SUMMARY
Social play activities among juveniles are thought to contribute to the development of social and emotional skills in humans and animals. Conversely, social play deficits are observed in developmental neuropsychiatric disorders. Importantly, many of these disorders show sex differences in incidence, course of the disease, and severity of symptoms. We hypothesized that sex differences in the neural systems controlling social behavior can contribute to these differences. We therefore studied the involvement of the sexually dimorphic vasopressin and oxytocin systems, which have been implicated in these disorders, in juvenile social play behavior. Single-housed 5-week-old juvenile male and female rats were exposed to an unknown age-and sex-matched conspecific for 10 min in their home cage and social play behaviors were recorded. We found no consistent sex differences in level or elements of social play in vehicle-treated rats. However, intracerebroventricular injection of the specific vasopressin 1a receptor (V1aR) antagonist (CH2)5Tyr(Me2) AVP significantly reduced social play behaviors in males, while increasing them in females. Intracerebroventricular injection of the specific oxytocin receptor antagonist des-Gly-NH2,d(CH2)5[Tyr(Me)2,Thr4]OVT did not alter social play in either sex. To locate the effects of V1aR blockade on social play, we targeted the lateral septum, a sexually dimorphic brain region showing denser vasopressin fibers in males than in females and abundant expression of V1aR in both sexes. Surprisingly, blockade of V1aR in the lateral septum increased social play behaviors in males, but decreased them in females. These findings suggest sex- and brain region-specific roles for vasopressin in the regulation of social play behavior in juvenile rats.
Keywords: female, juvenile, lateral septum, male, oxytocin, play-fighting, sex difference, social play, vasopressin, V1a receptor
INTRODUCTION
Social play (also referred to as play-fighting or rough-and-tumble play) is predominantly displayed by juvenile animals, including human children (Panksepp, 1981; Bekoff & Byers, 1998; Pellis & Iwaniuk, 2000; Burghardt, 2005). Social play is thought to contribute to the development of social and emotional skills in humans and animals (Baldwin, 1986; Pellegrini, 1988; Vanderschuren et al., 1997; Bekoff & Byers, 1998; Van den Berg et al., 1999; Guralnick et al., 2006; Cordoni & Palagi, 2011). Conversely, social play deficits are observed in neurodevelopmental disorders such as autism spectrum disorders (ASD), early-onset schizophrenia, and attention-deficit/hyperactivity disorder (Alessandri, 1992; Moller & Husby, 2000; Jordan, 2003). Importantly, many of these disorders show sex differences in incidence, course of the disease, and severity of symptoms. For example, autism spectrum disorders (ASD) typically appear early in development and are four to eight times more common in males than in females (Fombonne, 2003; Beaudet, 2012). However, little is known about the neural basis of sex-biases in neurodevelopmental disorders.
The neuropeptides vasopressin (AVP) and oxytocin (OXT) have been found to modulate various social behaviors such as pair bonding, aggression, and social recognition in adult rodents (Donaldson & Young, 2008; Veenema & Neumann, 2008; Goodson & Thompson, 2010), and social trust, social cooperation, and social cognition in adult humans (Kosfeld et al., 2005; Guastella et al., 2010; Rilling et al., 2012). In contrast, the involvement of AVP and OXT in juvenile social play behaviors is less well explored. Importantly, the AVP and OXT systems are sexually dimorphic (De Vries, 2008). For example, compared to females, males have more AVP-expressing cells in the bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA) and denser AVP-axonal projections to limbic brain regions, especially to the lateral septum (LS) (De Vries et al 1981; van Leeuwen et al., 1985; Szot & Dorsa, 1993). This sex difference is found in many mammalian species (De Vries & Panzica, 2006) and already exists in juveniles (De Vries et al., 1981). In addition, the synthesis of OXT in hypothalamic brain regions is significantly higher in female than in male mice (Haussler et al., 1990) while OTR binding densities in several brain regions is higher in male than in female rats (Uhl-Bronner et al., 2005; Dumais, Veenema, unpublished observation). These findings suggest that AVP and OXT modulate social behaviors in sexually dimorphic ways, but do not necessarily suggest that these neuropeptides cause sex differences in behavior (De Vries, 2004). For example, AVP facilitates partner preference in male, but not in female, prairie voles (Cushing et al., 2001). Moreover, reduced anxiety is found in V1a receptor knockout male, but not female, mice (Bielsky et al., 2004, 2005). In humans, AVP has sex-specific effects on social communication (Thompson et al., 2006) and V1aR polymorphisms correlate with pair-bonding behavior in men, but not in women (Walum et al., 2008). Sex differences in behavioral or brain responses were also found after manipulations of the OXT brain system in voles (Insel & Hulihan, 1995) and after intranasal OXT application in humans (Kubzansky et al., 2012; Lische et al., 2012).
To test the hypothesis that AVP and OXT also affect social behavior in sexually dimorphic ways during development, we studied the effects of acute pharmacological manipulations of the AVP and OXT systems on social play in 5-week-old male and female rats. We first studied the effects of intracerebroventricular (ICV) blockade of AVP V1a receptor (V1aR) or ICV blockade of the OXT receptor (OTR) on social play behaviors. To test the hypothesis that the sexually dimorphic effects of ICV injections of V1aR blockade were mediated by the sexually dimorphic projections of the BST and MeA, we specifically targeted the LS, which shows denser AVP fibers in males than in females (De Vries et al., 1981) and abundant expression of V1aR in male and female juvenile rats (Veenema et al., 2012).
METHODS
Animals
Three-week-old Wistar rats were obtained from Charles River (Raleigh, NC) and maintained under standard laboratory conditions (12 h light/dark cycle, lights off at 14:00 h, 22°C, 50% humidity, food and water ad libitum). Rats were housed in same-sex groups of four in standard rat cages (48 x 27 x 20 cm) unless otherwise mentioned. The experiments were conducted in accordance with the guidelines of the NIH and approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Cannulation
After one week of daily handling to familiarize rats with the injection procedure, juvenile (33 days of age) males and females were anesthetized with isoflurane (Butler Schein Animal Health, Dublin, OH) and mounted on a stereotaxic frame with the tooth bar set at −4.5 mm. Guide cannulae (21 gauge for lateral ventricle, 22 gauge for LS; Plastics One, Roanoke, VA) were implanted stereotaxically 2 mm dorsal to the targets. Coordinates in mm from bregma were: lateral ventricle (+1.0 AP, −1.6 LM; −1.6 DV); LS (+0.4 AP, −1.0 LM, −3.6 DV; Paxinos & Watson, 2007). For septal injections, cannulae were implanted under an angle of 10° from the midsagittal plane to avoid damage to the sagittal sinus. Cannulae were fixed to the skull with two stainless steel screws and Cerebond adhesive and closed with a dummy cannula (Plastics One, Roanoke, VA). After surgery, rats were individually housed in standard rat cages (48 x 27 x 20 cm). At the end of the experiments, rats were killed with CO2, and either blue ink (ICV) or charcoal (LS) was injected as marker to check proper placement of the cannulae visually (ICV) or histologically on Nissl-stained coronal brain sections (LS; see Fig. 1B).
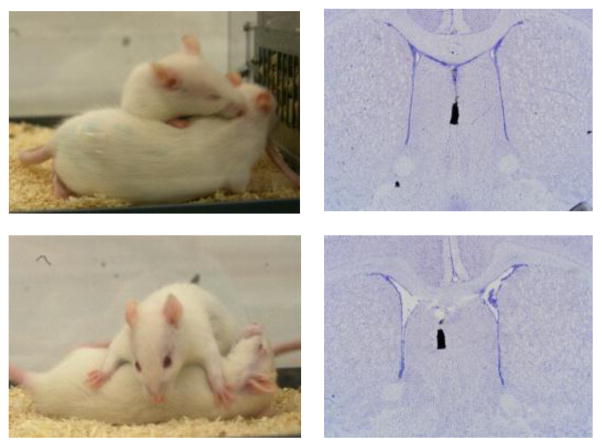
Figure 1.
(A) Pictures illustrate behavioral elements/postures of social play in juvenile rats: top figure shows an attack towards the nape of the neck of the intruder rat; bottom picture shows pinning and supine positions. (B) Pictures of Nissl-stained coronal brain sections illustrate the injection location in the septum using charcoal as marker. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Social play behavior test
Social play was assessed in 5-week-old juvenile rats, as social play is the highest at this age (Panksepp, 1981; Pellis & Pellis, 1990) following a procedure described in Veenema & Neumann (2009). Briefly, during the first hour of the dark phase, rats (‘residents’) were exposed in their home cage to an unknown age- and sex-matched ‘intruder’ rat for 10 min. All tests were videotaped and behavior was measured by a researcher blind to the treatment condition using JWatcher (http://www.jwatcher.ucla.edu/). The following behaviors were scored for the resident according to Veenema & Neumann (2009): duration of social play (the total amount of time spent in playful social interactions including nape attacks, pinning, and supine poses), social investigation (sniffing the anogenital and head/neck regions), and non-social exploration, and numbers of nape attacks (nose attacks or nose contacts towards the nape of the neck of the intruder), pins (the resident holds the intruder on its back in a supine position), and supine poses (the resident is pinned by the intruder). Images depicting a nape attack, pinning pose, and supine pose are shown in Fig 1A. More detailed descriptions of these characteristic behavioral postures observed during social play in rats can be found in Pellis & Pellis (2009) and Trezza et al. (2010).
Experimental procedures
Two days after cannulation, rats were exposed to the social play test in their home cage. This restricted recovery period matches that used for other procedures applied routinely in our lab, e.g., intracerebral microdialysis. Importantly, we do not find effects of surgery and cannula or probe implantation after two days on behavior in various behavioral tests (Beiderbeck et al., 2007; Veenema et al., 2010, 2012; Lukas et al., 2011, 2013). Rats received an injection 20 min before the social play test. The injection was given into the lateral ventricle (Exp. 1) or LS (Exp. 2) via an injector cannula that extended 2 mm beyond the guide cannula and was connected via polyethylene tubing to a Hamilton syringe. After keeping the injector cannula in place for 30 s following injection to allow for tissue uptake, it was replaced by a dummy cannula. Intruder rats did not focus on the cannulae of the experimental rats during the social play tests. Time of administration and concentrations of drugs were based on previous studies showing behavioral effects in rats (Lukas et al., 2011, 2013; Veenema et al., 2012).
Experiment 1: Effects of ICV injections of V1aR and OTR antagonists on social play
Juveniles received an injection into the lateral ventricle of either Ringer’s solution (vehicle) (pH 7.4; 5 μl Ringer; males n=8; females n=10), the specific V1aR antagonist d(CH2)5Tyr(Me2)AVP (Manning et al., 2008; 0.75 μg/5 μl Ringer; males n=11; females n=8), or the specific OTR antagonist des-Gly-NH2,d(CH2)5[Tyr(Me)2,Thr4]OVT (Manning et al., 2008; 0.75 μg/5 μl Ringer; males n=9; females n=8) and were tested for social play 20 min later.
Experiment 2: Effects of septal injections of V1aR antagonist and synthetic AVP on social play
Rats received an injection into the septum of either Ringer’s solution (vehicle) (0.5 μl Ringer; males n=7; females n=7), the V1aR antagonist (10 ng/0.5 μl Ringer; males n=7; females n=7) or synthetic AVP (200 pg/0.5 μl Ringer; males n=7; females n=6) and were tested for social play 20 min later.
Statistics
Social play behaviors were analyzed using a two-way ANOVA (sex x treatment). Bonferroni post-hoc tests were used to test for differences among groups. Data are presented as means + SEM. Significance was set at p < 0.05.
RESULTS
Experiment 1: Effects of ICV injections of V1aR and OTR antagonists on social play
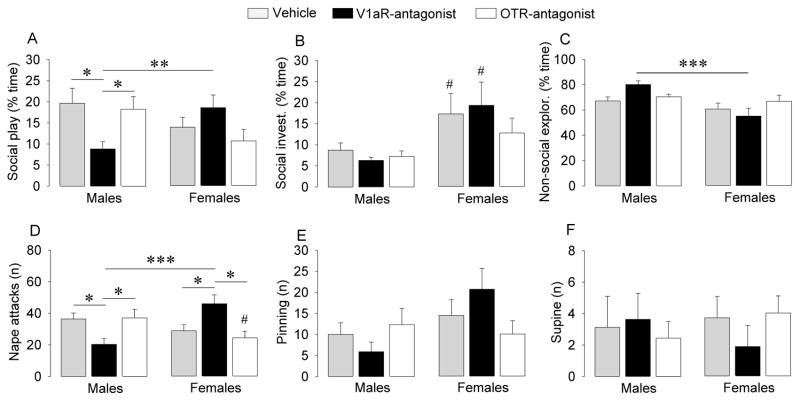
No sex differences were found for social play behaviors in vehicle-treated rats (Fig. 2). ICV injection of the V1aR antagonist altered social play behaviors in sex-specific ways as reflected by sex x treatment effects for duration of social play (F2,50=4.68, p < 0.05) and number of nape attacks (F2,50=11.0, p < 0.001), as well as by a strong tendency towards a sex x treatment effect for number of pins (F2,50=3.09, p = 0.054). Post hoc testing indicated that V1aR antagonist-treated males showed a significant decrease in duration of social play and number of nape attacks (Fig. 2A and 2D; p < 0.05 versus vehicle as well as versus OTR antagonist groups). In contrast, V1aR antagonist-treated females showed a significant increase in number of nape attacks (Fig. 2A and D; p < 0.05 versus vehicle as well as versus OTR antagonist groups). Hence, V1aR antagonist treatment induced robust sex differences in social play behaviors with males showing lower levels of social play (p < 0.005) and fewer nape attacks (p < 0.001) than females (Fig. 2A and 2D). There was also a sex difference in social investigation (F1,50=16.2, p < 0.001) with post hoc tests indicating higher levels in vehicle and V1aR antagonist-treated females than in male counterparts (p < 0.05; Fig. 2B) and a sex difference in non-social exploration (F1,50=12.7, p < 0.001), with post hoc tests indicating lower levels in V1aR antagonist-treated females versus V1aR antagonist-treated males (p < 0.001; Fig. 2C). ICV injection of the OTR antagonist did not affect social and non-social behaviors, except that females showed less nape attacks than males (p < 0.05; Fig. 2D).
Figure 2.
ICV injections of a V1aR but not an OTR antagonist reduced duration of social play (A) and number of nape attacks (D) in juvenile male rats but enhanced number of nape attacks in juvenile female rats (F2,50=4.68, p < 0.05 and F2,50=11.0, p < 0.001, respectively), and tended to do so for the number of pins (E; F2,50=3.09, p = 0.054). Social play, social investigation (B), and non-social exploration (C) are expressed as percentage of total time. Bars indicate means + SEM. *: p < 0.05, **: p < 0.005, *** p < 0.001, #: p < 0.05 versus male counterparts; Bonferroni post hoc tests.
Experiment 2: Effects of septal injections of V1aR antagonist and synthetic AVP on social play
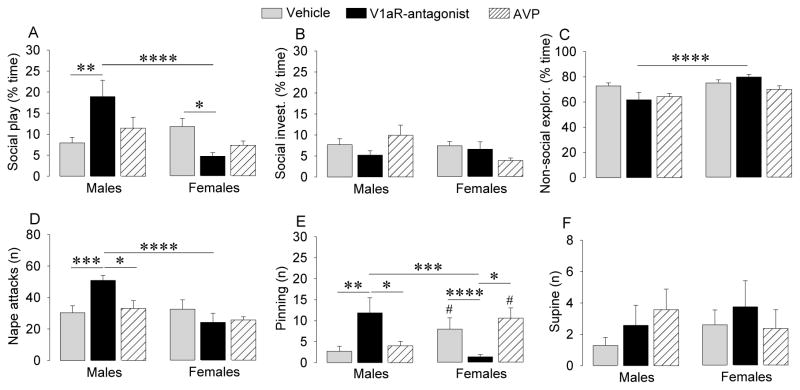
To test whether the sexually dimorphic effects of intraventricular injections of V1aR antagonist were due to their actions on sexually dimorphic projections from the BST and MeA, we targeted the LS. Interestingly, this generally yielded effects opposite to those of intraventricular V1aR antagonist injections. Sex x treatment effects were found for duration of social play (F2,34=9.39, p < 0.005), number of nape attacks (F2,34=5.82, p < 0.01), and number of pins (F2,34=12.2, p < 0.001). Post hoc testing confirmed that, in males, V1aR antagonist treatment significantly increased duration of social play (p < 0.01 versus vehicle; Fig. 3A), number of nape attacks (p < 0.005 versus vehicle; p < 0.05 versus AVP-treated males ; Fig. 3D)and number of pins (p < 0.01 versus vehicle; p < 0.05 versus AVP-treated males; Fig. 3E). In contrast, V1aR antagonist treatment in females significantly reduced duration of social play (p < 0.05 versus vehicle; Fig. 3A) and number of pins (p < 0.001 versus vehicle; p < 0.05 versus AVP-treated females; Fig. 3E). Hence, V1aR antagonist injections into the septum induced robust sex differences in social play behaviors with males showing higher levels of social play (p < 0.001; Fig. 3A), more nape attacks (p < 0.001; Fig. 3D), and more pins (p < 0.005; Fig. 3E) than females. Administration of AVP into the LS did not result in changes in social play behaviors (Fig. 3). No sex differences were found for social play behaviors in vehicle-treated or AVP-treated rats, except for a sex difference in the number of pins, with more pins in females than in males (p < 0.05; Fig 3E). Finally, there was a sex difference in non-social exploration (F1,34=10.1, p < 0.005), with post hoc tests indicating higher levels in V1aR antagonist-treated females versus V1aR antagonist-treated males (p < 0.001; Fig. 3C).
Figure 3.
Septal injections of a V1aR antagonist enhanced duration of social play (A), number of nape attacks (D), and number of pins (E) in juvenile male rats but reduced these parameters in juvenile female rats (F2,34=9.39, p < 0.005, F2,34=5.82, p < 0.01 and F2,34=12.2, p < 0.001, respectively). Social play, social investigation (B), and non-social exploration (C) are expressed as percentage of total time. Bars indicate means + SEM. *: p < 0.05, **: p < 0.01, *** p < 0.005, **** p < 0.001, #: p < 0.05 versus male counterparts; Bonferroni post hoc tests.
DISCUSSION
We demonstrated that central AVP regulates juvenile social play behavior in brain region- and sex-specific ways. Specifically, ICV injections of V1aR antagonist significantly reduced social play behaviors in males, but increased these behaviors in females. Conversely, septal injections of V1aR antagonist increased social play behaviors in males, while decreasing them in females. This suggests that the neurochemical underpinnings of social play differ between males and females.
Given that OXT has been implicated in a variety of social behaviors in adult animals, we were surprised to find hardly any effects of ICV injections of a specific OTR antagonist on social play behaviors, even though the dose that we used impaired social recognition in adult male rats (Lukas et al., 2013) and in juvenile male rats (Veenema, unpublished observation). Moreover, the V1aR antagonist increased as well as decreased social play depending on where it was injected. Therefore, potential effects of the OTR antagonist on different brain regions might have canceled each other out after ICV injections. In support, ICV injections of OTR antagonist failed to inhibit partner preference in male prairie voles (Winslow et al., 1993), but injections into the lateral septum did (Liu et al., 2001).
In the behavioral paradigm that we used, we did not find sex differences in the duration and frequency of social play behaviors in vehicle-treated juvenile rats, except that females showed more pins than males in the second experiment. This is in line with some, but not all studies. Males show more social play than females when social play is recorded among cage-mates and under undisturbed conditions (Poole & Fish, 1976; Meaney & Stewart, 1981; Parent & Meaney, 2008). However, these sex differences in social play disappear when rats are briefly isolated and tested for social play in resident-intruder settings (Panksepp & Beatty, 1980; Panksepp, 1981; Thor & Holloway, 1984). In fact, play-soliciting behaviors were found to be higher in females than in males when playing with an unfamiliar partner (Cirulli et al., 1996). These findings may indicate that sex differences in the level and or elements of social play depend on the social context. Importantly, we found that V1aR blockade induced sex differences in social play in a resident-intruder setting, as ICV administration of a specific V1aR antagonist reduced the duration of social play in males but not in females and reduced the number of nape attacks in males while increasing the number of nape attacks in females. This suggests that AVP stimulates social play in males while inhibiting it in females.
AVP released from cells in the BNST and MeA is a logical candidate for playing such a dimorphic role. AVP cells in the BNST and MeA are more numerous and have denser projections in male than in female juvenile rats (De Vries et al., 1981; Szot & Dorsa, 1993). Circumstantial evidence supports the involvement of these cells in social play. For example, a prenatal immune challenge reduced social play as well as AVP mRNA expression in the BNST and MeA, but did so in males only (Taylor et al., 2012). Likewise, silencing MeCP2 expression in the MeA reduced social play (Kurian et al., 2008) as well as AVP mRNA expression in the MeA (Forbes-Lorman et al., 2012) in males but not in females.
To test whether AVP projections of the BNST or MeA modulate social play, we targeted the LS, because this area receives projections from AVP-expressing neurons in the BNST and MeA that are denser in males than in females (De Vries & Buijs, 1983; Van Leeuwen et al., 1985; Caffe et al., 1987). Contrary to ICV injections, septal injections of V1aR antagonist increased social play behaviors in males while decreasing them in females. This indicates that the effects of ICV injections of V1aR antagonist on social play cannot be explained by an effect on the LS, even though the LS borders with the lateral ventricles. Instead, the widespread distribution of V1aR throughout the brain, including many areas that border the ventricles (Tribollet et al., 1988), suggests that the effects of ICV injections on social play reflect a net inhibition of V1aR activation in multiple brain regions.
ICV injections are, therefore, likely to interfere with AVP released from sources other than the BNST and MeA that may control social play. In juvenile male hamsters, for example, AVP cells in the nucleus circularis and the SON showed enhanced c-Fos immunolabeling after social play, suggesting increased activity (Cheng et al., 2008). Furthermore, V1aR blockade in the anterior hypothalamus (which is a possible projection site of AVP cells in the nucleus circularis and SON; Cheng et al., 2008) decreased the number of attacks and bites during social play in juvenile male hamsters (Cheng & Delville, 2009). It is not known whether, in rats, these systems are involved in social play as well, but if they are, ICV injections of V1aR antagonists would have likely interfered with their actions. Although these systems are not sexually dimorphic to the extent that BNST and MeA projections are, they may interact with systems that are sexually dimorphic, which may explain the different effects in males and females.
Although septal injections of V1aR antagonists affected social play, we did not see an effect of AVP itself. Using the same concentration and injection procedure, we found previously that septal AVP injections extended social recognition in female juvenile rats and in male and female adult rats (Veenema et al., 2012). It could be that a higher concentration of AVP in the septum is required to alter social play behaviors. Alternatively, it may be that endogenous AVP already influences play behavior, but not social recognition, at maximum levels. This suggests that social recognition and social play may be modulated by AVP acting on distinct neural pathways or that there are differences in how these behaviors trigger the co-release of other neurotransmitters that may, in turn, modulate the effects of AVP on these behaviors.
Our studies indicate for the first time the involvement of AVP in the modulation of social play in juvenile rats. Previous studies have shown that social play in juvenile rats is modulated by a wide variety of neuroactive agents, including opioids, dopamine, cannabinoids, norepinephrine, serotonin, and GABA (Vanderschuren et al., 1997; Siviy, 1998; Trezza et al., 2010). Notably, in none of these studies, sex appears to be considered as a factor. It would be of interest in future studies to explore possible interactions of AVP with these systems in the modulation of social play and whether such interactions would explain sex-specific regulation of social play by the septal AVP system.
Whereas injections of V1aR antagonist had opposing effects on social play in male and female juveniles, such injections affected social discrimination in a similar direction (Veenema et al., 2012). V1aR blockade in the LS, for example, made juvenile males as well as females spend more time investigating a familiar as opposed to a novel conspecific, albeit that these effects were significantly stronger in males than in females (Veenema et al., 2012). Moreover, whereas in the current study septal AVP injections did not alter social play in either sex, it facilitated social recognition in female, but not male juveniles (Veenema et al., 2012). These findings demonstrate the specificity of the behavioral changes observed after acute pharmacological manipulations of the AVP system on social play and social recognition, two distinct forms of social behavior. These findings further demonstrate that AVP is an important modulator of juvenile social behaviors and affects these behaviors in sex- and brain region-specific ways and may also depend on context. Along with other studies in adult animals and humans, this suggests that the role of the AVP system in regulating social behaviors is highly complex in juvenile animals as well.
Acknowledgments
We thank Dr. Maurice Manning for providing the V1aR and OTR antagonists and Chido Kativhu and Caroline J. Smith for technical assistance.
ROLE OF FUNDING SOURCE
This research was funded by the German Research Foundation VE453/4-1 and the Brain and Behavior Research Foundation 17382 to AHV and NSF Grant IBN 9421658 and NIH Grant RO1 MH047538 to GJD. Resources had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
CONTRIBUTORS
Dr. Veenema and Mr. Bredewold designed and performed the experiments and analyzed the data. Drs. Veenema and De Vries wrote the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessandri SM. Attention, play, and social behavior in ADHD preschoolers. J Abnorm Child Psychol. 1992;20:289–302. doi: 10.1007/BF00916693. [DOI] [PubMed] [Google Scholar]
- Baldwin JD. Behavior in infancy: Exploration and play. In: Mitchell G, Erwin J, editors. Comparative primate biology, Volume 2, Part A. Behavior, conservation, and ecology. Alan R. Liss; New York: 1986. pp. 295–326. [Google Scholar]
- Beaudet AL. Neuroscience. Preventable forms of autism? Science. 2012;338:342–3. doi: 10.1126/science.1229178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bekoff M, Byers JA. Animal play. Cambridge University Press; Cambridge, UK: 1998. [Google Scholar]
- Bielsky IF, Hu SB, Young LJ. Sexual dimorphism in the vasopressin system: lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behav Brain Res. 2005;164:132–136. doi: 10.1016/j.bbr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Burghardt GM. The Genesis of Animal Play: Testing the Limits. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Delville Y. Vasopressin facilitates play fighting in juvenile golden hamsters. Physiol Behav. 2009;98:242–246. doi: 10.1016/j.physbeh.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Terranova ML, Laviola G. Affiliation in periadolescent rats: behavioral and corticosterone response to social reunion with familiar or unfamiliar partners. Pharmacol Biochem Behav. 1996;54:99–105. doi: 10.1016/0091-3057(95)02169-8. [DOI] [PubMed] [Google Scholar]
- Cordoni G, Palagi E. Ontogenetic trajectories of chimpanzee social play: similarities with humans. PLoS One. 2011;6:e27344. doi: 10.1371/journal.pone.0027344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The prevalence of autism. JAMA. 2003;289:87–89. doi: 10.1001/jama.289.1.87. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7:230–238. doi: 10.4161/epi.7.3.19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010;67:1220–1222. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guralnick MJ, Connor RT, Neville B, Hammond MA. Promoting the peer-related social development of young children with mild developmental delays: effectiveness of a comprehensive intervention. Am J Ment Retard. 2006;111:336–356. doi: 10.1352/0895-8017(2006)111[336:PTPSDO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Haussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat. 1990;3:271–276. [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Jordan R. Social play and autistic spectrum disorders: a perspective on theory, implications and educational approaches. Autism. 2003;7:347–360. doi: 10.1177/1362361303007004002. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: Male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38:916–926. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Moller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26:217–232. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Beatty WW. Social deprivation and play in rats. Behav Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Pellegrini AD. Elementary school children’s rough-and-tumble play and social competence. Dev Psychol. 1988;24:802–806. [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Iwaniuk AN. Comparative analyses of the role of postnatal development on the expression of play fighting. Dev Psychobiol. 2000;36:136–47. [PubMed] [Google Scholar]
- Pellis SM, Pellis V. The playful brain: venturing to the limits of neuroscience. Oneworld Publications; Oxford, UK: 2009. [Google Scholar]
- Poole T, Fish J. Investigation of individual, age and sexual differences in play of Rattus norvigicus (Mammalia: Rodentia) J Zool. 1976;179:249–260. [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM. Neurobiological substrates of play behavior: Glimpses into the structure and function of mammalian playfulness. In: Bekoff M, Byers JA, editors. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge University Press; Cambridge, UK: 1998. pp. 221–242. [Google Scholar]
- Szot P, Dorsa DM. Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Dev Brain Res. 1993;73:177–183. doi: 10.1016/0165-3806(93)90136-x. [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3:15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Sex and social play in juvenile rats (Rattus norvegicus) J Comp Psychol. 1984;96:276–284. [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135:147–154. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61:50–56. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]