Abstract
The objectives of this study were to (a) evaluate and compare the ability of ex vivo-generated induced regulatory T cells (iTregs) and freshly isolated natural Tregs (nTregs) to reverse/attenuate preexisting intestinal inflammation in a mouse model of chronic colitis and (b) quantify the Tregtargeted gene expression profiles of these two Treg populations. We found that ex vivo-generated iTregs were significantly more potent than nTregs at attenuating preexisting colitis. This superior therapeutic activity was associated with increased accumulation of iTregs within the mesenteric lymph nodes and large and significant reductions in interleukin (IL)-6 and IL-17A expression in the colons of iTreg- versus nTreg-treated mice. The enhanced immunosuppressive activity of iTregs was not because of increased expression or stability of Foxp3 as iTregs and nTregs obtained from the mesenteric lymph nodes, and colons of reconstituted mice expressed similar levels of this important transcription factor. In addition, we observed a total of 27 genes that were either upregulated or downregulated in iTregs when compared with nTregs. Although iTregs were found to be superior at reversing established disease, their message levels of IL-10 and IL-35 and surface expression of the gut-homing molecules CCR9 and α4β7 were significantly reduced when compared with nTregs. Taken together, our data demonstrate that ex vivo-generated iTregs are significantly more potent than nTregs at attenuating preexisting gut inflammation despite reduced expression of classical regulatory cytokines and gut-homing molecules. Our data suggest that the immunosuppressive activity of iTregs may be because of their ability to directly or indirectly decrease expression of IL-6 and IL-17A within the inflamed bowel.
Keywords: Crohn’s disease, iTregs, Foxp3, cytokines, IL-17, IL-6, T cells
There is a growing body of experimental and clinical data suggesting that the chronic intestinal inflammation observed in the inflammatory bowel diseases (Crohn’s disease and ulcerative colitis) may originate as a dysregulated immune response to enteric bacterial antigens in genetically susceptible individuals.1,2 There is also good evidence to suggest that, in the absence of appropriate regulatory mechanisms, naive T cells become primed and polarized to yield colitogenic effector T helper (Th)1/Th17 cells via their interaction with enteric antigen-loaded dendritic cells within the gutdraining mesenteric lymph nodes and intestinal lamina propria.3 Unfettered generation and expansion of these disease-producing effector cells result in the enhanced production of Th1 and Th17 cytokines (e.g., interferon [IFN]-γ, interleukin [IL]-17, and IL-22) in the case of Crohn’s disease or the overproduction of Th2 cytokines (IL-5 and IL-13) in ulcerative colitis.4 Studies from numerous laboratories using a variety of immune-manipulated and genetically engineered animal models of chronic intestinal inflammation strongly suggest that regulatory T cells (Tregs; CD4+Foxp3+) are important in regulating and suppressing potentially aggressive immune responses toward autoantigens and commensal enteric antigens.5–8 Two major subsets of Tregs have been identified including natural Tregs (nTregs) that are generated within the thymus and induced Tregs (iTregs) that are produced in the periphery from conventional (CD4+Foxp3 −) T cells in a transforming growth factor (TGF)-β- and IL-2-dependent manner.3
Natural Tregs represent approximately 10% to 15% of the peripheral CD4+ T cells in a healthy mouse (<2% in humans) and are enriched within the CD4+CD25+ subset.5,7,9,10 These Tregs develop in the thymus and, by virtue of their ability to recognize self and foreign antigens, are thought to play critical roles in the preservation of self-tolerance and prevention of immunopathology following chronic immune stimulation. Historically, surface expression of the IL-2 receptor-α chain (CD25) has been useful for identifying nTregs; however, it should be noted that not all CD25+ T cells are nTregs and not all nTregs express CD25. To date, the most specific marker of nTregs is the forkhead winged helix transcription factor Foxp3.6,8,9 Foxp3 is a member of the forkhead box family and has been shown to be both necessary and sufficient for nTreg generation and activity. Rudensky and coworkers8–10 have demonstrated that Foxp3 expression identifies nTregs as the major naturally occurring regulatory cells in mice. Natural Tregs have been shown to suppress various immune responses by a number of different mechanisms including secretion of regulatory cytokines (e.g., IL-10, TGF-β, and IL-35), competition for essential cytokines and growth factors (e.g., IL-2), and contact-dependent mechanisms.7,10–13
Although much of the published literature has focused on thymus-derived nTregs, there is an accumulating literature demonstrating that conventional CD4+Foxp3 − T cells may be converted to Foxp3-expressing iTregs in a TGF-β-dependent manner in vitro and in vivo.14–19 Powrie and coworkers have found that the intestine and the gut-draining mesenteric lymph nodes are major sites for the generation of iTregs.20,21 They report that functionally specialized intestinal dendritic cells (CD103+ dendritic cells) convert, in an antigen-specific and retinoic acid (RA)-dependent manner, conventional CD4+Foxp3− T cells to Foxp3-expressing iTregs that exhibit enhanced expression of CCR9 and α4β7. It is thought that increased expression of these “gut homing” adhesion molecules may imprint these regulatory cells such that they will traffic to small and/or large intestine where they prevent/limit enteric antigen-driven inflammation.15 In addition, recent studies suggest that iTreg generation may be important for regulating allergic/Th2 inflammation at additional mucosal interfaces.22 Generation of iTregs in situ produces regulatory cells that are phenotypically and functionally very similar, if not identical, to thymic-derived nTregs. Currently, there are no specific surface markers that reliably distinguish iTregs from nTregs. Several studies have reported that cotransfer of small numbers of nTregs or iTregs with naive (CD45RBhigh) T cells into SCID or RAG−/− recipients dramatically attenuates the development of chronic intestinal inflammation that develops in the T-cell transfer model of chronic colitis.23–32 Although nTregs are capable of attenuating the preexisting colitis induced by adoptive transfer of CD45RBhigh T cells into SCID or RAG−/− recipients, this generally requires the transfer of relatively large numbers of nTregs and may take several weeks or months to occur.12,33–35
Although there is a great deal of excitement regarding the therapeutic potential of Tregs to treat inflammatory bowel disease and other chronic inflammatory/autoimmune diseases, there are several unanswered questions and concerns with the use of these lymphocytes as a cell-based therapeutic strategy. Some investigators have suggested that Tregs are unstable in vitro and in vivo and may convert to pathogenic effector (Th1/Th17) T cells under physiological or inflammatory conditions.36–40 However, a recent study demonstrates that Foxp3 expression and suppressive activity is maintained following adoptive transfer of nTregs into recipients under physiological conditions or into mice following radiation-induced lymphopenia, inflammation, or bacterial infection.41 Another concern related to Treg-based therapy in the treatment of chronic inflammatory disorders is the relative paucity of nTregs and iTregs.42–44 For example, it has been estimated that approximately 25 to 50 L of blood would be required to prepare suitable numbers of high purity Tregs (5 × 108 cells) to treat graftversus-host disease.45 Thus, isolation of sufficient numbers of peripheral nTregs and/or iTregs for therapeutic use is untenable.
We have recently reported a novel ex vivo method for generating large numbers of Foxp3-expressing iTregs from conventional CD4+Foxp3 − T cells.30 This method requires polyclonal T-cell activation in the absence of costimulation but presence of IL-2, TGF-β, and all-trans RA. We found that these iTregs were significantly more potent than freshly isolated nTregs at suppressing T-cell activation in vitro and as effective as nTregs at preventing the development of chronic colitis in vivo. To our knowledge, only 1 study has directly compared the ability of ex vivo-generated iTregs versus nTregs to reverse/attenuate preexisting intestinal inflammation. Haribhai et al.46 showed that nTregs were much more effective than ex vivo-generated iTregs at attenuating established colitis in mice. However, these investigators used iTregs that were generated using culture conditions that differed significantly from those described in our protocol. Furthermore, they did not quantify and compare the suppressive properties of the ex vivo-generated iTregs versus freshly isolated nTregs in vitro. Therefore, we aimed to (a) evaluate and compare the ability of ex vivo-generated iTregs versus freshly isolated nTregs to reverse/attenuate preexisting intestinal inflammation in a mouse model of chronic colitis and (b) quantify and compare the targeted gene expression profiles of these 2 Treg populations. Data presented in the current study demonstrate that iTregs produced using our published protocol30 are superior to freshly isolated nTregs at attenuating preexisting gut inflammation despite having reduced expression of the classical regulatory cytokines (IL-10 and IL-35) and gut-homing molecules. The possible mechanisms responsible for this enhanced suppressive activity are discussed.
MATERIALS AND METHODS
Animals
B6.SJL-Ptprca Pepcb/BoyJ (CD45.1; C57BL/6), B6.129S7-Rag1tm1Mom/J (RAG1−/− C57BL/6), and B6.Cg-Foxp3tm2Tch/J (Foxp3-GFP; C57BL/6) mice were originally acquired from The Jackson Laboratory (Bar Harbor, ME). The mice were bred at the animal care facility at Louisiana State University Health Sciences Center, Shreveport, and given standard laboratory rodent chow and water ad libitum. All mice were maintained on 12/12-hour light/dark cycles in standard animal cages with filter tops under specific pathogen-free conditions. All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center, and Shreveport, and performed according to the criteria outlined by the National Institutes of Health.
Isolation of nTregs and Ex Vivo Generation of iTregs
Freshly isolated nTregs were prepared from the spleens of Foxp3-GFP mice following CD4 negative selection, staining with CD4-APC (clone: GK1.5), and sorting for CD4+GFP+ T cells. For some studies nTregs were activated in vitro with plate-bound CD3 mAb (10 µg/mL; eBioscience, San Diego, CA) and soluble CD28 mAb (1 µg/mL; Biolegend, San Diego, CA) in the presence of IL-2 (1000 IU/mL; Chiron, Emeryville, CA) for 7 days and then resorted for CD4+Foxp3GFP+ cells. Induced Tregs were generated as described previously.30 Briefly, CD4+ cells were prepared from congenically marked CD45.2+ Foxp3-GFP mice using negative selection and incubated for 4 days with plate-bound CD3 mAb in the presence of IL-2 (135 U/mL), TGF-β (20 ng/mL; R&D Systems, Minneapolis, MN) and all-trans RA (1 nM; Acros, Geel, Belgium). At 4 days after conversion of CD4+Foxp3− T cells in vitro, T cells were stained with a CD4-APC antibody from eBioscience, and cells were sorted for the CD4+GFP+ population using FACSAria (BD Biosciences, San Jose, CA) to 95% to 99% purity.
Suppression Assay
CD4+CD25− responder cells were isolated by first negatively selecting for CD4+ T cells using the EasySep CD4+ T-cell enrichment kit (Stemcell Technologies, Vancouver, Canada) and then removing CD25+ cells by labeling them with biotinylated CD25 mAb (BD Biosciences) and magnetic streptavidin-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and passing the cells through a magnetic column (Miltenyi Biotec). The responder cells were activated with plate-bound CD3 mAbs and soluble CD28 mAbs (10 and 1 µg/mL, respectively) or concanavalin A (1 µg/mL) for 4 days with increasing concentrations of freshly isolated nTregs or activated nTregs. Proliferation was measured by adding [3H]-thymidine for the last 24 hours of the incubation period and measuring the thymidine incorporation with a Wallac 1409 scintillation counter (EG&G Wallac Inc, Gaithersburg, MD).
Real-time Polymerase Chain Reaction
RNA was isolated from sorted (>97%) CD4+Foxp3+ T cells or colon using the RNeasy Mini kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s protocol. cDNA was generated using qScript cDNA supermix (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer’s protocol. Real-time polymerase chain reaction (PCR) was performed using the Treg-targeted 96-gene qPCR StellARray (Lonza, Basel, Switzerland), where primer-coated 96 well plates were incubated with the cDNA in 3 biological replicates or for 3 separate colons for each group (selected colons with median blinded histopathology scores for each group) and with PerfeCTa SYBR Green FastMix (Quanta) for amplification in an iCycler (Bio-Rad, Hercules, CA). Real-time PCR data from iTregs and nTregs was analyzed with the Global Pattern Recognition analysis tool v.2.0 (provided by Lonza). Real-time PCR data from colon samples was analyzed using the standard ΔΔCt method47 with 18S rRNA used as internal control for data normalization. The ΔCt values for each gene were calculated using 18S rRNA as a reference gene. Following that, an unpaired t test was used to compare the ΔCt values between the groups using GraphPad Prism software (GraphPad Software Inc, La Jolla, CA).
FACS Analysis
Cells were washed with phosphate-buffered saline (PBS) containing 4% fetal bovine serum and incubated with FcR-block Anti-Mouse CD16/CD32 (clone: 93) for 15 minutes at 4°C. After a washing step, the cells were incubated in a predetermined concentration of antibodies in a total volume of 100 µL. The following antibody clones were used: CCR9-PE (CW-1.2), α4β7-APC (DATK-32), αE-APC (2E7), Foxp3-PE (FJK-16s), CD45.2-PerCP-Cy5.5 (104), CD45.1-PE-Cy7 (A20), CD11b-APC (M1/70), CD4-PerCP-Cy5.5 (RM4-5), Foxp3 eFluor450 (FJK-16s), CD4-PE (GK1.5), and CD4-PE-Cy7 (GK1.5) from eBioscience; CD62L-APC (MEL-14), CTLA-4-PE (UC10-4F10-11), α4-PE (R1-2), GITR-PE-Cy7 (DTA-1), and CD4-Pacific Blue (RM4-5) from BD Biosciences; and Helios-Alexa Fluor 647 (22F6) and CCR6-APC (29-2L17) from Biolegend. Foxp3, CTLA-4, and Helios were stained as intracellular antigens. Cells were analyzed on the FACS-LSRII or FACS-Calibur (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR).
T-cell Transfer Model of Chronic Colitis
Chronic colitis was induced via adoptive transfer of congenically marked CD4+CD45RBhigh T-cells obtained from CD45.1 donors using our previously published method.48 Briefly, spleens were removed from donor (CD45.1) mice and teased into singlecell suspensions in PBS with 4% fetal bovine serum (FACS buffer) using the frosted sides of glass microslides. CD4+ T cells were enriched by negative selection using a Dynal Mouse CD4 Negative Isolation Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Enriched CD4+ T cells were labeled with anti-CD4 and anti-CD45RB antibodies and purified into CD4+CD45RBhigh cells by 2-color sorting on a FACS Aria (Becton-Dickinson, San Jose, CA). The CD45RBhigh population was defined as the 40% of cells exhibiting the brightest CD45RB staining and was found to be >98% pure on postsort analysis. RAG1−/− mice were injected (intraperitoneal) with 0.5 × 106 cells of CD4+CD45RBhigh suspended in 500 µL of PBS. Clinical evidence of disease (e.g., weight loss and loose stools/diarrhea) was monitored and recorded weekly from the time of the injection. Previous studies from our laboratory and others have determined that chronic colitis develops at 3 to 4 weeks after T-cell transfer.12,48 Therefore, at 4 weeks after the transfer of the CD4+CD45RBhigh cells, 1.0 × 106 CD45.2+CD4+Foxp3+ nTregs or iTregs in 500 µL of PBS were injected (intraperitoneal) into these mice. Five hundred µL of PBS was used as the control.
Macroscopic and Histopathologic Evaluation of Chronic Colitis
At 8 weeks after T-cell reconstitution or when animals lost >20% of their original body weight, RAG1−/− mice were killed, and the colons were removed, cleaned of fecal material, and scored for macroscopic evidence of inflammation using our previously published scoring criteria.48 Briefly, normal colonic morphology was assigned a score of 0, mild bowel wall thickening in the absence of visible hyperemia was assigned a score of 1, moderate bowel wall thickening and hyperemia was given a score of 2, severe bowel wall thickening with rigidity and marked hyperemia was assigned a score of 3, and severe bowel wall thickening with rigidity, hyperemia, and colonic adhesions was given a score of 4. Colonic length and weight were also measured to calculate weight-to-length ratios, which provide a quantitative index of inflammation.48 In addition to macroscopic inflammation, representative sections of distal colon were fixed overnight in 10% PBS-formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Blinded histopathologic evaluation of the colons using our published scoring criteria was performed by a pathologist unaware of the treatment.48
Lymphocyte Isolation and Analysis
Lymphocytes were obtained from the spleen, mesenteric lymph nodes (MLNs), and colonic lamina propria (cLP) and analyzed by flow cytometry as previously described.49,50 Briefly, spleens and MLNs were removed from RAG1−/− mice reconstituted with CD4+CD45RBhigh T cells (which had been treated with iTregs, nTregs, or PBS) and teased into a single-cell suspension using the frosted ends of 2 glass slides in 4% FACS buffer on ice. cLP mononuclear cells were prepared by the digestion of the finely minced intestinal pieces remaining after intestinal intraepithelial lymphocyte isolation in RPMI-1640, 4% fetal bovine serum, and collagenase type VIII (200 U/mL; Sigma, St. Louis, MO) for 40 minutes at 250 rpm in a 37°C shaker. Cells from the cLP were further enriched by centrifugation over a 44%/70% Percoll gradient. The cell pellet was washed and resuspended in FACS buffer, and viable cells were counted using a solution of 0.4% Trypan blue in PBS. Absolute numbers of CD4+ T cells in the spleen, MLNs, and cLP of reconstituted animals were calculated by multiplying the total number of viable cells isolated from each tissue by the percentage of total cells positive for CD4 as determined by flow cytometric analysis.
RESULTS
Ex Vivo-generated iTregs but not nTregs Attenuate Preexisting Colitis
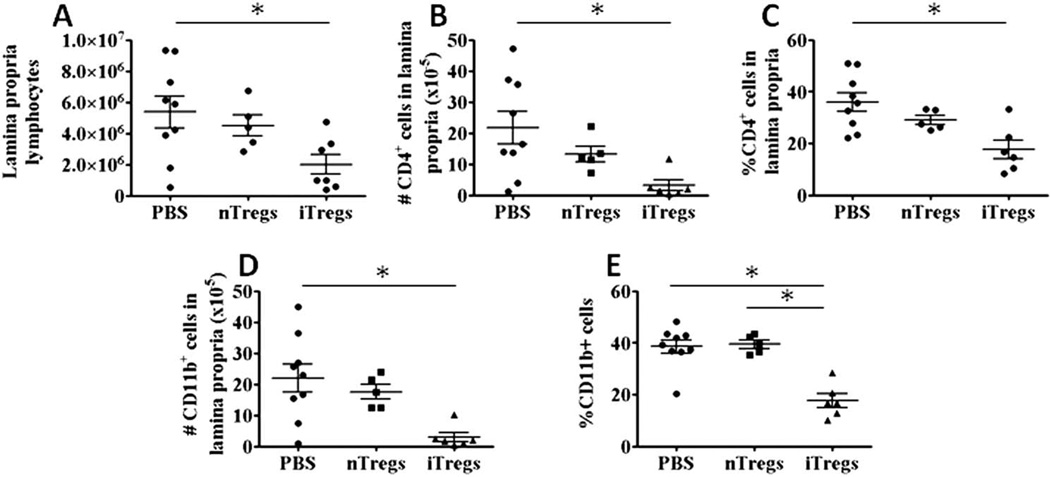
Previous studies have demonstrated that small numbers of nTregs or iTregs are effective at preventing the development of chronic gut inflammation.23–25,27–29,31,32 Although some reports have shown that nTregs are also effective at reversing preexisting colitis in mice,12,33–35 there have been no reports documenting a therapeutic efficacy of ex vivo-generated iTregs in attenuating established disease. Indeed, Haribhai et al.46 reported that iTregs are incapable of attenuating established colitis in mice. Therefore, we wished to evaluate and directly compare the ability of iTregs generated using our published protocol to freshly isolated nTregs to reverse/attenuate preexisting colitis. To do this, we injected (intraperitoneal) flowsorted 1 × 106 CD4+Foxp3+ iTregs, 1 × 106 nTregs, or PBS into RAG1−/− mice that had received CD4+CD45RBhigh T cells 4 weeks previously. Previous studies from our laboratory and others have determined that chronic colitis develops within 3 to 4 weeks after transfer of CD4+CD45RBhigh T cells.12,48 All mice receiving iTregs or nTregs survived for the 8-week observation period, whereas several of the PBS-injected mice had to be killed at earlier time points because of excessive weight loss (>15% of original weight). We found that colons obtained from mice treated with either PBS or nTregs exhibited macroscopic evidence of robust colonic inflammation including increases in both gross colon scores (Fig. 1A) and colon weight-to-length ratios at 8 weeks after CD45RBhigh T-cell transfer (4 weeks after PBS or Treg injection)48 (Fig. 1B). Blinded histopathologic analyses confirmed moderate-tosevere colitis in both the PBS- and nTreg-treated mice, which was characterized by bowel wall thickening, massive lymphocyte and myeloid cell infiltration, goblet cell loss, crypt abscesses, and epithelial cell injury (Fig. 1C, D). Treatment of colitic mice with iTregs remarkably and significantly attenuated colonic inflammation as judged by significant reductions in gross colon scores (Fig. 1A), colon weight-to-length ratios (Fig. 1B), and blinded histopathologic scores (Fig. 1C, D). In addition, weight loss did not appear to be as great in the iTreg-treated group when compared with the group treated with nTregs (Fig. 1E). Treatment of mice with iTregs also appeared to suppress the extraintestinal inflammation that occurs in the lungs of these mice when compared with PBS- or nTreg-treated animals51; however, this reduction was not statistically significant (Fig. 1F). Flow cytometric analyses confirmed that iTreg-mediated abrogation of preexisting colitis was associated with decreases in total lymphocytes within the colon when compared with PBS-treated mice (Fig. 2A). Indeed, colons obtained from mice treated with iTregs exhibited significantly lower numbers of CD4+ T cells and a reduction in the percentage of CD4+ T-cells in the lamina propria when compared with the PBS-treated mice but not in nTreg-treated mice (Fig. 2B, C). A similar pattern was observed for CD11b+ myeloid cells, in which we observed significantly fewer myeloid cells in the lamina propria of mice treated with iTregs when compared with PBS-treated mice and less frequent when compared with PBS-treated mice and nTreg-treated mice (Fig. 2D, E). Previous data from our laboratory clearly show that iTregs possess enhanced suppressive activity in vitro when compared with freshly isolated nTregs.30 To ascertain whether the enhanced suppressive activity of iTregs versus nTregs was function of T-cell activation, we activated freshly isolated nTregs in vitro and compared their suppressive activity to that of freshly isolated nTregs in vitro. We observed no significant difference in suppressive activity of activated versus freshly isolated nTregs (Fig. 3)
FIGURE 1.
Ex vivo-generated iTregs reverse preexisting colitis in mice. Chronic colitis was induced in Rag1−/− mice by adoptive transfer of CD4+CD45RBhigh T cells as described in the Methods section. Four weeks after T-cell transfer, 1 × 106 flow-purified iTregs, nTregs, or PBS were injected (intraperitoneal). At 8 weeks after T-cell transfer, mice were killed, and tissue was evaluated for macroscopic and histopathologic evidence of inflammation: (A) gross colon score; (B) colon weight-to-length ratio; and (C,D) blinded histopathologic score of colon; (E) percentage of original body weight; (F) blinded histopathologic score of lungs. Data are presented as the mean ± SEM; *P < 0.05.
FIGURE 2.
Ex vivo-generated iTregs reduce the infiltration of different populations of leukocytes into the colons of mice with preexisting colitis. Induction and treatment of mice with established colitis is described in the Methods section: (A) absolute numbers of lamina propria lymphocytes; numbers (B) and percentages (C) of CD4+ T cells within the colon; numbers (D) and percentages (E) of CD11b+ myeloid cells within the colon. The number of animals in each group was PBS (N = 9), nTregs (N = 5), and iTregs (N = 6). Data are presented as the mean ± SEM; *P < 0.05.
FIGURE 3.
Activated nTregs have equivalent suppressive capability as fresh nTregs in vitro. CD4+CD25− responder cells were activated with Concanavalin A (1 µg/mL) for 4 days in the presence of fresh or activated nTregs. Proliferation was determined by measuring 3H-thymidine uptake during the last 24 hours of culture. Each condition was performed in a triplicate, and this is a representative from 2 experiments. Data are presented as the mean ± SEM.
Tissue Distribution of and Foxp3 Expression in Ex Vivo-generated iTregs and nTregs
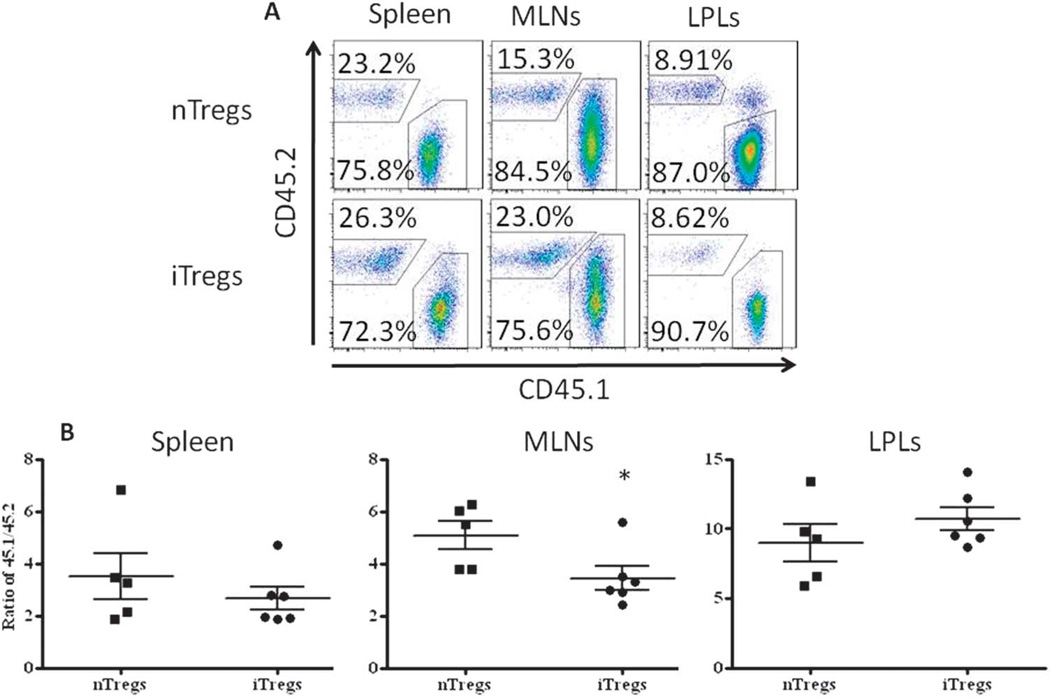
Using the congenic markers CD45.1 and CD45.2 to track the T cells originating from the CD45RBhigh population and those originating from either Treg population, we were able to quantify the accumulation of each population within the spleen, MLNs, and cLP. We found that the percentages of CD4+ T cells originating from the CD45RBhigh population (CD45.1) were significantly higher than either Treg population (CD45.2) within all 3 tissues and that the progeny of the CD45RBhigh population were more prevalent in the colon when compared with the spleen and MLNs (Fig. 4A). In addition, we observed a significantly greater frequency of nTreg and iTreg progeny within the spleen and MLNs when compared with the colon, with the frequency of iTregs significantly greater than nTregs in the MLNs (Fig. 4A). Despite their superior ability to reverse/attenuate chronic colitis, the frequency of iTregs within the colon was similar to that observed for nTregs (Fig. 4A; 8.62% versus 8.91%, respectively). Not surprisingly, when these data were expressed as the ratio of CD45.1/CD45.2, we observed a significant decrease in this ratio in the MLNs of mice treated with iTregs versus nTregs (Fig. 4B). No significant differences were noted between iTreg- and nTreg-treated groups in the spleen and cLP (Fig. 4B). Some investigators have suggested that nTregs and possibly iTregs may become unstable in vivo and convert to potentially pathogenic effector (Th1/Th17) T cells under inflammatory conditions.40,52 Although others have demonstrated that Foxp3 expression and the suppressive activity of nTregs or iTregs in vivo during the development of inflammation in different mouse models41,52 are quite stable, we wished to quantify Foxp3 expression at 4 weeks after the adoptive transfer of iTregs or nTregs into mice with established disease. Gating on the Treg population (CD4+CD45.2+) in 3 different tissues, we found that 60% to 68% of the nTreg progeny isolated from the spleen, MLNs, and colon expressed Foxp3 with 56% to 63% of the iTreg progeny expressing Foxp3 in the same 3 tissues (Fig. 5A). Of note, we did observe a small but significant decrease in iTreg Foxp3 expression in T cells obtained from the spleen when compared with nTreg Foxp3 expression (Fig. 5B) Taken together, these data suggest that: (a) Foxp3 expression in iTregs and nTregs is remarkably stable over several weeks when transferred into mice with preexisting inflammation and (b) the inability of nTregs to attenuate established disease is not because of decreased Foxp3 expression compared with iTregs.
FIGURE 4.
Flow cytometric analyses of iTregs and nTregs in the spleen, MLNs, and colonic lamina propria lymphocytes (LPLs) of colitic mice treated with iTregs or nTregs: (A) representative flow cytometry scatter plots; (B) ratio of CD45.1 to CD45.2 cells. Data are presented as the mean ± SEM; *P < 0.05.
FIGURE 5.
Foxp3 expression by iTregs and nTregs in the spleen, MLNs, and colonic lamina propria lymphocytes (LPLs) of colitic mice treated with iTregs or nTregs at 8 weeks after CD45RBhigh T cell transfer. The flow cytometric data were generated from cells gated on CD4+CD45.2+: (A) representative flow cytometry images of iTregs or nTregs obtained from the spleen, MLNs, and LPLs; (B) Foxp3 expression of the CD45.2+ cells from each animal. Data are presented as the mean ± SEM; *P < 0.05.
Alterations in Colonic Cytokine Expression following Treg Treatment
A number of different mechanisms have been reported to be used by Tregs to suppress T-, B-, and natural killer-cell activation11–13; however, the mechanisms responsible for their immunosuppressive activity in models of chronic inflammation are only now beginning to be elucidated. As a first step toward defining the mechanisms by which iTregs but not nTregs reverse/ attenuate established intestinal inflammation at 4 weeks after Treg transfer (8 weeks after CD45RBhigh T cell transfer), we quantified and compared the expression of a variety of different proinflammatory and regulatory cytokines in the colons of mice treated with PBS, iTregs, or nTregs. For the first analysis, we compared the expression profiles of iTreg-treated mice with that obtained from PBS-treated animals. We found that the expression of IFN-γ, TNF-α, and IL-1β in the colons of iTreg-treated mice was reduced by 90%, 75%, and 99%, respectively (P < 0.05) when compared with PBS-treated mice (Fig. 6A). Although we observed a marked reduction in IL-6 expression as well, this difference was not statistically significant.
FIGURE 6.
Cytokine gene expression in the colons of mice treated with PBS, nTregs, or iTregs at 8 weeks after CD45RBhigh T cell transfer. Messenger RNA was quantified using real-time PCR: (A) cytokine expression of iTreg- versus PBS-treated mice; (B) cytokine expression of nTreg- versus PBS-treated mice; (C) cytokine expression of iTregversus nTreg-treated mice. *P < 0.05. Error bars represent 95% confidence intervals.
For our second analysis, we compared the cytokine expression profile of mice treated with nTregs with that of PBS-treated animals. We found that only IL-1β and TGF-β were significantly reduced in the colons of nTreg-treated mice versus the PBS-treated group (Fig. 6B). A trend for increased expression of IL-17A in nTreg-treated versus PBS-treated mice was noted; however, this increase was not statistically significant. Surprisingly, when we compared the mRNA expression profiles of iTreg- versus nTreg-treated mice, we found no significant differences in the expression of the different Th1 and Th2 cytokines including IL-2, LTα, IFN-γ, TNF-α, IL-1β, and IL-13 (Fig. 6C). However, adoptive transfer of iTregs into mice with preexisting disease resulted in large and significant reductions (>98%) in expression of both IL-6 and IL-17A (P < 0.05) when compared with the nTreg-treated group.
Gene and Protein Expression Profiles of Ex Vivo-generated iTregs and nTregs
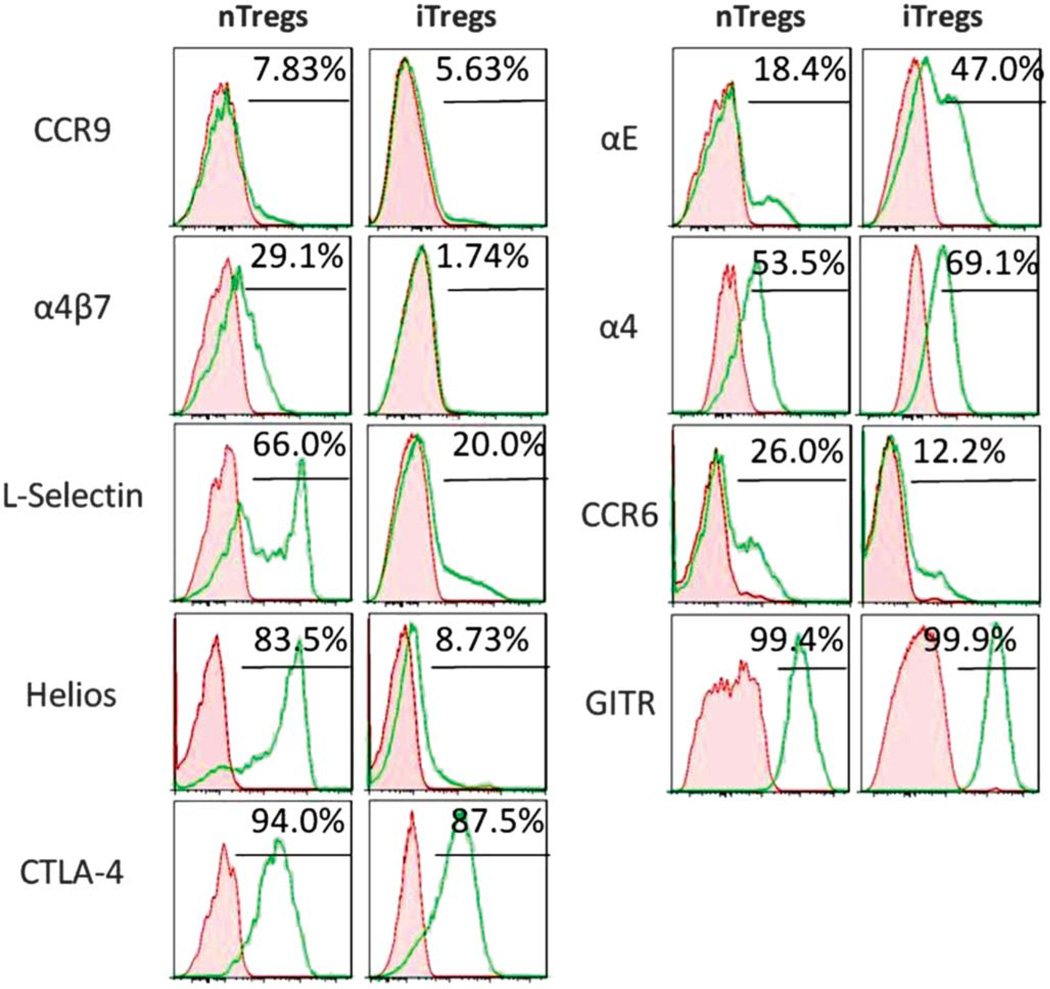
In an attempt to identify the genetic basis for the enhanced immunosuppressive function of iTregs in vivo, we compared the gene expression profile and protein expression of ex vivogenerated iTregs with that of freshly isolated nTregs. Using a customized Treg-targeted 96-gene qPCR array, we found that a total of 27 genes were significantly altered (P < 0.05; either upregulated or downregulated) in iTregs compared with nTregs. The mRNA transcripts for Foxp3 (Table 1) and message and protein levels of CTLA-4 (Ctla4; Table 1 and Fig. 7) and GITR (Tnfrsf18; Table 1 and Fig. 7) were similar in both iTreg and nTreg populations. Helios, a member of the Ikaros family of transcription factors, has been described as a marker for nTregs.53 However, more recent reports have challenged the conclusion and suggest that Helios can be induced in iTregs under specific activation conditions.54 We found that >83% of nTregs expressed Helios whereas >9% of iTregs expressed this protein. It is, therefore, clear that our method of generating iTregs does not induce Helios. In addition, we found that iTregs expressed significantly higher levels of the cytolytic enzymes granzyme A (Gzma, 18-fold) and B (Gzmb, ~5-fold) when compared with nTregs (Table 2). Furthermore, iTregs expressed significantly greater message for genes proposed to be important in Treg-mediated suppressive activity, Foxp3 expression, and Treg homing including galectin-1 (Lgals1, ~6-fold),55 Runx1 (14-fold),56 Hsp60 (Hspd1, >3-fold),57 and CCR8 (>4-fold)58 (Table 2). Finally, iTregs were found to express significantly greater amounts of mRNA for CCL4 (>130-fold) and CD8a (>8-fold) when compared with nTregs (Table 2).
TABLE 1.
Canonical Treg Gene Expression by iTregs and nTregs
| Gene | Expression (Fold-difference from iTregs Compared with nTregs) |
Significance (P) |
|---|---|---|
| Foxp3 | 1.0 | >0.05 |
| Ctla4 | −21.3 | >0.05 |
| Tnfrsf18 | −22.5 | >0.05 |
Real-time PCR was performed using the Treg-targeted 96-gene qPCR StellARray (Lonza), where primer-coated 96-well plates were incubated with the cDNA in 3 biological replicates and analyzed with the Global Pattern Recognition (GPR) analysis tool v.2.0 (provided by Lonza).
FIGURE 7.
Surface expression of various Treg-associated proteins. Freshly isolated nTregs and ex vivo-generated iTregs were prepared from Foxp3-GFP mice as described in the Methods section. Cells were stained and gated on CD4+Foxp3+ cells. Representative histograms from 3 separate experiments where protein levels were quantified using flow cytometry. The red-shaded area represents isotype antibody controls whereas the green lines represent protein-specific antibodies.
TABLE 2.
Increased Gene Expression in iTregs Compared with nTregs
| Gene | Expression (Fold-increase in iTregs over nTregs) |
Significance (P) |
|---|---|---|
| Ccl4 | 130 | <0.05 |
| Gzma | 18 | <0.05 |
| Runx1 | 14 | <0.05 |
| Cd8a | 7.5 | <0.05 |
| Lgals1 | 5.7 | <0.05 |
| Gzmb | 4.7 | <0.05 |
| Ccr8 | 4.5 | <0.05 |
| Hspd1 | 3.3 | <0.05 |
In addition to enhanced expression of several genes that have been suspected to be important for Treg-mediated immune regulation and trafficking in vivo, we also observed some rather surprising results for several other genes thought to play crucial roles in Treg-mediate immunosuppression and/or trafficking. For example, we observed large and significant decreases in expression of IL-10 (15-fold), Ebi3 (IL-35 subunit; 19-fold), and IL-6Rα (3.9-fold) and decreased expression of the gut-homing molecules CCR9 (>7-fold) and CCR6 (24-fold) (Table 3). Although small but significant amounts of CCR9 protein expression were observed on the surface of both iTregs and nTregs, we found a significant reduction in the surface expression of CCR6 on iTregs versus nTregs (Fig. 7). In addition, mRNA transcripts for the α4 (Itga4) and β7 integrins (Itgb7) were reduced by 12-fold (P < 0.05; Table 3) and 6.5-fold (P < 0.06), respectively, in iTregs when compared with nTregs. Indeed, we found that iTreg surface expression of the gut-homing integrin α4β7 was very low (<2%) when compared with its expression on nTregs (29%) (Fig. 7). We also quantified surface expression of the α4 integrin (CD49d) on iTregs and nTregs to ascertain whether the absence of α4β7 was because of decreased expression of the α4 subunit. Surprisingly, we observed substantial surface expression of α4 (CD49d) on iTregs (69.1%) that was slightly increased when compared with nTregs (53.5%) (Fig. 7). It is known that αE integrin (CD103) may compete with α4 for binding to the β7 integrin and, therefore, presumably reduce the expression of α4β7 as has been shown to be a mechanism for other integrins.59 In fact, we observed large and significant increases in expression of both αE integrin message (Itgae; Table 2) and protein (Fig. 7), suggesting an explanation for the virtual absence of α4β7 on iTregs. Finally, we wished to examine Treg surface expression of L-selectin (CD62L) as this adhesion molecule has been shown to be important for Treg trafficking to lymphoid tissue such as the mesenteric lymph nodes.60 Not surprisingly, we observed significantly lower surface expression of L-selectin on iTregs versus nTregs, suggesting an activated phenotype for our ex vivo-generated iTregs (Fig. 7). In addition, it is worth noting that we failed to observe significant IL-17A mRNA expression in iTregs despite the well-characterized role of TGF-β in generating both iTregs and Th17 cells. In contrast to the results obtained with iTregs, we did observed significant IL-17A expression in 2 of the 3 nTreg preparations; however, these differences were not statistically different from iTreg expression.
TABLE 3.
Decreased Gene Expression in iTregs Compared with nTregs
| Gene | Expression (Fold-decrease in iTregs over nTregs) |
Significance (P) |
|---|---|---|
| Il18 | −600 | <0.05 |
| Cd7 | −290 | <0.05 |
| Il7 | −100 | <0.05 |
| Cd200r1 | −96 | <0.05 |
| Sell | −46 | <0.05 |
| Icosl | −38 | <0.05 |
| Il7r | −29 | <0.05 |
| Ccr6 | −24 | <0.05 |
| Ebi3 | −19 | <0.05 |
| Il10 | −15 | <0.05 |
| Cxcr4 | −14 | <0.05 |
| Cd86 | −13 | <0.05 |
| Itga4 | −12 | <0.05 |
| Ccr9 | −7.2 | <0.05 |
| Stat5b | −5.9 | <0.05 |
| Cd27 | −5.0 | <0.05 |
| Folr4 | −4.0 | <0.05 |
| Il6ra | −3.9 | <0.05 |
| Il2rb | −2.8 | <0.05 |
DISCUSSION
There is an accumulating literature demonstrating that adoptive transfer of freshly isolated nTregs or iTregs can prevent the development of the chronic inflammation that arises in a variety of different animal models of autoimmune or chronic inflammatory disease.15,23,26,52 Although some investigators have reported that nTregs or iTregs are effective at attenuating or reversing established inflammation,34,46,52 there are also several studies demonstrating that nTregs or iTregs are either ineffective or only mildly effective at suppressing preexisting inflammation in several different animal models.46,52,61–63 Indeed, a recent study reported that freshly isolated nTregs are much more effective than ex vivogenerated iTregs at attenuating preexisting colitis in mice.46 We were surprised by these data given our previous observations that polyclonal activation of conventional CD4+ T cells in the absence of costimulation but presence of IL-2, TGF-β, and RA generates Foxp3-expressing iTregs that are significantly more potent than nTregs in suppressing T cell activation in vitro and equally effective at attenuating the development of chronic colitis.30 Therefore, we wished to evaluate and compare the therapeutic efficacies and gene expression profiles of our ex vivo-generated iTregs versus nTregs in a well-defined mouse model of chronic intestinal inflammation. We found that, in contrast to the report by Haribhai et al.,46 iTregs generated using our published protocol30 were superior to nTregs at reversing preexisting intestinal inflammation in a mouse model of chronic colitis. The enhanced suppressive activity of iTregs correlated with their increased expression of several genes suspected to be involved in mediating the suppressive activity of Tregs (e.g., galectin-1 and granzymes A and B) in vitro and in vivo in addition to the downregulation of tissue IL-6 and IL-17A in the colons of iTreg-treated mice.
The reasons for the contrasting results are not clear at the present time but several possible explanations exist. First, Haribhai et al.46 generated their iTregs using polyclonal activation in the presence of costimulation but absence of IL-2 and RA, whereas our protocol requires that T-cell activation occur in the presence of IL-2 and RA but absence of costimulation. In addition, we used substantially larger amounts (4-fold larger) of TGF-β and CD3 mAb compared with those used by Haribhai et al.30,46 Furthermore, we have previously demonstrated iTregs generated using our protocol are significantly more potent at suppressing T-cell proliferation in vitro than are nTregs, whereas Haribhai et al. did not report the suppressive activity of their ex vivo-generated iTregs in vitro. Another possible explanation for our differing results may be because that the flow-purified iTregs that were used for all of our studies contained sufficient numbers of contaminating nTregs to promote functional synergy between the 2 Treg populations as described by Haribhai et al.46 We do not believe that this explanation can account for the dramatic therapeutic differences we observed for the following reason. First, one would have to assume that the ~10% contaminating cells present in our negatively selected, starting population of enriched (>90%) CD4+ T cells would be entirely nTregs and that these nTregs would proliferate to a much greater extent than iTregs over the 4-day incubation period such that the ratio of nTregs:iTregs would equal or exceed the 1:1 ratio reported to be required for functional synergy of these 2 Treg populations in vivo.46 Although possible, we believe that this is highly unlikely given the fact that nTregs are incapable of proliferating in vitro at the concentration of IL-2 (135 U/mL) that was used for our conversion assays.64 Data presented in the current study are consistent with another report demonstrating that iTregs are more potent than nTregs in suppressing chronic joint inflammation in a mouse model of arthritis.65 Another novel finding presented in the current study suggests that ex vivo-generated iTregs may have the ability to suppress the extraintestinal inflammation that accompanies the development of colitis.48,51 For example, we observed a trend for decreased lung inflammation in iTreg- versus nTreg-treated mice (Fig. 1F).
In addition to the novel observation that iTregs possess potent immunosuppressive activity in vivo, we found that freshly isolated, flow-purified nTregs were incapable of reversing established colitis at 8 weeks after the adoptive transfer of CD45RBhigh T cells (Fig. 1). These data are surprising given that both Mottet et al.34 and Haribhai et al.46 have reported that nTregs were very effective at suppressing preexisting intestinal inflammation in a similar T-cell transfer model of chronic colitis. On the surface, there seems to be little difference in the study design between our study and those of Mottet et al. and Haribhai et al. However, on closer examination, several major differences are apparent that may account for the discrepant results. One major difference is that the mice were killed at 8 weeks after T-cell transfer in our studies whereas Mottet et al.34 and Haribhai et al.46 killed their mice at 16 and 17 weeks after T-cell transfer, respectively. Of note, Mottet et al. stated that the “colitic mice receiving 106 CD4+CD25+ T cells typically started to recover 2 wk after the transfer with gradual disappearing of clinical signs.”34 Although we did not observe significant attenuation of colonic inflammation in the nTreg-treated mice at week 8 compared with PBS-treated animals, we cannot exclude the possibility that nTregs may promote a slow resolution of colonic inflammation over a protracted period of time. Nevertheless, our data would suggest that iTregs are more effective than nTregs at mediating a more rapid attenuation of chronic colitis.
Another novel observation that was made in the current study was that our ex vivo conversion protocol generates iTregs with low level expression of the classical “gut-homing” adhesion molecules CCR9 and α4β7 (Fig. 7). These data are rather surprising as they contrast significantly with other studies reporting that ex vivo-generated iTregs express significant levels of both CCR9 and α4β7.14,66,67 The most likely explanation for these contrasting results is the different methods used for the conversion of conventional T cells to iTregs in vitro. For example, we induce conversion of CD4+ T cells via T-cell receptor crosslinking in the presence of IL-2, TGF-β, and RA but in the absence of costimulation whereas the other studies used polyclonal activation with costimulation.14,66,67 It is possible that certain costimulatory signals positively regulate the expression of these adhesion molecules. In addition, the lack of surface expression of the guthoming molecules on iTregs may be because of the different concentrations of RA and/or TGF-β used in previous studies.14,66,67 Regardless of the exact reasons for these differing results, it appears surprising that α4β7- and CCR9-deficient iTregs are capable of reversing preexisting intestinal inflammation. However, it should be noted that there is little direct evidence demonstrating that T cell-associated α4β7 is required for trafficking to the healthy or inflamed colon. Indeed, the bulk of the literature suggests that T cells use α4β7 for their preferential homing to the small intestine via its interaction with endothelial mucosal addressin cell adhesion molecule-1.19,68 Kang et al.19 generated iTregs with enhanced expression of α4β7 and observed a 10- to 15-fold greater accumulation of these cells in the small bowel than control iTreg expressing low levels of α4β7.19 Similar results have been published with α4β7-expressing Th17 cells, which preferentially migrate to the small intestine but not to the colon.68 Another interesting yet perplexing observation that was made in the current study was that, despite decreased expression of the α4 gene (Table 2) and reduced surface expression of α4β7 (Fig. 7), we observed substantial and slightly increased surface expression of the α4 integrin on iTregs compared with nTregs (Fig. 7). Although the mechanisms responsible for these seemingly conflicting data are not readily apparent, it may be that, early on during the 4-day culture of iTregs, α4 message is in fact transcribed, translated, and then degraded whereas the α4 protein remains stably associated with the β1 integrin in the form of the very late antigen (VLA)-4 adhesion molecule (α4β1). Studies to address this possibility are currently underway.
Although some studies have suggested that CCR9 may play an important role in T-cell trafficking to the small intestine,19,68 there is little direct evidence demonstrating that this chemokine receptor is a major molecular determinant for T-cell trafficking to the colon.69 Indeed, data presented in the current study clearly demonstrate that ex vivo-generated iTregs with little or no surface expression of CCR9 are capable of homing to the inflamed colon (Fig. 4). The specific T cell-associated adhesion molecules (or combinations thereof) that is/are required for homing of iTregs (or nTregs) to the inflamed colon remain to be identified. A recent study suggests that CCR6 may play a role in the trafficking of nTregs to the inflamed colon.70 However, we present data in the current study demonstrating large and significant reductions in CCR6 gene and protein expression (Fig. 7 and Table 3). It is quite possible that iTregs (and/or nTregs) may use additional adhesion molecules such as lymphocyte function-associated antigen 1 (LFA-1) and/or α4β1 (VLA-1 and VLA-4) to enter the inflamed bowel given the fact that several endothelial counter receptors (e.g., intercellular adhesion molecule-1, vascular cell adhesion molecule-1 , and mucosal addressin cell adhesion molecule-1) are dramatically upregulated in the inflamed microcirculation.71
The mechanisms responsible for the enhanced suppressive effects of iTregs versus nTregs in vivo remain to be elucidated. We demonstrate in the current study that the expression of the proinflammatory cytokines IL-6 and IL-17A in colons of iTregtreated mice is markedly and significantly reduced (>90%) when compared with nTreg-treated animals. Because IL-6 is known to be required for the generation of Th17 cells,72 we speculate that iTreg-mediated reductions in colonic IL-6 may prevent or limit the generation of IL-17A-producing Th17 effector cells. The mechanisms by which iTregs decrease IL-6 production in the colon are not clear at the present time but may be because of galectin-1-mediated apoptosis of IL-6-producing effector cells (see below). Another possible mechanism that may account for the differences in therapeutic efficacy of iTregs versus nTregs is increased stability of Foxp3 expression in ex vivo-generated iTregs. Although some investigators have suggested that Tregs are unstable in vivo and may convert to pathogenic effector T cells under physiological or inflammatory conditions,40 other laboratories have demonstrated that Treg-associated Foxp3 expression and suppressive function are remarkably stable following adoptive transfer in vivo and Tregs do not produce proinflammatory cytokines in the context of autoimmune inflammation.41,73 In addition, Zheng et al.74 has reported that iTregs are resistant to conversion into Th17 effector cells when treated with IL-6 whereas nTregs are not. Furthermore, recent studies clearly show that IL-2, a cytokine known to be upregulated in chronic gut inflammation, together with T-cell receptor stimulation significantly increases the demethylation of the Treg-specific demethylated region of the Foxp3 locus, thereby inducing epigenetic imprinting and stabilization of Foxp3 expression in vivo.75 Data presented in the current study suggest that Foxp3 expression in iTregs and nTregs is remarkably stable over several weeks when transferred into mice with preexisting inflammation and the inability of nTregs to attenuate established disease is not because of decreased Foxp3 expression compared with iTregs (Fig. 5). Another possible mechanism that may account for the increased immune-suppressive activity of iTregs in vivo is their increased accumulation within the MLNs (Fig. 4). It may be that iTregs have a greater propensity to traffic to the inflamed MLNs where they suppress or limit the generation and/or expansion of disease producing effector cells. Alternatively, iTregs may promote a tolerogenic state for APCs as has been suggested in another model of chronic inflammation.65 Alternatively, trafficking of iTregs and nTregs to the MLNs in mice with established disease may be similar but iTregs expand to a greater extent resulting in increased suppression of effector cell function and/or proliferation. Studies to differentiate between these 2 possibilities are currently underway.
Additional potential mechanisms that may be responsible for the enhanced therapeutic activity of iTregs are their greatly increased expression of galectin-1 and granzymes A and B. Although not as well studied as the classical regulatory cytokines (e.g., IL-10, TGF-b, and IL-35), these 3 genes have been suggested to play important roles in the immune-suppressive activity of Tregs. For example, Perillo et al.76 showed that galectin-1 induces apoptosis of activated but not resting T-cells in vitro. This could account for the enhanced suppressive function of our iTregs because the majority of T cells within the inflamed colon are activated/effector T cells such as Th1 and Th17 cells. In addition, galectin-1 has been suggested to promote Treg survival and proliferation in vivo.77 Secretion of cytolytic enzymes such as granzymes A and B has also been considered to be important for the immunomodulatory function of Tregs.13 Again, the roles of each of these genes in iTreg-mediated reversal of preexisting colitis are the subject of future studies.
In conclusion, we present data in this study that demonstrate that, for the first time, ex vivo-generated iTregs produced using our recently published protocol30 are superior to freshly isolated nTregs at attenuating preexisting gut inflammation despite having reduced expression of the classical regulatory cytokines (IL-10 and IL-35) and gut-homing molecules. The mechanisms responsible for this remarkable immunomodulatory activity have not been definitively identified but seem to be associated with the upregulation of several different genes that may directly or indirectly decrease expression of IL-6 and IL-17A within the inflamed bowel.
Acknowledgments
Supported by NIH Grants R21AI085140, R21NS059724, and 8P20 GM103433-10.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 6.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol. 2012 doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–1287. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 12.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4 +CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson MJ, Pino-Lagos K, Rosemblatt M, et al. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilate AM, Lafaille JJ. Induced CD4(+)Foxp3(+) regulatory T cells in immune tolerance. Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantini MC, Dominitzki S, Rizzo A, et al. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, Hall JA, Sun CM, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SG, Lim HW, Andrisani OM, et al. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 24.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 25.Endharti AT, Okuno Y, Shi Z, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cellinduced colitis. J Immunol. 2011;186:41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 26.Fantini MC, Becker C, Tubbe I, et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foussat A, Cottrez F, Brun V, et al. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J Immunol. 2003;171:5018–5026. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto M, Nakano M, Terabe F, et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 29.Housley WJ, Adams CO, Nichols FC, et al. Natural but not inducible regulatory T cells require TNF-{alpha} signaling for in vivo function. J Immunol. 2011 doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson F, Robinson-Jackson SA, Gray L, et al. Ex vivo generation of regulatory T cells: characterization and therapeutic evaluation in a model of chronic colitis. Methods Mol Biol. 2011;677:47–61. doi: 10.1007/978-1-60761-869-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke NR, Patterson SJ, Hamilton MJ, et al. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol. 2009;183:975–983. doi: 10.4049/jimmunol.0803749. [DOI] [PubMed] [Google Scholar]
- 32.Wohler J, Bullard D, Schoeb T, et al. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. 2009;46:2424–2428. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 34.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4 +CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 35.Piconese S, Pittoni P, Burocchi A, et al. A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. Eur J Immunol. 2010;40:2902–2913. doi: 10.1002/eji.201040505. [DOI] [PubMed] [Google Scholar]
- 36.Osorio F, LeibundGut-Landmann S, Lochner M, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs . Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L, Kitani A, Fuss I, et al. Cutting edge: regulatory T cells induce CD4 +CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 39.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubtsov YP, Niec RE, Josefowicz S, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 43.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83–41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 46.Haribhai D, Lin W, Edwards B, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan JS, Reed A, Chen F, et al. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostanin DV, Furr KL, Pavlick KP, et al. T cell-associated CD18 but not CD62L, ICAM-1, or PSGL-1 is required for the induction of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1706–G1714. doi: 10.1152/ajpgi.00573.2006. [DOI] [PubMed] [Google Scholar]
- 50.Ostanin DV, Pavlick KP, Bharwani S, et al. T cell-induced inflammation of the small and large intestine in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G109–G119. doi: 10.1152/ajpgi.00214.2005. [DOI] [PubMed] [Google Scholar]
- 51.Koboziev I, Karlsson F, Zhang S, et al. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis. 2011;17:1229–1245. doi: 10.1002/ibd.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan Q, Fan H, Quesniaux V, et al. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabransky DJ, Nirschl CJ, Durham NM, et al. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garin MI, Chu CC, Golshayan D, et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 56.Klunker S, Chong MM, Mantel PY, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caramalho I, Lopes-Carvalho T, Ostler D, et al. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soler D, Chapman TR, Poisson LR, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 59.DeNucci CC, Pagan AJ, Mitchell JS, et al. Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit. J Immunol. 2010;184:2458–2467. doi: 10.4049/jimmunol.0902407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venturi GM, Conway RM, Steeber DA, et al. CD25+CD4+ regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J Immunol. 2007;178:291–300. doi: 10.4049/jimmunol.178.1.291. [DOI] [PubMed] [Google Scholar]
- 61.Bagavant H, Tung KS. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175:944–950. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 62.Huter EN, Stummvoll GH, DiPaolo RJ, et al. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou X, Kong N, Wang J, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong N, Lan Q, Chen M, et al. Antigen-specific transforming growth factor beta-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore C, Sauma D, Morales J, et al. Transforming growth factor-beta and all-trans retinoic acid generate ex vivo transgenic regulatory T cells with intestinal homing receptors. Transplant Proc. 2009;41:2670–2672. doi: 10.1016/j.transproceed.2009.06.130. [DOI] [PubMed] [Google Scholar]
- 67.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Kang SG, HogenEsch H, et al. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 70.Kitamura K, Farber JM, Kelsall BL. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol. 2010;185:3295–3304. doi: 10.4049/jimmunol.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cromer WE, Mathis JM, Granger DN, et al. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011;17:578–593. doi: 10.3748/wjg.v17.i5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 73.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q, Kim YC, Laurence A, et al. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perillo NL, Pace KE, Seilhamer JJ, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 77.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230:144–159. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]