Abstract
Objectives
Transfusion of stored red blood cells (RBCs) is associated with increased morbidity and mortality in trauma patients. Plasma hemoglobin scavenges nitric oxide (NO), which can cause vasoconstriction, induce inflammation and activate platelets. We hypothesized that transfusion of RBCs stored for prolonged periods would induce adverse effects (pulmonary vasoconstriction, tissue injury, inflammation, and platelet activation) in lambs subjected to severe hemorrhagic shock, and that concurrent inhalation of NO would prevent these adverse effects.
Design
Animal study.
Setting
Research laboratory at the Massachusetts General Hospital, Boston, MA.
Subjects
Seventeen awake Polypay-breed lambs.
Interventions
Lambs were subjected to 2 h of hemorrhagic shock by acutely withdrawing 50% of their blood volume. Lambs were resuscitated with autologous RBCs stored for 2 h or less (fresh) or 39±2 (mean±SD) days (stored). Stored RBCs were administered with or without breathing NO (80 ppm) during resuscitation and for 21 h thereafter.
Measurements and Main Results
We measured hemodynamic and oxygenation parameters, markers of tissue injury and inflammation, plasma hemoglobin concentrations, and platelet activation. Peak pulmonary arterial pressure was higher after resuscitation with stored than with fresh RBCs (24±4 vs. 14±2 mmHg, p<0.001) and correlated with peak plasma hemoglobin concentrations (R2=0.56, p=0.003). At 21 h after resuscitation, pulmonary myeloperoxidase activity was higher in lambs resuscitated with stored than with fresh RBCs (11±2 vs. 4±1 U/g, p=0.007). Furthermore, transfusion of stored RBCs increased plasma markers of tissue injury and sensitized platelets to adenosine diphosphate activation. Breathing NO prevented the pulmonary hypertension, and attenuated the pulmonary myeloperoxidase activity, as well as tissue injury and sensitization of platelets to adenosine diphosphate.
Conclusions
Our data suggest that resuscitation of lambs from hemorrhagic shock with autologous stored RBCs induces pulmonary hypertension and inflammation, which can be ameliorated by breathing NO.
Keywords: blood transfusion, hemorrhagic shock, nitric oxide, inflammation, pulmonary hypertension, resuscitation
Introduction
Hemorrhage and organ failure following trauma are the leading causes of death in patients younger than 45 years in the U.S. (1). Transfusion of red blood cells (RBCs) can restore blood oxygen carrying capacity after hemorrhagic shock (HS) (2). During extended storage of human blood (up to 42 days) RBCs undergo progressive biochemical, mechanical, and functional changes, altering their physiological properties (3, 4). These pathological changes result in shedding of hemoglobin (Hb)-containing microparticles (5) and hemolysis of RBCs during storage and after transfusion (6). When oxy-Hb is released from RBCs, it can scavenge nitric oxide (NO) (7) resulting in vasoconstriction (8), inflammation, platelet activation (9), and organ injury (6).
Several studies have associated transfusion of RBCs stored for prolonged periods with increased morbidity and mortality in severely injured patients (10–16). Weinberg et al. reported that trauma patients receiving packed RBCs stored beyond 14 days during the first 24 h of hospitalization had a higher mortality risk compared to patients receiving RBCs stored for less than 14 days (15). This difference in mortality was only observed when patients received 3 or more RBC units. Thus, not only the age, but also the quantity of transfused stored RBCs seems to negatively influence the clinical outcome. Other prospective randomized studies have reported no differences in clinical outcomes when comparing transfusion of fresh and stored RBCs in critically ill patients and healthy volunteers (17–21). Thus, whether stored blood transfusions are deleterious, and if so, in which patients, remains uncertain.
Nitric oxide relaxes vascular smooth muscle cells, modulates immune responses, and inhibits platelet aggregation (22). When inhaled, NO selectively dilates the pulmonary circulation, but does not produce systemic vasodilation or hypotension in a wide range of species, including humans (23) and lambs (24). The absence of systemic vasodilation while breathing NO would be vital for resuscitation of hypotensive patients suffering from HS.
For this study, we adapted an ovine model of HS (25–27) to investigate transfusion of autologous RBCs. We previously reported that transfusion of 300 ml of packed autologous RBCs stored for 40 days transiently increased the mean pulmonary arterial pressure (PAP) and pulmonary vascular resistance index (PVRI) in lambs (28). This pulmonary vasoconstrictor effect was potentiated by partially inhibiting NO synthase (NOS) with NG-nitro-L-arginine methyl-ester (L-NAME) and prevented by breathing 80 ppm NO during transfusion.
Our group recently reported that resuscitation of mice from HS with stored syngeneic RBCs adversely impacted clinical outcome (29). Breathing NO reduced inflammation and tissue injury and improved short-term survival rates after HS in mice resuscitated with stored RBCs.
In the current study, we hypothesized that HS would sensitize lambs to the adverse effects of stored RBC transfusion, inducing severe pulmonary vasoconstriction, tissue injury, inflammation, and platelet activation. We further hypothesized that inhalation of NO during and after transfusion of stored RBCs might prevent these adverse effects. We report that resuscitation from HS with stored RBCs induced greater pulmonary vasoconstriction and tissue injury, as well as greater pulmonary myeloperoxidase activity, plasma Hb concentrations, and platelet sensitization than did resuscitation with fresh RBCs. Breathing NO prevented the pulmonary vasoconstriction and attenuated the tissue injury, pulmonary myeloperoxidase activity, and platelet sensitization in lambs transfused with stored RBCs.
Materials and Methods
Experimental Protocol for Autologous RBC Storage and Transfusion in Awake Lambs Subjected to HS
All experiments were approved by the Subcommittee on Research Animal Care, Massachusetts General Hospital, Boston, MA. The Guide for the Care and Use of Laboratory Animals (U.S. National Research Council, 2011) was followed for animal handling. We studied seventeen 3- to 4-month-old Polypay-breed lambs (New England Ovis, Dover, NH) weighing 28 to 35 kg. For animals receiving stored RBCs, one unit of blood was obtained at 42 days and another unit at 35 days prior to transfusion, with procedures as previously described (28). Briefly, lambs were anesthetized with an intramuscular injection of ketamine (20 mg/kg; Hospira, Inc., Lake Forest, IL), and blood (450 ml) was drawn from an external jugular vein into a Double Blood-Pack Unit (Fenwal, Inc., Lake Zurich, IL) containing citrate-phosphate-dextrose-adenine (CPDA) solution. Blood was leukoreduced, using an integrated RS2000 leukoreduction filter. Red blood cells were separated from plasma and resuspended in additive solution containing saline, adenine, glucose and mannitol (AS-1, Adsol Solution, Fenwal, Inc.). Animals resuscitated with fresh RBCs were transfused with their non-leukoreduced whole blood that was withdrawn to induce HS into bags containing CPDA solution. The detailed composition of resuscitation solutions can be found in Table 1.
Table 1.
Lamb body weight, amount of blood shed, and detailed composition of resuscitation solutions.
|
|
||||
|---|---|---|---|---|
| Fresh (n=6) | Stored (n=6) | Stored + iNO (n=5) | p value (one-way ANOVA) | |
| Lamb body weight (kg) | 30 ± 1 | 32 ± 1 | 31 ± 1 | 0.14 |
| Blood shed (ml/kg) | 35 ± 1 | 33 ± 1 | 35 ± 1 | 0.27 |
| Blood shed (% TBV) | 50 ± 2 | 47 ± 1 | 50 ± 1 | 0.22 |
| Resuscitation solution infused over 1 h (ml/kg) | 40 ± 2 | 38 ± 2 | 39 ± 2 | 0.26 |
| Erythrocytes (ml) | 315 ± 4 | 330 ± 4 | 330 ± 3 | |
| Erythrocyte supernatant (ml) | 0 ± 0 | 270 ± 4 | 270 ± 3 | |
| Lactated Ringer’s solution (ml) | 300 ± 0 | 600 ± 0 | 600 ± 0 | |
| Plasma (ml) | 585 ± 4 | 0 ± 0 | 0 ± 0 | |
| Hematocrit of resuscitation solution (%) | 26 ± 2 | 28 ± 2 | 28 ± 2 | 0.18 |
| Free hemoglobin of resuscitation solution (μM) | 7 ± 2 | 29 ± 10 * | 47 ± 35 * | <0.001 |
iNO = inhaled nitric oxide, TBV = total blood volume.
p<0.05 values differ from fresh red blood cells. All data mean ± SD.
On the day of the HS experiment, anesthesia was induced by breathing 5% isoflurane (Baxter, Deerfield, IL) in oxygen via mask. After endotracheal intubation, animals underwent a tracheostomy and were instrumented with a carotid artery catheter and a 7F pulmonary artery catheter (Edwards Lifesciences, Irvine, CA). After surgery, animals were placed in a custom-made Babraham metabolic cage (30) and allowed to recover from anesthesia for 2 h. Lambs breathed spontaneously via the tracheostomy at an inspired oxygen fraction (FiO2) of 0.25. The heart rate, mean arterial pressure (MAP), PAP, and central venous pressure were monitored continuously. Pulmonary capillary wedge pressure was intermittently measured every 15–30 min. Cardiac output was assessed by thermodilution. Cardiac index (CI), PVRI, and stroke volume were calculated using standard formulae. Hemodynamic data were collected until sacrifice at 21 h after completing resuscitation.
Three groups of lambs were studied. In all animals, the most severe form of HS (Class 4) (31) was induced by withdrawing more than 40% of the lamb’s total blood volume (32). Blood was withdrawn via the carotid artery catheter at a rate of 0.66 ml·kg−1·min−1, until MAP was less than 50 mmHg. Mean arterial pressure was maintained around 50 mmHg for a total of 120 min. Additional 60 ml of blood were withdrawn whenever MAP increased above 50 mmHg for more than 5 min. After 120 min of HS, animals were resuscitated with RBCs warmed to 37°C (transfused over 30 min) and lactated Ringer’s solution (Hospira, Inc.; Table 1). Following the resuscitation period, lactated Ringer’s solution containing 5% dextrose (Hospira, Inc.) was continuously infused at 3 ml·kg−1·h−1.
For resuscitation, one group (n=6) of lambs received whole blood collected into CPDA, which had been withdrawn to induce HS (fresh RBCs). A second group of lambs (n=6) was transfused with blood stored in AS-1 for an average of 39 days before transfusion (stored RBCs). A third group (n=5) of lambs was transfused with stored RBCs while breathing 80 parts per million (ppm) NO (Medical-Technical Gases, Medford, MA). Nitric oxide was administered during resuscitation and for 21 h thereafter. Inspiratory concentrations of NO were measured using a Sievers NO Analyzer (GE Analytical Instruments, Boulder, CO).
Lambs were continuously monitored and were sedated with an i.v. bolus of 0.01–0.02 mg/kg midazolam (Hospira, Inc.) if they appeared distressed. Plasma glucose concentrations were monitored using a OneTouch Ultra blood glucose monitor (LifeScan, Inc., Milpitas, CA). Plasma glucose concentrations below 80 mg/dl were treated with an i.v. injection of 5 ml of 50% dextrose (Hospira, Inc.).
Biochemical Analysis of Blood Samples
Arterial and mixed-venous blood samples were measured before induction of HS, every 30 min during HS, every 15 min during resuscitation, and at 0.5, 1, 2, 3, 9, 15, and 21 h after resuscitation. Samples from the blood storage bags were obtained immediately following transfusion. Blood gas tensions, Hb concentrations, and pH were analyzed using an ABL800 Flex blood gas analyzer (Radiometer Medical, Copenhagen, Denmark). Oxygen delivery index (DO2I) and oxygen consumption index (VO2I) were calculated using standard formulae.
The concentration of Hb in plasma and in the supernatant of the transfusate was determined by spectral deconvolution (5). Absorption spectra of plasma were measured from 500 to 700 nm using a Libra S70 spectrophotometer (Biochrom, Cambridge, United Kingdom) with cuvettes of 1 cm path length. The measured spectra were calibrated to remove background signals: Absorbance values at 675 nm and 700 nm from each spectrum were connected and extrapolated. The entire range below the line connecting these two values was subtracted from the measured spectrum, and calibrated spectra were used to determine the concentrations of oxy-Hb, met-Hb, and deoxy-Hb (33). The spectra from 550 to 700 nm were deconvoluted using a least squares analysis with Excel 2007 Solver (Microsoft, Redmond, WA).
Lactate levels were measured using a Lactate Plus monitoring system (Nova Biomedical, Waltham, MA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine phosphokinase (CPK) activity, and creatinine concentrations were determined using kits obtained from Bioassay Systems (Hayward, CA). Serum iron concentrations were measured using a commercially available kit (Genzyme, Cambridge, MA).
Lung Myeloperoxidase Activity
Twenty to thirty mg of lung tissue were homogenized in 0.5 ml of 50 mM potassium phosphate (pH 7.4), and centrifuged at 12,000g for 20 min. Lung homogenates were resuspended in 0.5 ml of hexadecyl-trimethyl-ammonium bromide (Sigma-Aldrich, St. Louis, MO) in 50 mM potassium phosphate (pH 6.2), homogenized, sonicated, freeze-thawed, and sonicated again. The solution was centrifuged, and 20 μl of the supernatant were mixed with 70 μl of o-dianisidine dihydrochloride (0.357 mg/ml; Sigma-Aldrich) in Hanks’ balanced salt solution and 60 μl of 0.1% hydrogen peroxide in 50 mM potassium phosphate (pH 6.2). Myeloperoxidase activity (U/g lung) was determined by measuring absorption at 450 nm.
Lung Wet to Dry Ratio
At necropsy, one piece of the lower lobe of the left lung (15–20 g) was excised and immediately weighed (34). The tissue was dried in a microwave oven for 40 min and reweighed. Values are expressed as the ratio of wet to dry weight.
Quantitation of Gene Expression Levels
At 21 h after resuscitation ended, animals were anesthetized and euthanized by intravenous injection of 50 ml of 20% KCl solution. Tissue samples were obtained from the lung, liver, and kidney, snap frozen in liquid nitrogen, and stored at −80°C.
RNA was extracted from tissues using Trizol (Invitrogen, Carlsbad, CA), and complementary DNA was synthesized using MMLV-RT (Invitrogen). Real-time amplification of transcripts was detected using a Mastercycler ep Realplex (Eppendorf, Hamburg, Germany). The relative expression of target transcripts was normalized to levels of 18S ribosomal RNA. Primer pairs were used to detect transcripts encoding heme oxygenase (HO)-1, TCCCTGCGTCCCTCCCTTCTG, AGGGTCCAGGGAAGACCCCG; myeloperoxidase, GCTGAGGCGGGACACAACCC, CCCAGTTCCGTTTCCGGGGC; interleukin-6 (IL-6), CAGAAAATAAGCTGAAACTTCCA, ATGTCAGTGTGTGTGGCTGGAG; and tumor necrosis factor-α (TNF-α), GGCTCTCCTGTCTCCCGT, GTTGGCTACAACGTGGGC.
Assessment of Platelet Activation by Flow Cytometry
Venous blood (3 ml) was withdrawn into Vacutainer tubes (BD, Franklin Lakes, NJ) containing 3.2% sodium citrate before induction of HS and at the end of transfusion. Platelet-rich plasma was obtained by centrifuging blood at 170g at room temperature for 20 min. Five μL of platelet-rich plasma were incubated with 2 μL anti-P-selectin (CD62P) antibody (ab54427; Abcam, Inc., Cambridge, MA) and 53 μL HEPES-buffered saline for 30 min at room temperature. To assess the sensitivity of platelets to pro-thrombotic stimuli, additional 5 μL samples of platelet-rich plasma were incubated with 2 μL anti-CD62P antibody and increasing concentrations of adenosine diphosphate (ADP, Sigma-Aldrich). All samples were centrifuged at 1500g at room temperature for 8 min, and the supernatant was discarded. Pellets were resuspended and incubated 20 min with 2 μL of fluorescein isothiocyanate-labeled secondary antibody (ab11588; Abcam, Inc.) and 58 μL HEPES-buffered saline. Platelets were fixed with 0.5% formyl saline and the number of platelets expressing CD62P was determined with a FACSCalibur flow cytometer (BD).
In separate experiments, we tested whether NO synthesis by platelet NOS is involved in ovine platelet activation by Hb. Platelets were isolated from the blood of healthy lambs (n=5) as described above. Platelet-rich plasma was incubated with increasing concentrations of ADP, with or without L-NAME (1 mM) and ovine Hb (50 μM). Data were analyzed using FlowJo 7.6 software (Tree Star, Inc., Ashland, OR).
Statistical Analysis
All data are expressed as mean values ± SD. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA). A Kolmogorov-Smirnov test was used to verify normality of measured variables. For hemodynamic measurements, a two-way ANOVA with repeated measures was used to compare differences between groups at various time points. When the interaction p value between time and condition was significant, comparisons were made at each individual time point using a one-way ANOVA with post-hoc Bonferroni-adjusted comparison testing. Within-group comparisons were performed using a paired t-test. For comparisons of mRNA levels an independent t-test was applied to compare differences between two groups, and corrected for multiple comparisons using Bonferroni adjustment. Differences in platelet activation were compared using a Kruskal-Wallis test. p values < 0.05 were considered significant.
Results
Hemoglobin Concentrations are Restored to Baseline Values After Resuscitation from HS with Fresh or Stored RBCs
Hemoglobin concentrations did not differ at baseline between any of the 3 groups (Supplemental Digital Content 1). In accordance with criteria defining Class 4 HS (31), the amount of blood withdrawn from the lambs was greater than 40 % of the estimated blood volume (Table 1). Hemoglobin concentrations decreased to a similar extent in all 3 groups during HS and returned to baseline values at the end of resuscitation with either fresh or stored RBCs (Supplemental Digital Content 1). During 1 h of resuscitation, lambs received 1200 ml of resuscitation solutions. The hematocrit of the solutions used for resuscitation did not differ between the 3 groups (Table 1).
Resuscitation from HS with Stored RBCs Induces Pulmonary Hypertension
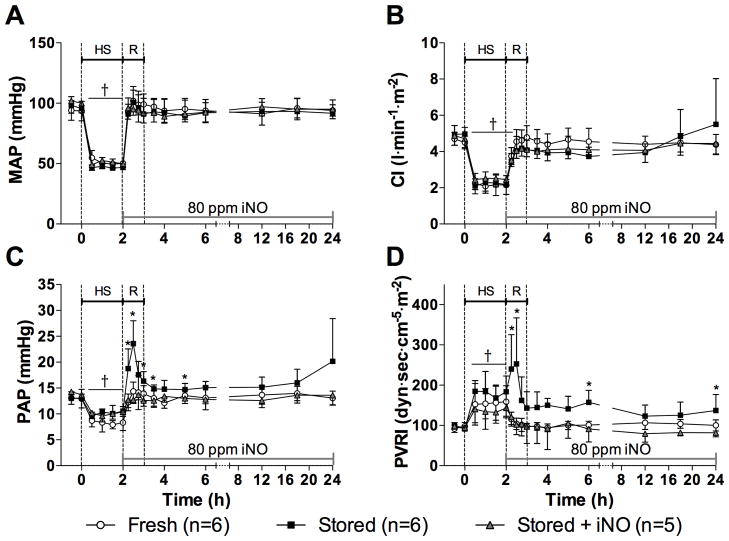
Hemorrhagic shock decreased MAP by 49±4%, and MAP remained below baseline values for 120 min (p<0.001, Fig. 1A). Similarly, HS reduced CI to 48±4% of baseline values (p<0.001, Fig. 1B). The reduction of CI was due to a reduced stroke volume during HS (Supplemental Digital Content 2). During resuscitation, both MAP and CI returned to baseline values in all groups (Fig. 1A and 1B).
Figure 1.
(A) Mean arterial pressure (MAP), (B) cardiac index (CI), (C) mean pulmonary arterial pressure (PAP), and (D) pulmonary vascular resistance index (PVRI) before, during and after resuscitation (R) from hemorrhagic shock (HS). *p<0.05 values of both fresh red blood cells (RBCs) and stored RBCs + inhaled nitric oxide (iNO) differ from stored RBCs. †p<0.05 values differ from baseline (0 min) in all 3 groups. All data mean ± SD.
Mean PAP decreased 28±3% during HS (Fig. 1C). Resuscitation with fresh RBCs restored PAP to baseline values (14±2 mmHg), whereas resuscitation with stored RBCs increased PAP to 24±4 mmHg (p<0.001, values differ). In lambs resuscitated with fresh RBCs, PVRI did not increase during transfusion (104±5 dyn·sec·cm−5·m−2, Fig. 1D). In lambs resuscitated with stored RBCs, PVRI increased to 253±43 dyn·sec·cm−5·m−2 during transfusion (p=0.01, differs vs. fresh RBCs) and remained elevated until 3 h after resuscitation. Inhalation of NO prevented the increase of PAP and PVRI both during and after resuscitation with stored RBCs (Fig. 1C and 1D).
Transfusion of Stored RBCs Alters Oxygenation Parameters
Hemorrhagic shock decreased SvO2 and DO2I, whereas SaO2, PaO2 and VO2I remained unchanged (Supplemental Digital Content 1). After completing resuscitation with either fresh or stored RBCs, SvO2 and DO2I returned to baseline values. At 6 and 12 h, DO2I was lower in lambs resuscitated with stored than with fresh RBCs (p<0.05). Breathing NO did not prevent the reduction in DO2I after transfusion of stored RBCs.
At 8 to 10 h after transfusion, two of the six lambs resuscitated with stored RBCs developed progressive systemic hypoxemia and tachypnea. The PaO2 decreased to less than 60 mmHg in both lambs breathing at FiO2 0.25, resulting in PaO2/FiO2 ratios below 200. Mean PAP markedly increased to values of 24 and 35 mmHg in these two lambs, which developed severe acute respiratory failure at the end of study.
Lactate Levels Increase During HS
Plasma concentrations of lactate did not differ between the 3 groups at baseline and increased during HS to a similar extent in all 3 groups (Supplemental Digital Content 3). Lactate concentrations returned to baseline levels at the end of resuscitation in lambs transfused with fresh RBCs, but remained elevated for 1 h after resuscitation in lambs transfused with stored RBCs. In animals breathing NO during and after resuscitation with stored RBCs, lactate concentrations returned to baseline levels 30 min after resuscitation ended.
Tissue Injury is Increased After Resuscitation with Stored RBCs
The plasma concentrations of creatinine and plasma activity of ALT, AST, and CPK did not differ between the 3 groups at baseline (Table 2). Plasma CPK activity at 3, 6, 12 and 24 h was higher in lambs receiving stored RBCs than in those receiving fresh RBCs (p=0.002). Inhalation of NO prevented the increase in the activity of CPK associated with transfusion of stored RBCs. At 12 and 24 h, plasma AST activity levels were higher in animals resuscitated with stored RBCs than in those transfused with either fresh RBCs or stored RBCs while breathing NO (p=0.01).
Table 2.
Organ function parameters and markers of tissue injury.
|
|
||||||
|---|---|---|---|---|---|---|
| 0 h | 3 h (HS+R) | 6 h | 12 h | 24 h | ||
| Creatinine (mg/dl) | Fresh | 0.72 ± 0.15 | 0.73 ± 0.23 | 0.61 ± 0.29 | 0.63 ± 0.17 | 0.65 ± 0.14 |
| Stored | 0.63 ± 0.15 | 0.76 ± 0.09 | 1.00 ± 0.67 | 0.64 ± 0.22 | 0.90 ± 0.74 | |
| Stored + iNO | 0.76 ± 0.25 | 0.73 ± 0.07 | 0.57 ± 0.10 | 0.93 ± 0.62 | 0.39 ± 0.15 | |
| AST (U/l) | Fresh | 38 ± 4 | 40 ± 8 | 47 ± 10 | 55 ± 23 | 53 ± 21 |
| Stored | 38 ± 6 | 40 ± 8 | 51 ± 8 | 84 ± 48 # | 112 ± 53 *# | |
| Stored + iNO | 39 ± 7 | 34 ± 7 | 37 ± 7 | 44 ± 14 | 45 ± 23 | |
| ALT (U/l) | Fresh | 7 ± 4 | 7 ± 3 | 9 ± 4 | 10 ± 6 | 12 ± 6 |
| Stored | 8 ± 3 | 7 ± 4 | 11 ± 7 | 14 ± 8 | 20 ± 10 | |
| Stored + iNO | 6 ± 3 | 4 ± 3 | 5 ± 3 | 6 ± 3 | 8 ± 4 | |
| CPK (U/l) | Fresh | 68 ± 25 | 157 ± 122 | 206 ± 167 | 179 ± 168 | 73 ± 101 |
| Stored | 93 ± 59 | 258 ± 166# | 292 ± 154# | 224 ± 156 | 256 ± 187# | |
| Stored + iNO | 92 ± 49 | 108 ± 61 | 140 ± 68 | 115 ± 105 | 30 ± 19 | |
ALT = alanine aminotransferase, AST = aspartate aminotransferase, CPK = creatine phosphokinase, HS = hemorrhagic shock, R = resuscitation.
p<0.05 values differ from both fresh red blood cells (RBCs) and stored RBCs + inhaled nitric oxide (iNO);
p<0.05 values differ from baseline (0 h). All data mean ± SD.
Pulmonary Neutrophil Accumulation and Edema are Associated with Transfusion of Stored RBCs
The pulmonary myeloperoxidase activity in lambs resuscitated with stored RBCs was higher than that of lambs transfused with fresh RBCs (11±2 vs. 4±1 U/g, p=0.007; Fig. 2). In animals breathing NO during and after resuscitation with stored RBCs, the pulmonary myeloperoxidase activity (8±1 U/g) did not differ from that of lambs resuscitated with fresh RBCs (p=0.09).
Figure 2.
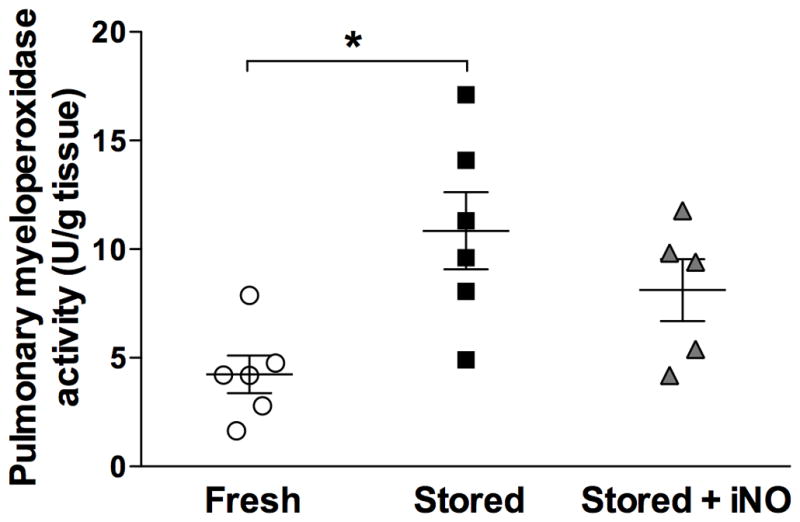
Pulmonary myeloperoxidase activity at 21 h after resuscitation from hemorrhagic shock. *p<0.05 values of fresh RBCs differ from stored RBCs. All data mean ± SD. iNO = inhaled nitric oxide.
Lung wet to dry weight ratios 21 h after resuscitation did not differ between any of the groups (fresh RBCs 4.9±0.5, stored RBCs 5.3±0.2, and stored RBCs with inhaled NO 4.8±0.4; p=0.23). However, both lambs that developed delayed-onset severe acute respiratory failure and systemic hypoxemia after transfusion of stored RBCs had markedly elevated lung wet to dry weight ratios (6.0 and 6.1).
Pulmonary and hepatic gene expression levels of myeloperoxidase, IL-6, and TNF-α did not differ between lambs transfused with fresh or stored RBCs (Supplemental Digital Content 4).
Plasma Hb Concentrations are Increased up to 21 h After Transfusion of Stored RBCs
Cell-free Hb concentrations were higher in supernatant solutions of stored RBCs than in those of fresh RBCs (Table 1). Plasma Hb concentrations increased in lambs transfused with stored RBCs, with or without breathing NO (Fig. 3), but not in animals transfused with fresh RBCs. For the first 15 h after resuscitation, plasma Hb concentrations of lambs receiving stored RBCs did not differ from those of lambs breathing NO during and after stored RBC transfusion. At 21 h after resuscitation, plasma Hb concentrations of lambs transfused with stored RBCs were higher than those at baseline. However, when lambs were treated with inhaled NO, plasma Hb concentrations had returned to baseline levels at 21 h after resuscitation.
Figure 3.
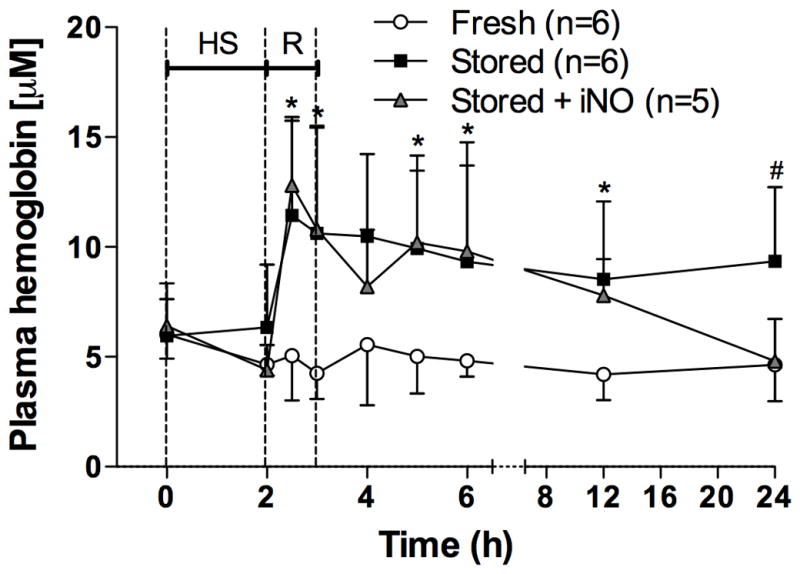
Plasma hemoglobin concentrations before, during, and after resuscitation (R) from hemorrhagic shock (HS). *p<0.05 values of both stored red blood cells (RBCs) and stored RBCs + inhaled nitric oxide (iNO) differ from fresh RBCs. #p<0.05 values of both fresh RBCs and stored RBCs + iNO differ from stored RBCs. All data mean ± SD.
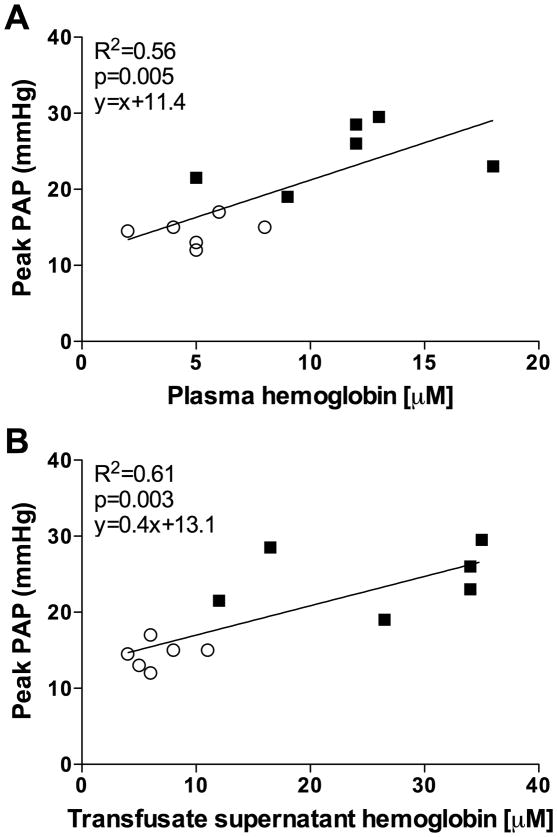
Peak mean PAP at the end of transfusion correlated with peak plasma Hb concentrations at the end of transfusion (R2=0.56, p=0.005, Fig. 4A) and with the concentration of Hb in the supernatant of the transfusate at the end of the storage period (R2=0.61, p=0.003; Fig. 4B).
Figure 4.
Correlation of (A) plasma hemoglobin concentrations and (B) transfusate supernatant hemoglobin concentrations with peak mean pulmonary areterial pressue (PAP) at the end of transfusion. Open circles: lambs resuscitated with fresh red blood cells (RBCs); black squares: lambs resuscitated with stored RBCs.
Stored Blood Transfusion Increases Hepatic HO-1 Gene Expression Levels and Serum Iron Concentrations
At 21 h after resuscitation, hepatic HO-1 mRNA levels were higher in lambs resuscitated with stored than with fresh RBCs (p<0.001, Supplemental Digital Content 4), but pulmonary HO-1 mRNA levels did not differ (p=0.23).
Furthermore, at 21 h after resuscitation, serum iron concentrations were higher in lambs resuscitated with stored RBCs than in those resuscitated with fresh RBCs (80±20 vs. 44±7 μg/dl, p=0.03). Serum iron concentrations in lambs receiving stored RBCs did not differ between animals breathing gas supplemented with NO and those not breathing NO (102±19 vs. 80±20 μg/dl, p=0.09).
Hemorrhagic Shock Sensitizes Ovine Platelets to ADP Stimulation
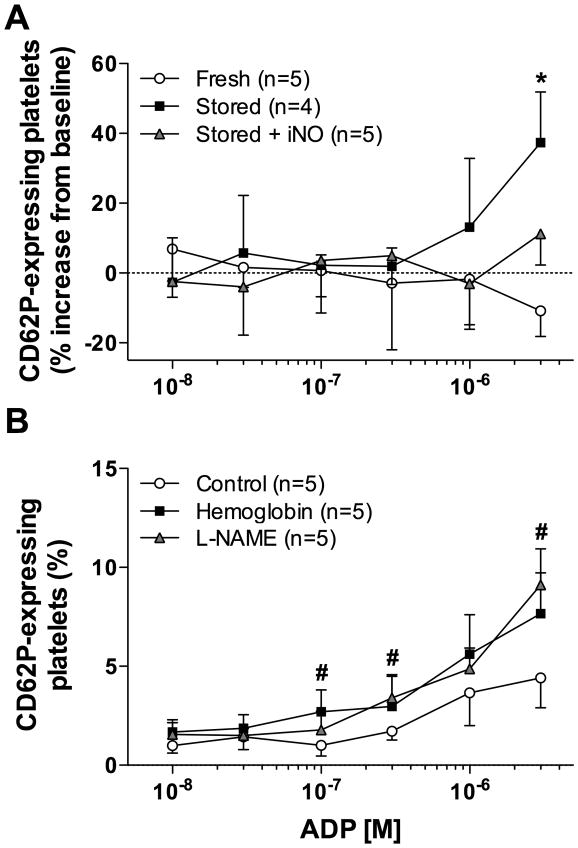
There was no difference in the percentage of circulating platelets expressing CD62P before HS or after resuscitation from HS with either fresh or stored RBCs (data not shown). Furthermore, no differences in CD62P expression were observed in platelets after stimulation with low concentrations of ADP ex vivo. However, when platelets were incubated with 3×10−6 M ADP ex vivo, more platelets from lambs resuscitated with stored RBCs expressed CD62P than those from lambs resuscitated with fresh RBCs (p=0.004; fig. 5A). In lambs resuscitated with stored RBCs, breathing NO attenuated the ability of ADP to increase the fraction of platelets expressing CD62P.
Figure 5.
Platelet activation determined by flow cytometric detection of cell-surface P-selectin (CD62P) after incubation with increasing doses of adenosine diphosphate (ADP). (A) Relative platelet activation after resuscitation with fresh or stored red blood cells (RBCs) with or without inhaled nitric oxide (iNO). (B) Platelet activation after ex vivo treatment with NG-nitro-L-arginine methyl-ester (L-NAME; 1mM) or ovine hemoglobin (50 μM). *p<0.05 values of both fresh RBCs and stored RBCs + iNO differ from stored RBCs. #p<0.05 values of control differ from both L-NAME and hemoglobin. All data mean ± SD.
In order to elucidate whether NO synthesis by platelet NOS is involved in ovine platelet activation by Hb, we first incubated naïve ovine platelets in vitro with either L-NAME or Hb and then treated them with increasing concentrations of ADP. Platelet activation by ADP was more pronounced in platelets incubated with L-NAME or Hb than in naïve platelets (p<0.05). Both L-NAME and Hb treatment increased the expression of CD62P on platelets to a similar extent (fig. 5B).
Discussion
We adapted our previously described ovine transfusion model (28) to investigate the effects of transfusing stored RBCs in awake lambs subjected to HS. Resuscitation from HS with stored RBCs produced transient pulmonary hypertension, which correlated with peak concentrations of plasma Hb at the end of transfusion. Furthermore, resuscitation from HS with stored RBCs increased circulating markers of tissue injury (AST and CPK) and pulmonary myeloperoxidase activity, and sensitized platelets to stimulation with ADP. Inhalation of NO prevented pulmonary vasoconstriction, and attenuated tissue injury and the increase in pulmonary myeloperoxidase and platelet activity after resuscitation from HS with stored RBCs.
We studied an established model of ovine HS (25–27), producing systemic hypoperfusion as reflected by an increase in plasma lactate levels during HS. Similar to our study, Fischer et al. maintained MAP near 50 mmHg over 2 h, and observed a comparable decrease in CI and DO2I during HS (25). Fischer et al. reported that resuscitation from HS with diaspirin cross-linked Hb induced systemic and pulmonary hypertension, whereas we found that resuscitation from HS with stored RBCs only induced transient pulmonary, but not systemic, hypertension. The plasma Hb concentrations after resuscitation with diaspirin cross-linked Hb reported by Fischer et al. were more than 100-fold higher than those in our lambs after transfusion of 600 ml of stored RBCs. The different responses of the systemic and pulmonary vascular systems in these two studies illustrate the remarkable sensitivity of the lamb’s pulmonary vasculature to the scavenging of NO by plasma Hb.
Similar to the results observed in our previous lamb study (28), transfusion of stored RBCs increased both the PAP and PVRI, whereas transfusion of fresh RBCs did not. However, equivalent concentrations of plasma Hb after stored blood transfusion induced a greater PAP and PVRI in animals subjected to 2 h of HS than in healthy lambs. The pulmonary vasoconstrictor effect in animals subjected to HS and stored blood transfusion was not only more pronounced, but pulmonary vasoconstriction and hypertension lasted longer after HS than in healthy lambs. Animal models and human studies of HS have shown that reperfusion injury produces systemic and pulmonary endothelial dysfunction, thereby decreasing the vascular bioavailability of NO (35–38). Consequently, endothelial dysfunction and reduced vascular bioavailability of NO after HS might have contributed to the increased pulmonary vasoconstrictor response caused by transfusion of stored RBCs. Moreover, pulmonary vasoconstriction might have been facilitated by an increase of pulmonary vascular permeability after HS, resulting in greater exposure of pulmonary smooth muscle cells to plasma Hb (39, 40). In addition, the lambs in this study received 2 units of RBCs (roughly equivalent to 4 units of RBCs transfused into a human adult), as compared to 1 unit in our previous study (28). Thus, the greater volume load could have resulted in higher pulmonary vascular pressures.
A recent prospective randomized trial in premature infants comparing fresh RBC transfusion to standard blood banking practice showed no difference in major neonatal morbidities (21). Pulmonary hemodynamic parameters, however, were not evaluated. Extrapolating from our study of 3-month-old lambs, the neonatal pulmonary circulation might be sensitive even to small increases in plasma Hb concentrations.
Previous animal studies have shown detrimental pulmonary effects after transfusion of stored RBCs (28, 41). In the current lamb study, pulmonary myeloperoxidase activity was higher after resuscitation with stored than with fresh RBCs. Myeloperoxidase activity, which correlates with sequestration and activation of neutrophils in lung tissue, is also elevated in ovine acute lung injury and sepsis (42). In contrast, pulmonary mRNAs encoding myeloperoxidase, IL-6, and TNF-α were not elevated 21 h after resuscitation with stored RBCs. Lange et al. reported increased pulmonary IL-6 mRNA levels in sheep at 8 h, but not 12, 18, or 24 h after induction of acute lung injury and sepsis (42). Thus, the mRNA levels of myeloperoxidase, IL-6, and TNF-α may have been elevated at earlier time points in our model.
Villagra et al. reported that plasma Hb concentrations and platelet activation were greater in patients with sickle cell disease than in healthy volunteers and that platelet CD62P expression correlated with markers of intravascular hemolysis (43). We did not find differences in CD62P expression in unstimulated platelets of lambs resuscitated with fresh or stored RBCs. However, ovine platelets were more readily activated by ADP after resuscitation from HS with stored than with fresh RBCs. This platelet-sensitizing effect might be induced by scavenging of systemic or platelet-derived NO by Hb. We demonstrated that inhibition of platelet NOS by L-NAME increased CD62P expression to a similar extent as did incubation of platelets with purified Hb. These data suggest that decreased bioavailability of NO after resuscitation with stored RBCs might be one mechanism that increases the sensitivity of ovine platelets to ADP.
Transfusion of blood components is associated with an increased risk of developing transfusion-related acute lung injury (TRALI), which occurs within 6 h after transfusion (44). Tung et al. reported that transfusion of xenogeneic supernatant from stored but not fresh RBCs contributed to the development of TRALI in endotoxin-treated sheep (34). In our study, two lambs resuscitated with stored RBCs developed severe hypoxemia with tachypnea starting at 8 to 10 h after transfusion. These two animals had the highest lung wet to dry ratios of all the animals studied, indicating severe pulmonary edema. No other animal transfused with either fresh RBCs or breathing inhaled NO during transfusion with stored RBCs developed respiratory dysfunction or pulmonary edema. Experimental studies report that inhaled NO reduced pulmonary capillary leakage and the accumulation of macrophages and neutrophils in isolated rat lungs subjected to ischemia-reperfusion injury (45, 46). In the current study, breathing NO prevented pulmonary vasoconstriction and neutrophil accumulation associated with transfusion of stored RBCs to resuscitate HS. Hidalgo et al. reported that sequestered neutrophils can interact with platelets, thereby mediating endothelial injury and impeding microcirculatory flow (47). Taken together, these data suggest that inhaled NO might exert its systemic effects by reducing neutrophil-platelet interactions.
One limitation of the current study is that lambs resuscitated with fresh RBCs were not precise controls for those resuscitated with stored RBCs. Due to the 2 h time limitation, fresh blood was not leukoreduced. Leukoreduction of 1 unit of sheep blood using standard human leukoreduction filters takes up to 1 h, possibly due to differences in the size and surface antigen expression of ovine and human leukocytes (48). Withdrawal of 2 units of blood within 2 days of the HS experimental procedure would have induced a severe stress in the lambs. Furthermore, if 2 units were donated within days of the experimental procedure, the blood Hb levels at baseline would have been markedly reduced. Because of the short processing time and the need to have all groups begin at equal Hb concentrations, we believed it preferable to transfuse the blood removed acutely to induce HS.
Studies in animal models can only suggest noxious mechanisms or processes that may be occurring in transfused patients. Results obtained by transfusing different species may not apply to patients due to biochemical, physiological, and anatomical differences. We will have to wait for the results of various large, prospective, and randomized trials of stored blood transfusion to draw final conclusions. Yet, in the meantime, we can elucidate possible mechanisms and effects to search for in the human studies.
Conclusions
Resuscitation from HS with stored RBCs in lambs induced severe pulmonary hypertension, caused tissue injury, increased pulmonary myeloperoxidase activity, and sensitized platelets to ADP. Breathing NO ameliorated these adverse effects. Our data suggest that the use of fresh RBCs for resuscitation from HS might improve the clinical outcome in trauma patients. Furthermore, inhaled NO should be tested to prevent possible adverse hemodynamic and inflammatory effects when patients with HS receive stored RBC transfusions.
Supplementary Material
Acknowledgments
Source of Funding: This study was supported by funds of the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA. Dr. Malhotra was supported by the Fellow-to-Faculty Transition Award 11FTF7290032 from the American Heart Association (Dallas, TX). Dr. Bloch was supported by the National Institute of Health R01 grant HL074352 (Bethesda, MD), and has received grants from Ikaria Inc., Clinton, NJ, to study inhaled nitric oxide. Dr. Stowell consulted for Haemonetics, NHLBI. He also received travel reimbursement from the Australian New Zealand Society of Hematology. Dr. Zapol receives royalties from patents on inhaled nitric oxide licensed by Massachusetts General Hospital to Linde Corporation, Munich, Germany, and Ikaria Inc.; and has applied for a U.S. patent entitled, “Attenuation of artificial oxygen carrier-induced vasoconstriction,” Provisional Patent Application 60/864,734.
The authors would like to thank Patricia DiVito, Joan Arsenault, and Michele Pariseau (Blood Transfusion Service of the Department of Pathology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts) for assistance with blood processing; Richard Hurley, D.V.M., Julie Hurley, D.V.M., Jay Mariacher, Caitlin Dubois, and Lisa Quinn (New England Ovis, Rollinsford, New Hampshire) for providing advice on sheep-handling techniques and for breeding and husbandry of the lambs; and Francine E. Lui, Ph.D. (Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts) for providing purified ovine hemoglobin.
Footnotes
All experiments were performed at the Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Conflicts of Interest
The remaining authors report no conflicts of interest.
References
- 1.Causes of Death by Age Group. [Accessed October 17, 2012]; Available at: http://www.cdc.gov/Injury/wisqars/pdf/10LCD-Age-Grp-US-2009-a.pdf.
- 2.Tsai AG, Hofmann A, Cabrales P, et al. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gevi F, D’Alessandro A, Rinalducci S, et al. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012 doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azarov I, Liu C, Reynolds H, et al. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–33579. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu B, Raher MJ, Volpato GP, et al. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W, Jin R, Zhang J, et al. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116:1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- 11.Keller ME, Jean R, LaMorte WW, et al. Effects of age of transfused blood on length of stay in trauma patients: a preliminary report. J Trauma. 2002;53:1023–1025. doi: 10.1097/00005373-200211000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Murrell Z, Haukoos JS, Putnam B, et al. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282–274. [DOI] [PubMed] [Google Scholar]
- 14.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg JA, McGwin G, Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juffermans NP, Vlaar AP, Prins DJ, et al. The age of red blood cells is associated with bacterial infections in critically ill trauma patients. Blood Transfus. 2012;10:290–295. doi: 10.2450/2012.0068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 18.Hebert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 19.Weiskopf RB, Feiner J, Toy P, et al. Fresh and stored red blood cell transfusion equivalently induce subclinical pulmonary gas exchange deficit in normal humans. Anesth Analg. 2012;114:511–519. doi: 10.1213/ANE.0b013e318241fcd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berra L, Coppadoro A, Yu B, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y. The multiple actions of NO. Pflugers Arch. 2010;459:829–839. doi: 10.1007/s00424-009-0773-9. [DOI] [PubMed] [Google Scholar]
- 23.Rossaint R, Falke KJ, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 24.Frostell C, Fratacci MD, Wain JC, et al. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 25.Fischer SR, Burnet M, Traber DL, et al. Plasma volume expansion with solutions of hemoglobin, albumin, and Ringer lactate in sheep. Am J Physiol. 1999;276:H2194–2203. doi: 10.1152/ajpheart.1999.276.6.H2194. [DOI] [PubMed] [Google Scholar]
- 26.Norberg A, Brauer KI, Prough DS, et al. Volume turnover kinetics of fluid shifts after hemorrhage, fluid infusion, and the combination of hemorrhage and fluid infusion in sheep. Anesthesiology. 2005;102:985–994. doi: 10.1097/00000542-200505000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Frithiof R, Eriksson S, Bayard F, et al. Intravenous hypertonic NaCl acts via cerebral sodium-sensitive and angiotensinergic mechanisms to improve cardiac function in haemorrhaged conscious sheep. J Physiol. 2007;583:1129–1143. doi: 10.1113/jphysiol.2007.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron DM, Yu B, Lei C, et al. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei C, Yu B, Shahid M, et al. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–1202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison FA. Proceedings: The Babraham metabolism cage for sheep. J Physiol. 1974;242:20P–22P. [PubMed] [Google Scholar]
- 31.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansard SL. Residual organ blood volume of cattle, sheep and swine. Proc Soc Exp Biol Med. 1956;91:31–34. doi: 10.3181/00379727-91-22160. [DOI] [PubMed] [Google Scholar]
- 33.Zijlstra WG, Buursma A, Assendelft OWV. Zeist: VSP BV. 2000. Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application. [Google Scholar]
- 34.Tung JP, Fraser JF, Nataatmadja M, et al. Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI) Crit Care. 2012;16:R19. doi: 10.1186/cc11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage SA, Fitzpatrick CM, Kashyap VS, et al. Endothelial dysfunction after lactated Ringer’s solution resuscitation for hemorrhagic shock. J Trauma. 2005;59:284–290. doi: 10.1097/01.ta.0000179453.89769.1c. [DOI] [PubMed] [Google Scholar]
- 36.Douzinas EE, Orfanos SE, Livaditi O, et al. Hypoxemic resuscitation prevents pulmonary capillary endothelial dysfunction induced by normoxemic resuscitation from hemorrhagic shock. Crit Care Med. 2009;37:869–875. doi: 10.1097/CCM.0b013e31819b81ec. [DOI] [PubMed] [Google Scholar]
- 37.Causey MW, Salgar S, Singh N, et al. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J Vasc Surg. 2012;55:1096–1103. e1051. doi: 10.1016/j.jvs.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 38.Mathru M, Lang JD. Endothelial dysfunction in trauma patients: a preliminary communication. Shock. 2005;24:210–213. doi: 10.1097/01.shk.0000174936.51903.7d. [DOI] [PubMed] [Google Scholar]
- 39.Wessel DL, Adatia I, Giglia TM, et al. Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation. 1993;88:2128–2138. doi: 10.1161/01.cir.88.5.2128. [DOI] [PubMed] [Google Scholar]
- 40.Dull RO, DeWitt BJ, Dinavahi R, et al. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol. 2004;97:1930–1937. doi: 10.1152/japplphysiol.00102.2004. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. doi: 10.1182/blood-2012-10-462945. epub 2012/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lange M, Szabo C, Traber DL, et al. Time profile of oxidative stress and neutrophil activation in ovine acute lung injury and sepsis. Shock. 2012;37:468–472. doi: 10.1097/SHK.0b013e31824b1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villagra J, Shiva S, Hunter LA, et al. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 45.Chetham PM, Sefton WD, Bridges JP, et al. Inhaled nitric oxide pretreatment but not posttreatment attenuates ischemia-reperfusion-induced pulmonary microvascular leak. Anesthesiology. 1997;86:895–902. doi: 10.1097/00000542-199704000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Gazoni LM, Tribble CG, Zhao MQ, et al. Pulmonary macrophage inhibition and inhaled nitric oxide attenuate lung ischemia-reperfusion injury. Ann Thorac Surg. 2007;84:247–253. doi: 10.1016/j.athoracsur.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Hidalgo A, Chang J, Jang JE, et al. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hein WR, Mackay CR. Other surface antigens identified on sheep leukocytes. Vet Immunol Immunopathol. 1991;27:115–118. doi: 10.1016/0165-2427(91)90090-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.