Abstract
Purpose
To compare bellows-gated late gadolinium enhancement (LGE) with standard navigator-gated (NAV-gated) LGE for left atrial (LA) imaging, to eliminate the inflow artifacts associated with NAV-gating.
Materials and Methods
Eleven subjects, including 6 patients with atrial fibrillation (AF), were imaged with a 3D free-breathing NAV-gated and bellows-gated LGE. Motion compensation was compared by blinded grading of image sharpness and motion ghosting (0=worst, 2=best). Inflow artifacts in the right inferior PV (RIPV) and right superior PV (RSPV) were characterized on the same scale (0=none, 2=prominent). In patients, each PV was divided into four quadrants circumferentially in order to assess agreement about scar presence on both image sets.
Results
Respiratory compensation was not different (1.7± 0.5 vs. 1.6± 0.5, sharpness, 1.6± 0.5 vs. 1.6± 0.5, ghosting, p=NS) for bellows- and NAV-gated images. For NAV-gated LGE, inflow artifacts were more prominent in the RSPV than the RIPV (1.2±0.8 vs. 0.7±0.5, p=0.046). Visually, inflow artifacts both obscured and mimicked the true scar. Disagreement on the presence of scar was found in 18% of the assessed quadrants, with 25% disagreement for RSPV quadrants (p=0.01).
Conclusion
Bellows-gated LGE provides similar respiratory compensation as NAV-gating, without inflow artifacts, leading to improved assessment of scar presence.
Keywords: bellows, late gadolinium enhancement, artifacts, NAV-gating, Atrial fibrillation, respiratory compensation
INTRODUCTION
Late gadolinium enhancement (LGE) MRI can visualize pre-existing (LA) left atrial fibrosis (1) or post-ablation scar (2) in patients with atrial fibrillation (AF), using an inversion recovery gradient echo sequence (3) with increased spatial resolution (1,2). To achieve high spatial resolution, long scan times with right hemi-diaphragm (RHD) navigator-gated (NAV-gated) imaging are used. However, the inversion pulse required for LGE also nulls the liver at the time of data acquisition (optimal myocardial inversion time (TI)), so that NAV-gating, which relies on monitoring the lung-liver interface, is hindered. A NAV-restore pulse (4) (applied immediately after the initial inversion pulse to reinvert the region of the liver on the right side of the heart (the RHD) permits gating, and is invaluable for applications such as LGE of the left ventricular myocardium. However, right-sided pulmonary vein (PV) blood magnetization is also reinverted, and flows into the LA during the time between the inversion pulse and the data acquisition, generating artifacts (see Figures 1-3). Left-sided pulmonary vein blood is not reinverted, and no prominent inflow artifacts are observed on the left side.
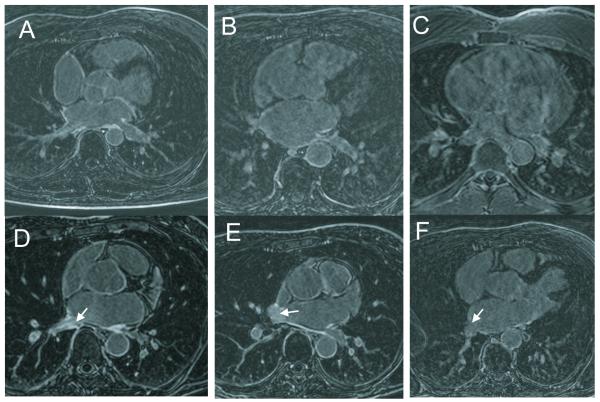
Figure 1.
A-C. Examples of images receiving ghosting/sharpness scores of D) 2/2, E) 1/1, F) 0,1, a poor quality image. D-F. Examples of images receiving scores of (A) 2 (prominent) (B) 1, and (C) 0 (absent) for presence of inflow artifacts (arrows).
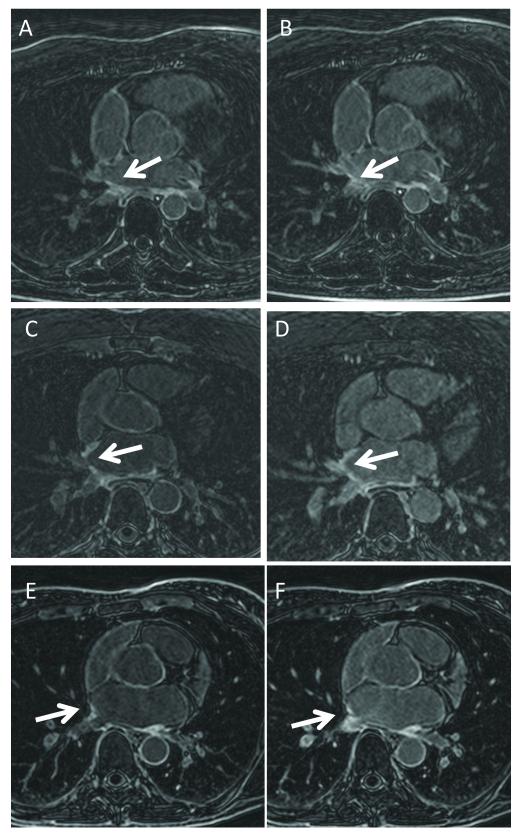
Figure 3.
Bellows-gated (A, C, E) and NAV-gated (B, D, F) LA LGE, in three post-ablation patients. Image quality is similar. The NAV artifacts can either mimic scar, or mask true scar. In A) scar is apparent in the inferior aspect of the RIPV, while in B) scar is also apparent, but it is not clear if the enhancement is true scar or inflow artifact, due to the surrounding blood-pool enhancement. In C) RSPV scar is apparent, but in D) the scar appears more extensive, due to artifacts. In E) RIPV scar is present, but it is unclear whether this scar is real or artifactual in F) due to the enhancement of the entire ostium.
These artifacts can be partially addressed by using a NAV restore with a smaller diameter (3.6 cm instead of 6cm). Another solution is to eliminate the NAV restore, and either apply the NAV earlier before data acquisition(5) (e.g. 100ms prior to data acquisition), or directly after data acquisition(6), which are both times when the liver magnetization is non-zero since the liver had a shorter or longer time to recover from the inversion pulse. However, NAV-gating monitoring is optimum when coincident with the acquisition of central k-space (7), so that an early or postponed NAV pulse might reduce the quality of respiratory gating.
Bellows-gating is an early respiratory compensation approach, with recent data suggesting good correlation with the superior-inferior diaphragmatic motion (8-11). Here, we present an alternative approach for eliminating PV blood inflow artifacts, by use of bellows-gated LGE, in which the NAV is not used, and the flow related NAV restore artifacts are thus absent. We hypothesize that bellows-gating will provide similar image quality for this moderately high resolution application with elimination of NAV restore artifacts.
MATERIALS AND METHODS
MR Imaging
All imaging was performed on a 1.5T Philips Achieva (Philips Healthcare, Best, NL). Subjects provided written informed consent. Eleven subjects (5 healthy subjects, 3 female, 21±1 years, and 6 AF patients post-ablation, 1 female, 58±10 years) were imaged successively with two identical 3D LGE sequences but different respiratory compensation techniques-one using RHD NAV-gating and a second using respiratory bellows gating. LGE LA imaging was performed 10 to 30 minutes after injection of 0.2mmol/Kg of gadolinium contrast agent (Gd-DTPA), using a 3D free-breathing inversion recovery gradient echo sequence, ECG-gated at mid-diastole (TR/TE/θ= 5.3ms/2.6ms/15°) covering the entire LA in the axial orientation, with spatial resolution of 1.4 × 1.4 × 4 mm3, and 100-150 ms acquisition window, as previously described (2). Both scans required 270 ± 97 heart-beats to complete data acquisition (4.5 minutes at 100% efficiency, 60 bpm). The inversion time was chosen based on a Look-Locker TI scout to null left ventricular myocardial signal (12).
Respiratory motion suppression was performed using 1) RHD NAV-gating with a 5mm window and no tracking and 2) bellows-gating. The NAV pulse prescribed at the dome of the RHD. The 3.0cm diameter NAV and 3.6cm diameter NAV-restore were pencil-beam excitation pulses (13) placed ~50ms prior to the central k-space data acquisition. The NAV restore used a 180° (inversion) pulse applied immediately after the standard non-selective 180° RF pulse used for T1-weighting. The bellows was commercially provided (Philips Healthcare, Best, The Netherlands), consisting of a pressure-sensing pillow, which was semi-tightly wedged between the subject’s upper abdomen and a hard surface (i.e., the ECG sensor box), by an experienced technologist following our standard clinical subject preparation. The bellows-gated scan used the commercially provided bellows-gating technique. Pressure readings were measured producing a signal with an arbitrary scaling. Data was accepted if the pressure value was close (within the 50% of the peak to peak signal) to the end expiratory level.
Analysis
For all studies (N=11), in order to compare motion compensation quality of the bellows- and NAV-gated images, image sharpness and ghosting were evaluated on a scale of 0 to 2 (0=blurry, ghosting obscuring anatomy, 1=some blur, visible ghosts, 2=sharp, no ghosting) in a blinded fashion by a single experienced observer. Because observing the inflow artifact unblinds the reader, images were analyzed in a single slice in which no inflow artifact was observable. Figure 1A-C shows examples of images graded with scores 0-2.
For all studies (N=11), the appearance of inflow artifacts in the right PVs (right inferior PV (RIPV) and right superior PV (RSPV) separately) were noted (0=none, 1=slight, 2=prominent) in the NAV-gated LGE volumes by a single experienced observer. The appearance of any subtle enhancement in the left PVs was also examined, to confirm the absence of artifacts in left PVs. Inflow artifact was noted as a region in the LA or PV ostia where blood-pool (and not just wall) enhancement was present. A score of zero was assigned If the blood pool enhancement was absent, a score of 1 if ostial blood pool enhancement was less intense than any scar, and a score of 2, if blood pool enhancement was of similar or greater intensity. In subjects without scar, valves were used as a fibrotic structure. Artifact was confidently identified using the bellows gated images—in which no artifact existed--as reference to distinguish between scar and artifact. Figure 1D-F shows examples of images graded with scores 0-2.
For post-ablation patient images (N=6), in order to compare scarring at well matched locations on both data sets, an assessment of the presence of scar in all four PVs, in each of the four circumferential quadrants of each PV (superior, inferior, anterior, posterior portions of the PV) was performed using the 3D volume of slices, blinded to results of matching data set. A total of 96 quadrants were studied (6 patients × 4 PVs × 4 quadrants). Care was taken to compensate for the NAV artifact in these assessments, by attempting to discern between artifacts and true enhancement in each reading. For each discordant sector, the image data sets were compared side by side to visually determine the cause of discordance, including inflow artifacts, motion blurring, and scar-blood contrast.
Statistics
Differences in image quality, and differences in inflow artifact grades between the RSPV and RIPV, were compared using a Wilcoxon signed rank test. Using Fischer’s exact test, disagreement in scar presence was compared for each PV, for the bellows- and NAV-gated images.
RESULTS
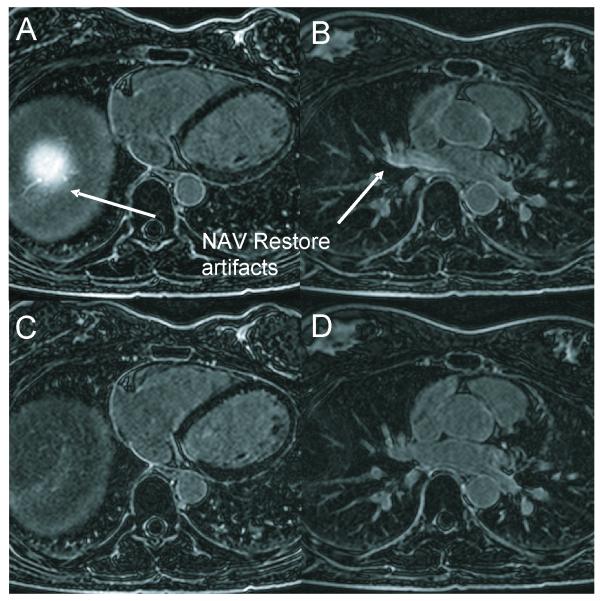
Figure 2 compares bellows- and NAV-gating in a healthy subject. Note the dome of the right diaphragm is “bright” due to the NAV-restore, and the right PV blood is also bright (Fig 1A), some of which flows into the LA (Fig 1B); this is not observed in the bellows-gated images (Fig 1C,D). Figure 3 compares bellows- and NAV-gating in 3 post-ablation AF patients. Scarring is apparent around the PV ostia, with ambiguous hyperenhancement resulting from the inflow artifacts (arrows) in the NAV-gated images.
Figure 2.
Comparison of LA LGE in a single healthy subject. A,B) Separate slices from NAV-gated LA LGE, showing the artifact resulting from the NAV-restore artifacts on the liver (A), and in the right superior PV (B). C,D) Separate slices from Bellows-gated LA LGE, with similar quality to (A,B), but without the NAV artifacts. The excited column of tissue shown in A) includes PV blood which flows into the LA, obscuring scar in the right PVs.
Table 1 summarizes the comparison of bellows- and NAV-gated LA LGE. Respiratory gating efficiencies for the bellows (49±15%) and the NAV (54±16%) were similar (p=0.5). On blinded assessment of slices free of inflow artifact, image quality (sharpness/ghosting) was similar (1.7± 0.5 vs. 1.6± 0.5, for sharpness of bellows vs. NAV, p=0.32, and 1.6± 0.5 vs. 1.6± 0.5 for ghosting of bellows vs. NAV, p=NS). Inflow artifacts were not present in the left-sided veins. The average inflow artifact score was higher in the RSPV than the RIPV (1.2 ± 0.8 vs. 0.7 ± 0.5, p=0.046). For the blinded qualitative grading of scarring in each PV and each quadrant, 17 of the 96 (18%) quadrants were in disagreement between bellows-gated and NAV-gated images (with one showing scar, the other not), including 1/24 LIPV quadrants, 5/24 LSPV, 5/24 RIPV, and 6/24 RSPV quadrants (p=0.01 for RSPV). In the majority (65%) of disagreements, scar was observed on bellows-gated images but not on NAV-gated images. For the right PVs, nine of the eleven disagreements (82%) were in quadrants which were graded as unscarred in the NAV-gated images, but scarred in the bellows gated images. Furthermore, on a direct image comparison, it was estimated that in 8/17 cases the cause of discordance was a combination of low scar-blood contrast and the presence of inflow artifacts, with the remaining differences due to low scar-blood contrast (5/17) alone or motion artifacts (4/17).
Table 1.
Comparison of bellows-gating and NAV-gated imaging (0=worst, no artifact, 2=best, prominent artifact).
| Method | Bellows | NAV |
|---|---|---|
| Gating Efficiency | 49±15% | 54±16% |
| Sharpness | 1.7 ± 0.5† | 1.6 ± 0.5† |
| Ghosting | 1.6 ± 0.5 | 1.6 ± 0.7 |
| Inflow Artifacts due to NAV | ||
| RSPV | 0 | 1.2 ± 0.8* |
| RIPV | 0 | 0.7 ± 0.5* |
RIPV=right inferior PV, RSPV=right superior PV.
P=0.046
p=0.31.
DISCUSSION
Bellows-gated LGE of the LA provides similar respiratory compensation (motion suppression, sharpness and scan efficiency) to that of NAV-gated LGE, and eliminates NAV-related inflow artifacts. Moghari et al. using NAV-gating with a delay between imaging and the NAV pulse (5) found similar results: adequate respiratory compensation for this moderately high resolution application, and eliminated inflow artifacts. The bellows-gated approach has the additional advantage over NAV of simple implementation, and has similar gating efficiency. The RSPV has more prominent inflow artifacts from NAV-gating compared to the RIPV, which impacts scar assessment. While these inflow artifacts can both increase apparent scarring, or mask real scar, there was a general trend towards the artifact obscuring the real scar in the RSPV, thus underestimating the circumferential scarring in the RSPV. Potential reasons that the RSPVs exhibit the more artifacts than the RIPV may be increased flow in the RSPV, or increased intersection of the RSPV blood with the reinverted column.
The comparison of bellows- and NAV-gated images of scarring in each sector of each PV found that almost half of all discordance between the image sets was due to the inflow artifacts especially in low contrast images. Therefore, the reduction of this artifact is critical. However, other causes of discordance were due to a low contrast between blood and scar and motion artifacts.
Our study was small, though it is unlikely that more subjects would affect the result that NAV-gated and bellows-gated respiratory motion compensation was comparable for these moderate resolution images. The order of imaging was fixed (NAV-gated LGE followed by bellows-gated LGE), as required by the clinical setting. This may have resulted in different contrast and signal to noise ratio for the two studies (either better or worse for the later study, depending on the time of the first study relative to the injection). However, we did not focus on absolute image quality comparison, but rather on the effect of inflow artifacts on scar appearance, and the adequacy of bellows-gating for respiratory compensation. Scar presence was analyzed by a single blinded experienced observer, and artifacts resulted in underestimation of scarring. A different observer might err towards over-estimation of scar due to artifacts. Future work might focus on optimizing the bellows-gating method with respect to the efficiency and motion-compensation quality.
In conclusion, 3D LA LGE imaging using bellows-gating can provide similar respiratory compensation as NAV-gating, with the advantage of absent RIPV and RSPV inflow artifacts. This leads to improved assessment of scar presence.
Acknowledgements
The authors thank the subject volunteers and Kraig V. Kissinger, RT and Beth Goddu, RT, and Lois Geopfert, Nurse of the BIDMC Cardiac MR Center.
Funding Sources: This work was supported by in part by grants from the NIH (NHLBI R21 HL098573 and R21 HL103463).
References
- 1.McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52(15):1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 2.Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243(3):690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 4.Spuentrup E, Buecker A, Karassimos E, Gunther RW, Stuber M. Navigator-gated and real-time motion corrected free-breathing MR Imaging of myocardial late enhancement. Rofo. 2002;174(5):562–567. doi: 10.1055/s-2002-28271. [DOI] [PubMed] [Google Scholar]
- 5.Moghari MH, Peters DC, Smink J, et al. Pulmonary vein inflow artifact reduction for free-breathing left atrium late gadolinium enhancement. Magn Reson Med. 66(1):180–186. doi: 10.1002/mrm.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badger TJ, Oakes RS, Daccarett M, et al. Temporal left atrial lesion formation after ablation of atrial fibrillation. Heart Rhythm. 2009;6(2):161–168. doi: 10.1016/j.hrthm.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Spuentrup E, Manning WJ, Botnar RM, Kissinger KV, Stuber M. Impact of navigator timing on free-breathing submillimeter 3D coronary magnetic resonance angiography. Magn Reson Med. 2002;47(1):196–201. doi: 10.1002/mrm.10026. [DOI] [PubMed] [Google Scholar]
- 8.Madore B, Farneback G, Westin CF, Duran-Mendicuti A. A new strategy for respiration compensation, applied toward 3D free-breathing cardiac MRI. Magnetic resonance imaging. 2006;24(6):727–737. doi: 10.1016/j.mri.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Moller A, Zikic D, Botnar RM, et al. Dual cardiac-respiratory gated PET: implementation and results from a feasibility study. Eur J Nucl Med Mol Imaging. 2007;34(9):1447–1454. doi: 10.1007/s00259-007-0374-9. [DOI] [PubMed] [Google Scholar]
- 10.Santelli C, Nezafat R, Goddu B, et al. Respiratory bellows revisited for motion compensation: preliminary experience for cardiovascular MR. Magn Reson Med. 65(4):1097–1102. doi: 10.1002/mrm.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spincemaille P, Nguyen T, Prince M, Wang Y. Quantitative study of motion detection performance of center of k-space measurements; Proceedings of the 15th Annual Meeting of the ISMRM; Berlin, Germany. 2007.p. 1826. [Google Scholar]
- 12.Look D, Locker D. Time savings in measure of NMR and EPR relaxation times. Rev Sci Instrum. 1970;41:250–251. [Google Scholar]
- 13.Nehrke K, Bornert P, Groen J, Smink J, Bock JC. On the performance and accuracy of 2D navigator pulses. Magnetic resonance imaging. 1999;17(8):1173–1181. doi: 10.1016/s0730-725x(99)00043-0. [DOI] [PubMed] [Google Scholar]