypersensitive FD patients (had lower gastric distension pressures) showed significant brain activations in regions not noted in healthy subjects and normosensitive FD patients.92 Interestingly, this study failed to show activation of the medial pain system, i.e., insula, ACC, and thalamus, which suggests that in FD patients, there may be a dysfunction in activating the descending anti-nociceptive pathways of the medial pain system, and/or no additional cortical activity volume recruitment as distension increases.92 These findings support the presence of aberrant brain activity in FD patients and its possible role in symptom severity and global outcome, with some similarities observed between FD and IBS in terms of areas of brain activation (i.e., insula, ACC and thalamus).
In FM, there is also evidence of altered central activation patterns compared to controls.94 In a functional magnetic resonance imaging (fMRI) study conducted by Gracely et al.,95 FM patients had increased activations of the primary and secondary somatosensory cortex, insula, and ACC compared to “pain free” subjects using non-painful and painful thermal stimuli as well as painful pressure stimuli.95,96 In a PET study conducted in FM patients with and without coexistent IBS, increased regional cerebral blood flow to the midcingulate cortex was seen in response to noxious somatic stimuli in FM patients with IBS and to visceral stimuli in IBS-only patients.97 This study suggests that stimulus-specific enhancement of midcingulate responses to sensory stimuli occurs in both FM and IBS and may be associated with cognitive enhancement of either visceral (IBS) or somatic (IBS+FM) sensory input. Studies utilizing a dopamine receptor antagonist and opioid receptor agonists in FM patients suggest that a possible dysfunction in the central dopaminergic and opioidergic systems exist that results in ongoing widespread pain.98 In addition, neuroimaging studies postulate dysregulation of the central nervous system and its sensory pathways with exaggerated neural responses to afferent sensory stimuli, augmented sensory processing, and/or increased anticipation, attention, and memories from pain derived from abnormal cognitive and sensory processing.96
Neuroimaging studies have also been performed in TMD patients. A small study used voxel-based morphometry and MRI of the brain to assess changes in the brain structure or morphology in TMD patients. Decreased gray matter volume in regions, including the left ACC, right anterior insular cortex, and superior temporal gyrus were found.99 In addition, decreased white matter volume in the prefrontal cortex was noted. These findings signify possible alterations of the central pain system as a contributing cause of TMD.99 Jiang et al.100 conducted a fMRI study to assess brain activation patterns during evoked clenching in three sets of patients: TMD patients, patients with atypical facial pain, and healthy subjects. Patients with TMD and atypical facial pain showed different areas of brain activation: TMD patients showed activation of the postcentral gyrus, cingulate gyrus, and prefrontal cortices, whereas atypical facial pain patients had activation of the thalamus and ACC. This study noted that different pain pathways are activated even when the same stimulus is applied,100 suggesting a possible intrinsic dysfunction of the pain pathway or central processing of pain. Furthermore, magnetoencephalography was performed in TMD patients and demonstrated evidence of altered brain responses to innocuous tactile stimulation above the masseter muscle compared to healthy controls.101 These findings support the presence of abnormal central pain processing in TMD.
Neuroimaging studies are limited in IC/PBS. Using an acoustic startle paradigm, increased central nervous system excitability in response to a visceral-related threat was seen in female PBS/IC patients compared to healthy controls.102 In a similarly designed paradigm, comparable findings were found in IBS patients compared to controls.103
In CFS patients, low perfusion of the brainstem was observed compared to healthy controls using brain single-photon emission computed tomography (SPECT) imaging.78 In another study, PET scans of the brain demonstrated decreased metabolism in the right mediofrontal cortex and brainstem in CFS patients compared to healthy controls.79 Another brain perfusion imaging study was conducted in CFS patients without current depressive symptoms, patients with depression, and healthy controls. Both CFS and depression patients had increased perfusion to the right thalamus, pallidum and putamen, while CFS patients also had increased perfusion to the left thalamus.104 Despite the limitations that depression patients were taking tricyclic antidepressants and that a high proportion of CFS patients had a prior history of depression and were on antidepressants, this study suggested a possible role of an overactive thalamus associated with hypervigilance and hyperattentiveness in CFS patients.104 However, neuroimaging studies have reported contradictory results in CFS,105,106 and therefore the role of structural or functional changes in the brain in the pathogenesis of CFS is not yet well understood.
In summary, brain imaging is an innovative research tool to study the role of CNS alterations in IBS and other functional somatic syndromes. Neuroimaging modalities, analytical methodology, and types of stimuli differed in many of these studies. However, in response to painful stimuli, there were similar brain regions, which were activated in the different functional pain syndromes. These included sensory processing regions (i.e., thalamus, insula), and cognitive and affective processing regions (i.e., ACC). Therefore, these studies suggest there are shared alterations in central circuits involved in sensory perception that may contribute to the etiology of these syndromes.
Peripheral immune activation
There are multiple components within the immune system such as T lymphocytes, mast cells, cytokines, and toll-like receptors (TLR) that have been postulated to play a role in pathophysiology of IBS. Mediators released by immune cells, such as mast cells, have been proposed to increase the sensitivity of primary sensory afferents in IBS patients and to lower the pain threshold. Barbara et al.107 found that the mean area of colonic mucosa occupied by mast cells in close proximity to sensory neurons was significantly greater than that in controls. Furthermore, they also showed that the number of mast cells in the vicinity of nerve fibers positively correlated with severity and frequency of abdominal pain/discomfort in IBS patients.108 In addition, soluble factors, such as proteases, histamine, and serotonin, released from mucosal biopsies of IBS patients were shown to excite human submucosal neurons.109 However, in a study by Klooker et al.,110 both hypersensitive and normosensitive IBS patients had fewer mast cells in the rectum and descending colon compared to healthy controls. In addition, tryptase and histamine release in the supernatant of rectal biopsies were similar among IBS patients with visceral hypersensitivity, IBS patients with visceral normosensitivity, and controls.110
While elevated levels of certain cytokines from serum or stimulated peripheral blood mononuclear cells have been reported in IBS patients compared to controls, proinflammatory cytokines in the colonic mucosa are not significantly elevated although lower levels of the anti-inflammatory cytokine interleukin, IL-10, have been reported.111 In one study, IBS patients had increased plasma levels of IL-6 and IL-8, whereas those with IBS and comorbid conditions such as FM, premenstrual dysmorphic disorder, and CFS also had increased plasma levels of IL-1 β and TNF-α.112 Other recent studies found increased mRNA expression of TLR 3,4, and 5 in the colonic mucosa of rats with colonic hypersensitivity exposed to early life stress.113 Compared to healthy controls, IBS patients had increased mRNA expression of TLR 4 and 5 and decreased expression of TLR 7 and 8 in the colonic mucosa.114 Taken together with the finding of increased fecal levels of human β-defensin-2 in IBS patients compared to healthy controls,115 the innate immune system may play a role in IBS pathogenesis.114 Chronic stress has been postulated to induce immunologic responses in IBS patients that in turn can lead to altered gut function, including sensory, motor, and secretory changes.113 Differing views on the role of immunologic responses in pathophysiology of IBS suggest that IBS is a heterogenous condition and there are likely phenotypic subgroups primarily characterized by different biologic mechanisms.
Immune system involvement in the pathogenesis of FD has been studied.116,117 Eosinophils were found in higher concentrations in the duodenal mucosa of patients with FD compared to healthy controls, but there was no difference in the amount of eosinophils in the stomach.118 A proposed mechanism of the pathophysiologic role of eosinophils in FD is immune activation with cytokine release that can result in pain, neural excitation, and muscle spasm.116 For example, when an allergen or infection disturbs the duodenum, eosinophils are activated, which in turn, release cytokines and other degranulation products such as nerve growth factors and major basic proteins.119 These degranulation factors can directly act on sensory nerves and muscarinic receptors that can lead to neurologic dysfunction and increased smooth muscle contractions.119 In addition, immune system dysfunction following an infectious gastroenteritis has also been associated with FD. Duodenal biopsies showed increased macrophages and areas of focally concentrated T-cells in “post-infectious FD.”120 These studies suggest that immune dysregulation exists in FD, although further studies are needed.
Although limited, immune system dysfunction has also been studied in FM.121,122 A study by Blanco et al.121 demonstrated evidence of tissue injury from skin biopsies in FM patients, including the presence of proinflammatory cytokines, increased mast cells, IgG deposits, and expression of nociceptive glutamate NMDA receptors. They postulated a potential role of mast cells in releasing proinflammatory products that then results in “CNS hypersensitivity” along with fatigue, pain, and other local and systemic symptoms of FM.121 Another study hypothesized that corticotropin-releasing hormone (CRH) and substance P trigger the release of mast cells resulting in an enhanced inflammatory state.122 Although there are studies that support the presence of elevated cytokines and immune dysfunction in fibromyalgia,123-126 other studies do not.127 Additional elucidation is needed.
In TMD, immune dysfunction has been hypothesized to play a pathophysiologic role.128,129 The presence of elevated levels of immunoglobulins in temporomandibular joint synovial fluids was thought to result in an inflammatory reaction where complement activation ultimately leads to articular cartilage damage through increased blood vessel permeability, which allows entry of lysosomal enzymes.128 Another study found a positive correlation between joint effusion at the tempormandibular joint and levels of cytokines, such as IL-1, IL-6 and tumor necrosis factor (TNF) receptors I and II, in TMD patients suggesting that joint effusions in TMD are inflammatory.129
Immune system involvement, particularly that involving mast cells, has been proposed in IC/PBS.130,131 Mastocytosis, or increased mast cells, has been shown in the bladder of a subset of patients with IC.130,132 Mastocytosis has been more commonly demonstrated in IC/PBS with Hunner’s ulcers than IC/PBS without ulcers, however, this discrepancy may be due to methodological differences.131 Two studies demonstrated increased mast cells in the bladder of IC/PBS patients although there were differences in location (detrusor, submucosal layer) and type of IC/PBS.133,134 It has been speculated that mast cells within the bladder uroepithelium can release neurotransmitters and neuropeptides, which then activate C-fiber afferent sensory nerves, which ultimately results in visceral hypersensitivity and hyperalgesia.131 Another proposed mechanism is that mast cells release granules (i.e., IL-6, glycoprotein-like substances), which cause vasodilatation, inflammation, muscle contraction, and inflammation.133,135
Chronic fatigue syndrome has also been associated with mast cells and immunologic dysfunction.77,136 Overproduction of proinflammatory cytokines (e.g., TNF-α),137 other inflammatory markers (i.e., C-reactive protein [CRP], beta2-microglobulin),74 and reduction of natural-killer cells have been demonstrated in CFS compared to healthy controls. These immune system markers are thought to be associated with the flu-like symptoms and fatigue seen in CFS.136,138 However, other studies demonstrated that when body mass index and other patient factors (age, depression, etc.) were taken into account, there were no major differences in levels of inflammatory markers (i.e., CRP, IL-6) between controls and CFS patients.139,140 Interestingly, in a subset of patients with CFS, IBS, and FM, no IgE-mediated food hypersensitivity was found.141
In summary, the immune system seems to play a pathophysiologic role in at least a subset of patients with these functional somatic syndromes. Common immune markers of interest are mast cells and proinflammatory cytokines such as TNF-α, IL-1 and IL-6. Although enhanced immune activation has been hypothesized to have a pathophysiologic role in symptom development, further studies demonstrating a convincing causal association between immune markers and symptom expression are needed.
Post-infectious etiology
Post-infectious IBS (PI-IBS) is by description the onset of IBS symptoms following an infectious gastroenteritis.142 This form of IBS is more commonly characterized by non-constipation predominant IBS with findings of elevated immune cells in rectal biopsies.142 In a recent review, the patient reported incidence of PI-IBS ranged from 4% to 36%.143 PI-IBS has been reported following bacterial infections, such as Salmonella, Shigella, and Campylobacter, although it has also been associated with viral144 and parasitic infections.145 Proposed risk factors for PI-IBS include female gender, severe infection, and chronic stress at the time of the infection.146
Although there is less extensive data than in IBS, infectious agents have also been associated with the onset of FD.117,147,148 Suzuki et al.147 proposed that H. pylori-associated FD is due to abnormal ghrelin or leptic secretion, altered expression of muscle-specific microRNAs, and duodenal inflammatory cell infiltration. However, the association between H. pylori and FD has been questioned because some studies do not show eradication of FD symptoms after H. pylori eradication.149,150 Giardia lamblia infection has been also associated with FD.148 Patients who experienced a G. lamblia infection completed structured surveys and questionnaires 12–30 months later and 24% patients met Rome II diagnostic criteria for FD.148 Another study conducted in Spain found a five-fold increase in the incidence of dyspepsia following Salmonella gastroenteritis.151 The mechanism underlying postinfectious functional dyspepsia is still not clear, but persistent T-cell aggregates, increased duodenal macrophages, immune activation, and impaired gastric accommodation with dysfunction in gastric nitrergic neurons have been hypothesized to have etiologic roles.117
The onset of FM has also been associated with infection.152 Associations between FM and hepatitis C, HIV, parvovirus, and chronic Lyme disease have been reported.153-156 However, other studies failed to demonstrate a relationship between infections such as hepatitis C and FM.157,158 Despite reported associations between infection and FM, no definite causal–effect relationship has been described.
Studies assessing a relationship between infection and TMD are limited. Case reports of tuberculosis in the temporomandibular joint has been reported,159,160 although it is not known if this infection is associated with the persistent pain observed in the TMD patients.159,161 In pediatric patients with TMD (from infancy to 18 years of age), approximately 18.4% had a prior history of infection of the surrounding structures of the temporomandibular joint such as chronic otitis media, staphylococcus infection, or parotid gland infection leading to septic arthritis.162 Furthermore, TMD symptoms in HIV patients receiving antiviral treatment has been reported,163 suggesting a possible association between HIV and TMD. As most studies are small and limited, more information is needed to demonstrate if a “post-infectious TMD” syndrome exists.
Although IC/PBS is clinically diagnosed in the absence of a urinary tract infection, an association with prior urinary tract infections has been found.164,165 Furthermore, gynecological infections have also been demonstrated in patients with IC/PBS.166 In a study by Berger et al.,165 bacteriuria was found in IC/PBS, although no symptom improvement was seen with resolution of bacteriuria with antibiotics and symptom flares were not associated with episodes of bacteriuria.165,167 Thus, a clear etiologic link between infection and IC/PBS has not been confirmed.
Infectious agents, including bacteria, protozoa, and viruses, have long been suspected as playing a causal role in CFS, which has also been labeled “post-infectious fatigue syndrome.”168,169 Mycoplasma infection has been associated with CFS.170 Postulated pathophysiologic mechanisms to explain this relationship include dysregulation of the synthetase/RNase L antiviral pathway and immune system dysregulation (e.g., mycoplasmas acting as T- and B-cell activators).170 Also, viruses such as human herpes virus and a xenotropic murine leukemia related virus have been shown to be associated CFS. Viruses are hypothesized to cause CFS in part due to their damaging effects on T-cell memory function.171,172 In addition, about 5% of patients who have had Giardia enteritis subsequently developed a post-infectious fatigue syndrome. Similar to that observed with PI-IBS, these patients were found to have experienced more stressful life events prior to getting the Giardia infection.173 Another study showed increased risk of having CFS and IBS after following a cohort of a population exposed to acute giardiasis outbreak, with relative risk of having both CFS and IBS post exposure as 6.8 (95% CI 5.3-8.5).174 However, not all studies were able to find a close association between infection and CFS.175 Therefore, although it may be still premature to state that there is a causal relationship, an association appears to exist.
In summary, infection has been proposed as a pathophysiologic factor contributing to the development of these functional somatic syndromes. It appears that infectious source can be bacterial, viral, or parasitic.
Neuroendocrine dysregulation
Given the stress-sensitive nature of IBS and other functional pain syndromes, hypothalamic-pituitary-adrenal (HPA) axis function at basal states and in response to hormone challenge or stress have been studied. Although alterations in HPA axis activity have been reported in IBS patients, some studies report an increased response whereas others show no or a blunted response at baseline or provoked by stress or hormone challenge,176 In one study evaluating the basal circadian rhythm of ACTH and cortisol in women with IBS (with or without FM) and healthy women, both IBS and IBS+FM patients demonstrated blunted ACTH and elevated cortisol levels suggesting a dysregulated HPA axis compared to controls.177 In patients with IBS and healthy controls, early adverse life events, such as childhood abuse and parental loss, were associated with an enhanced cortisol response to a visceral stressor.178
In FD, studies evaluating neuroendocrine dysregulations have been performed.179 In a relatively small study by Bohmelt et al.,179 HPA axis function was compared in patients with IBS, non-ulcer dyspepsia, or IBS with coexistent non-ulcer dyspepsia, and healthy controls. Results suggested attenuated pituitary and adrenocortical activity in the patient group who had lower salivary cortisol levels and a less robust response to CRH.179 In a study by Mutsuura et al.,180 a relationship between HPA axis function and mood was reported in FD and other functional syndromes (patients had a diagnosis of either FD, IBS, chronic tension headaches, CFS, chronic pain, or other functional GI disorder) compared to healthy controls. In the patient group, there was a negative correlation with depressive mood and morning free salivary cortisol although a positive correlation was observed in healthy controls.180 Although these studies showed some evidence of HPA axis dysregulation in FD patients, limitations included combining FD with other functional syndrome, differences in medications used by these patients, and underlying comorbid depression/anxiety/or stress state, which may affect cortisol levels.
In FM patients, abnormalities in the HPA axis have been more extensively studied.181,182 For example, in a recent study, free salivary cortisol levels were measured at multiple times of the day in FM patients and healthy controls, who also completed questionnaires assessing psychophysiological measures, such as sleep disturbances and perceived stress. FM patients demonstrated significantly lower cortisol levels throughout the day as well as higher scores on the psychophysiological assessments (i.e., more perceived stress, sleeping problems).182 Although some studies showed blunted cortisol levels in FM patients, other studies did not demonstrate statistically significant decreased cortisol levels.183,184 Differences in methodology, patient characteristics, and other factors could explain the contrary results.
Studies have investigated the role of the HPA axis in TMD, although to a lesser extent than IBS and FM. Elevated cortisol levels were observed in TMD patients.185,186 However, in another study, morning free salivary cortisol levels did not differ between TMD patients and healthy controls.187
In IC/PBS, HPA axis function involvement has been studied as well. In a study by Lutgendorf et al.,188 24-h urine cortisol and free salivary cortisol were measured at various times of the day and did not differ between IC/PBS patients and healthy controls. However, within the IC/PBS group, there was a negative correlation between cortisol level and urinary symptoms, particularly urinary urgency.188 Interestingly, cats with naturally occurring IC demonstrated smaller adrenal gland size at autopsy with decreased adrenal reserve and increased CRF activity, suggesting enhanced negative feedback of glucocorticoids at the adrenal level.189,190
There has also been interest in HPA axis function in CFS, because symptoms of fatigue are shared by CFS and Addison’s disease and are explained in part by low cortisol levels.191,192 Papadopoulos and Cleare191 summarized the four main features of low cortisol levels in CFS: a mild degree of hypocortisolism, attenuated diurnal variation of cortisol, enhanced negative feedback, and blunted HPA axis responsiveness.
Basal and stimulated HPA axis functions are highly dynamic and affected by a multitude of factors, and thus, it is not entirely surprising that results can be inconsistent. However, studies overall suggest that HPA axis dysregulation exists in IBS and other related functional syndromes. It is possible that early dysregulation of the HPA axis results in elevated HPA axis hormone levels, whereas more prolonged dysregulation leads to ultimately blunted cortisol levels due to ineffective HPA axis responsiveness.
Genetic factors
A genetic disposition to developing IBS or other chronic functional pain syndromes has been advocated. Multiple candidate genes are being studied, but those that have been linked to IBS include genes that encode for serotonin-related proteins (e.g., SERT, 5HT3-receptor), noradrenergic signaling markers (e.g., alpha-adrenergic receptor), and immunologic markers (e.g., IL-10).193-195 Relatives of IBS patients are two to three times more likely to have IBS than others.196 Furthermore, family case studies of IBS patients have shown a familial aggregation of IBS, however, these studies were limited by confounding factors such as environmental exposure and shared disease susceptibility genes for other IBS risk factors (e.g., depression, anxiety).193 Although a few candidate gene polymorphisms may be associated with IBS, studies are limited by relatively small sample sizes, lack of reproducibility in larger studies, and the heterogeneity and lack of reliability of the clinical phenotype of IBS.196
In FD, genetics involvement in the pathophysiology is also being studied. Interleukin and migratory inhibiting factor genes were found to be associated with the FD subset of patients with H. pylori.197 Other genes of interest in FD are serotonin receptor promoter genes, catechol-o-methyltransferase (COMT), cyclooxygenase-1, and G protein activated MPA kinase pathway (GNβ3).198 However, a clear link between specific genetic polymorphisms and FD symptoms has yet to be demonstrated.
Genetic factors have been studied in FM. Proposed genes of interest include those associated with COMT, serotonin receptors, and mu-opioid receptor (OPRM1).199,200 Gene x experience interactions were found with genetic polymorphisms of COMT and OPRM1. Specific genotypes were associated with changes in daily positive affect regardless of increased daily pain ratings.199
Investigations attempting to identify a cluster of genes, which play a role in pain syndromes, particularly TMD, are ongoing. Genes of interest include those that encode for COMT,201 beta-2 adrenergic receptor,202 and estrogen receptor.203
In IC/PBS, familial clustering204 and twin studies204 suggest that a genetic susceptibility to developing IC/PBS exists. Although there are concerns for confounding factors in genetic association studies in IC/PBS, the presence of a greater concordance of IC/PBS in monozygotic compared to dizygotic twins supports that genetic factors play a role in IC/PBS and that not all of the inheritance pattern is attributed to environmental factors or comorbid conditions.
There are various genes of interest in FM and CFS, such as those linked to COMT and glucocorticoid and mineralocorticoid receptors.205 Also, glutamate receptor genes involved in the circadian rhythm have been studied in CFS.206 In a recent meta-analysis, 11 genes were identified, which may be associated with CFS symptoms.207 For example, the symptom of fatigue in CFS has been hypothesized to be associated to the gene WAVE3, which is involved in regulation of brain cytokines.207 One limitation of gene association studies in CFS is the presence of co-morbidities that may confound results.208-210
Currently, genetic studies suggest that individual genes have relatively small effects and multiple genes combined with environmental and developmental factors may collectively increase the vulnerability to developing these functional pain syndromes.
CONCLUSION
In summary, defining the exact pathophysiologic mechanisms underlying IBS and other functional pain syndromes still remains elusive. Various mechanisms such as enhanced pain perception, altered brain activation, dysregulations in immunologic and neuroendocrine function, and genetic factors appear to be involved to some extent. Whether one proposed mechanism predominates over another, and whether there is one unifying mechanism explaining these functional disorder requires further study. However, when examining some of the GI and non-GI functional disorders, key unifying features can be observed. First, these functional disorders tend to overlap within the same individual. Second, studies have demonstrated common pathophysiologic disturbances, such as the presence of central sensitization associated with enhanced pain perception. Third, these disorders are often responsive to similar treatment interventions, such as antidepressants and psychological and behavioral therapies. Studies are increasingly supportive of the possibility that these disorders are multifactorial and patient populations may cluster into phenotypic subgroups that are characterized by a unique set of pathophysiologic mechanisms and treatment responses.
Abbreviations
- ACC
anterior cingulate cortex
- CFS
chronic fatigue syndrome
- FD
functional dyspepsia
- FGID
functional gastrointestinal disorders
- FM
fibromyalgia
- fMRI
functional magnetic resonance imaging
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- IC/PBS
interstitial cystitis/painful bladder syndrome
- IL
interleukin
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- TMD
temporomandibular joint disorder
- TNF
tumor necrosis factor
Footnotes
FUNDING
This work has been in part supported by the National Institutes of Health grants R01 AR46122 and P50 DK64539 to Dr. Chang.
DISCLOSURES
Drs. Kim and Chang have nothing to disclose.
Correction added after online publication 21 August 2012: Copyright line for the reuse of Figures 1 and 2 modified from “Permission obtained from Functional Somatic Syndromes, Eds: EA Mayer and C Bushnell” to “Adapted from229. This figure has been reproduced with permission of the International Association for the Study of Pain ® (IASP ®). The figure may not be reproduced for any other purpose without permission.”
REFERENCES
- 1.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–96. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimentel M. The prevalence of small intestinal bacterial overgrowth in irritable bowel syndrome: IBS vs healthy controls (not historical definitions) Gut. 2008;57:1334–5. [PubMed] [Google Scholar]
- 4.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–59. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 7.Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Tack J. Factors associated with co-morbid irritable bowel syndrome and chronic fatigue-like symptoms in functional dyspepsia. Neurogastroenterol Motil. 2011;23:524–e202. doi: 10.1111/j.1365-2982.2010.01667.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Hibi T. Overlap syndrome of functional dyspepsia and irritable bowel syndrome - are both diseases mutually exclusive? J Neurogastroenterol Motil. 2011;17:360–5. doi: 10.5056/jnm.2011.17.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221–7. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead WE, Palsson O, Jones KR. Systemic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AS, Panahian-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: responses to a Web-based survey. J Sex Marital Ther. 2003;29(Suppl. 1):45–58. doi: 10.1080/713847126. [DOI] [PubMed] [Google Scholar]
- 12.Wu EQ, Birnbaum H, Mareva M, et al. Interstitial Cystitis: cost, treatment and co-morbidities in an employed population. Pharmacoeconomics. 2006;24:55–65. doi: 10.2165/00019053-200624010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Vege SS, Locke GR,, 3rd, Weaver AL, Farmer SA, Melton LJ,, 3rd, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004;79:1501–6. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 14.Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith G, Levin JS. Effect of sleep quality on symptoms of irritable bowel syndrome. Dig Dis Sci. 1993;38:1809–14. doi: 10.1007/BF01296103. [DOI] [PubMed] [Google Scholar]
- 16.Mahadeva S, Goh KL. Anxiety, depression and quality of life differences between functional and organic dyspepsia. J Gastroenterol Hepatol. 2011;26(Suppl. 3):49–52. doi: 10.1111/j.1440-1746.2011.06656.x. [DOI] [PubMed] [Google Scholar]
- 17.da Silva RA, Pinheiro RT, Horta BL, Moraes I, Faria AD. Functional dyspepsia and depression as an associated factor. Arq Gastroenterol. 2006;43:293–8. doi: 10.1590/s0004-28032006000400010. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen AY, Jonkers DM, Leue C, et al. Dysfunctional cognitions, anxiety and depression in irritable bowel syndrome. J Clin Gastroenterol. 2010;44:e236–41. doi: 10.1097/MCG.0b013e3181eed5d8. [DOI] [PubMed] [Google Scholar]
- 19.Fietta P, Manganelli P. Fibromyalgia and psychiatric disorders. Acta Biomed. 2007;78:88–95. [PubMed] [Google Scholar]
- 20.Cohen H, Neumann L, Haiman Y, Matar MA, Press J, Buskila D. Prevalence of post-traumatic stress disorder in fibromyalgia patients: overlapping syndromes or post-traumatic fibromyalgia syndrome? Semin Arthritis Rheum. 2002;32:38–50. doi: 10.1053/sarh.2002.33719. [DOI] [PubMed] [Google Scholar]
- 21.Cella M, White PD, Sharpe M, Chalder T. Cognitions, behaviours and co-morbid psychiatric diagnoses in patients with chronic fatigue syndrome. Psychol Med. 2012;9:1–6. doi: 10.1017/S0033291712000979. [DOI] [PubMed] [Google Scholar]
- 22.Dansie EJ, Furberg H, Afari N, et al. Conditions comorbid with chronic fatigue in a population-based sample. Psychosomatics. 2012;53:44–50. doi: 10.1016/j.psym.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher RM, Marbach JJ, Raphael KG, Dohrenwend BP, Cloitre M. Is major depression comorbid with temporomandibular pain and dysfunction syndrome? A pilot study. Clin J Pain. 1991;7:219–25. doi: 10.1097/00002508-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol. 2008;180:1378–82. doi: 10.1016/j.juro.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins KE, Eberhart N, Hilton L, et al. Depressive disorders and panic attacks in women with bladder pain syndrome/interstitial cystitis: a population-based sample. Gen Hosp Psychiatry. 2011;33:143–9. doi: 10.1016/j.genhosppsych.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henningsen P, Derra C, Turp JC, Hauser W. Functional somatic pain syndromes: summary of hypotheses of their overlap and etiology. Schmerz. 2004;18:136–40. doi: 10.1007/s00482-003-0299-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–93. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 28.Heitkemper M, Jarrett M. Irritable bowel syndrome: does gender matter? J Psychosom Res. 2008;64:583–7. doi: 10.1016/j.jpsychores.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Lange M, Karpinski N, Krohn-Grimberghe B, Petermann F. Patients with fibromyalgia: gender differences. Schmerz. 2010;24:262–6. doi: 10.1007/s00482-010-0924-0. [DOI] [PubMed] [Google Scholar]
- 30.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther. 2010;32:738–55. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleibeuker JH, Thijs JC. Functional dyspepsia. Curr Opin Gastroenterol. 2004;20:546–50. doi: 10.1097/00001574-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Talley NJ, Choung RS. Whither dyspepsia? A historical perspective of functional dyspepsia, and concepts of pathogenesis and therapy in 2009. J Gastroenterol Hepatol. 2009;24(Suppl. 3):S20–8. doi: 10.1111/j.1440-1746.2009.06067.x. [DOI] [PubMed] [Google Scholar]
- 34.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–6. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mearin F, Calleja JL. Defining functional dyspepsia. Rev Esp Enferm Dig. 2012;103:640–7. doi: 10.4321/s1130-01082011001200006. [DOI] [PubMed] [Google Scholar]
- 36.Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–37. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 38.Raspe H. Rheumatism epidemiology in Europe. Soz Praventivmed. 1992;37:168–78. doi: 10.1007/BF01624572. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 40.Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72:930–47. [PMC free article] [PubMed] [Google Scholar]
- 41.Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–83. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 42.Dworkin SF, Huggins KH, Wilson L, et al. A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain. 2002;16:48–63. [PubMed] [Google Scholar]
- 43.Carmeli E, Sheklow SL, Blommenfeld I. Comparative Study of Repositioning Splint Therapy and Passive Manual Range of Motion Techniques for Anterior Displaced Temporomandibular Discs with Unstable Excursive Reduction. Physiotherapy. 2001;2001:26–36. [Google Scholar]
- 44.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28-29, 1987. J Urol. 1988;140:203–6. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 45.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 46.Moutzouris DA, Falagas ME. Interstitial cystitis: an unsolved enigma. Clin J Am Soc Nephrol. 2009;4:1844–57. doi: 10.2215/CJN.02000309. [DOI] [PubMed] [Google Scholar]
- 47.Avellaneda Fernandez A, Perez Martin A, Izquierdo Martinez M, et al. Chronic fatigue syndrome: aetiology, diagnosis and treatment. BMC psychiatry. 2009;9(Suppl. 1):S1. doi: 10.1186/1471-244X-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fluge O, Bruland O, Risa K, et al. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS One. 2011;6:e26358. doi: 10.1371/journal.pone.0026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Q, Verne GN. New insights into visceral hypersensitivity–clinical implications in IBS. Nat Rev Gastroenterol Hepatol. 2011;8:349–55. doi: 10.1038/nrgastro.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naliboff BD, Munakata J, Fullerton S, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–12. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q, Fillingim RB, Riley JL,, 3rd, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454–61. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–75. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Dorn SD, Palsson OS, Thiwan SI, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–9. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caldarella MP, Giamberardino MA, Sacco F, et al. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782–9. doi: 10.1111/j.1572-0241.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 56.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 57.Casellas F, Lopez-Vivancos J, Badia X, Vilaseca J, Malagelada JR. Impact of surgery for Crohn’s disease on healthrelated quality of life. Am J Gastroenterol. 2000;95:177–82. doi: 10.1111/j.1572-0241.2000.01681.x. [DOI] [PubMed] [Google Scholar]
- 58.Verne GN, Himes NC, Robinson ME, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 59.Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin J Pain. 2007;23:323–30. doi: 10.1097/AJP.0b013e318032e496. [DOI] [PubMed] [Google Scholar]
- 60.Mertz H, Fullerton S, Naliboff B, Mayer EA. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814–22. doi: 10.1136/gut.42.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152–9. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 62.Tack J, Caenepeel P, Corsetti M, Janssens J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology. 2004;127:1058–66. doi: 10.1053/j.gastro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Holtmann G, Goebell H, Talley NJ. Functional dyspepsia and irritable bowel syndrome: is there a common pathophysiological basis? Am J Gastroenterol. 1997;92:954–9. [PubMed] [Google Scholar]
- 64.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 65.Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Arch Phys Med Rehabil. 2011;92:653–6. doi: 10.1016/j.apmr.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diers M, Koeppe C, Yilmaz P, et al. Pain ratings and somatosensory evoked responses to repetitive intramuscular and intracutaneous stimulation in fibromyalgia syndrome. J Clin Neurophysiol. 2008;25:153–60. doi: 10.1097/WNP.0b013e31817759c5. [DOI] [PubMed] [Google Scholar]
- 67.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185–93. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 68.Chun A, Desautels S, Slivka A, et al. Visceral algesia in irritable bowel syndrome, fibromyalgia, and sphincter of oddi dysfunction, type III. Dig Dis Sci. 1999;44:631–6. doi: 10.1023/a:1026682113096. [DOI] [PubMed] [Google Scholar]
- 69.Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12:T61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–51. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 72.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983–7. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 73.Mukerji G, Waters J, Chessell IP, Bountra C, Agarwal SK, Anand P. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006;6:31. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchwald D. Fibromyalgia and chronic fatigue syndrome: similarities and differences. Rheum Dis Clin North Am. 1996;22:219–43. doi: 10.1016/s0889-857x(05)70270-2. [DOI] [PubMed] [Google Scholar]
- 75.Nijs J, Meeus M, Van Oosterwijck J, et al. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur J Clin Invest. 2012;42:203–12. doi: 10.1111/j.1365-2362.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 76.Vecchiet J, Cipollone F, Falasca K, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335:151–4. doi: 10.1016/s0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 77.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–9. doi: 10.1016/j.pain.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 78.Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM. 1995;88:767–73. [PubMed] [Google Scholar]
- 79.Tirelli U, Chierichetti F, Tavio M, et al. Brain positron emission tomography (PET) in chronic fatigue syndrome: preliminary data. Am J Med. 1998;105:54S–8S. doi: 10.1016/s0002-9343(98)00179-x. [DOI] [PubMed] [Google Scholar]
- 80.Mountz JM, Bradley LA, Modell JG, et al. Fibromyalgia in women Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum. 1995;38:926–38. doi: 10.1002/art.1780380708. [DOI] [PubMed] [Google Scholar]
- 81.Ohman L, Simren M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39:201–15. doi: 10.1016/j.dld.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17:604–12. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Ducrotte P. Irritable bowel syndrome: from the gut to the brain-gut. Gastroenterol Clin Biol. 2009;33:703–12. doi: 10.1016/j.gcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 86.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–8. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 87.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 88.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–72. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mearin F, Cucala M, Azpiroz F, Malagelada JR. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991;101:999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- 90.Chua AS. Reassessment of functional dyspepsia: a topic review. World J Gastroenterol. 2006;12:2656–9. doi: 10.3748/wjg.v12.i17.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Oudenhove L, Vandenberghe J, Dupont P, et al. Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study. Am J Gastroenterol. 2010;105:913–24. doi: 10.1038/ajg.2010.39. [DOI] [PubMed] [Google Scholar]
- 92.Vandenberghe J, Dupont P, Van Oudenhove L, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684–93. doi: 10.1053/j.gastro.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 93.Zeng F, Qin W, Liang F, et al. Abnormal resting brain activity in patients with functional dyspepsia is related to symptom severity. Gastroenterology. 2011;141:499–506. doi: 10.1053/j.gastro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Caro XJ, Winter EF, Dumas AJ. A subset of fibromyalgia patients have findings suggestive of chronic inflammatory demyelinating polyneuropathy and appear to respond to IVIg. Rheumatology. 2008;47:208–11. doi: 10.1093/rheumatology/kem345. [DOI] [PubMed] [Google Scholar]
- 95.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 96.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–78. [PubMed] [Google Scholar]
- 97.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003;98:1354–61. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- 98.Ceko M, Bushnell MC, Gracely RH. Neurobiology underlying fibromyalgia symptoms. Pain Res Treat. 2012;2012:585419. doi: 10.1155/2012/585419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerstner G, Ichesco E, Quintero A, Schmidt-Wilcke T. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: a voxel-based morphometry study. J Orofacl Pain. 2011;25:99–106. [PubMed] [Google Scholar]
- 100.Jiang T, Li J, Jin Z, Wang YW, Feng HL, Ishikawa T. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = [Comparison of atypical orofacial pain and temporomandibular disorders synovitis pain processing in the human brain using functional magnetic resonance imaging] Chin J Stomatol. 2006;41:670–3. [PubMed] [Google Scholar]
- 101.Alonso AA, Koutlas IG, Leuthold AC, Lewis SM, Georgopoulos AP. Cortical processing of facial tactile stimuli in temporomandibular disorder as revealed by magnetoencephalography. Exp Brain Res. 2010;204:33–45. doi: 10.1007/s00221-010-2291-6. [DOI] [PubMed] [Google Scholar]
- 102.Twiss C, Kilpatrick L, Craske M, et al. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol. 2009;181:2127–33. doi: 10.1016/j.juro.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naliboff BD, Waters AM, Labus JS, et al. Increased acoustic startle responses in IBS patients during abdominal and nonabdominal threat. Psychosom Med. 2008;70:920–7. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacHale SM, Lawrie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550–6. doi: 10.1192/bjp.176.6.550. [DOI] [PubMed] [Google Scholar]
- 105.Puri BK, Jakeman PM, Agour M, et al. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3-T MRI study. Br J Radiol. 2012;85:e270–3. doi: 10.1259/bjr/93889091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perrin R, Embleton K, Pentreath VW, Jackson A. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol. 2010;83:419–23. doi: 10.1259/bjr/85621779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 108.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 109.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–34. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–21. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 111.Chang L, Adeyemo M, Karagiannidis I, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262–72. doi: 10.1038/ajg.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scully P, McKernan DP, Keohane J, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–43. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 113.McKernan DP, Nolan A, Brint EK, et al. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS One. 2009;4:e8226. doi: 10.1371/journal.pone.0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329–36. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 115.Langhorst J, Junge A, Rueffer A, et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404–10. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 116.Walker MM, Warwick A, Ung C, Talley NJ. The role of eosinophils and mast cells in intestinal functional disease. Curr Gastroenterol Rep. 2011;13:323–30. doi: 10.1007/s11894-011-0197-5. [DOI] [PubMed] [Google Scholar]
- 117.Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251–7. doi: 10.5056/jnm.2010.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175–83. doi: 10.1016/j.cgh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 119.Walker MM, Salehian SS, Murray CE, et al. Implications of eosinophilia in the normal duodenal biopsy - an association with allergy and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:1229–36. doi: 10.1111/j.1365-2036.2010.04282.x. [DOI] [PubMed] [Google Scholar]
- 120.Kindt S, Tertychnyy A, de Hertogh G, Geboes K, Tack J. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil. 2009;21:832–e56. doi: 10.1111/j.1365-2982.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 121.Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403–12. doi: 10.1007/s10067-010-1474-7. [DOI] [PubMed] [Google Scholar]
- 122.Lucas HJ, Brauch CM, Settas L, Theoharides TC. Fibromyalgia–new concepts of pathogenesis and treatment. Int J Immunopathol Pharmacol. 2006;19:5–10. [PubMed] [Google Scholar]
- 123.Ross RL, Jones KD, Bennett RM, Ward RL, Druker BJ, Wood LJ. Preliminary Evidence of Increased Pain and Elevated Cytokines in Fibromyalgia Patients with Defective Growth Hormone Response to Exercise. Open Immunol J. 2010;3:9–18. doi: 10.2174/1874226201003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology. 2001;40:743–9. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 125.Wang H, Buchner M, Moser MT, Daniel V, Schiltenwolf M. The role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 months. Clin J Pain. 2009;25:1–4. doi: 10.1097/AJP.0b013e31817e13a3. [DOI] [PubMed] [Google Scholar]
- 126.Togo F, Natelson BH, Adler GK, et al. Plasma cytokine fluctuations over time in healthy controls and patients with fibromyalgia. Exp Biol Med. 2009;234:232–40. doi: 10.3181/0808-RM-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia-Lozano JR, Capilla-Sevilla C, Garcia-Lopez O, Moreno-Gallego I. Correlation between cytokines and anxious-depressive symptoms in patients with fibromyalgia. Reumatol Clin. 2008;4:136–9. doi: 10.1016/S1699-258X(08)71822-9. [DOI] [PubMed] [Google Scholar]
- 128.Chang H, Israel H. Analysis of inflammatory mediators in temporomandibular joint synovial fluid lavage samples of symptomatic patients and asymptomatic controls. J Oral Maxillofac Surg. 2005;63:761–5. doi: 10.1016/j.joms.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 129.Kaneyama K, Segami N, Sun W, Sato J, Fujimura K. Levels of soluble cytokine factors in temporomandibular joint effusions seen on magnetic resonance images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:411–8. doi: 10.1016/j.tripleo.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 130.Theoharides TC, Pang X, Letourneau R, Sant GR. Interstitial cystitis: a neuroimmunoendocrine disorder. Ann N Y Acad Sci. 1998;840:619–34. doi: 10.1111/j.1749-6632.1998.tb09601.x. [DOI] [PubMed] [Google Scholar]
- 131.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 132.Larsen S, Thompson SA, Hald T, et al. Mast cells in interstitial cystitis. Br J Urol. 1982;54:283–6. doi: 10.1111/j.1464-410x.1982.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 133.Larsen MS, Mortensen S, Nordling J, Horn T. Quantifying mast cells in bladder pain syndrome by immunohistochemical analysis. BJU Int. 2008;102:204–7. doi: 10.1111/j.1464-410X.2008.07576.x. discussion 207. [DOI] [PubMed] [Google Scholar]
- 134.Feltis JT, Perez-Marrero R, Emerson LE. Increased mast cells of the bladder in suspected cases of interstitial cystitis: a possible disease marker. J Urol. 1987;138:42–3. doi: 10.1016/s0022-5347(17)42981-8. [DOI] [PubMed] [Google Scholar]
- 135.Park CS, Bochner BS. Potential targeting of siglecs, mast cell inhibitory receptors, in interstitial cystitis. Int Neurourol J. 2011;15:61–3. doi: 10.5213/inj.2011.15.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aoki T, Miyakoshi H, Usuda Y, Herberman RB. Low NK syndrome and its relationship to chronic fatigue syndrome. Clin Immunol Immunopathol. 1993;69:253–65. doi: 10.1006/clin.1993.1178. [DOI] [PubMed] [Google Scholar]
- 137.Moss RB, Mercandetti A, Vojdani A. TNF-αlpha and chronic fatigue syndrome. J Clin Immunol. 1999;19:314–6. doi: 10.1023/a:1020595709352. [DOI] [PubMed] [Google Scholar]
- 138.Patarca R. Cytokines and chronic fatigue syndrome. Ann N Y Acad Sci. 2001;933:185–200. doi: 10.1111/j.1749-6632.2001.tb05824.x. [DOI] [PubMed] [Google Scholar]
- 139.Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–37. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 140.Nater UM, Youngblood LS, Jones JF, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 141.Berstad A, Undseth R, Lind R, Valeur J. Functional bowel symptoms, fibromyalgia and fatigue: A food-induced triad? Scand J Gastroenterol. 2012 doi: 10.3109/00365521.2012.690045. doi:10.3109/00365521.2012.690 045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebocontrolled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77–84. doi: 10.1046/j.1365-2036.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 143.Schwille-Kiuntke J, Frick JS, Zanger P, Enck P. Post-infectious irritable bowel syndrome-a review of the literature. Z Gastroenterol. 2011;49:997–1003. doi: 10.1055/s-0031-1281581. [DOI] [PubMed] [Google Scholar]
- 144.Porter CK, Gormley R, Tribble DR, Cash BD, Riddle MS. The Incidence and gastrointestinal infectious risk of functional gastrointestinal disorders in a healthy US adult population. Am J Gastroenterol. 2011;106:130–8. doi: 10.1038/ajg.2010.371. [DOI] [PubMed] [Google Scholar]
- 145.Wood NJ. Infection: Giardia lamblia is associated with an increased risk of both IBS and chronic fatigue that persists for at least 3 years. Nat Rev Gastroenterol Hepatol. 2011;8:597. doi: 10.1038/nrgastro.2011.176. [DOI] [PubMed] [Google Scholar]
- 146.Spiller RC. Inflammation as a basis for functional GI disorders. Best Pract Res Clin Gastroenterol. 2004;18:641–61. doi: 10.1016/j.bpg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 147.Suzuki H, Matsuzaki J, Hibi T. What is the difference between Helicobacter pylori-associated dyspepsia and functional dyspepsia? J Neurogastroenterol Motil. 2011;17:124–30. doi: 10.5056/jnm.2011.17.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hanevik K, Dizdar V, Langeland N, Hausken T. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 2009;9:27. doi: 10.1186/1471-230X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Talley NJ, Janssens J, Lauritsen K, Racz I, Bolling-Sternevald E, The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months’ follow up. BMJ. 1999;318:833–7. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blum AL, Talley NJ, O’Morain C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia Omeprazole plus Clarithromycin and Amoxicillin Effect One Year after Treatment (OCAY) Study Group. N Engl J Med. 1998;339:1875–81. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 151.Mearin F, Perez-Oliveras M, Perello A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 152.Ablin JN, Shoenfeld Y, Buskila D. Fibromyalgia, infection and vaccination: two more parts in the etiological puzzle. J Autoimmun. 2006;27:145–52. doi: 10.1016/j.jaut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 153.Simms RW, Zerbini CA, Ferrante N, Anthony J, Felson DT, Craven DE. Fibromyalgia syndrome in patients infected with human immunodeficiency virus The Boston City Hospital Clinical AIDS Team. Am J Med. 1992;92:368–74. doi: 10.1016/0002-9343(92)90266-e. [DOI] [PubMed] [Google Scholar]
- 154.Buskila D, Shnaider A, Neumann L, et al. Musculoskeletal manifestations and autoantibody profile in 90 hepatitis C virus infected Israeli patients. Semin Arthritis Rheum. 1998;28:107–13. doi: 10.1016/s0049-0172(98)80043-7. [DOI] [PubMed] [Google Scholar]
- 155.Dinerman H, Steere AC. Lyme disease associated with fibromyalgia. Ann Intern Med. 1992;117:281–5. doi: 10.7326/0003-4819-117-4-281. [DOI] [PubMed] [Google Scholar]
- 156.Buyukkose M, Kozanoglu E, Basaran S, Bayramoglu O, Yarkin F. Seroprevalence of parvovirus B19 in fibromyalgia syndrome. Clin Rheumatol. 2009;28:305–9. doi: 10.1007/s10067-008-1044-4. [DOI] [PubMed] [Google Scholar]
- 157.Narvaez J, Nolla JM, Valverde-Garcia J. Lack of association of fibromyalgia with hepatitis C virus infection. J Rheumatol. 2005;32:1118–21. [PubMed] [Google Scholar]
- 158.Palazzi C, D’Amico E, D’Angelo S, Nucera A, Petricca A, Olivieri I. Hepatitis C virus infection in Italian patients with fibromyalgia. Clin Rheumatol. 2008;27:101–3. doi: 10.1007/s10067-007-0737-4. [DOI] [PubMed] [Google Scholar]
- 159.Ranganathan LK, Mathew GC, Gandhi S, Manohar M. Tuberculosis of temporomandibular joint presenting as swelling in the preauricular region. J Oral Maxillofac Surg. 2012;70:e28–31. doi: 10.1016/j.joms.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 160.Helbling CA, Lieger O, Smolka W, Iizuka T, Kuttenberger J. Primary tuberculosis of the TMJ: presentation of a case and literature review. Int J Oral Maxillofac Surg. 2010;39:834–8. doi: 10.1016/j.ijom.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 161.Gandhi S, Ranganathan LK, Bither S, Koshy G. Tuberculosis of temporomandibular joint: a case report. J Oral Maxillofac Surg. 2011;69:e128–30. doi: 10.1016/j.joms.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 162.Allori AC, Chang CC, Farina R, Grayson BH, Warren SM, McCarthy JG. Current concepts in pediatric temporomandibular joint disorders: Part 1. Etiology, epidemiology, and classification. Plast Reconstr Surg. 2010;126:1263–75. doi: 10.1097/PRS.0b013e3181ebe207. [DOI] [PubMed] [Google Scholar]
- 163.Fiorentino PM, Piancino MG, Debernardi C, Attard N. Temporomandibular joint disorders during HIV infection: a case report. J Orofac Pain. 2009;23:174–6. [PubMed] [Google Scholar]
- 164.Warren JW, Brown V, Jacobs S, Horne L, Langenberg P, Greenberg P. Urinary tract infection and inflammation at onset of interstitial cystitis/painful bladder syndrome. Urology. 2008;71:1085–90. doi: 10.1016/j.urology.2007.12.091. [DOI] [PubMed] [Google Scholar]
- 165.Berger RE. Re: Prevalence and Impact of Bacteriuria and/or Urinary Tract Infection in Interstitial Cystitis/Painful Bladder Syndrome. J Urol. 2011;185:155. doi: 10.1016/S0022-5347(11)60074-8. [DOI] [PubMed] [Google Scholar]
- 166.Li GZ, Zhang N, Du P, et al. Risk factors for interstitial cystitis/painful bladder syndrome in patients with lower urinary tract symptoms: a Chinese multi-center study. Chin Med J. 2010;123:2842–6. [PubMed] [Google Scholar]
- 167.Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:551–4. doi: 10.1007/s00192-006-0184-9. [DOI] [PubMed] [Google Scholar]
- 168.Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325–37. doi: 10.1055/s-0031-1287654. [DOI] [PubMed] [Google Scholar]
- 169.Chia J, Chia A, Voeller M, Lee T, Chang R. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J Clin Pathol. 2010;63:165–8. doi: 10.1136/jcp.2009.070466. [DOI] [PubMed] [Google Scholar]
- 170.Nijs J, Nicolson GL, De Becker P, Coomans D, De Meirleir K. High prevalence of Mycoplasma infections among European chronic fatigue syndrome patients Examination of four Mycoplasma species in blood of chronic fatigue syndrome patients. FEMS Immunol Med Microbiol. 2002;34:209–14. doi: 10.1111/j.1574-695X.2002.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 171.Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26:24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 172.Mikovits JA, Lombardi VC, Pfost MA, Hagen KS, Ruscetti FW. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Virulence. 2010;1:386–90. doi: 10.4161/viru.1.5.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Naess H, Nyland M, Hausken T, Follestad I, Nyland HI. Chronic fatigue syndrome after Giardia enteritis: clinical characteristics, disability and long-term sickness absence. BMC Gastroenterol. 2012;12:13. doi: 10.1186/1471-230X-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;61:214–9. doi: 10.1136/gutjnl-2011-300220. [DOI] [PubMed] [Google Scholar]
- 175.Henrich TJ, Li JZ, Felsenstein D, et al. Xenotropic murine leukemia virusrelated virus prevalence in patients with chronic fatigue syndrome or chronic immunomodulatory conditions. J Infect Dis. 2010;202:1478–81. doi: 10.1086/657168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–5. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamicpituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Videlock EJ, Adeyemo M, Licudine A, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–62. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67:288–94. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- 180.Mutsuura H, Kanbara K, Fukunaga M, et al. Depression and anxiety correlate differently with salivary free cortisol in the morning in patients with functional somatic syndrome. Appl Psychophysiol Biofeedback. 2009;34:291–8. doi: 10.1007/s10484-009-9110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Crofford LJ. The hypothalamic-pituitary-adrenal stress axis in fibromyalgia and chronic fatigue syndrome. Z Rheumatol. 1998;57(Suppl. 2):67–71. doi: 10.1007/s003930050239. [DOI] [PubMed] [Google Scholar]
- 182.Riva R, Mork PJ, Westgaard RH, Ro M, Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med. 2010;17:223–33. doi: 10.1007/s12529-010-9097-6. [DOI] [PubMed] [Google Scholar]
- 183.Tak LM, Cleare AJ, Ormel J, et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183–94. doi: 10.1016/j.biopsycho.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 184.Tanriverdi F, Karaca Z, Unluhizarci K, Kelestimur F. The hypothalamopituitary-adrenal axis in chronic fatigue syndrome and fibromyalgia syndrome. Stress. 2007;10:13–25. doi: 10.1080/10253890601130823. [DOI] [PubMed] [Google Scholar]
- 185.Yang D, Ye L. Temporomandibular disorders and declarative memory. Med Hypotheses. 2011;76:723–5. doi: 10.1016/j.mehy.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 186.Da Silva Andrade A, Gamero GH, Pereira LJ, Junqueira Zanin IC, Gaviao MB. Salivary cortisol levels in young adults with temporomandibular disorders. Minerva Stomatol. 2008;57:109–16. [PubMed] [Google Scholar]
- 187.Nilsson AM, Dahlstrom L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk related temporomandibular disorders. Acta Odontol Scand. 2010;68:284–8. doi: 10.3109/00016357.2010.494620. [DOI] [PubMed] [Google Scholar]
- 188.Lutgendorf SK, Kreder KJ, Rothrock NE, et al. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. J Urol. 2002;167:1338–43. [PubMed] [Google Scholar]
- 189.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172:1242–8. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- 190.Westropp JL, Welk KA, Buffington CA. Small adrenal glands in cats with feline interstitial cystitis. J Urol. 2003;170:2494–7. doi: 10.1097/01.ju.0000095566.63870.66. [DOI] [PubMed] [Google Scholar]
- 191.Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. 2011;8:22–32. doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- 192.Demitrack MA, Dale JK, Straus SE, et al. Evidence for impaired activation of the hypothalamic-pituitaryadrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–34. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 193.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138:1276–85. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Camilleri M. Evolving concepts of the pathogenesis of irritable bowel syndrome: to treat the brain or the gut? J Pediatr Gastroenterol Nutr. 2009;48(Suppl. 2):S46–8. doi: 10.1097/MPG.0b013e3181a1174b. [DOI] [PubMed] [Google Scholar]
- 195.Schmulson M, Pulido-London D, Rodriguez O, et al. Lower Serum IL-10 Is an Independent Predictor of IBS Among Volunteers in Mexico. Am J Gastroenterol. 2012;107:747–53. doi: 10.1038/ajg.2011.484. [DOI] [PubMed] [Google Scholar]
- 196.Saito YA. The role of genetics in IBS. Gastroenterol Clin North Am. 2011;40:45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Arisawa T, Tahara T, Shibata T, et al. Genetic polymorphisms of molecules associated with inflammation and immune response in Japanese subjects with functional dyspepsia. Int J Mol Med. 2007;20:717–23. [PubMed] [Google Scholar]
- 198.Holtmann G, Talley NJ. Hypothesis driven research and molecular mechanisms in functional dyspepsia: the beginning of a beautiful friendship in research and practice? Am J Gastroenterol. 2006;101:593–5. doi: 10.1111/j.1572-0241.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 199.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychol. 2010;29:134–42. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bondy B, Spaeth M, Offenbaecher M, et al. The T102C polymorphism of the 5-HT2A-receptor gene in fibromyalgia. Neurobiol Dis. 1999;6:433–9. doi: 10.1006/nbdi.1999.0262. [DOI] [PubMed] [Google Scholar]
- 201.Slade GD, Diatchenko L, Ohrbach R, Maixner W. Orthodontic Treatment, Genetic Factors and Risk of Temporomandibular Disorder. Semin Orthod. 2008;14:146–56. doi: 10.1053/j.sodo.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Diatchenko L, Anderson AD, Slade GD, et al. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:449–62. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. Int J Oral Maxillofac Surg. 2007;36:391–4. doi: 10.1016/j.ijom.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 204.Warren JW, Keay SK, Meyers D, Xu J. Concordance of interstitial cystitis in monozygotic and dizygotic twin pairs. Urology. 2001;57:22–5. doi: 10.1016/s0090-4295(01)01120-7. [DOI] [PubMed] [Google Scholar]
- 205.Light KC, White AT, Tadler S, Iacob E, Light AR. Genetics and Gene Expression Involving Stress and Distress Pathways in Fibromyalgia with and without Comorbid Chronic Fatigue Syndrome. Pain Res Treat. 2012;2012:427869. doi: 10.1155/2012/427869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smith AK, Fang H, Whistler T, Unger ER, Rajeevan MS. Convergent genomic studies identify association of GRIK2 and NPAS2 with chronic fatigue syndrome. Neuropsychobiology. 2011;64:183–94. doi: 10.1159/000326692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pihur V, Datta S. Meta analysis of Chronic Fatigue Syndrome through integration of clinical, gene expression, SNP and proteomic data. Bio-information. 2011;6:120–4. doi: 10.6026/97320630006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kaiser J. Biomedicine. Genes and chronic fatigue: how strong is the evidence? Science. 2006;312:669–71. doi: 10.1126/science.312.5774.669. [DOI] [PubMed] [Google Scholar]
- 209.Buchwald D, Herrell R, Ashton S, et al. A twin study of chronic fatigue. Psychosom Med. 2001;63:936–43. doi: 10.1097/00006842-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 210.Walsh CM, Zainal NZ, Middleton SJ, Paykel ES. A family history study of chronic fatigue syndrome. Psychiatr Genet. 2001;11:123–8. doi: 10.1097/00041444-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 211.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 212.Thompson WG. Irritable bowel syndrome: prevalence, prognosis and consequences. CMAJ. 1986;134:111–3. [PMC free article] [PubMed] [Google Scholar]
- 213.El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643–54. doi: 10.1111/j.1365-2036.2004.01897.x. [DOI] [PubMed] [Google Scholar]
- 214.Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457–64. doi: 10.4065/mcp.2010.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Boneva RS, Maloney EM, Lin JM, et al. Gynecological history in chronic fatigue syndrome: a population-based case-control study. J Womens Health. 2011;20:21–8. doi: 10.1089/jwh.2009.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Poveda Roda R, Bagan JV, Diaz Fernandez JM, Hernandez Bazan S, Jimenez Soriano Y. Review of temporomandibular joint pathology Part I: classification, epidemiology and risk factors. Med Oral Patol Oral Cir Bucal. 2007;12:E292–8. [PubMed] [Google Scholar]
- 217.Nacul LC, Lacerda EM, Pheby D, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med. 2011;9:91. doi: 10.1186/1741-7015-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.LeResche L, Mancl LA, Drangsholt MT, Huang G, Von Korff M. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007;129:269–78. doi: 10.1016/j.pain.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. J Urol. 1999;161:549–52. [PubMed] [Google Scholar]
- 220.Wang A, Liao X, Xiong L, et al. The clinical overlap between functional dyspepsia and irritable bowel syndrome based on Rome III criteria. BMC Gastroenterol. 2008;8:43. doi: 10.1186/1471-230X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. Br J Rheumatol. 1991;30:220–2. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- 222.Pace FMG, Bollani S, Sarzi-Puttini P, Bianchi Porro G. Visceral Sensitivity in Patients with Fibromyalgia and in Normal Patients. Gastroenterology. 1997;112:A802. [Google Scholar]
- 223.Sperber AD, Dekel R. Irritable Bowel Syndrome and Co-morbid Gastrointestinal and Extra-gastrointestinal Functional Syndromes. J Neurogastroenterol Motil. 2010;16:113–9. doi: 10.5056/jnm.2010.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Goldenberg DL, Simms RW, Geiger A, Komaroff AL. High frequency of fibromyalgia in patients with chronic fatigue seen in a primary care practice. Arthritis Rheum. 1990;33:381–7. doi: 10.1002/art.1780330311. [DOI] [PubMed] [Google Scholar]
- 225.Gomborone JE, Gorard DA, Dewsnap PA, Libby GW, Farthing MJ. Prevalence of irritable bowel syndrome in chronic fatigue. J R Coll Physicians Lond. 1996;30:512–3. [PMC free article] [PubMed] [Google Scholar]
- 226.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–7. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 227.Clemens JQ, Brown SO, Kozloff L, Calhoun EA. Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol. 2006;175:963–6. doi: 10.1016/S0022-5347(05)00351-4. discussion 967. [DOI] [PubMed] [Google Scholar]
- 228.Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123–31. doi: 10.1016/j.juro.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chang L, Drossman D. Chapter 5: Irritable Bowel Syndrome and Related Functional Disorders. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes: Presentation and Pathophysicology. IASP Press; Seattle, WA: 2009. pp. 87–120. [Google Scholar]