Abstract
Rare autosomal dominant mutations result in familial Alzheimer's disease (FAD) with a relatively consistent age of onset within families. This provides an estimate of years until disease onset (relative age) in mutation carriers. Increased AD risk has been associated with differences in functional magnetic resonance imaging (fMRI) activity during memory tasks, but most of these studies have focused on possession of apolipoprotein E allele 4 (APOE4), a risk factor, but not causative variant, of late‐onset AD. Evaluation of fMRI activity in presymptomatic FAD mutation carriers versus noncarriers provides insight into preclinical changes in those who will certainly develop AD in a prescribed period of time. Adults from FAD mutation‐carrying families (nine mutation carriers, eight noncarriers) underwent fMRI scanning while performing a memory task. We examined fMRI signal differences between carriers and noncarriers, and how signal related to fMRI task performance within mutation status group, controlling for relative age and education. Mutation noncarriers had greater retrieval period activity than carriers in several AD‐relevant regions, including the left hippocampus. Better performing noncarriers showed greater encoding period activity including in the parahippocampal gyrus. Poorer performing carriers showed greater retrieval period signal, including in the frontal and temporal lobes, suggesting underlying pathological processes. Hum Brain Mapp 34:3308–3319, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: early onset, functional magnetic resonance imaging, PSEN1, APP, mutation, hippocampus, medial temporal lobe, volume
INTRODUCTION
Pathological brain changes occur many years before Alzheimer's disease (AD) is diagnosable through cognitive testing [Braak and Braak, 1991; Thal et al., 2002]. Neuroanatomical and neurophysiological differences in this early stage may be observed through functional and structural magnetic resonance imaging (fMRI and sMRI) in those with mild cognitive impairment (MCI) [Dickerson and Sperling, 2008; Frisoni et al., 2010], or an increased genetic risk for AD as determined either by possession of an apolipoprotein E ε4 allele (APOE4) [Bookheimer and Burggren, 2009], or mutations that confer AD in an autosomal dominant manner [Quiroz et al., 2010; Ringman et al., 2007, 2011a].
There is no perfect predictor of late‐onset AD, making incipient disease difficult to ascertain, except retrospectively. However, carriers of rare genetic mutations in the presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) genes inherit an early onset form of familial AD (FAD) in a fully penetrant autosomal dominant manner [Bertram and Tanzi, 2008]. In FAD, age of onset varies between families, but is typically similar within a family [Fox et al., 1997; Murrell et al., 2006], allowing the incipient AD stage to be estimated via “relative age.” We used relative age (the number of years before median age of disease diagnosis in a family) as a surrogate for incipient disease stage in mutation carriers. This allows the earliest, preclinical stages of the disease (before massive structural damage to the brain has occurred) to be studied prospectively.
Many studies have found that adults with increased genetic risk for AD have greater [Bassett et al., 2006; Bondi et al., 2005; Bookheimer et al., 2000; Filippini et al., 2009; Han et al., 2007; Quiroz et al., 2010] or less [Bassett et al., 2006; Bondi et al., 2005; Johnson et al., 2006; Quiroz et al., 2010; Trivedi et al., 2006] regional fMRI activity during episodic memory tasks in the medial temporal lobe (MTL). In some cases, higher AD genetic risk was associated with both higher and lower fMRI signal in the same study depending on MTL subregion and brain hemisphere [Bondi et al., 2005; Quiroz et al., 2010]. Of the studies that found MTL effects, only one [Quiroz et al., 2010] measured genetic risk for AD using FAD mutations rather than either APOE genotype or a family history of late‐onset AD. In that study, which used a novelty encoding task, mutation noncarriers showed more fMRI activity than carriers in some AD‐related regions, such as the left parahippocampal gyrus and precuneus, while carriers showed more fMRI activity than noncarriers in other AD‐related regions such as right posterior cingulate, inferior and superior parietal lobules, hippocampus, and dorsolateral prefrontal cortex [Quiroz et al., 2010]. The task that we employed was different from that used by the previous study, but we hypothesized based on their work and others, that we would find fMRI activity differences between mutation carriers and noncarriers in AD‐related regions, especially the MTL.
AD risk conferred by APOE4 may affect the brain via both common and distinct mechanisms as FAD mutations do. For instance, PSEN1 and APP mutations generally increase beta amyloid (Aβ) production, its aggregation, or the proportion of Aβ42 to Aβ40 [Brouwers et al., 2008]. APOE4 is also associated with greater Aβ aggregation, as well as clearance, but may also result in different tau phosphorylation and aggregation, inflammation, and lipid metabolism [Verghese et al., 2011]. Here we investigated the understudied relationship between FAD mutations and functional brain activity in those with preclinical AD.
We also evaluated in which brain regions fMRI activity was best associated with task performance after controlling for relative age and education. Retrieval success for episodic memory tasks has been shown to associate with fMRI activity in the MTL, posterior midline, medial prefrontal cortex, and posterior parietal cortex [Heun et al., 2006; Huijbers et al., 2010; Tsukiura et al., 2011], all regions that are preferentially affected in AD. We hypothesized that those who perform better on our memory task would show greater fMRI signal in these regions versus those who perform worse, particularly in mutation carriers. Identifying ways in which the brain activity is modified in those having probable differences in amyloid processing, and clarifying which regions are most important to a cognitive task given possible early disease processes offers insight into integral disease processes and how they affect cognition. These insights may help guide future prevention and intervention efforts.
MATERIALS AND METHODS
Subjects
Subjects were 17 first‐degree relatives of PSEN1 or APP mutation carriers (age 19–46; mean 32.2 years) (Table 1). Eight mutation carriers had the PS1A431E mutation and one had the APPV717I mutation. Noncarriers were from families having the PS1A431E mutation (4), the APPV717I mutation (2), and the PS1L235V mutation (2). A χ2 test showed that the mutation carriers were not different from the noncarriers (P = 0.45) in terms of how many were from an APP mutation‐ versus a PSEN1 mutation‐carrying family. All participants underwent extensive clinical, cognitive, biochemical, and imaging evaluations. Genotyping of FAD mutations was performed at UCLA as described previously [Braskie et al., 2012]. No mutation carriers were demented or had mild cognitive impairment [Petersen, 2004], using criteria described previously in detail [Braskie et al., 2012]. Subjects were normotensive and had no history of head injury requiring hospitalization, or of drug or alcohol dependency. No subject was taking medications designed to improve cognition. We excluded subjects having fMRI scans with excessive motion (>2 mm) or severe artifacts, subjects whose normalized (z score) pretest score on the scanner task exceeded an arbitrary threshold of 1.25 points different from the normalized posttest score, and those for whom the full pretest data were not available. Subjects were selected from 27 young to middle‐aged adults scanned while performing a verbal paired associates task. Of those, 10 were excluded: 6 for having technically inadequate scans, 1 for lacking a pretest, 2 for having a pretest not representative of posttest score, and 1 for far exceeding the characteristic age of the family's disease diagnosis.
Table 1.
Demographic characteristics of subjects
| Mutation carriers | Mutation noncarriers | |
|---|---|---|
| No. subjects | 9 | 8 |
| Age (yr)a, b | 29.8 ± 5.6 | 35.0 ± 8.5 |
| Relative age (yr)a, b | −14.0 ± 5.5 | −11.5 ± 11.6 |
| Males/femalesa | 1/8 | 1/7 |
| Family gene risk (PSEN1/APP)a | 8/1 | 6/2 |
| APOE4+a | 2 | 1 |
| Education (yr)a, b | 12.9 ± 3.1 | 12.5 ± 4.5 |
| Mini‐Mental State Exam (MMSE)a | 28.8 ± 1.0 | 28.5 ± 1.3 |
| Cognitive Abilities Screening Instrument (CASI)a | 93.0 ± 4.0 | 92.3 ± 5.8 |
| Word pair pretest score (out of 42)a | 27.2 ± 12.2 | 24.9 ± 9.6 |
| Word pair posttest score (out of 7)a | 6.0 ± 1.4 | 5.9 ± 0.8 |
A two‐tailed t‐test did not show a significant difference between mutation carriers and noncarriers.
Listed as mean ± standard deviation.
Subjects were of Mexican descent, residing either in Mexico or the United States. All MRI scans took place at UCLA on a single scanner. All subjects spoke sufficient Spanish to perform Spanish language cognitive testing. A fluently bilingual psychometrician performed all neuropsychological assessments, all of which were available and validated in Spanish. All subjects (except for a single subject who had undergone previous clinical testing) and the psychometrician were blind to subjects' genetic status.
Seventeen of these subjects were part of a prior novelty encoding fMRI study examining the effect of APOE genotype and familial mutation in familial AD mutation carriers [Ringman et al., 2011a]. Sixteen were evaluated previously in an article that related estimated time until AD diagnosis to fMRI activity during a novelty encoding task [Braskie et al., 2012]. Those studies did not include the current verbal paired associates task data, nor did they relate fMRI signal to task performance.
Study procedures were approved by the Institutional Review Boards at UCLA and the National Institute of Neurology and Neurosurgery in Mexico City. All subjects provided written, informed consent. Subjects were told that they would be tested for the FAD mutation for which they were at risk but in the context of the research protocol would not learn the result.
Imaging Procedures
Subjects underwent fMRI scanning (3T Siemens Allegra) using an echo‐planar imaging scan sequence while performing a verbal paired associates task (30 −3.0/1.0 mm gap slices, aligned to the anterior–posterior commissure plane; 3.1 × 3.1 mm in‐plane resolution; TR = 2,500 ms; TE = 35 ms; flip angle = 90°). For coregistration to the Montreal Neurological Institute (MNI) standard brain, we also acquired a T2‐weighted matched bandwidth scan (TR = 5,000 ms; TE = 33 ms; 4 averages) and a whole brain axial T1‐weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) three‐dimensional MRI sequence (1 mm slices/0 mm gap; TR = 1,900 ms; TE = 4.38 ms; TI = 1,100 ms; flip angle 15°; 1 × 1 mm in‐plane resolution).
Memory Activation Task
During fMRI scanning, subjects performed a Spanish language version of a verbal paired associates task shown previously to be sensitive to genetic risk for AD [Bookheimer et al., 2000]. In this task, subjects learned pairs of words, and were asked to recall the second words in the pairs, given the first words as cues.
Behavioral data were derived from an alternative pretest version of the task, administered on a laptop computer before scanning. The total number of correct retrievals for each subject was summed across six trials to arrive at a total possible score of 42. To include only subjects whose pretest scores were good indicators of the posttest scores, we converted pretests and post‐tests to z scores and excluded subjects for whom the absolute value of the difference was >1.25. Nineteen subjects who had usable scans were included in this calculation, and of those, two were excluded based on this criterion. The two subjects who exceeded this threshold had values of 2.3 (for someone who performed at the top of the lowest quartile on the pretest but got a 0 on the posttest, possibly indicating inattention to the task in the scanner) and 1.9 (for someone who was in the lowest quartile on the pretest, but got a perfect score on the posttest, possibly indicating an initial misunderstanding of pretest instructions). The next highest difference score was 1.1. Using two‐tailed t‐tests, the absolute value of the difference between pretest and post‐test z scores was not significantly different between mutation carriers and noncarriers either before excluding the two subjects who had above‐threshold difference values (P = 0.68) or in our final subjects sample (P = 0.33).
During the scan, subjects encoded seven pairs of unrelated words presented aurally in Spanish (MacStim presentation software version 3.1; WhiteAnt Occasional Publishing). Each word pair was presented for 3.75 s, followed by 1.25 s after each pair during which no word was presented. Each 35 s encoding block was followed by a 35 s distracter task period during which a “+” and “o” were alternately presented. Subjects were to press a button when they saw the “+.” During the 35 s retrieval blocks, participants heard the first word of each pair, and during the following 1.25 s, they attempted to recall the second word silently. The scan began and ended with 35 s of blank screen. After the scan, subjects were verbally tested to assess learning of the stimuli.
Functional MRI Data Analysis
We used FSL 4.1.4 tools (FMRIBs Software Library; available at: http://www.fmrib.ox.ac.uk/fsl) to analyze the fMRI data. Removal of nonbrain material, motion correction, and coregistration to MPRAGE scans and a standard MNI template brain were performed as described previously [Braskie et al., 2012]. FMRI scans were processed with a high‐pass temporal filter of 110 s (based on one complete task cycle) and spatial smoothing with a Gaussian kernel of 5 mm full width half maximum. Motion parameters were added to the model to reduce the effect of motion on the data.
We used FSLs FMRIBs Improved Linear Model (FILM) [Woolrich et al., 2001] to perform individual statistical analyses. For each subject, we contrasted fMRI activity that occurred while the subject attempted to learn or recall word pairs with fMRI activity while they performed the distracter task. Encoding versus distraction, and retrieval versus distraction will be called encoding and retrieval periods, respectively. All voxel‐wise analyses (both individual and group comparisons) were performed using a threshold of z > 2.3, adjusted to P < 0.05 using Gaussian Random Field theory‐based cluster thresholding to correct for multiple comparisons. We compared the fMRI activity for mutation carriers versus noncarriers during encoding and retrieval blocks using FSL tool FMRIBs Local Analysis of Mixed Effects (FLAME) [Beckmann et al., 2003], and controlled for age, pretest score, and education across all subjects. Here, we evaluated group differences in activity with any differences that might exist between groups for age, task performance, and education removed from the analysis. We therefore compared mutation carriers and noncarriers in one analysis while including these as covariates of no interest demeaned across all subjects.
We next evaluated where fMRI signal best described task performance (pretest score) after adjusting for relative age and education within both the mutation carrier and noncarrier groups. This effectively identifies voxels in which performance (given education level, incipient disease stage, and age) was correlated with fMRI response. Relative age was calculated as actual age minus the median age of AD diagnosis in the subject's family. The pretest scores across all subjects included in the study were significantly correlated with performance on the posttest (r = 0.67; P = 0.003), and thus, the pretest was a good indicator of task performance in the scanner. We used the pretest values rather than the post‐test values as an indicator of task performance because the pretest scores spanned a wider range and had no obvious ceiling effect. For this analysis, mutation carriers and noncarriers were directly compared within the same design. Relative age and pretest scores were covariates of no interest that were demeaned within each group. Because we were not evaluating the effects of education within group, but rather wished to remove the effects of varying education across groups, years of education were included as a covariate of no interest demeaned across groups.
All voxel‐wise analyses were performed using the statistical parametric mapping within the FSL software. We used multiple regression (P < 0.05) to perform additional tests in a region of interest (ROI) analyses of the MTL (hippocampus and parahippocampal gyrus [PHG]).
Region of Interest (ROI) Analyses
For our functional analyses, we used the Harvard‐Oxford standard ROIs [Desikan et al., 2006] to delineate right and left hippocampus and PHG in MNI space. FMRI signal in the MTL is sensitive to AD‐related differences during memory tasks [Dickerson et al., 2005; Hamalainen et al., 2007; Petrella et al., 2007; Remy et al., 2005]. Registration of whole brains to a template brain may not sufficiently align hippocampal subregions to detect functional differences across subjects. Thus, for each subject, we used FSL featquery to evaluate mean signal within right and left hippocampus ROIs during encoding and retrieval.
Because we found a significant relationship between pretest score and PHG fMRI activity in noncarriers, but not carriers, we also performed an ROI analysis to determine whether there was a trend toward significance in carriers. The ROI was limited to PHG voxels that were significantly associated with pretest score in the noncarriers after adjusting for relative age and education. We used FSLs featquery tool to determine each subject's mean fMRI percent signal change in the ROI. We performed multiple regression on the mean values, adjusting for relative age and education.
Structural Analyses
Structural ROIs of right and left hippocampus were automatically created using FSL FIRST software, a subcortical brain segmentation program that uses Bayesian shape and appearance models [Patenaude et al., 2007]. Hippocampal masks were created based on individual MPRAGE scans in native space and then manually refined as needed by a single trained reviewer blind to mutation group status. We obtained volumes for right and left hippocampus and intracranial volume (ICV; derived from the skull‐stripped MPRAGE scans) using the fslstats function in FSL. To adjust for head size, we divided hippocampal volumes by ICV for each subject. We evaluated the hippocampal volumes in two ways. First, multiple regression evaluated whether hippocampal volumes as a percentage of ICV were different between groups, controlling for sex and age. Second, as an additional test to determine whether atrophy may be responsible for our fMRI group differences in the hippocampus, we correlated raw left hippocampal volume with the mean retrieval period fMRI activity in those hippocampal voxels that showed significant group differences.
We also calculated gray matter volumes within each brain. To do this, we first normalized each T1‐weighted image to the MNI standard template brain using FSLs nonlinear image registration tool (FNIRT), and visually inspected the results. We then segmented each brain into gray and white matter and cerebrospinal fluid using FSLs automated segmentation tool (FAST) [Zhang et al., 2001]. Normalized gray matter volume for each individual was calculated using the fslstats tool.
To determine whether our fMRI group differences were above and beyond any contribution of gray matter or hippocampal atrophy, we performed two additional analyses. Total normalized gray matter volume and bilateral hippocampal volumes were in turn included as covariates of no interest in our fMRI analyses where we compared group activation differences during memory retrieval while controlling for age, pretest score, and education.
RESULTS
Mutation carriers were not different from noncarriers in terms of sex (P = 0.93), actual age (P = 0.15), relative age (P = 0.57), pretest score for the verbal paired associates task used in the scanner (P = 0.67), education (P = 0.84), or Mini‐Mental State Exam (MMSE) score (P = 0.62) as determined using two‐tailed unpaired t‐tests (Table 1).
The range of age of dementia diagnosis between families was relatively narrow: from 40 to 54 years old. Therefore, in all subjects, relative age was strongly correlated with chronological age (r = 0.82; P = 0.000047); voxel associations with relative age also reflected chronological age to some extent. In mutation carriers, relative age additionally reflects how close the subject is to exhibiting AD symptoms, with a less negative (higher) number indicating more imminent disease.
When all subjects were considered without regard for group, fMRI activity was greater during both encoding and retrieval versus distraction in frontal (especially in the left hemisphere), parietal and temporal cortex and cerebellum, and additionally during retrieval versus distraction in occipital cortex, the cingulate gyrus, the insula, striatum, and thalamus (Table 2).
Table 2.
Regions of greater fMRI response: Encoding or retrieval versus distraction—all subjects
| Laterality | Brodmann area | Peak MNI coordinates | Max. z score | |
|---|---|---|---|---|
| Encoding | ||||
| Frontal lobe | ||||
| Precentral g. | R/L | 4 | −54, −10, 44 | 4.34 |
| Superior frontal g. | L | 8 | −10, 22, 48 | 2.76 |
| Medial frontal g. | R/L | 6 | −6, 6, 56 | 4.54 |
| Medial frontal g. | L | 32 | −6, 14, 48 | 3.35 |
| Middle frontal g. | L | 46 | −50, 24, 22 | 3.26 |
| Inferior frontal g. | L | 13 | −44, 28, 2 | 4.45 |
| Inferior frontal g. | L | 47 | −42, 32, −6 | 4.66 |
| Parietal lobe | ||||
| Postcentral g. | R/L | 2 | −50, −20, 50 | 3.44 |
| Superior parietal lobule | L | 7 | −36, −60, 52 | 3.24 |
| Precuneus | L | 39 | −34, −66, 40 | 3.76 |
| Inferior parietal lobule | R/L | 40 | 40, −34, 52 | 3.85 |
| Temporal lobe | ||||
| Superior temporal g. | R/L | 21 | −64, −16, −4 | 5.56 |
| Superior temporal g. | R/L | 22 | −62, −8, 0 | 5.49 |
| Superior temporal g. | R/L | 41 | −44, −32, 10 | 5.38 |
| Superior temporal g. | R/L | 42 | −58, −24, 6 | 5.87 |
| Cerebellum | L | −12, −50, −12 | 3.39 | |
| Retrieval | ||||
| Frontal lobe | ||||
| Precentral g. | R/L | 6 | 56, −4, 40 | 3.84 |
| Medial frontal g. | R/L | 6 | 6, 6, 56 | 5.70 |
| Medial frontal g. | R/L | 32 | −4, 10, 48 | 5.18 |
| Inferior frontal g. | L | 9 | −58, 12, 26 | 4.36 |
| Inferior frontal g. | L | 46 | −52, 30, 8 | 4.78 |
| Parietal lobe | ||||
| Postcentral g. | R/L | 3 | 48, −18, 64 | 3.06 |
| Postcentral g. | R | 5 | 42, −38, 64 | 3.40 |
| Precuneus | L | 7 | −16, −78, 40 | 3.54 |
| Inferior parietal lobule | R/L | 40 | 42, −36, 52 | 3.77 |
| Cingulate g. | R/L | 32 | −8, 26, 28 | 4.91 |
| Insula | R/L | 13 | 32, 18, 0 | 4.58 |
| Temporal lobe | ||||
| Superior temporal g. | R/L | 22 | −64, −32, 6 | 5.24 |
| Superior temporal g. | R/L | 41 | 60, −22, 10 | 4.93 |
| Superior temporal g. | R/L | 42 | 66, −28, 8 | 4.97 |
| Middle temporal g. | R/L | 21 | −52, −32, −2 | 3.94 |
| Occipital lobe | ||||
| Cuneus | L | 7 | −4, −76, 34 | 3.30 |
| Cuneus | L | 18 | −4, −76, 22 | 3.14 |
| Cuneus | L | 19 | −8, −78, 40 | 2.88 |
| Caudate | R/L | −12, −4, 14 | 5.16 | |
| Putamen | R/L | 26, −4, 8 | 3.02 | |
| Thalamus | R/L | 16, −22, 16 | 3.92 | |
| Cerebellum | R/L | 32, −62, −28 | 4.60 |
Sample MNI coordinates reflect activation peaks for a region.
When fMRI activity was compared between mutation carriers and noncarriers, controlling for age, scanner task pretest score, and education, noncarriers showed more signal during retrieval in several regions known to be affected in early AD: inferior parietal cortex, precuneus, posterior middle temporal gyrus, and hippocampus (Figs. 1 and 2). Additional differences were found in the insula, brainstem, and thalamus (Table 3). After controlling for gray matter volume by including it as a covariate of no interest, noncarriers still showed more signal during memory retrieval in all four AD‐related regions: inferior parietal cortex, precuneus, posterior middle temporal gyrus, and hippocampus. When average bilateral hippocampal volume as a percentage of ICV was included as a covariate of no interest, noncarriers still showed more signal during memory retrieval in all of those regions except for the hippocampus. Mutation carriers did not show greater fMRI signal than noncarriers in any region. There were no significant effects of group during encoding. As an additional test to determine whether our group differences were actually limited to the retrieval period or may simply be subthreshold during encoding, we performed the same comparison for an additional contrast: retrieval versus encoding. No voxels were significantly different between groups for this contrast.
Figure 1.
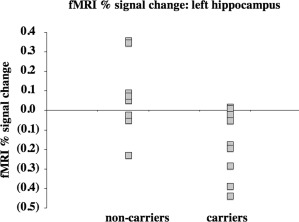
After controlling for age, pretest score, and education across all subjects, percent signal change during memory retrieval was higher in mutation noncarriers versus carriers in the left hippocampus (R 2 [full model] = 0.69; P [full model] = 0.005; P [partial contribution of group] = 0.01).
Figure 2.
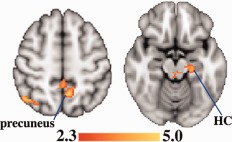
After controlling for age, pretest score, and education across all subjects, fMRI signal during memory retrieval was greater in mutation noncarriers versus carriers in regions that are affected in AD. These regions included the precuneus and hippocampus. Significant voxels (z ≥ 2.3; cluster thresholding corrected for multiple comparisons to adjust the image‐wise threshold to P < 0.05) are highlighted to represent z scores. Coordinates for the axial direction in MNI space are 52 mm (left image) and −16 mm (right image). The brain is displayed in radiological convention (left = right). HC = hippocampus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Regions in which fMRI response during memory retrieval is significantly greater in mutation noncarriers versus carriers after controlling for age, pretest scores, and education
| Laterality | Brodmann area | Peak MNI coordinates | Max. z score | |
|---|---|---|---|---|
| Temporal lobe | ||||
| Middle temporal g. | R | 19 | 40, −54, 18 | 3.73 |
| Hippocampus | L | −22, −24, −16 | 3.04 | |
| Parietal lobe | ||||
| Precuneus | RL | 7 | −8, −56, 50 | 3.53 |
| Angular g. | R | 39 | ||
| Supramarginal g. | R | 40 | 46, −60, 50 | 3.36 |
| Insula | R | 13 | 60, −30, 22 | 3.14 |
| Brain stem | RL | 10, −28, −12 | 3.13 | |
| Thalamus | R | 24, −26, 0 | 3.06 |
Sample MNI coordinates reflect activation peaks for a region.
To identify brain activity that is important for performance, within each group we also examined the relationship between fMRI activity and pretest score, controlling for relative age and education. We found that in noncarriers, increased fMRI activity during encoding was associated with higher memory pretest score in medial frontal and occipital cortex, temporal cortex (including in the bilateral PHG), insula, cerebellum, and brainstem (Table 4; Fig. 3). No voxels were significantly associated with pretest score in carriers during encoding. During retrieval, lower pretest scores for carriers were associated with greater fMRI activity in frontal and temporal cortex, anterior cingulate, and insula (Table 4, Fig. 4). FMRI activity was not significantly associated with lower scores in the noncarriers or with higher scores in the carriers.
Table 4.
Regions in which fMRI response significantly correlates with pretest scores controlling for relative age and education
| Laterality | Brodmann area | Peak MNI coordinates | Max. z score | |
|---|---|---|---|---|
| Encoding—noncarriers—greater fMRI signal with better pretest score | ||||
| Frontal lobe | ||||
| Inferior frontal g. | R | 45 | 50, 20, 0 | 3.40 |
| Inferior frontal g. | R | 47 | 44, 20, −6 | 3.01 |
| Temporal lobe | ||||
| Fusiform g. | RL | 37 | 28, −44, −16 | 3.06 |
| Parahippocampal g. | RL | 19 | 22, −52, −6 | 3.39 |
| Superior temporal g. | R | 22 | 54, 18, −8 | 3.48 |
| Occipital lobe | ||||
| Fusiform g. | RL | 19 | −26, −70, −6 | 2.93 |
| Lateral occipital cortex | R | 19 | −48, −78, 0 | 3.36 |
| Lingual g. | RL | 18 | 16, −72, −4 | 3.29 |
| Lingual g. | RL | 19 | 18, −50, −4 | 3.36 |
| Middle occipital cortex | L | 19 | −44, 76, 4 | 2.95 |
| Insula | R | 13 | 50, 16, 0 | 3.19 |
| Cerebellum | RL | −12, −54, −6 | 3.34 | |
| Brainstem | RL | −16, −26, −4 | 2.58 | |
| Retrieval—carriers—greater fMRI activity with worse pretest score | ||||
| Frontal lobe | ||||
| Dorsolateral prefrontal cortex | R | 9 | 52, 32, 30 | 3.34 |
| Dorsolateral prefrontal cortex | R | 46 | 48, 34, 14 | 3.41 |
| Middle frontal g. | R | 10 | 36, 44, 14 | 3.31 |
| Paracentral lobule | RL | 31 | 2, −8, 46 | 3.97 |
| Superior frontal g. | R | 6 | 10, 0, 66 | 3.68 |
| Temporal lobe | ||||
| Middle temporal g. | RL | 22 | 52, −48, 2 | 3.36 |
| Superior temporal g. | RL | 13 | 54, −18, 6 | 3.42 |
| Superior temporal g. | L | 21 | −64, −8, −4 | 4.14 |
| Superior temporal g. | RL | 22 | 66, −38, 20 | 3.75 |
| Superior temporal g. | RL | 41 | 58, −22, 8 | 3.33 |
| Superior temporal g. | RL | 42 | 62, −32, 6 | 3.29 |
| Transverse temporal g. | L | 41 | −38, −28, 12 | 3.13 |
| Cingulate g. | RL | 24 | 2, −14, 38 | 3.78 |
| Insula | L | 13 | −44, −28, 20 | 3.15 |
Sample MNI coordinates reflect activation peaks for a region.
Figure 3.
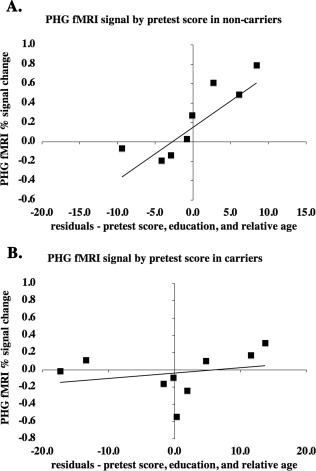
After controlling for relative age and education within group, mutation noncarriers (A) with higher pretest scores showed significantly greater encoding period fMRI activity in bilateral PHG (R 2 [full model] = 0.89; P [full model] = 0.02; P [partial contribution of pretest] = 0.01). Mutation carriers (B) did not show a significant relationship between pretest score and average fMRI activity in bilateral PHC (R 2 [full model] = 0.73; P [full model] = 0.07; P [partial contribution of pretest] = 0.34), although a strong trend toward significance existed in left PHG.
Figure 4.
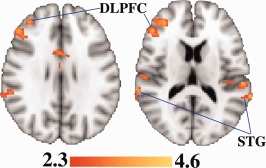
After controlling for relative age and education within each group, fMRI signal during memory retrieval was higher in mutation carriers having lower memory pretest scores. Highlighted regions include the dorsolateral prefrontal cortex (DLPFC) and superior temporal gyrus (STG), both of which were previously implicated in APOE4‐related fMRI differences using a similar task [Bookheimer et al., 2000]. Significant voxels (z ≥ 2.3; cluster thresholding corrected for multiple comparisons to adjust the image‐wise threshold to P < 0.05) are highlighted to represent z scores. Coordinates for the axial direction in MNI space are 14 mm (left image) and 28 mm (right image). The brain is displayed in radiological convention (left = right). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We found that after controlling for relative age and education, mutation noncarriers (but not carriers) who showed greater voxel‐wise encoding period fMRI activity in bilateral posterior parahippocampal gyrus performed better on the scanner task pretest. The peak activity was found in Brodmann area (BA) 19, but the region of significant activity included voxels in BA 35 (perirhinal cortex) and BA 36 (parahippocampal cortex). Because the MTL is a particular focus of studies that evaluate early AD‐related fMRI changes [Dickerson et al., 2005; Johnson et al., 2006; Kircher et al., 2007], we additionally used an ROI approach to evaluate fMRI activity in the PHG. We evaluated whether PHG fMRI signal truly responded differently between groups during memory encoding or whether a subthreshold trend existed in mutation carriers. As determined in the voxel‐wise analyses, after controlling for relative age and education in noncarriers, encoding period fMRI activity was greater with better task performance in bilateral PHG (R 2 [full model] = 0.89; P [full model] = 0.02; P [partial contribution of pretest] = 0.01). Pretest score did not significantly relate to average fMRI activity in bilateral PHG in mutation carriers (R 2 [full model] = 0.73; P [full model] = 0.07; P [partial contribution of pretest] = 0.34). However, a strong trend toward greater fMRI activity in better performing subjects existed in the left PHG (R 2 [full model] = 0.76; P [full model] = 0.054; P [partial contribution of pretest] = 0.047), but not the right PHG (R 2 [full model] = 0.65; P [full model] = 0.13; P [partial contribution of pretest] = 0.95).
We evaluated mean fMRI signal in bilateral hippocampus during encoding and retrieval using an ROI approach. During retrieval, when relative age, pretest scores, and education were controlled across all subjects, mutation carriers showed significantly less activity in bilateral hippocampus than non‐carriers (R 2 [full model] = 0.62; P [full model] = 0.01; P [partial contribution of group] = 0.04). FMRI activity was not different between groups in bilateral hippocampus during encoding (R 2 [full model] = 0.31; P [full model] = 0.30). We then evaluated right and left hippocampus separately, controlling for relative age, pretest score, and education. During retrieval, mutation carriers showed significantly less fMRI activity in the left (R 2 [full model] = 0.69; P [full model] = 0.005; P [partial contribution of group] = 0.01), but not the right hippocampus (R 2 [full model] = 0.47; P [full model] = 0.09; P [partial contribution of group] = 0.22). However, after controlling for relative age and education during memory retrieval within each group, left hippocampal activity was not significantly associated with pretest score for carriers (R 2 [full model] = 0.57; P [full model] = 0.20) or noncarriers (R 2 [full model] = 0.57; P [full model] = 0.29). To evaluate the contribution of atrophy to our hippocampal group fMRI activity differences, we related the mean fMRI activity in significant left hippocampal voxels to the raw hippocampal volume values across all subjects. Raw left hippocampal volume was not significantly correlated with mean fMRI activity (R 2 = 0.003; P = 0.85). Right (R 2 [full model] = 0.17; P [full model] = 0.48), and left hippocampal volumes as a percentage of ICV were not significantly different between groups after adjusting for age and sex (R 2 [full model] = 0.21; P [full model] = 0.38).
DISCUSSION
Despite similar cognitive abilities and task performance, during memory retrieval, FAD mutation carriers showed less fMRI activity than noncarriers in regions that included the left hippocampus (after controlling for age, pretest score, and education).
As we hypothesized, many of the regions in which we saw group differences—such as hippocampus, temporal cortex, precuneus, and inferior parietal lobule—show early structural changes in AD [Braak and Braak, 1991; Chetelat et al., 2010; McDonald et al., 2009]. When we controlled for total gray matter volumes by including them as a covariate of no interest, the variance in fMRI signal was accounted for by group classification above and beyond any variance in gray matter volume in all four of these regions. We had similar results when we controlled for average bilateral hippocampal volume in the same way, but the difference in fMRI signal between the two groups was no longer statistically significant in the hippocampus, suggesting that hippocampal atrophy may contribute to the effect. However, hippocampal volume did not differ between groups and mean activity in significant voxels of the left hippocampus was not related to raw left hippocampal volumes, suggesting that atrophy is relatively unlikely to explain the entire effect. Damage to the entorhinal cortex—a region of very early AD pathology accumulation [Braak and Braak, 1991]—results in hypometabolism in most of these regions in nonhuman primates [Meguro et al., 1999], so our group differences may represent incipient AD processes. Group activity was significantly different only during retrieval, but when retrieval and encoding were contrasted, the groups did not differ, suggesting that similar, subthreshold effects may exist during encoding.
Presymptomatic mutation carriers showed less fMRI activity in the hippocampus versus noncarriers. Several previous fMRI studies of AD risk in cognitively intact adults found hippocampal differences associated with AD genetic risk [Bassett et al., 2006; Bondi et al., 2005; Bookheimer et al., 2000; Filippini et al., 2009; Han et al., 2007; Johnson et al., 2006; Quiroz et al., 2010; Trivedi et al., 2006]. In most of these studies, the focus was on greater MTL activity in higher risk adults, which most frequently occurred in the right hemisphere [Bassett et al., 2006; Bondi et al., 2005; Filippini et al., 2009; Han et al., 2007; Quiroz et al., 2010]. However, three of these studies also found, as we did, that lower AD risk related to greater left hippocampal and/or parahippocampal activity [Bassett et al., 2006; Bondi et al., 2005; Quiroz et al., 2010], and two additional studies found that lower risk adults had greater activity in the right [Trivedi et al., 2006] or bilateral [Johnson et al., 2006] hippocampus.
In a prior novelty encoding study, we found no group hippocampal differences in an overlapping sample of FAD mutation carriers [Braskie et al., 2012; Ringman et al., 2011a], even when those subjects [Braskie et al., 2012] were reanalyzed to control for age and education across all subjects (R 2 [full model] = 0.05; P [full model] = 0.78). In our current intentional encoding and retrieval task, we found that FAD mutation carriers showed less fMRI activity in the left hippocampus (during retrieval only) than our noncarriers. Our prior novelty task used incidental rather than intentional encoding, and there was no memory recall component. It is possible that this recall task is more sensitive to mutation status differences in a small sample. The only previous AD risk study that we know of that separately evaluated where low AD risk was associated with higher fMRI during memory recall (rather than recognition), likewise found higher fMRI signal in low risk adults in the left MTL [Bassett et al., 2006]. Alternatively, our results may arise from resting state metabolism differences; the novelty encoding task, whose baseline activity involves viewing repeated pictures, may be less sensitive to such differences than the word pair task, which uses a simple reaction task as a baseline. However, preclinical FAD mutations are associated with reduced hippocampal resting state metabolism relative to controls [Mosconi et al., 2006], which would more likely result in greater task‐induced fMRI hippocampal activity rather than the lower activity we saw here in carriers. Therefore, this explanation is less likely.
Within each group, we examined how fMRI activity related to memory pretest score, controlling for relative age and education. In mutation noncarriers, relative age closely reflects chronological age, while in carriers, it represents both chronological age and nearness to disease symptoms. This analysis demonstrates how fMRI activity relates to task performance given age, incipient disease stage, and level of education. During encoding, better performing mutation noncarriers, but not carriers, showed greater fMRI signal in several frontal, occipital, and temporal regions including bilateral PHG (Table 4) after adjusting for relative age and education. A correlation between memory performance and MTL activity only in those having relatively lower AD risk is not without precedent [Trivedi et al., 2006]. To identify possible subthreshold effects, we further performed ROI analysis in both carriers and noncarriers on significant PHG voxels (Fig. 3). PHG activity was not significantly correlated with pretest score in mutation carriers, but there was a strong trend toward a positive relationship between the two in left PHG only. Therefore, noncarriers engaged PHG in both hemispheres to successfully perform the task, but in carriers, who may have more MTL pathology, this relationship was disrupted, particularly in the right hemisphere. Right MTL has previously been identified as a site of early AD genetic risk differences in fMRI signal during memory tasks [Bassett et al., 2006; Bondi et al., 2005; Filippini et al., 2009; Han et al., 2007]. Although there were no significant performance differences between groups, it is possible that the mutation carriers already have mild, preclinical memory deficits not detectable by our task. A task that evaluates reaction time as well as correctness of response in a challenging task may be useful to highlight cognitive differences between mutation carriers and noncarriers in future studies.
In contrast with our hypothesis, in mutation carriers, memory performance was worse in those who showed greater retrieval period fMRI activity in several regions, including dorsolateral prefrontal (DLPFC) and superior temporal gyrus (STG) (Table 4). It is possible that detrimental processes were affecting these regions in some mutation carriers, interfering with the subjects' ability to perform the task and increasing relative fMRI signal beyond an optimal level. Examples of processes that result in greater fMRI activity in high‐risk subjects who perform worse may include inflammation, dedifferentiation (a decreased specificity of regional recruitment) [Li and Lindenberger, 1999; Logan et al., 2002], or an excitotoxic response to nearby amyloid pathology [Sperling et al., 2010]. The concept of intermediate levels of fMRI activity being needed to optimally perform a memory task has been explored previously [Liu et al., 2010].
Decreased cerebrospinal fluid (CSF) levels of Aβ42, and increased levels of total τ and phosphorylated τ181 are associated with incipient [Mattsson et al., 2009] and established [Andreasen et al., 2001] AD. Prior research has shown that in an FAD mutation carrier, cerebrospinal fluid levels of Aβ42, total τ and phosphorylated τ181 were already changing between 17 and 22 years before the average age of AD diagnosis in the patient's family [Ringman et al., 2011b]. Mutation carriers in our study were between 22 and 5 years younger than the median age of AD diagnosis in their families (mean relative age of 14), suggesting that most of these subjects were likely to already have initial AD changes underway at the time of our study. The regions affected here were both implicated previously in APOE4‐related fMRI differences using a similar task [Bookheimer et al., 2000], suggesting that activity there was relevant to the task and varied with AD risk. Our DLPFC results were limited to the right hemisphere, consistent with the hemispheric encoding/retrieval asymmetry (HERA) model [Tulving et al., 1994], in which right PFC is more engaged than left during episodic memory retrieval.
Our results may be influenced by blood perfusion or resting metabolism, both of which influence fMRI signal and may differ in presymptomatic FAD mutation carriers [Johnson et al., 2001; Kennedy et al., 1995; Mosconi et al., 2006; Scholl et al., 2011]. A prior study of late‐onset AD risk found increased resting‐state perfusion and (during memory encoding) lower fractional BOLD and perfusion in the MTL of APOE4 carriers [Fleisher et al., 2009]. Future studies in FAD mutation carriers that examine such factors alongside task‐related fMRI would be useful in determining their contributions to fMRI signal variations. We are limited by our small sample size, which may unduly increase the effects of random variations in the population. Therefore, our results must be seen as merely suggestive. Verifying these results in a larger sample would aid in interpreting these data. Additionally, despite our efforts to recruit similar numbers of male and female subjects, our sample was ∼88% female. Mutation carriers and noncarriers had a similar percentage of male subjects. Future studies having more male subjects would be useful in determining the extent to which our results are generalizable to samples more representative of the population.
The generalizability of our results to late‐onset AD is uncertain. Similar retrospective studies that control for relative age in older adults who later develop AD may be useful in determining how fMRI signal relates to cognitive performance. Comparing amyloid deposition (determined through positron emission tomography) with performance and fMRI signal in studies of those at risk for late‐onset AD also may address this question. Different genetic risk factors, including those that cause FAD may affect fMRI signal in the brain in varying ways and through different mechanisms. In the current study, we have examined genetic risk from three different FAD mutations as one entity. We have also compared our results with results from previous articles whose focus was late‐onset AD risk, primarily APOE4. We do so because although genetic risk factors may be manifested differently through fMRI signal, there are also similarities among these risk factors. For instance, APP and PSEN1 mutation carriers and APOE4 carriers are all likely to have increased total Aβ or proportion of Aβ42 to Aβ40 in the brain with respect to noncarriers, regardless of whether this is due to increased production, aggregation, or decreased clearance [Brouwers et al., 2008; Verghese et al., 2011]. A focus on the similarities, rather than the differences between how these variants affect brain structure and function may improve the generalizability of results, and also may help identify which gene effects on the brain have the greatest impact on cognition and disease onset or progression.
CONCLUSION
Presymptomatic FAD mutation carriers showed less retrieval period fMRI signal than noncarriers in several AD‐related regions (after adjusting for age, memory test score, and education). During memory encoding, greater fMRI signal in several regions, including bilateral PHG, related to better memory performance in mutation noncarriers. There was also a strong trend toward left PHG only relating to memory performance in mutation carriers, suggesting a disruption of this relationship in the right PHG. Greater fMRI activity was not anywhere correlated with better memory performance in mutation carriers. However, in some regions, including DLPFC and STG, greater retrieval period activity was associated with worse memory performance, suggesting that these regions are both important to performance and disrupted in mutation carriers, possibly by incipient AD‐related processes.
REFERENCES
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K (2001): Evaluation of CSF‐tau and CSF‐Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 58:373–379. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL (2006): Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain 129:1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM (2003): General multilevel linear modeling for group analysis in FMRI. Neuroimage 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE (2008): Thirty years of Alzheimer's disease genetics: The implications of systematic meta‐analyses. Nat Rev Neurosci 9:768–778. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG (2005): fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S, Burggren A (2009): APOE‐4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annu Rev Clin Psychol 5:343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW (2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E (1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol (Berl) 82:239–259. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Medina LD, Rodriguez‐Agudelo Y, Geschwind DH, Macias‐Islas MA, Cummings JL, Bookheimer SY, Ringman JM (2012): Increased fMRI signal with age in familial Alzheimer's disease mutation carriers. Neurobiol Aging 33:424.e11–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Sleegers K, Van Broeckhoven C (2008): Molecular genetics of Alzheimer's disease: an update. Ann Med 40:562–583. [DOI] [PubMed] [Google Scholar]
- Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, Ellis KA, Szoeke C, Martins RN, O'Keefe GJ, Salvado O, Masters CL, Rowe CC; Australian Imaging Biomarkers and Lifestyle Research Group (2010): Relationship between atrophy and beta‐amyloid deposition in Alzheimer disease. Ann Neurol 67:317–324. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand‐Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA (2005): Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA (2008): Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: Insights from functional MRI studies. Neuropsychologia 46:1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proc Natl Acad Sci USA 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB (2009): Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging 30:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Kennedy AM, Harvey RJ, Lantos PL, Roques PK, Collinge J, Hardy J, Hutton M, Stevens JM, Warrington EK, Rossor MN (1997): Clinicopathological features of familial Alzheimer's disease associated with the M139V mutation in the presenilin 1 gene. Pedigree but not mutation specific age at onset provides evidence for a further genetic factor. Brain 120:491–501. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM (2010): The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 6:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H (2007): Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 28:1889–1903. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey‐Bloom J, Salmon DP, Thal LJ, Bondi MW (2007): Verbal paired‐associate learning by APOE genotype in non‐demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 28:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W (2006): Successful verbal retrieval in elderly subjects is related to concurrent hippocampal and posterior cingulate activation. Dement Geriatr Cogn Disord 22:165–172. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Daselaar SM (2010): Dissociating the “retrieval success” regions of the brain: Effects of retrieval delay. Neuropsychologia 48:491–497. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Lopera F, Jones K, Becker A, Sperling R, Hilson J, Londono J, Siegert I, Arcos M, Moreno S, Madrigal L, Ossa J, Pineda N, Ardila A, Roselli M, Albert MS, Kosik KS, Rios A (2001): Presenilin‐1‐associated abnormalities in regional cerebral perfusion. Neurology 56:1545–1551. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA (2006): The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci 26:6069–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AM, Rossor MN, Frackowiak RS (1995): Positron emission tomography in familial Alzheimer disease. Alzheimer Dis Assoc Disord 9:17–20. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT (2007): Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry 78:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S‐C, Lindenberger U.1999. Cross‐level unification: a computational exploration of the link between deterioration of neurotransmitter systems dedifferentiation of cognitive abilities in old age In: Nilsson L‐G, Markowitsch HJ, editors.Cognitive Neuroscience of Memory.Hogrefe & Huber Publishing,Seattle. [Google Scholar]
- Liu X, Qin S, Rijpkema M, Luo J, Fernandez G (2010): Intermediate levels of hippocampal activity appear optimal for associative memory formation. PLoS One 5:e13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL (2002): Under‐recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 33:827–840. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B, Blennow K (2009): CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302:385–393. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema‐Notestine C, Hagler DJ Jr, Holland D, Koyama A, Brewer JB, Dale AM (2009): Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology 73:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro K, Blaizot X, Kondoh Y, Le Mestric C, Baron JC, Chavoix C (1999): Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non‐human primate as shown by PET. Implications for Alzheimer's disease. Brain 122:1519–1531. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, Caffarra P, Pupi A (2006): Hypometabolism exceeds atrophy in presymptomatic early‐onset familial Alzheimer's disease. J Nucl Med 47:1778–1786. [PubMed] [Google Scholar]
- Murrell J, Ghetti B, Cochran E, Macias‐Islas MA, Medina L, Varpetian A, Cummings JL, Mendez MF, Kawas C, Chui H, Ringman JM (2006): The A431E mutation in PSEN1 causing familial Alzheimer's disease originating in Jalisco State, Mexico: An additional fifteen families. Neurogenetics 7:277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith S, Kennedy D, Jenkinson M (2007): FIRST‐FMRIB's integrated registration and segmentation tool. In: Thirteenth Annual Meeting of the Organization for Human Brain Mapping (HBM). Organization for Human Brain Mapping annual meeting (2007) Chicago, IL, USA.
- Petersen RC (2004): Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, Doraiswamy PM (2007): Cortical deactivation in mild cognitive impairment: High‐field‐strength functional MR imaging. Radiology 245:224–235. [DOI] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillon G, Lopera F, Stern CE (2010): Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol 68:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W (2005): Verbal episodic memory impairment in Alzheimer's disease: A combined structural and functional MRI study. Neuroimage 25:253–266. [DOI] [PubMed] [Google Scholar]
- Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, Schaffer B, Varpetian A, Tseng B, Ortiz F, Fitten J, Cummings JL, Bartzokis G (2007): Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain 130:1767–1776. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Medina LD, Braskie M, Rodriguez‐Agudelo Y, Geschwind DH, Macias‐Islas MA, Cummings JL, Bookheimer S (2011a)Effects of risk genes on BOLD activation in presymptomatic carriers of familial Alzheimer's disease mutations during a novelty encoding task. Cereb Cortex 21:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Taylor K, Teng E, Coppola G, Gylys K (2011b)Longitudinal change in CSF biomarkers in a presymptomatic carrier of an APP mutation. Neurology 76:2124–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl M, Almkvist O, Axelman K, Stefanova E, Wall A, Westman E, Langstrom B, Lannfelt L, Graff C, Nordberg A (2011): Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol Aging 32:1388–1399. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA (2010): Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med 12:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H (2002): Phases of A beta‐deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC (2006): Reduced hippocampal activation during episodic encoding in middle‐aged individuals at genetic risk of Alzheimer's disease: a cross‐sectional study. BMC Med 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, Akitsuki Y, Taki Y, Kawashima R (2011): Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face‐name associations. J Cogn Neurosci 23:200–213. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S (1994): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91:2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM (2011): Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 10:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20:45–57. [DOI] [PubMed] [Google Scholar]


