Abstract
Prefrontal cortex (PFC) and posterior parietal cortex (PPC) are neural substrates for spatial cognition. We here review studies in which we tested the hypothesis that human frontoparietal cortex may function as a priority map. According to priority map theory, objects or locations in the visual world are represented by neural activity that is proportional to their attentional priority. Using functional magnetic resonance imaging (fMRI), we first identified topographic maps in PFC and PPC as candidate priority maps of space. We then measured fMRI activity in candidate priority maps during the delay periods of a covert attention task, a spatial working memory task, and a motor planning task to test whether the activity depended on the particular spatial cognition. Our hypothesis was that some, but not all, candidate priority maps in PFC and PPC would be agnostic with regard to what was being prioritized, in that their activity would reflect the location in space across tasks rather than a particular kind of spatial cognition (e.g., covert attention). To test whether patterns of delay period activity were interchangeable during the spatial cognitive tasks, we used multivariate classifiers. We found that decoders trained to predict the locations on one task (e.g., working memory) cross-predicted the locations on the other tasks (e.g., covert attention and motor planning) in superior precentral sulcus (sPCS) and in a region of intraparietal sulcus (IPS2), suggesting that these patterns of maintenance activity may be interchangeable across the tasks. Such properties make sPCS in frontal cortex and IPS2 in parietal cortex viable priority map candidates, and suggest that these areas may be the human homologues of the monkey frontal eye field (FEF) and lateral intraparietal area (LIP).
Keywords: attention, frontal eye field, lateral intraparietal area, parietal cortex, prefrontal cortex, priority map, topography, working memory
1. Introduction
The ability to reliably identify cortical maps has been essential to our understanding of the organizing principles of the brain and the functional architecture of specific neural areas (Hubel and Wiesel, 1959; Mountcastle, 1957). For example, the identification of subregions of visual cortex using topographic mapping (Engel et al., 1994; Wandell, 1999) and functional localizers (Kanwisher et al., 1997) has led to major advances. fMRI studies of human visual cortex have identified more than 12 topographic maps that provide an orderly tiling of visual space (Wandell et al., 2007). Recently, topographic maps have been discovered outside of occipital cortex, namely in association areas such as prefrontal cortex (PFC) (Hagler and Sereno, 2006; Kastner et al., 2007) and posterior parietal cortex (PPC) (Konen and Kastner, 2008; Saygin and Sereno, 2008; Schluppeck et al., 2005; Sereno et al., 2001; Silver et al., 2005; Swisher et al., 2007). Unlike retinotopic maps in early visual cortex, in which neighboring parts of the brain represent neighboring parts of the visual field, topographic maps in PFC and PPC are driven by attention to locations in space (Bressler and Silver, 2010).
Although PFC may be the most important area for higher cognition (Curtis and D’Esposito, 2004; Stuss and Knight, 2002), the inability to reliably define its subdivisions is a major factor that limits progress in understanding its functions; this factor is compounded by the large individual differences in PFC functional and structural neuroanatomy (Rajkowska and Goldman-Rakic, 1995; Van Essen, 2005). Theories suggest that PFC is specialized for several functions, including attentional control (Mesulam, 1990), working memory (Goldman-Rakic, 1987), and action selection (Passingham, 1993). Although nearly all theories of PFC emphasize its role in top-down executive control over posterior cortices (Miller and Cohen, 2001), the mechanisms of such control are unknown.
Important research questions are how PFC and PPC represent space and how spatial representations bias sensory and motor functions. Several lines of evidence have converged on a theory positing that activity in PFC and PPC constitutes maps of prioritized space (Bisley and Goldberg, 2010; Fecteau and Munoz, 2006; Itti and Koch, 2001; Serences and Yantis, 2006; Thompson and Bichot, 2005). According to this theory, the population activity of neurons in priority maps forms a rank-ordered or “prioritized” representation of important locations in the visual field (Bisley and Goldberg, 2010). Priority maps are theorized to be composed of populations of neurons organized topographically into a two-dimensional map of gaze-centered space (Itti and Koch, 2001). Such maps are continually sculpted by the saliency, or conspicuousness, of bottom-up information from early visual neurons about stimulus features (Itti and Koch, 2001), combined with goal-relevant, top-down information from higher association cortices (Serences and Yantis, 2006). The read-out of a priority map could be the mechanism by which competing representations of objects are selected in the visual system and competing representations of actions are selected in the motor system.
In a recent fMRI study (Jerde et al., 2012), we identified candidate priority maps by defining topographically organized areas of PFC and PPC. To identify topographic maps, we used a task in which covert attention was systematically shifted around the visual field. In three additional experiments using the same subjects, we compared changes in the blood oxygen level-dependent (BOLD) signal in the candidate priority maps as subjects maintained attention covertly in the periphery, maintained a location in working memory, and maintained a saccade plan. We predicted that if priority maps represent the location, and not the cause, of priority, then the spatiotemporal patterns of neural activity in priority maps would be indistinguishable across the spatial cognitive tasks. We here review the results of this study in the context of the growing body of literature that implicates the frontoparietal cortex in prioritizing space.
2. Common activation during working memory, attention, and intention
Persistent neural activity during the delay period between a sensory cue (such as the position of a briefly flashed spot of light) and a subsequent contingent motor response (such as the shift of gaze to a remembered location) is the most compelling evidence that this activity reflects a maintained spatial representation, e.g., working memory (Fuster and Alexander, 1971; Kubota and Niki, 1971). Such persistent activity is thought to link the prior stimulus cue with its contingent response (Fuster, 2001). Several features indicate that persistent activity is a mechanism for the maintenance of spatial working memory in humans and non-human primates. First, the BOLD signal persists in human brain areas homologous to non-human primate brain areas in which neuronal spiking persists, notably in PFC and PPC (Corbetta and Shulman, 2002; Curtis and D’Esposito, 2003; Funahashi et al., 1989; Goldberg et al., 2002; Snyder et al., 1997). Second, delay period activity is coupled to task performance, in that it persists as long as a spatial representation is actively maintained (Schluppeck et al., 2006; Srimal and Curtis, 2008). Furthermore, greater delay period activity predicts better performance on spatial working memory tasks (Curtis et al., 2004).
Traditionally, persistent activity has been posited to reflect the active maintenance of a working memory representation (Curtis and Lee, 2010). In a working memory task, for example, neurons that are selective for the presentation of the cue remain in an active state via persistent activity during the retention interval. Such delay period activity could, however, just as easily reflect the maintenance of spatial attention directed towards the prior location of the flashed cue, i.e., covert attention. Similarly, the delay period activity could reflect the preparation of a forthcoming saccade to the cued location, i.e., motor intention. Indeed, persistent activity in PFC and PPC has been reported during intervals in which animals attend covertly or prepare a motor response (Bisley and Goldberg, 2003a; Cisek and Kalaska, 2005; Cui and Andersen, 2007; Gottlieb et al., 1998; Schafer and Moore, 2007; Schall et al., 1995; Snyder et al., 1997; Thompson et al., 2005).
In three fMRI studies, we demonstrated that delay period activity showed a striking pattern of overlap in PFC and PPC while subjects maintained covert spatial attention (Ikkai and Curtis, 2008), maintained a spatial working memory representation (Srimal and Curtis, 2008), and maintained an oculomotor intention (Curtis and Connolly, 2008) (Fig. 1). Specifically, we observed delay period activity in all three tasks in dorsolateral PFC, superior (sPCS) and inferior (iPCS) precentral sulcus, dorsal IPS, and superior temporal sulcus. These results indicate that the neural mechanism supporting a variety of spatial cognitions is contingent upon persistent activity in PFC and PPC. A strong interpretation of these findings is that persistent activity in PFC and PPC reflects a single neural mechanism that is common to the maintenance of covert attention, the maintenance of a working memory representation, and the maintenance of a movement intention, and probably to other spatial cognitions (Ikkai and Curtis, 2011). Nonetheless, the nature of such a mechanism is unknown.
Fig. 1.
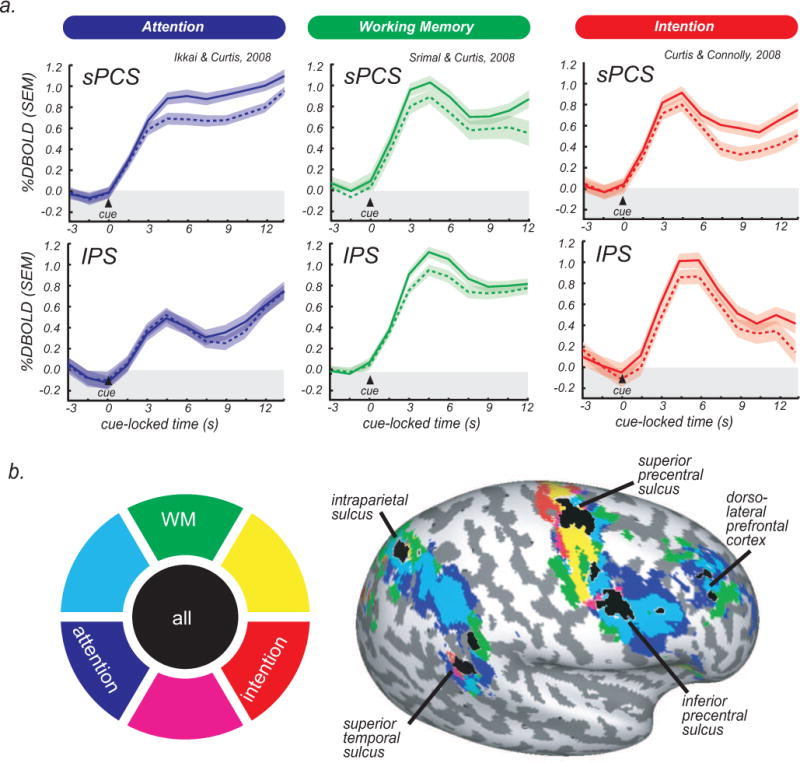
Time courses and activation in three event-related studies of spatial cognition. (a) Time courses (average, SEM) from the sPCS (top panels) and IPS (bottom panels) are time-locked to the presentation of the cue. Solid lines represent trials in which the locus of attention, memoranda, or direction of saccade was in the hemifield contralateral to the cortical hemisphere, and dashed lines represent ipsilateral trials. Note that both sPCS and IPS show activation that persists throughout the delay period and has a contralateral bias. (b) Significant delay period activity is projected on an inflated cortical sheet of the right hemisphere. The color wheel is the legend for the delay period activity; for example, areas activated for both attention and intention are depicted in magenta. Areas that show delay period activation for all three tasks are depicted in black and labeled.
3. Priority map theory
Space may be prioritized via persistent activity among neurons whose receptive fields include the behaviorally relevant location. In this conceptual framework, the activity of priority maps is theorized to tag locations in the environment that are salient and behaviorally relevant (Serences and Yantis, 2006). Beyond that, the theory is underspecified. Exactly what gets prioritized may be specific to a given cognitive function (e.g., working memory buffer or locus of attention) or effector system (e.g., gaze or reach plan). Alternatively, a priority map may be agnostic in that its activity is the same no matter what led to the prioritization in the map or how it will be used.
Theoretically, then, prioritized maps of space may contain only information about the locations of salient and behaviorally relevant information. Consider, for example, a working memory delay in which the position of a stimulus is 10° to the right. In terms of an attractor dynamics model, an attractor positioned within a topographic map at the cued location could be used to represent the prioritized spatial location (Compte et al., 2000). This map may be identical to a map during the planning of a saccade that is 10° to the right, and to a map during the maintenance of covert attention that is 10° to the right. The pattern of activity within the map of space may therefore be agnostic about the conditions that led to the prioritized location. Other brain areas could then read out the general map of prioritized space to implement the specific cognitive or behavioral demands. For example, downstream oculomotor areas (e.g., superior colliculus and brainstem saccade generator) may read out the priority maps of space in PFC and PPC to convert eye-centered retinotopic representations into the motor metrics for both memory- and visual-guided saccades (Bisley and Goldberg, 2003b; Sommer and Wurtz, 2001). Additionally, a read-out of the same priority map by posterior visual areas could bias the competition for neural representation toward neurons whose receptive fields match the peaks in the priority maps (Gregoriou et al., 2009; Moore and Armstrong, 2003).
Priority maps have two unique features that distinguish them from simpler maps of space (Thompson and Bichot, 2005). First, they can represent multiple locations simultaneously. And second, they can represent the varying levels of priority of multiple locations. Monkey electrophysiological studies of visual search indicate that FEF and LIP activity selects potential saccade goals based on the item’s bottom-up salience (e.g., contrast with respect to background) (Bichot et al., 2001b; Schall and Hanes, 1993; Thomas and Pare, 2007). The sudden onset of a visual stimulus in the neuron’s receptive field captures attention automatically even when it is behaviorally irrelevant (Yantis and Jonides, 1984). Later, FEF and LIP activity selects the location of behaviorally relevant stimuli, such as the target embedded within an array of distracters (Bichot et al., 2001a; Thomas and Pare, 2007). Therefore, the topographic pattern of activity in populations of FEF and LIP neurons is thought to encode the dynamic topography of prioritized locations. Theoretically, the topography takes the form of activations scaled by the salience and behavioral relevance of all items.
4. Topographic maps of space in prefrontal cortex and parietal cortex
Our experimental strategy was as follows. Utilizing a within-subject, multi-session design, we used topographic mapping to define candidate priority maps (e.g., putative FEF) (Jerde et al, 2012). In these topographic areas, we measured persistent activity in the same individuals during the delay periods of three separate experiments on covert attention, working memory, and motor preparation (Jerde et al., 2012).
Specifically, using phase-encoded fMRI methods (Engel, 2012; Engel et al., 1997; Engel et al., 1994), we identified four reliable topographic maps, IPS0–IPS3, along the dorsal IPS that matched those reported in previous studies, including IPS0/V7 (Tootell et al., 1998), IPS1 and IPS2 (Schluppeck et al., 2005; Sereno et al., 2001; Silver et al., 2005), and IPS3 (Konen and Kastner, 2008; Swisher et al., 2007). Beginning in V1 and terminating at the rostral end of the IPS, these topographic maps form a consecutive strip of alternating, inverted, and upright representations of the contralateral visual field. In PFC, we identified two reliable topographic maps in sPCS/iPCS that are consistent with previous reports (Hagler and Sereno, 2006; Kastner et al., 2007). We consider these subregions, given their spatial topography, to be candidate priority maps (Fig. 2a).
Fig. 2.
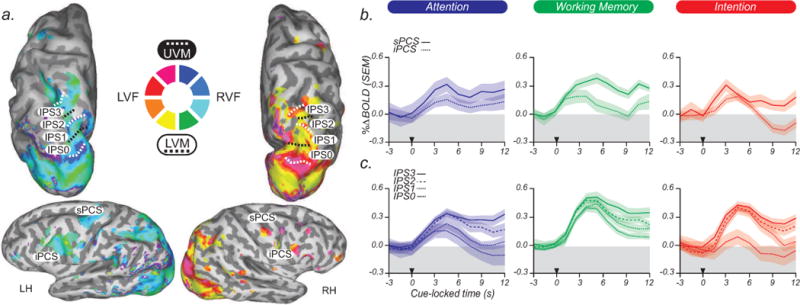
Topographic maps and time courses of activation. (a) Phase values are displayed on inflated cortices of one subject. As indicated in the central color wheel key, cool colors reflect the right visual field (RVF) and warm colors reflect the left visual field (LVF). The borders of topographic areas in parietal cortex are demarcated by dotted black lines reflecting the lower visual meridian (LVM) and dotted white lines reflecting the upper visual meridian (UVM). Four topographic areas are found along the caudal–rostral intraparietal sulcus (IPS0–IPS3). In the PFC, two topographic areas are found along the dorsal–ventral PCS (sPCS and iPCS). LH, left hemisphere; RH, right hemisphere. (b) Time courses from topographic areas along the PCS. Subject-averaged BOLD time courses time-locked to the cue (black triangle) show persistent delay period activity in sPCS, but not iPCS, while subjects covertly attended to a peripheral spatial location, maintained a spatial location in working memory, and planned an eye movement to a spatial position. (c) Persistent activity increases from posterior-to-anterior topographic IPS areas (IPS0–IPS3) during the same conditions.
We then asked whether these topographic areas contain populations of neurons whose activity prioritizes space. To answer this question, we measured delay period activity as the same subjects performed, on separate days, a covert attention task, a spatial working memory task, and a saccade planning task. The trial structure (e.g., timing, randomized delay lengths, number of trials, cues, feedback, spatial placement of cues and motor responses) was virtually identical across the tasks. All else being equal (i.e., the spatial priority), a theoretical priority map should not distinguish between these delay periods. Indeed, we found that activity in the same voxels persisted throughout the delay period irrespective of the task. Persistent activity increased from inferior-to-superior PCS areas (Fig. 2b) and from posterior-to-anterior IPS areas (Fig. 2c) during the maintenance of covert attention, the maintenance of a working memory representation, and the maintenance of a saccade plan. Additionally, activity was higher in the hemisphere contralateral to the locus of covert attention, the location of the memorandum, and the direction of the saccade plan, consistent with a lateralized representation of space. These data are consistent with our studies showing that activity persisted in PCS and IPS across these tasks (Curtis and Connolly, 2008; Ikkai and Curtis, 2008; Srimal and Curtis, 2008), and further demonstrate that the areas showing persistent activity in PFC and PPC are topographically organized. In general, these data support the priority map theory.
5. Decoding spatial priority
Unanswered questions of the priority map theory include what is being prioritized and how general is the prioritization (Fecteau and Munoz, 2006). Past studies, including our own, that have compared working memory and attention, or attention and oculomotor planning, have faced the null result problem: no differences in the patterns of brain activity (see Fig. 1) have been interpreted as though the same cortical areas performed the multiple computations (e.g., memory and attention). Here, we used multivariate decoding to sidestep the null result problem and substantially increase our inferential power. Specifically, in topographic PFC and PPC regions, we asked whether multivoxel patterns of delay period activity were interchangeable during covert attention, working memory, and motor planning.
Accordingly, multivoxel patterns of BOLD activity in topographic areas IPS0–IPS3 and sPCS/iPCS were assessed in a within-task decoding analysis (e.g., a classifier trained to discriminate the locus of covert attention was tested on its ability to predict the locus of covert attention), and a cross-task decoding analysis (e.g., a classifier trained on covert attention data predicts the location of spatial working memory and/or the location of motor intention) (Kriegeskorte, 2011). In the within-task decoding analysis, the pattern of delay period activity in several topographic areas could successfully decode the prioritized visual field for the three tasks, with the activity of sPCS, IPS2, and IPS3 being the best predictors. We consider the within-task decoding results as merely a proof of the feasibility of our methods. Although these results narrow our search to those three priority map candidates, they do not directly test our main hypothesis.
We next tested whether the activity in priority maps is interchangeable across various spatial tasks. That is, is such activity agnostic with regard to the nature of the priority? Priority map theory predicts that priority maps only tag the spatial coordinates of prioritized locations. To directly test this prediction, we performed cross-task decoding analyses by training classifiers on one task and testing their ability to generalize to the other two tasks. Activity in two candidate areas, sPCS and IPS2, predicted priority across the three tasks (Fig. 3). The predictability in the other topographic regions was less reliable, but was significant in some subjects, for some tasks. Further studies are necessary to elucidate the conditions under which these topographic areas are predictive of prioritized space and to what degree they might represent different types of information. We also must consider that our statistical power was not enough to reliably detect predictability in other topographic areas. Nonetheless, the relative strength of the effect in sPCS/IPS2 makes these two areas the strongest candidates to be priority maps. These results strongly support the prediction that the location, and not the cause, of priority is represented in the pattern of topographic activity in these areas.
Fig. 3.
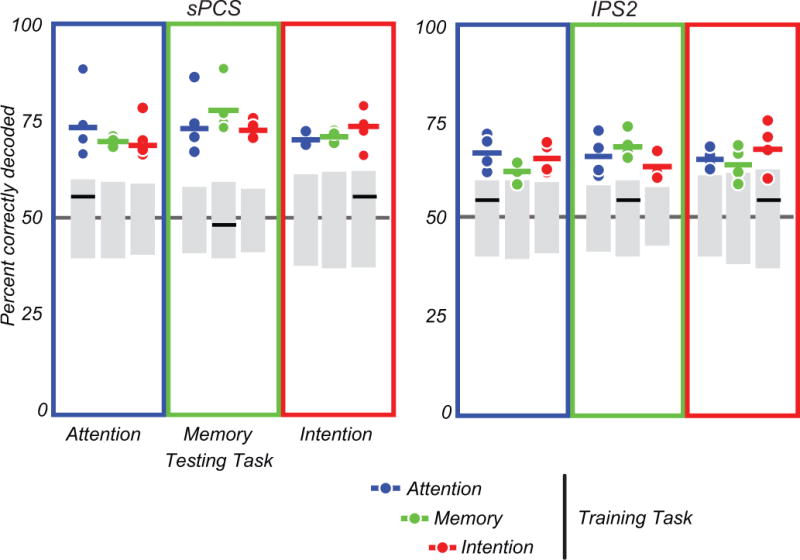
Multivariate decoding analysis. Classifier results for decoding the prioritized visual field for sPCS and IPS2 (see Jerde et al., 2012, for other areas). The percentage of correctly decoded trials is plotted. Each dot is an individual subject, and each colored horizontal line is the mean performance across subjects. The color of the dots indicates the task used to train the classifier. The color of the boxes indicates the task used to test the classifier. For within-task classification, dot and box colors match; for cross-task classification, dot and box colors do not match. The gray boxes represent the 2.5th and 97.5th percentile of the null distribution generated by random permutation analysis. Dots and bars beyond these cutoffs are significantly different from chance. The multivoxel patterns of delay period activity in sPCS and IPS2 predict the prioritized hemifield both within and across the three spatial cognitive tasks. The black horizontal bars indicate the mean performance of control analyses in which the mean signal difference of all voxels in the left and right hemisphere of topographic areas was used to predict the prioritized hemifield. See Jerde et al., 2012, for details.
6. Functions of sPCS and IPS2 in spatial cognition
The sPCS in prefrontal cortex and the IPS in posterior parietal cortex consistently show the most robust delay period activity across subjects, tasks, studies, and laboratories in human neuroimaging studies of spatial attention, spatial working memory, and saccade control (Ikkai and Curtis, 2011). These two areas contain the likely human homologues or evolved variants of monkey areas FEF and LIP.
Topographic sPCS may correspond to the monkey FEF. In humans, electrical stimulation of this area induces saccades to the contralateral visual field (Blanke et al., 1999), and lesions disrupt contraversive saccades (Gaymard et al., 1999; Rivaud et al., 1994). Moreover, robust and spatially selective BOLD activity in human sPCS is correlated with the selection, preparation, maintenance, and generation of saccades (Connolly et al., 2005; Corbetta et al., 1998; Curtis and Connolly, 2008; Curtis et al., 2004; Ford et al., 2005). In monkey FEF, neurons exhibit direction-selective presaccadic activity, and saccades can be elicited with little current (Bruce et al., 1985). FEF is thought to convert visual signals into potential saccade goals (Schall and Hanes, 1993). These same mechanisms may guide attention in the absence of eye movements (Awh et al., 2006; Thompson et al., 2005), and could be the means by which spatial representations are maintained in working memory (Armstrong et al., 2009).
On the long axis of the FEF, a topographic gradient of saccade amplitudes exists, with larger-amplitude saccades being more numerous in dorsal FEF, and smaller-amplitude saccades being more numerous in ventral FEF (Bruce and Goldberg, 1985). In addition, although FEF neurons represent all saccade directions, it is unknown whether they are topographically organized by angle. Bruce et al. (1985) reported that saccades of similar angles were elicited by microstimulation of nearby neurons, and angle appeared to systematically progress from the lip to the fundus of the arcuate sulcus. Although we reported an angular topographic map in the putative human FEF (Jerde et al., 2012), functional imaging studies have yet to find a topographic map of amplitude in this area.
Topographic IPS2 may correspond to the human homologue of monkey LIP. In humans, this view is consistent with suggestions from previous topographic mapping studies (Kastner et al., 2007; Schluppeck et al., 2005; Silver et al., 2005; Swisher et al., 2007) and recent neuropsychological data and theories (Gillebert et al., 2011; Ptak and Schnider, 2011; Vandenberghe et al., 2012). In monkeys, fMRI (Arcaro et al., 2011) and electrophysiological (Ben Hamed et al., 2001) data have provided evidence for a topographic map of contralateral visual space in area LIP. Monkey area LIP may correspond to IPS2 in the human, since both areas contain an inverted visual field representation in mid-IPS. Neurons in area LIP, like FEF, increase their firing rate when saccades are planned into their receptive field (Barash et al., 1991; Ipata et al., 2009). Moreover, their activity persists during the maintenance of covert attention (Bisley and Goldberg, 2003a) and throughout the retention intervals during spatial working memory (Gnadt and Andersen, 1988). Functional imaging studies of spatial cognition often activate a large portion of the IPS that probably includes IPS2 (Astafiev et al., 2003; Ikkai and Curtis, 2008; Schluppeck et al., 2006; Serences and Yantis, 2007; Srimal and Curtis, 2008). Furthermore, both FEF and LIP are densely interconnected with oculomotor and visual structures (Cavada and Goldman-Rakic, 1989a, b; Petrides and Pandya, 2006). They are thus ideally situated to receive the inputs necessary to construct a priority map that could be accessed by many brain areas to influence spatially guided behaviors.
Summary and conclusions
We used fMRI to test hypotheses about the nature of persistent activity in spatial cognitive tasks. We found that persistent activity in PFC and PPC carries information that can support a variety of spatial cognitions. Two topographic areas in particular, sPCS in frontal cortex and IPS2 in parietal cortex, function as prioritized maps of space for spatial cognition. Furthermore, these areas may be the human homologues of monkey areas FEF and LIP. The activity in sPCS and IPS2 could be read out by other brain areas depending on the spatial demands of perception, attention, working memory, motor planning, and other functions.
Overall, it is imperative to find efficient and reliable methods to identify topographic areas. The lack of such methods has severely limited our understanding of PFC and PPC. With improved methods of defining topographic areas, we could ask important theoretically-driven questions (Silver and Kastner, 2009). For example, why is the visual field represented in so many cortical areas? What is the spatial scale and organization of topography in the monkey brain (Kolster et al., 2009; Raffi and Siegel, 2005)? What details will be revealed in human brain maps as more sophisticated imaging techniques and higher field magnets become available (Gourtzelidis et al., 2005; Jerde et al., 2008; Olman et al., 2010)? What are the real-time dynamics of activity changes across topographic areas, as assessed by techniques such as magnetoencephalography (Medendorp et al., 2007; Simpson et al., 2011)? What is the relationship between topography and cognitive functions in naturalistic tasks that involve different effectors (e.g., eye and hand) and the intermixing of cognitions that is typical in daily behavior? Given the complexity of the brain, topographic representations of more nuanced and higher cognitive processes surely await discovery (Thivierge and Marcus, 2007).
We review our work testing theories of how space is prioritized.
Frontoparietal cortex activity persists during a variety of spatial tasks.
This is true in retinotopically defined portions of frontoparietal cortex.
Topographic sPCS and IPS2 cross predict spatial location across tasks.
sPCS and IPS2 are thus candidate priority maps of space.
Acknowledgments
This work was supported by National Institutes of Health Grants EY019221 (T.A.J.) and EY016407 (C.E.C.).
Abbreviations
- BOLD
blood oxygen level-dependent
- FEF
frontal eye field
- fMRI
functional magnetic resonance imaging
- iPCS
inferior precentral sulcus
- IPS
intraparietal sulcus
- LIP
lateral intraparietal area
- PFC
prefrontal cortex
- PPC
posterior parietal cortex
- sPCS
superior precentral sulcus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Trenton A. Jerde, Email: jerde@umn.edu.
Clayton E. Curtis, Email: clayton.curtis@nyu.edu.
References
- Arcaro MJ, Pinsk MA, Li X, Kastner S. Visuotopic organization of macaque posterior parietal cortex: a functional magnetic resonance imaging study. J Neurosci. 2011;31:2064–2078. doi: 10.1523/JNEUROSCI.3334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Chang MH, Moore T. Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci. 2009;29:15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Chenchal Rao S, Schall JD. Continuous processing in macaque frontal cortex during visual search. Neuropsychologia. 2001a;39:972–982. doi: 10.1016/s0028-3932(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Thompson KG, Chenchal Rao S, Schall JD. Reliability of macaque frontal eye field neurons signaling saccade targets during visual search. J Neurosci. 2001b;21:713–725. doi: 10.1523/JNEUROSCI.21-02-00713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003a;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. The role of the parietal cortex in the neural processing of saccadic eye movements. Adv Neurol. 2003b;93:141–157. [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Morand S, Thut G, Michel CM, Spinelli L, Landis T, Seeck M. Visual activity in the human frontal eye field. Neuroreport. 1999;10:925–930. doi: 10.1097/00001756-199904060-00006. [DOI] [PubMed] [Google Scholar]
- Bressler DW, Silver MA. Spatial attention improves reliability of fMRI retinotopic mapping signals in occipital and parietal cortex. Neuroimage. 2010;53:526–533. doi: 10.1016/j.neuroimage.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron. 2007;56:552–559. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci. 2004;4:528–539. doi: 10.3758/cabn.4.4.528. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lee D. Beyond working memory: the role of persistent activity in decision making. Trends Cogn Sci. 2010;14:216–222. doi: 10.1016/j.tics.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA. The development and use of phase-encoded functional MRI designs. Neuroimage. 2012;62:1195–1200. doi: 10.1016/j.neuroimage.2011.09.059. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Mantini D, Thijs V, Sunaert S, Dupont P, Vandenberghe R. Lesion evidence for the critical role of the intraparietal sulcus in spatial attention. Brain. 2011;134:1694–1709. doi: 10.1093/brain/awr085. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann NY Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Circuitry of the prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, Mountcastle VB, editors. Handbook of Physiology Sec 1 The Nervous System. New York, NY: Oxford University Press; 1987. pp. 373–417. [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Gourtzelidis P, Tzagarakis C, Lewis SM, Crowe DA, Auerbach E, Jerde TA, Ugurbil K, Georgopoulos AP. Mental maze solving: directional fMRI tuning and population coding in the superior parietal lobule. Exp Brain Res. 2005;165:273–282. doi: 10.1007/s00221-005-2298-6. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time locked to the shift and maintenance of spatial attention. Cereb Cortex. 2008;18:1384–1394. doi: 10.1093/cercor/bhm171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49:1428–1434. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Bisley JW, Goldberg ME. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp Brain Res. 2009;192:479–488. doi: 10.1007/s00221-008-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Jerde TA, Lewis SM, Goerke U, Gourtzelidis P, Tzagarakis C, Lynch J, Moeller S, Van de Moortele PF, Adriany G, Trangle J, et al. Ultra-high field parallel imaging of the superior parietal lobule during mental maze solving. Exp Brain Res. 2008;187:551–561. doi: 10.1007/s00221-008-1318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE. Prioritized maps of space in human frontoparietal cortex. J Neurosci. 2012;32:17382–17390. doi: 10.1523/JNEUROSCI.3810-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Arsenault JT, Ekstrom LB, Wald LL, Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci. 2008;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N. Pattern-information analysis: from stimulus decoding to computational-model testing. Neuroimage. 2011;56:411–421. doi: 10.1016/j.neuroimage.2011.01.061. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex. 2007;17:2364–2374. doi: 10.1093/cercor/bhl145. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Olman CA, Van de Moortele PF, Schumacher JF, Guy JR, Ugurbil K, Yacoub E. Retinotopic mapping with spin echo BOLD at 7T. Magn Reson Imaging. 2010;28:1258–1269. doi: 10.1016/j.mri.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE. The frontal lobes and voluntary action. New York: Oxford University Press; 1993. [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The attention network of the human brain: relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologia. 2011;49:3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Raffi M, Siegel RM. Functional architecture of spatial attention in the parietal cortex of the behaving monkey. J Neurosci. 2005;25:5171–5186. doi: 10.1523/JNEUROSCI.5201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Sereno MI. Retinotopy and attention in human occipital, temporal, parietal, and frontal cortex. Cereb Cortex. 2008;18:2158–2168. doi: 10.1093/cercor/bhm242. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Attention governs action in the primate frontal eye field. Neuron. 2007;56:541–551. doi: 10.1016/j.neuron.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GV, Weber DL, Dale CL, Pantazis D, Bressler SL, Leahy RM, Luks TL. Dynamic activation of frontal, parietal, and sensory regions underlying anticipatory visual spatial attention. J Neurosci. 2011;31:13880–13889. doi: 10.1523/JNEUROSCI.1519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol. 2001;85:1673–1685. doi: 10.1152/jn.2001.85.4.1673. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Pestent neural activity during the maintenance of spatial position in working memory. Neuroimage. 2008;39:455–468. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe function. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge JP, Marcus GF. The topographic brain: from neural connectivity to cognition. Trends Neurosci. 2007;30:251–259. doi: 10.1016/j.tins.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol. 2007;97:942–947. doi: 10.1152/jn.00413.2006. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Molenberghs P, Gillebert CR. Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia. 2012;50:1092–1103. doi: 10.1016/j.neuropsychologia.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Wandell BA. Computational neuroimaging of human visual cortex. Annu Rev Neurosci. 1999;22:145–173. doi: 10.1146/annurev.neuro.22.1.145. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform. 1984;10:601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]


