Abstract
Neurotransmitter release depends critically on the Ca2+ sensor synaptotagmin-1 and the SNARE proteins syntaxin-1, synaptobrevin and SNAP-25, which mediate membrane fusion by forming tight SNARE complexes that bridge the synaptic vesicle and plasma membranes. Interactions between the SNARE complex and the two C2 domains of synaptotagmin-1 (the C2A and C2B domains) are believed to play a key role in coupling Ca2+ sensing to membrane fusion, but the nature of these interactions is unclear, in part because of a paucity of data obtained by quantitative biophysical methods. Here we have analyzed synaptotagmin-1/SNARE complex interactions by monitoring the decrease in the intensities of one-dimensional 13C-edited 1H-NMR spectra of 13C-labeled fragments of synaptotagmin-1 upon binding to unlabeled SNARE complex. Our results indicate that there is a primary binding mode between synaptotagmin-1 and the SNARE complex that involves a polybasic region in the C2B domain and has a submicromolar affinity. Our NMR data, combined with precipitation assays, show that there are additional SNARE complex/synaptotagmin-1 interactions that lead to aggregation and that involve in part two arginines at the bottom of the C2B domain. Overall, this study shows the importance of disentangling the contributions of different types of interactions to SNARE complex/synaptotagmin-1 binding, and illustrate the usefulness of one-dimensional NMR methods to analyze intricate protein interactions.
Introduction
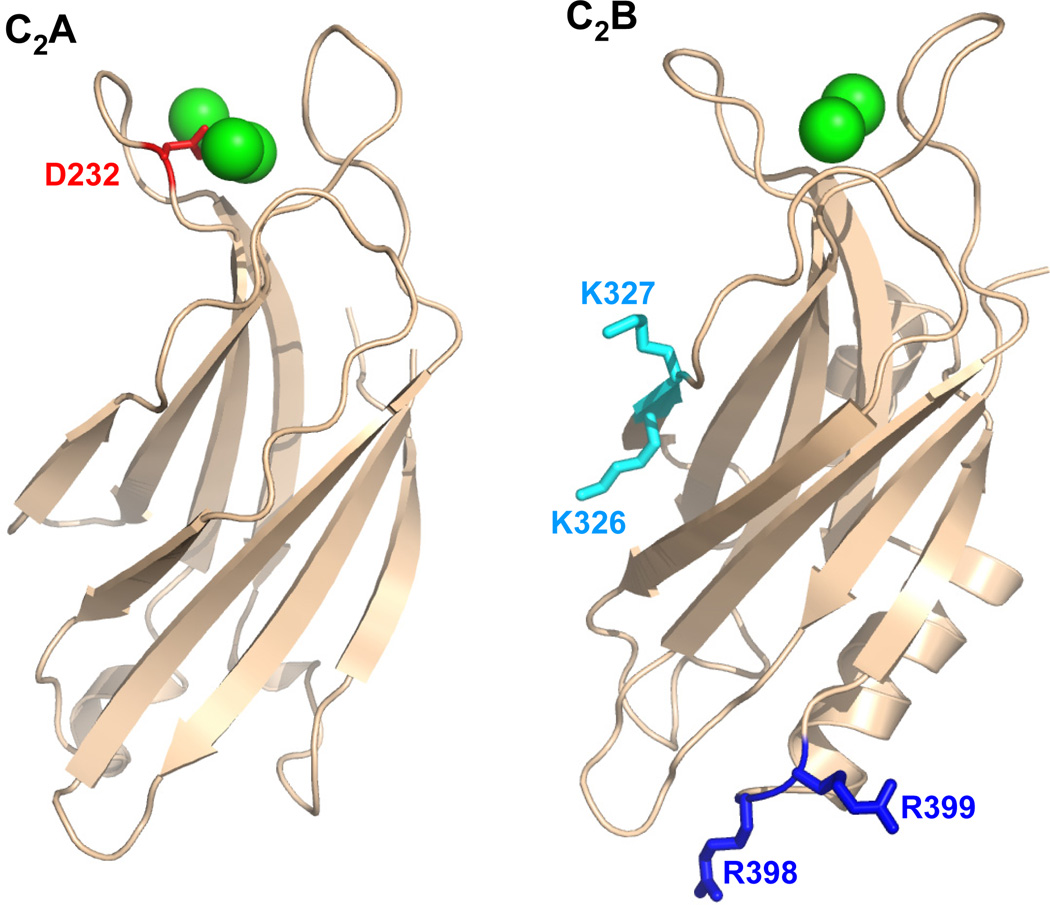
The release of neurotransmitters by Ca2+-evoked synaptic vesicle exocytosis is an exquisitely regulated process that is key for communication between neurons. Crucial components of the complex protein machinery that controls release are the synaptic vesicle protein synaptotagmin-1 and the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE)1 proteins synaptobrevin, syntaxin-1 and SNAP-25 [reviewed in (1–4)], which mediate Ca2+-triggered membrane fusion in a tight interplay with other key proteins (5). The three SNAREs form a four-helix bundle called the SNARE complex through their SNARE motifs (two from SNAP-25 and one each from synaptobrevin and syntaxin-1) (6;7). Assembly of the SNARE complex brings the synaptic vesicle and plasma membranes together (8), which is critical for membrane fusion. Synaptotagmin-1 contains two C2 domains (the C2A and C2B domains) that form most of its cytoplasmic region and adopt β-sandwich structures, binding three or two Ca2+ ions, respectively, through loops at the top of the sandwich (9–12) (Figure 1). These top loops also mediate Ca2+-dependent binding of synaptotagmin-1 to phospholipids (12–14). Point mutations that impair or enhance this activity lead to parallel effects on the efficiency of neurotransmitter release (15;16), demonstrating that synaptotagmin-1 is the major Ca2+ sensor for fast neurotransmitter release and showing the functional importance of Ca2+-dependent phospholipid binding to synaptotagmin-1.
Figure 1.
Ribbon diagrams of the synaptotagmin-1 C2A and C2B domains. Ca2+ ions are shown as green spheres. The side chains that were mutated are represented by stick models and labeled.
Ca2+ binding to the C2B domain is particularly crucial for synaptotagmin-1 function (17– 20), which may arise in part from its contribution to Ca2+-dependent phospholipid binding (21) and/or from its ability to bind simultaneously to two apposed membranes through the top loops and through two arginine side chains (R398 and R399) at the bottom of the domain (22) (Figure 1). Indeed, mutation of these two arginines almost completely abolishes neurotransmitter release and strongly impairs the ability of synaptotagmin-1 to cluster liposomes as well as to stimulate SNARE-dependent lipid mixing between liposomes (23). In addition, the C2B domain contains a polybasic region on the side of the β-sandwich (Figure 1) that was also implicated in binding to negatively charged phospholipids, including phosphoinositides (24), and mutations in this region also cause considerable (albeit more moderate) impairments in neurotransmitter release (25;26).
Synaptotagmin-1 function is widely believed to also depend on interactions with the SNAREs, which could provide a natural means to couple Ca2+ sensing to membrane fusion. However, it has been difficult to pinpoint how synaptotagmin-1/SNARE interactions mediate this coupling, in part because multiple types of such interactions were described. Initial work reported interactions between synaptotagmin-1 and syntaxin-1 that were Ca2+-dependent or Ca2+-independent, involved the C2A domain, the C2B domain, or both, and were ascribed to the syntaxin-1 SNARE motif or its N-terminal Habc domain (27–33). Later work also revealed binding of synaptotagmin-1 to SNAP-25, to syntaxin-1/SNAP-25 heterodimers and to the SNARE complex that again was Ca2+-dependent or Ca2+-independent, and ascribed preponderant roles to the synaptotagmin-1 C2A domain, the C2B domain or both, depending on the study (34–42). Several of the interactions that have been described probably arose from the promiscuity of these proteins (43) and hence may not physiologically relevant. However, interactions involving the SNARE complex are generally believed to play a role in neurotransmitter release because synaptotagmin-1 action occurs in the late steps of Ca2+-evoked exocytosis, when the SNARE complex is expected to be at least partially assembled. In fact, these interactions are likely key to couple Ca2+ sensing to membrane fusion but, unfortunately, they are still poorly understood. Thus, SNARE complex/synaptotagmin-1 binding was proposed to involve an acidic region in the middle of the SNAP-25 N-terminal SNARE motif (44) or an acidic region at the C-terminal half of the SNAP-25 C-terminal SNARE motif (36;41). Moreover, while some data suggest that binding involves the polybasic region of the C2B domain but not the two arginines at the bottom (23;41;44), other results suggested that both regions are involved (45), and a model built from single-molecule fluorescence spectroscopy data places the SNARE complex closest to the helix at the bottom of the C2B domain (46).
Some of the discrepancies between different studies might be due to the highly charged nature of synaptotagmin-1 and the SNARE complex, which may underlie binding in different modes, but inconsistencies may have also arisen from the frequent use of biochemical methods that, while powerful for initial exploratory studies, are prone to artifacts and/or are not adequate to obtain reliable quantitative data [see (47)]. In order to understand how SNARE complex/synaptotagmin-1 interactions control neurotransmitter release, it is critical to determine whether one major binding mode exists, what is the stoichiometry of binding, and how much non-specific interactions contribute to overall binding. For this purpose, it is necessary to use quantitative analytical methods that can define the relative contributions of different binding modes, as well as the effects of point mutations on binding. Unfortunately, these studies are hindered by the high tendency of SNARE complex/synaptotagmin-1 assemblies to precipitate in the presence of Ca2+ even at protein concentrations on the 10 µM range [(41); see also below].
In the research described here, we have explored the use of one-dimensional (1D) 13C-edited 1H-NMR spectroscopy (48) to analyze SNARE complex/synaptotagmin-1 interactions in solution quantitatively. Our results show that SNARE complex binding in solution is dominated by the synaptotagmin-1 C2B domain. Moreover, our data indicate that the polybasic region of the C2B domain constitutes the primary binding site for the SNARE complex, whereas the two arginines at the bottom of the C2B domain mediate additional, weaker interactions that lead to aggregation and precipitation of SNARE complex/synaptotagmin-1 assemblies. Overall, this study emphasizes the complexity of analyzing SNARE complex/synaptotagmin-1 interactions and illustrates the usefulness of 1D NMR methods to study protein complexes.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Constructs for expression of rat synaptotagmin-1 C2A domain (residues 140–267), C2B domain (residues 271–421) and C2AB fragment (residues 140– 421), as well as the SNARE motifs of rat synaptobrevin (residues 29–93), rat syntaxin-1A (residues 191–253), and human SNAP-25B (residues 11–82 and 141–203) were previously described (9;22;49;50). Mutations were performed using the QuickChange site-directed mutagenesis kit (Stratagene). All proteins were expressed as GST-fusions in Escherichia coli BL21(DE3) cells. Unlabeled proteins were expressed in LB broth, while uniform 13C- and 15N-labeling was achieved through expression in M9 minimal media with 13C6 -glucose as the sole carbon source and 15NH4Cl as the sole nitrogen source.
SNARE proteins were purified by affinity chromatography, followed by ion exchange and/or gel filtration as described (50). For synaptotagmin-1 fragments, the cell pellet was resuspended in cold Lysis Buffer (40 mM Tris-HCl pH 8.2, 200 mM NaCl, 2 mM DTT) with 1% Triton and protease inhibitors (1 mM each of Sigma Inhibitor cocktail, ABESF, EGTA, and EDTA). The suspension was frozen in liquid nitrogen and thawed at RT. The cells were passed four times through an EmulsiFlex-C5 cell homogenizer (Avestin) at 13,000 psi, spun at 19000 rpm for 30 minutes, and incubated for one hour at RT with 100 mg protamine sulfate (Sigma-Aldrich) per 35 ml supernatant. The mixture was spun again at 19000 rpm for 30 minutes, and the supernatant was passed through a 0.8 µm syringe filter before mixing with prewashed Glutathione Sepharose 4B beads (GE Healthcare) at a ratio of 1 ml of bead slurry per 1 L culture. Incubation was either three hours at RT or overnight at 4°C. The resin was extensively washed with 200 ml each of the following buffers: Lysis Buffer, Lysis Buffer with 50 mM CaCl2, and Lysis Buffer with 50 mM CaCl2 and 1 M NaCl. The resin was then equilibrated with Benzonase buffer (50 mM Tris-HCl, pH 8.0, 2 mM MgCl2, 2 mM DTT) before the addition of 20 ml Benzonase buffer and 5 µl Benzonase Nuclease (Novagen, 25 KUN) and rotation at RT for 3 hours. The Benzonase wash was discarded and the resin was extensively washed with high ionic strength buffer (1 M NaCl in benzonase buffer or TCB – see below). The resin was then equilibrated with Thrombin Cleavage buffer (TCB: 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, 2 mM DTT). Thrombin cleavage was carried out at RT for 3 hrs or at 4°C overnight in 10 ml TCB and 0.08 mg/ml thrombin (Sigma-Aldrich). The cleavage fraction was collected and elution was repeated with TCB until UV Abs280 was < 0.1 to recover the maximal amount of protein from the resin.
For the WT and mutant C2AB fragment, the elution fractions were combined and diluted with buffer A (50 mM NaAc pH 6.2, 5 mM CaCl2) so that [NaCl] ≤ 100 mM. Cation-exchange on a Source S column (GE Life Sciences) was performed in buffer A with a linear gradient from 0.1 to 0.7 M NaCl in 30 column volumes. This was followed by gel filtration on Superdex 75 (25 mM HEPES-NaOH pH 7.4, 125 mM NaCl). For the WT and mutant C2B domain, the elution fractions were concentrated after adding 2.5 mM EDTA, and samples were purified on a Superdex 75 column in gel filtration buffer (0.2 M phosphate, pH 6.3, 0.3 M NaCl). The gel filtration elution was buffer-exchanged using a 10 kDa centrifugal filter unit into 20 mM MES pH 6.3, and was then loaded onto a SourceS column where cation-exchange was carried out in 20 mM MES, pH 6.3, 20 mM CaCl2 using a linear gradient from 0.1 to 0.6 M NaCl in 40 column volumes. At least two distinct peaks normally emerged, both corresponding to protein of the correct molecular weight. Only the later fractions contain C2B domain devoid of acidic contaminants. These fractions were collected and buffer exchanged to the same final buffer into 25 mM HEPES-NaOH pH 7.4, 125 mM NaCl. For both the C2AB fragment and the C2B domain, 0.3 mM TCEP and 1 mM ABESF were added to the final purified proteins, but EDTA and EDTA-containing inhibitor cocktails were avoided.
Proper folding of the synaptotagmin-1 C2AB fragment mutants was confirmed through their 1H-15N TROSY-HSQC spectra. The SNARE motifs of synaptobrevin, syntaxin-1 and SNAP-25 were mixed in equimolar ratio and incubated overnight at 4°C to assemble the SNARE complex. Isolated SNARE motifs that did not incorporate into SNARE complexes were removed by extensive concentration-dilution in a 10 kDa Amicon centrifugal filter (50). The purity of the final SDS-resistant complex was verified SDS-PAGE and Coomassie blue staining.
NMR Spectroscopy
All NMR spectra were acquired at 25°C on Varian INOVA 500MHz or 600MHz spectrometers. Samples were prepared in 25 mM HEPES (pH 7.4) and 125 mM NaCl with 5% D2O. Standard conditions for titrations assays included 1 mM Ca2+ unless otherwise indicated. For Ca2+-free samples, 1 mM EDTA was added. 1D 13C-edited 1H-NMR spectra were obtained by acquiring the first trace of a 1H-13C heteronuclear single quantum coherence spectrum as previously described (48). Total acquisition times were 20–40 min for spectra acquired on cold probes and 1 hr for spectra acquired on room temperature probes. Spectra were analyzed with the VnmrJ software (Agilent Technologies Inc., Santa Clara, CA).
Titrations with SNARE complex
Samples contained a constant amount of uniformly 15N,13C-labeled C2AB fragment (2.4–3 µM) and the indicated concentrations of unlabeled SNARE complex. A new sample was prepared for each titration point, rather than adding SNARE complex to the same sample. For each point of the titration, we subtracted a 1D 13C-edited 1H-NMR spectrum of a sample containing 20 µM unlabeled SNARE complex scaled according to the concentration of SNARE complex corresponding to that point of the titration in order to account for the 1% natural abundance of 13C isotope. Assuming a 1:1 equilibrium-binding model where the C2AB fragment is considered as the protein and the SNARE complex as the ligand, the strongest methyl resonance (SMR) intensity resulting after the subtraction (I) can be expressed as a function of LT, the total SNARE complex concentration added by equation (1):
| (1) |
where If represents the SMR intensity of the free 15N,13C-labeled C2AB fragment, Ib is the SMR intensity of the 15N,13C-labeled C2AB fragment bound to the SNARE complex, PT is the total concentration of 15N,13C-labeled C2AB fragment, and Kd is the dissociation constant. The experimental data were fit to this equation using Sigma Plot (Systat Software Inc.) to derive the If, Ib and Kd parameters. After an initial fit, the calculated value of If was then used to normalize all the intensities, which allows comparison between data sets obtained at different times and different instruments. Hence, the value of If after the normalization is 1 and the Ib values are expressed as a fraction of If . The values of Ib and Kd described below and their errors were obtained by fitting separate data sets and then calculating the average and standard deviations of the Ib and Kd values obtained (2 to 4 data sets for each condition).
Synaptotagmin-1 fragment/SNARE complex precipitation assays
Samples containing 10 µM WT or mutant synaptotagmin-1 C2AB fragment or C2B domain were mixed with 10 or 20 µM SNARE complex under the same conditions as the NMR experiments (25 mM HEPES-NaOH, 125 mM NaCl, 1 mM Ca2+, pH 7.4). The total reaction volume was 50 µl. After 5 min incubation at room temperature, the samples were centrifuged at 13,000 rpm for 1.5 minutes in a benchtop centrifuge (Eppendorf AG 5415 D), and the supernatant was separated from the pellet. The pellet was resuspended in 50 µl buffer, and 5 µl of each of the supernatant and pellet fractions were analyzed by SDS PAGE using 15% polyacrylamide gels in Tris-Glycine-SDS running buffer, followed by Coomassie blue staining.
RESULTS
Ca2+enhancement of SNARE complex/synaptotagmin-1 binding
Over the years we have made many attempts to analyze interactions between a synaptotagmin-1 fragment spanning its two C2 domains (residues 140–421; referred to as the C2AB fragment) and a minimal SNARE complex formed by the SNARE motifs of synaptobrevin, syntaxin-1 and SNAP-25 (below referred to as the SNARE complex for simplicity). As mentioned above, these studies were hampered by the high tendency of SNARE complex/synaptotagmin-1 assemblies to aggregate in the presence of Ca2+ [(41); see also below]. Note in this context that the C2AB fragment as well as the individual C2A and C2B domains are highly soluble at physiological conditions in the absence and presence of Ca2+, remaining monomeric even at concentrations close to 1 mM (12;22;31;49;51), and that the SNARE complex that we use lacks a few residues at the C-terminus of syntaxin-1 to enhance its solubility, remaining monomeric at concentrations well above 100 µM (50;52). Thus, the insolubility of SNARE complex/C2AB fragment assemblies likely arises from charge neutralization, as the SNARE complex is highly acidic and the C2AB fragment contains abundant basic regions (particularly the C2B domain). During our studies we found that precipitation in the presence of 1 mM Ca2+ at physiological pH and ionic strength was largely avoided if the concentration of either the C2AB fragment or the SNARE complex was kept at 3 µM or below. Because of this constraint and because of the low enthalpies of SNARE complex/synaptotagmin-1 interactions, we were unable to obtain reliable quantitative data on these interactions by isothermal titration calorimetry (ITC). Thus, we explored the use of a method that relies on 1D 13C-edited 1H-NMR spectroscopy and that we developed as an alternative to ITC to study protein interactions quantitatively (48).
The method is based on measuring the intensity of the strongest methyl resonance (SMR) in 1D 13C-edited 1H-NMR spectra of a 13C-labeled protein, and quantifying the decrease in the SMR intensity upon binding to an unlabeled protein or macromolecule due to the resulting increase in the rotational correlation time and the corresponding resonance broadening. This method can be used with high sensitivity at low micromolar protein concentrations (even without a cryo-probe) because the methyl signals are not spread in additional dimensions as is common in multidimensional NMR experiments. As shown below, qualitative analysis of additional methyl resonances that are still observable at these low protein concentrations provides additional information to interpret the binding experiments. Although 15N-labeling is not necessary to acquire 1D 13C-edited 1H-NMR spectra, we used 15N,13C-labeled synaptotagmin-1 fragments in our experiments to allow verification of the purity of the fragments using 1H-15N HSQC spectra [(49), see also below]. Since SNARE complex/synaptotagmin-1 interactions are highly sensitive to the ionic strength (40), all experiments described in this study were performed with a constant ionic strength that resembles physiological conditions.
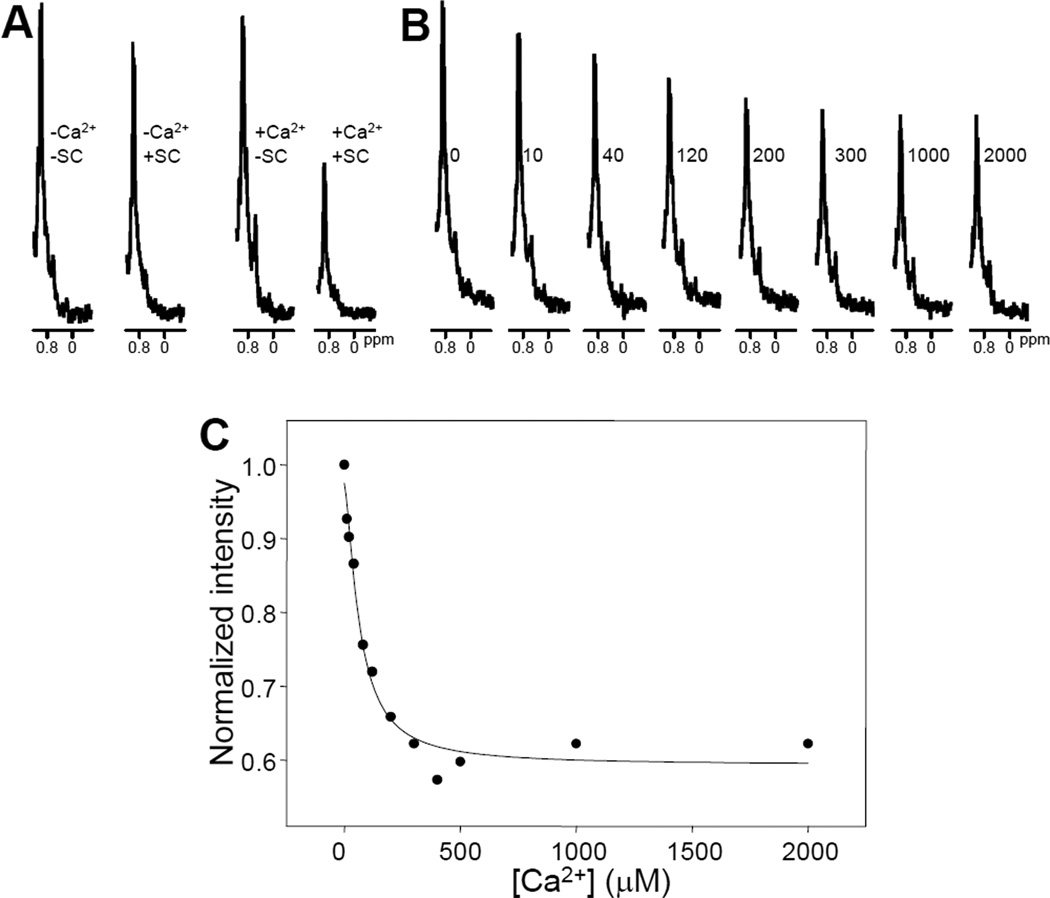
We first acquired 1D 13C-edited 1H-NMR spectra of 3 µM 15N,13C-labeled C2AB fragment in the absence and presence of a small excess (3.5 µM) of unlabeled SNARE complex. We observed that the SNARE complex induced a small decrease in SMR intensity when 1 mM EDTA was present (Figure 2A), indicating weak binding. However, a much stronger decrease in SMR intensity was observed in parallel experiments performed in the presence of 1 mM Ca2+ (Figure 2A). Since Ca2+ itself did not significantly affect the SMR intensity of the C2AB fragment, these results showed that, as expected, Ca2+ strongly enhances binding of the C2AB fragment to the SNARE complex. Ca2+ titrations in the presence of 3.5 µM SNARE complex revealed a progressive decrease in SMR intensity that saturates at about 300 µM Ca2+ (e.g. Figures 2B,C). Fitting of three independent experiments to a Hill equation yielded an average value of 58 ± 8 µM for the microscopic dissociation constant, and an average Hill coefficient of 1.2±0.3 (all errors are given as standard deviations). These results suggest that there is almost no cooperativity among the C2 domain Ca2+-binding sites in enhancing SNARE complex binding, as expected from the absence of cooperativity in intrinsic Ca2+ binding to the five sites of the synaptotagmin-1 C2 domains (10;12) if the SNARE complex does not contribute directly to coordinate the Ca2+ ions. However, the observed microscopic dissociation constant is considerably lower that the intrinsic dissociation constants of the individual Ca2+ binding sites of the C2B domain [300–600 µM; see (12)]. Because SNARE complex binding involves primarily the C2B domain (see below), these observations suggest that there is some cooperativity between Ca2+ binding and SNARE complex binding to the C2AB fragment. Such cooperativity may arise from long-range electrostatic interactions, as the SNARE complex is strongly negatively charged (7) and Ca2+-binding increases the positive charge of the C2 domains (12;31).
Figure 2.
A. Expansions of the region containing the SMR of 1D 13C-edited 1H-NMR spectra of 3 µM 15N,13C-labeled C2AB fragment acquired in the presence of 1 mM EDTA (-Ca2+) or 1 mM Ca2+ (+Ca2+), and in the absence (−SC) or presence (+SC) of 3.5 µM SNARE complex. B. analogous expansions of 1D 13C-edited 1H-NMR spectra of 3 µM 15N,13C-labeled C2AB fragment in the presence of 3.5 µM SNARE complex and the indicated concentrations of Ca2+ in µM units. C. Plot of the normalized SMR intensities observed in 1D 13C-edited 1H-NMR spectra of 3 µM 15N,13C-labeled C2AB fragment in the presence of 3.5 µM SNARE complex and variable concentrations of Ca2+. A subset of the data corresponding to this titration is shown in panel B. The curve shows the fit to a Hill equation.
Mutational analysis of SNARE complex/synaptotagmin-1 C2AB fragment interactions
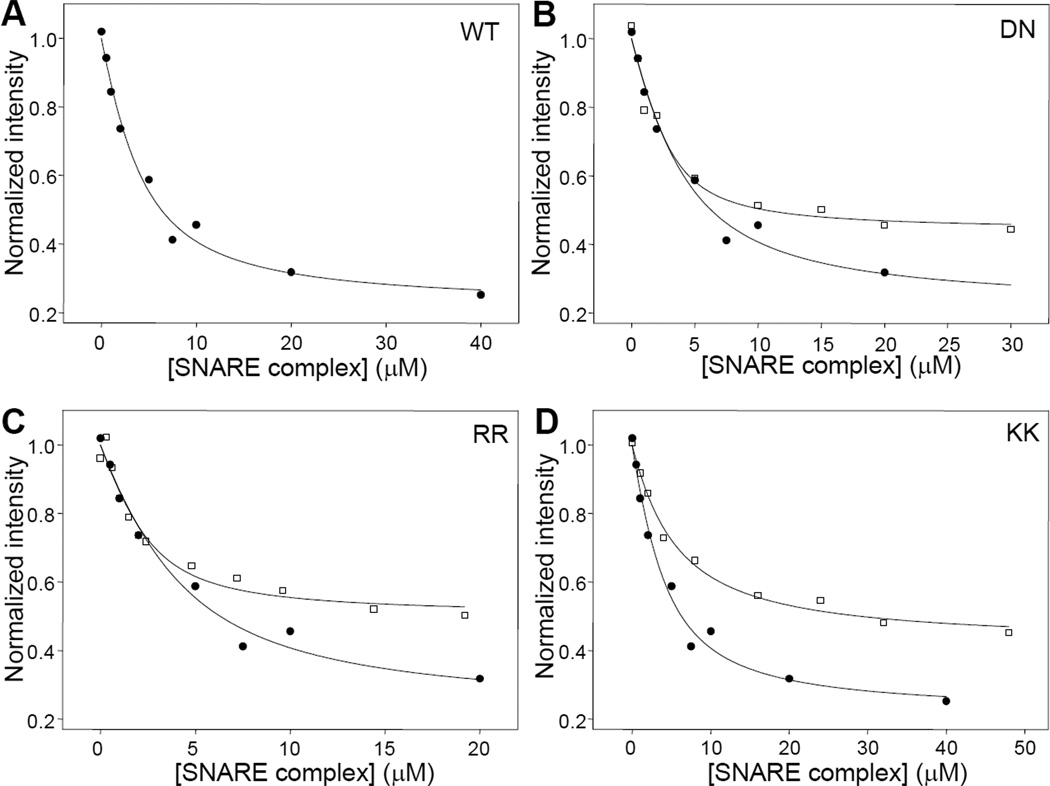
To study the affinity of the C2AB fragment for the SNARE complex, we performed titrations of 15N, 13Clabeled C2AB fragment with increasing amounts of unlabeled SNARE complex monitored with 1D 13C-edited 1H-NMR spectra. In the presence of 1 mM EDTA, we observed only modest decreases in the SMR intensity that were far from saturation at 40 µM SNARE complex and hence did not allow reliable measurement of the dissociation constant. However, titrations of 15N,13C-labeled C2AB fragment with unlabeled SNARE complex in the presence of 1 mM Ca2+ led to much stronger decreases in SMR intensity that appeared to be saturable at least to some degree (e.g. Figure 3A). To make the results obtained with different samples on different days or instruments comparable, all the data were normalized to the intensity at zero SNARE complex concentration (If). For this purpose, we first fitted each data set with the absolute intensities measured and obtained an intensity at zero SNARE complex concentration that was then used to normalize the data set. Curve fitting of multiple titrations assuming a standard protein to ligand binding model with a 1:1 stoichiometry (Equation 1) yielded an apparent Kd of 2.32 ± 0.15 µM (Table 1).
Figure 3.
Titrations of WT and mutant 15N,13C-labeled C2AB fragments with SNARE complex monitored by 1D 13C-edited 1H-NMR spectra. A. Plot of the SMR intensities observed in 1D 13C-edited 1H-NMR spectra of 3 µM WT C2AB fragment as a function of unlabeled SNARE complex concentration in the presence of 1 mM Ca2+. B-D. Plots for analogous titrations performed with DN (B), RR (C) and KK (D) mutant C2AB fragments (open squares), superimposed with a plot obtained for the WT C2AB fragment (closed circles; the same shown in A). The curves show the fits of the data obtained with equation (1).
Table 1.
Summary of apparent Kd and Ib values obtained from fitting the SNARE complex/synaptotagmin-1 fragment binding curves.
| C2AB WT | C2AB DN | C2AB RR | C2AB KK | |
|---|---|---|---|---|
| Kd (µM) | 2.32 ± 0.15 | 0.73 ± 0.33 | 0.94 ± 0.01 | 3.74 ± 1.29 |
| Ib | 0.29 ± 0.07 | 0.44 ± 0.01 | 0.48 ± 0.03 | 0.34 ± 0.07 |
| C2B WT | C2B RR | C2B KK | ||
| Kd (µM) | 0.80 ± 0.39 | 0.40 ± 0.30 | 3.78 ± 1.99 | |
| Ib | 0.17 ± 0.05 | 0.51 ± 0.04 | −0.03 ± 0.09 | |
We next investigated how SNARE complex/C2AB fragment binding as reported by 1D 13C-edited 1H-NMR spectra is affected by mutations that had previously been described to impair binding based on other analytical methods, in some cases with contradictory results. These mutations included: i) a D232N substitution in the C2A domain (referred to as DN mutation) that abolishes Ca2+ binding to two of its three Ca2+-binding sites (10) and was described to enhance neurotransmitter release as well as SNARE complex binding (53); ii) an R398Q,R399Q mutation at the bottom of the C2B domain (referred to as RR mutation) that abolishes neurotransmitter release (23) and was reported to impair SNARE binding in one study (45) but not another (23); and iii) a Lys326A,Lys327A mutation in the polybasic region of the C2B domain (referred to as KK mutation) that impairs neurotransmitter release (25;26) and was found to decrease binding to the SNARE complex (41;44) and to inositide polyphosphates (24;26).
Titrations of the 15N,13C-labeled C2AB fragment mutants with unlabeled SNARE complex (e.g. Figures 3B–D) yielded the following apparent Kd values: 0.73 ± 0.33 µM for the DN mutant, 0.94 ± 0.01 µM for the RR mutant, and 3.74 ± 1.29 µM for the KK mutant (Table 1). In principle, these results might suggest that the DN and RR mutations both increase the affinity of the C2AB fragment for the SNARE complex, while the KK mutation decreases binding. However, comparison of the titrations performed with the WT C2AB fragment with those performed with the DN and RR mutants (e.g. Figures 3B,C) showed that the fitted curves were undistinguishable for low SNARE complex concentrations (below 5 µM) and diverged at the higher concentrations. Hence, it became apparent from these comparisons that the differences in Kd values measured for the WT C2AB fragment and these two mutants arise from the differences yielded by the fitting algorithm for Ib, which is the normalized signal intensity extrapolated at infinite SNARE complex concentration. Thus, the Ib values obtained in the titrations were 0.29 ± 0.07 for the WT C2AB fragment, 0.44 ± 0.01 for the DN mutant, and 0.48 ± 0.03 for the RR mutant (Table 1). Assuming a 1:1 binding mode, which underlies equation 1, Ib is not expected to be altered by the mutations because it represents the normalized signal intensity corresponding to C2AB fragment fully bound to the SNARE complex. These observations suggest that the intensity values observed during the titrations at low SNARE complex concentrations for the WT C2AB fragment, as well as for the DN and RR mutants, reflect a primary, high affinity-binding mode that is not significantly affected by the DN and RR mutations. In addition, there appears to be one (or multiple) additional binding mode that is populated at higher SNARE complex concentrations and is impaired by the DN and RR mutations.
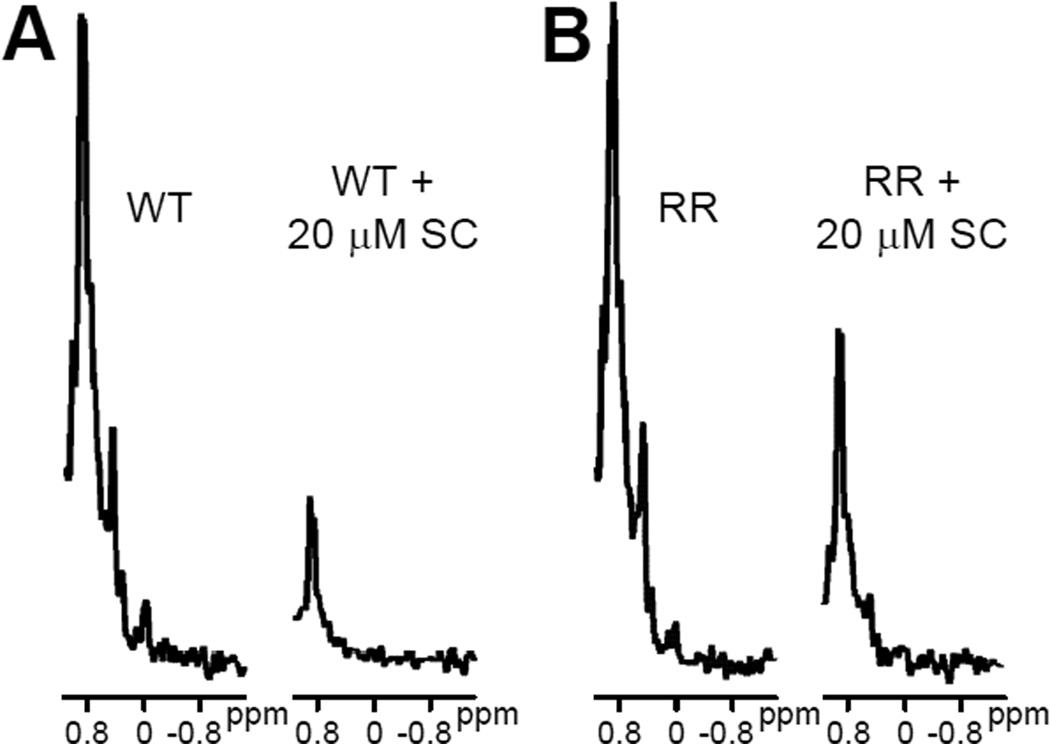
Further insights into this issue were obtained from comparison of the methyl region from 1D 13C-edited 1H-NMR spectra acquired without SNARE complex and with high SNARE complex concentrations (e.g. those in Figure 4). The SMR of the C2AB fragment includes a group of overlapped resonances that is centered around ca. 0.87 ppm, and we estimate that the overall intensity of the SMR depends on the individual intensities of resonances with maxima ranging from 0.81 to 0.93 ppm. Based on the assignments obtained for the synaptotagmin-1 C2A and C2B domains (12;54), this region includes approximately 48 methyl resonances. Among these resonances, 7 of them (15%) correspond to methyl groups that have high mobility because they are in flexible regions. Because the molecular weights of the C2AB fragment and the SNARE complex are 35 and 32 kDa, respectively, and because the SNARE complex is very elongated, formation of a 1:1 macromolecular assembly between them is expected to yield considerable broadening of the resonances from methyl groups in structured regions of the C2AB fragment, while resonances from methyl groups that remain highly mobile upon binding should be much less affected. Nevertheless, based on our experience in NMR analyses of the SNARE complex [e.g. (41;50;52)], resonances containing contributions from multiple methyl groups in structured regions (e.g. between 0.4 and 0.6 ppm) should still be detectable upon binding of 15N,13C-C2AB fragment to the SNARE complex with 1:1 stoichiometry. This is indeed what we observed for the C2AB fragment RR mutant at saturating SNARE complex concentrations (Figure 4B), suggesting that the results obtained for this mutant reflect 1:1 binding to the SNARE complex. However, only a sharp signal is observed at the position of the SMR for the WT C2AB fragment (3 µM) in the presence of 20 µM SNARE complex, with no detectable resonances at lower chemical shifts (Figure 4B).
Figure 4.
Expansions showing the methyl region of 1D 13C-edited 1H-NMR spectra of 3 µM WT (A) or RR mutant (B) C2AB fragment in 1 mM Ca2+ and in the absence or presence of 20 µM SNARE complex (SC).
These observations indicate that the additional binding mode(s) occurring for the WT C2AB at high SNARE complex concentrations involve the formation of large complexes and that the residual signal observed at the SMR position under these conditions corresponds to highly mobile methyl groups that are observable regardless of the molecular weight. This interpretation is further supported by the tendency of the WT C2AB fragment to precipitate with the SNARE complex when both are at concentrations in the 10 µM range (see below). Hence, the apparent Kd measured in the titrations performed with the WT C2AB fragment should not be considered reliable, and the corresponding Ib value does not correspond to 1:1 binding stoichiometry. Because the RR mutation disrupts, at least in part, formation of the larger complexes, the Kd measured for the RR mutant C2AB fragment (0.94 µM) can be considered a better estimate of the dissociation constant for the primary SNARE complex/C2AB fragment binding site, and 1:1 binding likely leads to an Ib value equal or larger to that measured for this mutant (0.48). Similar conclusions can be drawn for the DN mutant, although it appears that the DN mutation is less efficient at disrupting formation of large complexes than the RR mutation, as seen in the precipitation assays described below.
The Ib value obtained for the titrations performed with the KK mutant (0.34 ± 0.07, Table 1) was not significantly different from that obtained for the WT C2AB fragment (0.29 ± 0.07), although we cannot rule out some perturbation of the secondary binding mode(s) by the KK mutation. Hence, the increased Kd measured for the KK mutant (3.74 ± 1.29 µM) cannot arise from differences in Ib values. Note also that the titrations performed with the KK mutant exhibited a clear divergence from those performed with the WT C2AB fragment even at low SNARE concentrations (e.g. Figure 3D). Hence, these data show that the KK mutation impairs the primary binding mode between the C2AB fragment and the SNARE complex. Nevertheless, it is clear that the complications in data analysis caused by the secondary binding mode(s) hinder the measurement of reliable Kds and the quantification of the effects of mutations on the primary binding mode.
Contributions of the two synaptotagmin-1 C2 domains to SNARE complex binding
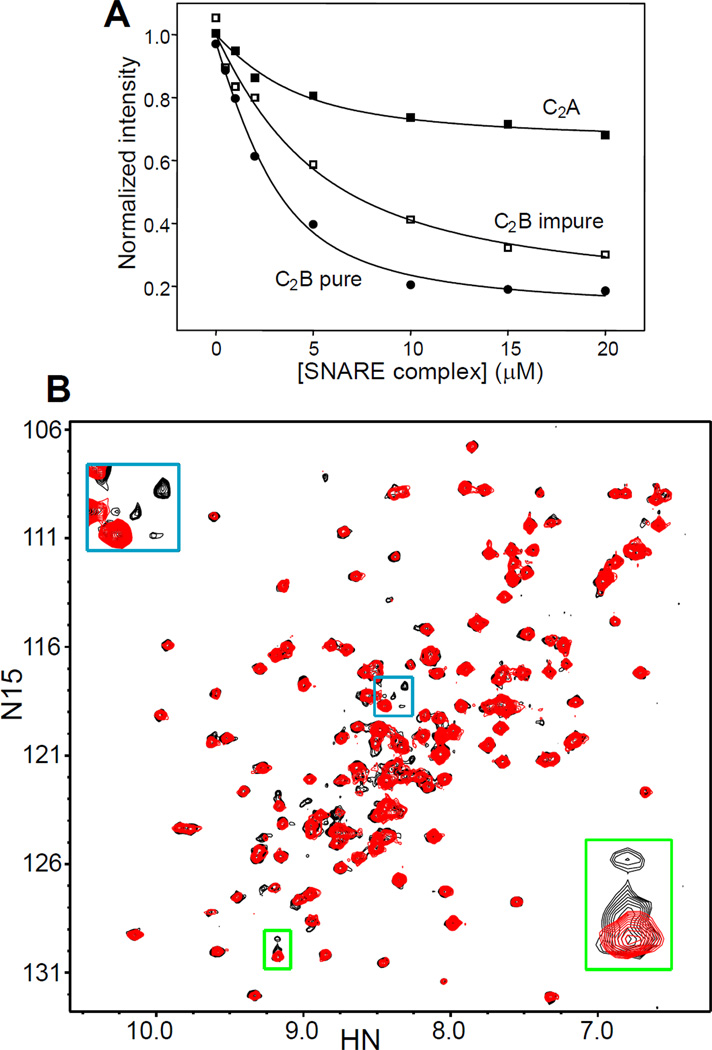
Our analyses with the C2AB fragment suggest that its primary binding site for the SNARE complex is located at the polybasic region of the C2B domain. To further test this conclusion and to dissect the contributions of the two synaptotagmin-1 C2 domains to SNARE complex, we performed titatrions of the isolated 15N,13C-labeled C2A domain and C2B domain with SNARE complex (Figure 5A). During these experiments it became apparent the importance of ensuring the purity of the isolated C2B domain, which has a particularly high tendency to bind polyacidic compounds via the same polybasic region that binds to the SNARE complex (49). For optimal purification, we have modified slightly the protocol that we published previously (49), including a treatment with Benzonase nuclease (see Experimental Procedures). The purity of 15N,13C-C2B domain samples obtained by this method was confirmed by the observation of a single set of cross-peaks in their 1H-15N HSQC spectra (e.g. Figure 5B, red contours), which constitutes the best method we found to ensure sample purity. Thus, a 15N,13C-C2B domain sample that was subjected to our purification protocol but omitting the final cation exchange column still exhibited multiplicity for some of the cross-peaks from residues near the polybasic region (Figure 5B, black contours), despite not having the UV maximum at 260 nm characteristic of nucleic acids. This cross-peak multiplicity shows that some contaminants remain bound to the polybasic region (49). Indeed, titatrions of this impure 15N,13C-C2B domain with SNARE complex monitored by 1D 13C-edited 1H-NMR spectra revealed a clearly weaker affinity (apparent Kd = 3.9 ± 1.0 µM) than analogous titrations with pure 15N,13C-C2B domain (apparent Kd = 0.80 ± 0.39 µM; Table 1) (see titration examples in Figure 5A).
Figure 5.
Analysis of the relative contributions of the synaptotagmin-1 C2 domains to SNARE complex binding. A. Plots of the SMR intensities observed in 1D 13C-edited 1H-NMR spectra of 3 µM synaptotagmin-1 C2A domain (solid squares), pure C2B domain (solid cirlces) and impure C2B domain (open squares) as a function of unlabeled SNARE complex concentration in the presence of 1 mM Ca2+. B. 1H-15N HSQC spectra of synaptotagmin-1 C2B domain that was fully purified (red contours) or that was purified by our standard protocol but omitting the final cation exchange chromatography (black contours). The insets show expansions of regions containing cross-peaks that are unique for the purified C2B domain but exhibit multiplicity in the impure C2B domain [see ref. (49)].
Titrations of 15N,13C-labeled C2A domain with SNARE complex revealed a gradual decrease in SMR intensity that appeared to be saturable (Figure 5A), and fitting of the data suggested an apparent Kd of ca. 2 µM. However, because the decreases in SMR intensity were rather small, it is unclear whether saturation was indeed reached and hence whether this Kd value is reliable. The small decreases in SMR intensity suggest the existence of a loose binding mode whereby a small basic patch of the C2A domain [perhaps in a Ca2+-binding loop; see (42)] contacts an acidic region or regions of the SNARE complex, resulting in only limited immobilization of the C2A domain. In contrast, the much stronger decrease in SMR intensity observed for the C2B domain (Figure 5A) suggests the formation of a bona fide macromolecular assembly with the SNARE complex with a more extensive binding surface and considerable immobilization of the C2B domain. This conclusion agrees with extensive evidence supporting the notion that synaptotagmin-1 binds to the SNARE complex primarily through the C2B domain (38;39;41;46).
The KK mutation impairs the primary binding mode and the RR mutation impairs aggregation of SNARE complex/synaptotagmin-1 assemblies
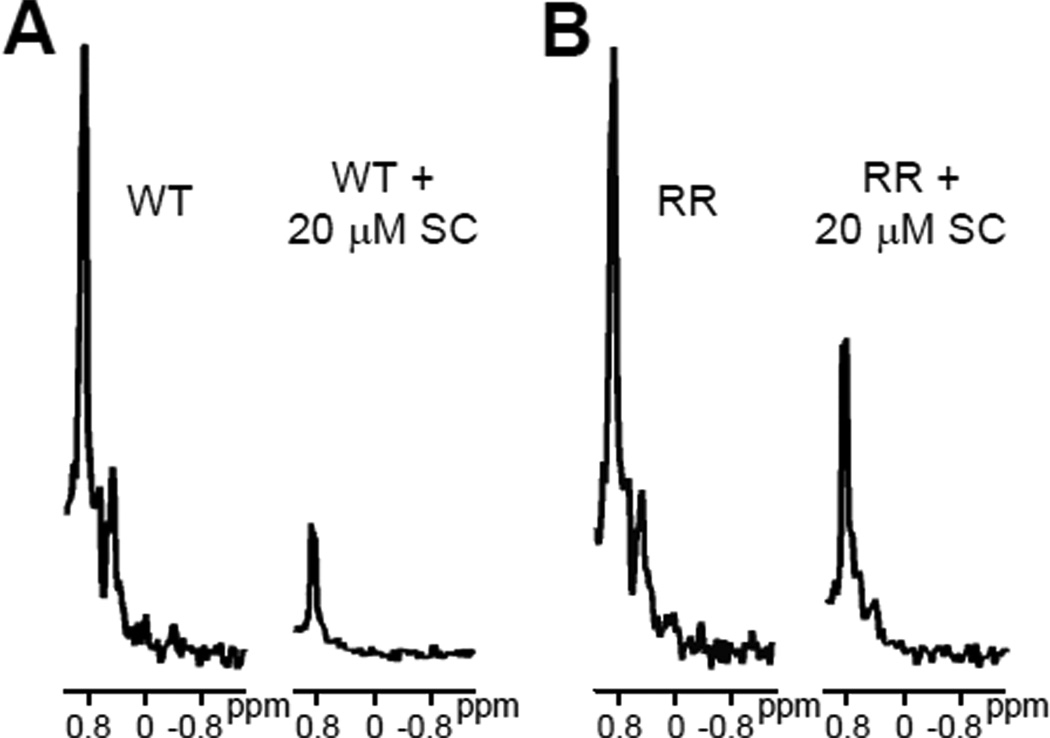
To investigate which region(s) of the C2B domain mediates binding to the SNARE complex, we performed titrations of 15N,13C-labeled RR and KK C2B domain mutants with the SNARE complex monitored by 1D 13C-edited 1H-NMR spectra (Figure 6). Interestingly, the results were similar to those obtained in the titrations of the same mutants of the C2AB fragment. Thus, in the titration performed with the C2B domain RR mutant we obtained an apparent Kd (0.40 ± 0.30 µM; Table 1) that was lower than that derived for the WT C2B domain, although we note that there is a considerable uncertainty in the mutant Kd because of intrinsic limitation of measuring Kd values below 1 µM by this method with the protein concentrations used. Nevertheless, the similarity with the results obtained for the C2AB fragment is also manifested by the observation that the titration of the C2B domain RR mutant saturated at substantially higher Ib value (0.51 ± 0.04; Table 1) than that observed for the WT C2B domain (0.17 ± 0.05). Moreover, comparison of the 1D 13C-edited 1H-NMR spectra acquired in the presence of 20 µM SNARE complex again showed that the resonances from methyl groups in structured regions (e.g. between 0.4 and 0.6 ppm) remained observable for the C2B domain RR mutant but not for the WT C2B domain (Figure 7). Hence, these results suggest that the higher SNARE complex concentrations lead to formation of large complexes for the WT C2B domain but such oligomerization is hindered by the RR mutation, thus leading to a 1:1 SNARE complex/C2B domain assembly.
Figure 6.
Titrations of WT and mutant 15N,13C-labeled C2B domain with SNARE complex monitored by 1D 13C-edited 1H-NMR spectra. A–B. Plots of the SMR intensities observed in 1D 13C-edited 1H-NMR spectra of 3 µM 15N,13C-labeled RR (A) or KK (B) mutant C2B domain as a function of unlabeled SNARE complex concentration in the presence of 1 mM Ca2+ (open squares), superimposed with a plot obtained for the WT C2B domain (closed circles). The curves show the fits of the data obtained with equation (1).
Figure 7.
Expansions showing the methyl region of 1D 13C-edited 1H-NMR spectra of 3 µM WT (A) or RR mutant (B) C2B domain in 1 mM Ca2+ and in the absence or presence of 20 µM SNARE complex (SC).
The results obtained with the C2B domain KK mutant also resembled those obtained with the C2AB fragment. Thus, the KK mutation in the C2B domain again increased the apparent Kd measured (3.78 ± 1.99 µM) with respect to the WT C2B domain Kd, without raising the Ib value. In fact, the calculated Ib for the C2B domain KK mutant was close to 0 (−0.03 ± 0.09; Table 1), which can be attributed to the uncertainty in this value arising when saturation is not reached at the highest SNARE complex concentrations. Overall, these results show that the KK mutation in the polybasic region impairs the major SNARE complex binding mode while having much less effect, if any, on the oligomerization.
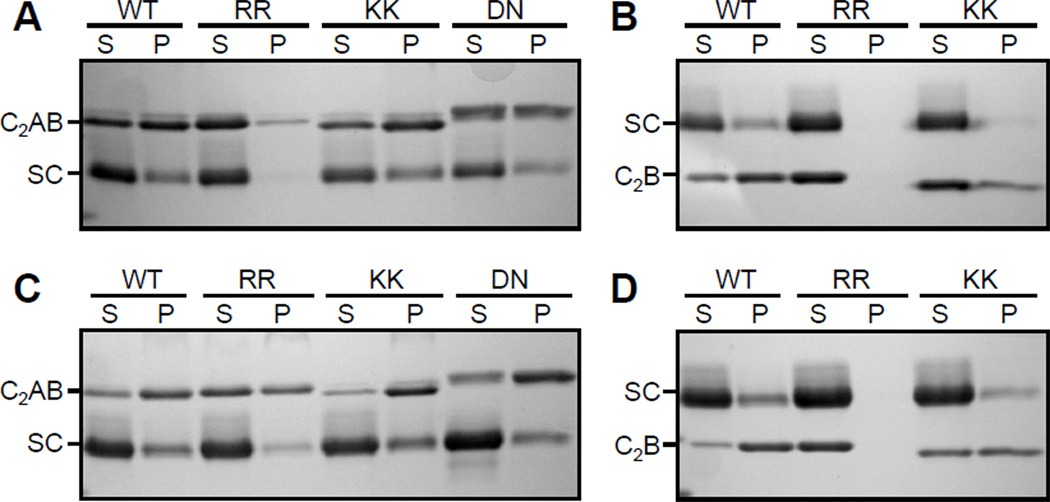
To test the conclusions emerging from the 1D NMR studies by another method, we performed precipitation assays with synaptotagmin-1 fragments and SNARE complex (Figure 8). For this purpose, we incubated 10 µM WT or mutant C2AB fragment with 10 or 20 µM SNARE complex, separated the precipitate from the soluble fraction by centrifugation, and analyzed the samples by SDS PAGE. The results showed that, under both conditions, a substantial fraction of the C2AB fragment precipitated and the RR mutation clearly decreased the precipitation (Figures 8A,C). The DN mutation appeared to somewhat decrease precipitation of the C2AB fragment with the SNARE complex, but to a much lesser extent than the RR mutation. In contrast, the KK mutation did not impair precipitation. Similar results were obtained in precipitation assays with the WT or mutant C2B domain and the SNARE complex, and in this case the inhibition of the precipitation by the RR mutation was even more dramatic (Figures 8B,D; note that the C2B domain runs below the SNARE complex in the gels, in contrast to the C2AB fragment). It is worth noting that the isolated C2AB fragment, the isolated C2B domain and the isolated SNARE complex are highly soluble and yield excellent NMR data at concentrations much higher than those used in these experiments (12;22;49;50;52). Hence, the precipitation results arise from the tendency of the synaptotagmin-1 fragments to aggregate with the SNARE complex.
Figure 8.
Analysis of the solubility of WT and mutant synaptotagmin-1 fragments in the presence of SNARE complex. Samples containing 10 µM C2AB fragment (A,C) or 10 µM C2B domain (B,D) were incubated with 10 µM (A,B) or 20 µM (C,D) SNARE complex for 5 min in the presence of 1 mM Ca2+. The soluble fractions (S) and the precipitates (P) were separated by centrifugation and analyzed by SDS PAGE followed by Coomassie blue staining. The positions of the C2AB fragment (C2AB), C2B domain (C2B) and SNARE complex (SC) are indicated. Note that the C2AB fragment runs above the SNARE complex (panels A,C) but the C2B domain runs below the SNARE complex (panels B,D) because of its smaller size. Note also that the KK and DN mutant C2AB fragments run slightly differently than the WT C2AB fragment, which most likely arises because the mutations change charged residues.
DISCUSSION
Synaptotagmin-1/SNARE interactions are widely believed to be critical for coupling Ca2+ sensing to membrane fusion during neurotransmitter release. Despite dozens of papers describing such interactions, it has been difficult to characterize them with quantitative biophysical methods and to define the binding sites involved. Because synaptotagmin-1 acts at the final, Ca2+-triggering step of release, its interactions with the SNARE complex are likely to be particularly important. Here we have used 1D 13C-edited 1H-NMR spectra to shed light into the nature of SNARE complex/synaptotagmin-1 interactions. Our results underline the difficulties involved in the analysis of such interactions, showing that the tendency of synaptotagmin-1 fragments to aggregate with the SNARE complex can severely hinder interpretation of the results. Moreover, our data reveal that the two arginines at the bottom of the C2B domain contribute to this aggregation tendency and strongly support the notion that the polybasic region of the C2B domain constitutes the primary binding site for the SNARE complex.
The propensity of SNARE complex/synaptotagmin-1 assemblies to aggregate in the presence of Ca2+ has hindered application of the standard two-dimensional (2D) heteronuclear NMR methods that can be used to readily map the binding sites involved in protein complexes (47). Even in the absence of Ca2+, analysis of SNARE complex/C2AB fragment interactions by TROSY-HSQC spectra revealed multiple binding sites (41) that probably reflect the formation of oligomeric assemblies at the protein concentrations used for these experiments (≥ 40 µM). Application of 1D NMR techniques has a clear disadvantage from the point of view of resolution, as it leads to loss of most of the residue specific information on binding. However, 1D NMR spectra exhibit a dramatic gain in sensitivity at regions where multiple resonances overlap, particular at the most intense methyl region that we refer to as SMR. Such gain in sensitivity, combined with the use of 13C-editing to select the 1H signals of only the 13C-labeled protein, allowed us to analyze SNARE complex interactions at concentrations of synaptotagmin-1 fragments in the 3 µM range.
While such concentrations of synaptotagmin-1 fragments prevent precipitation, our titrations monitored by 1D 13C-edited 1H-NMR spectra show that the higher SNARE complex concentrations still lead to the formation of large oligomeric complexes with the WT C2AB fragment or C2B domain, which can naturally be assumed to reflect the same phenomena that lead to precipitation at concentrations of synaptotagmin-1 fragments of 10 µM or higher. Our titrations also show that the RR and KK mutations in the bottom and the polybasic region of the C2B domain, respectively, have different effects on the underlying interactions. The RR mutation hinders formation of large complexes and strongly hinders precipitation, leading to titration curves that most likely correspond to SNARE complex/synaptotagmin-1 fragment binding with 1:1 stoichiometry. Hence, while the titrations performed with the WT synaptotagmin-1 fragments cannot be used to derive dissociation constants readily, those performed with the C2AB and C2B RR mutants provide more reliable data to estimate the affinity involved in the primary binding mode with the SNARE complex. It is still difficult to calculate accurate Kds from these titrations when the interaction under study is relatively tight because of the need to use concentrations of the unlabeled protein in the µM range, but the Kd values calculated from these titrations (0.94 ± 0.01 and 0.40 ± 0.30; Table 1) suggest that the actual dissociation constant is 1 µM or lower.
As concluded for the WT titrations, the Kds measured with the C2AB and C2B KK mutants cannot be considered reliable because the KK mutation does not have a clear effect in preventing aggregation, if any. However, it is clear that the KK mutation impairs the primary binding mode between synaptotagmin-1 and the SNARE complex based on the smaller decreases in SMR intensities observed at low SNARE complex concentrations for both the C2AB fragment (Figure 3D) and for the C2B domain (Figure 6B), compared to the titrations using WT fragments. These results and those obtained with the RR mutation strongly support the notion that the primary binding mode involves the polybasic region of the C2B domain but not the bottom region of the C2B domain. This conclusion agrees with some of the previous studies (23;41;44) but not others (45;46). Thus, while the existence of such a primary binding site as concluded from our data might seem trivial, it was not obvious from the available literature and is very important to understand how the functions of synaptotagmin-1 and SNAREs are coupled. Note also that much of the surface of the SNARE complex is highly negative (7;55) whereas most of the synaptotagmin-1 C2B domain and part of the C2A domain are highly positive upon Ca2+ binding (10;12;41). This observation, together with the high tendency of C2AB fragment/SNARE complexes to aggregate, and reports implicating different regions of both the SNARE complex and synaptotagmin-1 in binding (see Introduction), raised the possibility that the reported interactions might not be specific, or that there might be multiple binding modes with comparable affinities. Such features would strongly hinder the development of well-defined models for synaptotagmin-1/SNARE coupling as well as testing of these models. Our data now show that it is possible to disentangle the primary binding mode between synaptotagmin-1 and the SNARE complex from other interactions that favor oligomerization.
The contributions of the C2A domain to SNARE complex binding are still somewhat unclear. The titrations of the isolated C2A domain with SNARE complex (Figure 5A) indicate that the C2A domain does not have an extensive, intimate interaction with the SNARE complex, in contrast to the C2B domain. However, there appears to be some dynamic interaction that may involve a small surface of the C2A domain. This interaction may be disrupted by the DN mutation in the Ca2+-binding loops of the C2A domain. The DN mutation had a similar effect on the titrations of the C2AB fragment with the SNARE complex as that caused by the RR mutation (Figure 3), but the DN mutation did not impair precipitation with the SNARE complex, at least to the extent observed for the RR mutation (Figure 8). Nevertheless, it is still plausible that interactions of the C2A domain Ca2+-binding loops with the SNARE complex add to those involving the polybasic region and/or the bottom of the C2B domain to provide multivalency and hence favor formation of SNARE complex/C2AB fragment oligomers.
It is important to emphasize that the results presented here were obtained in the absence of membranes and that binding of synaptotagmin-1 to membranes most likely influences its interactions with the SNARE complex (40;41). Interestingly however, the conclusions derived from our data correlate well with results obtained in studies of how the synaptotagmin-1 C2AB fragment competes with complexin-I for binding to membrane-anchored SNARE complex, which showed that the KK mutation in the polybasic region of the C2B domain impairs complexin-I displacement by the C2AB fragment (41). In contrast, the RR mutation did not alter this activity but impaired the ability of the C2AB fragment or the C2B domain to bridge two membranes (22;23). All these results are consistent with a model whereby the polybasic region on the side of the synaptotagmin-1 C2B domain binds to the SNARE complex and this interaction places synaptotagmin-1 in an orientation that naturally allows binding of the Ca2+-binding loops at the top of both C2 domains to one membrane and the arginines at the bottom of the C2B domain to another, closely apposed membrane (41).
This model provides a clear explanation for the dramatic impairment of neurotransmitter release caused by the RR mutation and for the function of synaptotagmin-1 in cooperating with the SNAREs in inducing fast membrane fusion (22;23). Hence, it seems likely that membranes are the true physiological targets of the two arginines at the bottom of the C2B domain and of the C2A domain Ca2+-binding region, and that, in the absence of membranes, these positively charged regions are avid for binding to negatively charged surfaces such as those present around much of the SNARE complex. Such avidity could explain the participation of the two arginines and at the bottom of the C2B domain and of the C2A domain Ca2+-binding region in the aggregation of the C2AB fragment with the SNARE complex in solution. Note also that the observation of oligomerization and/or precipitation of protein complexes very often lacks physiological significance and just reflects insolubility. Nevertheless, we cannot rule out the possibility that SNARE complex/synaptotagmin-1 oligomers are functionally important and that disruption of such oligomers underlies the functional effects of the RR mutation. In fact, this alternative possibility is supported by the correlation between the strong effects of the RR mutation on C2AB fragment/SNARE complex aggregation and on neurotransmitter release. Clearly, multiple questions remain about how SNARE complex/synaptotagmin-1 interactions control neurotransmitter release. The results presented here show that disentangling the primary binding mode from additional interactions is crucial to address these questions, and that 1D NMR methods provide a powerful tool for this purpose.
Acknowledgments
We thank Yilun Sun for expert technical assistance and Alpay B. Seven for fruitful discussions.
Abbreviations
- 1D
one dimensional
- 2D
two dimensional
- HSQC
heteronuclear single quantum correlation
- NMR
nuclear magnetic resonance
- RT
room temperature
- SMR
strongest methyl resonance
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- TROSY
transverse relaxation optimized spectroscopy
- WT
wild type
Footnotes
This work was supported by grant I-1304 from the Welch Foundation and grant NS404944 from the National Institutes of Health (to J.R.). K.D.B. was supported in part by training grant T32 GM008297 from the National Institutes of Health.
REFERENCES
- 1.Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q.Rev. Biophys. 2005:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu.Rev. Cell Dev. Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizo J, Sudhof TC. The Membrane Fusion Enigma: SNAREs, Sec1/Munc18 Proteins, and Their Accomplices-Guilty as Charged? Annu.Rev. Cell Dev. Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat.Struct. Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- 7.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 8.Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 9.Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 10.Ubach J, Zhang X, Shao X, Sudhof TC, Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998:17, 3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao X, Fernandez I, Sudhof TC, Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 c(2)b-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 13.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J.Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Rizo J, Sudhof TC. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 16.Rhee JS, Li LY, Shin OH, Rah JC, Rizo J, Sudhof TC, Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 18.Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Chacon R, Shin OH, Konigstorfer A, Matos MF, Meyer AC, Garcia J, Gerber SH, Rizo J, Sudhof TC, Rosenmund C. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 2002;22:8438–8446. doi: 10.1523/JNEUROSCI.22-19-08438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin OH, Xu J, Rizo J, Sudhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16469–16474. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 23.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 2008;15:1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 25.Mackler JM, Reist NE. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J. Comp Neurol. 2001;436:4–16. [PubMed] [Google Scholar]
- 26.Li L, Shin OH, Rhee JS, Arac D, Rah JC, Rizo J, Sudhof T, Rosenmund C. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J. Biol. Chem. 2006;281:15845–15852. doi: 10.1074/jbc.M600888200. [DOI] [PubMed] [Google Scholar]
- 27.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC. Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 29.Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 30.Kee Y, Scheller RH. Localization of synaptotagmin-binding domains on syntaxin. J. Neurosci. 1996;16:1975–1981. doi: 10.1523/JNEUROSCI.16-06-01975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao X, Li C, Fernandez I, Zhang X, Sudhof TC, Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 33.Matos MF, Rizo J, Sudhof TC. The relation of protein binding to function: what is the significance of munc18 and synaptotagmin binding to syntaxin 1, and where are the corresponding binding sites? Eur. J. Cell Biol. 2000;79:377–382. doi: 10.1078/0171-9335-00063. [DOI] [PubMed] [Google Scholar]
- 34.Davis AF, Bai J, Fasshauer D, Wolowick MJ, Lewis JL, Chapman ER. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 35.Gerona RR, Larsen EC, Kowalchyk JA, Martin TF. The C terminus of SNAP25 is essential for Ca(2+)-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem. 2000;275:6328–6336. doi: 10.1074/jbc.275.9.6328. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 37.Rickman C, Davletov B. Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 2003;278:5501–5504. doi: 10.1074/jbc.C200692200. [DOI] [PubMed] [Google Scholar]
- 38.Rickman C, Archer DA, Meunier FA, Craxton M, Fukuda M, Burgoyne RD, Davletov B. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 2004;279:12574–12579. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- 39.Bowen ME, Weninger K, Ernst J, Chu S, Brunger AT. Single- molecule studies of synaptotagmin and complexin binding to the SNARE complex. Biophys. J. 2005;89:690–702. doi: 10.1529/biophysj.104.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Dai H, Shen N, Arac D, Rizo J. A Quaternary SNARE-Synaptotagmin- Ca(2+)-Phospholipid Complex in Neurotransmitter Release. J. Mol. Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch KL, Gerona RR, Larsen EC, Marcia RF, Mitchell JC, Martin TF. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell. 2007;18:4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Rickman C, Jimenez JL, Graham ME, Archer DA, Soloviev M, Burgoyne RD, Davletov B. Conserved prefusion protein assembly in regulated exocytosis. Mol. Biol. Cell. 2006;17:283–294. doi: 10.1091/mbc.E05-07-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaffaney JD, Dunning FM, Wang Z, Hui E, Chapman ER. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 2008;283:31763–31775. doi: 10.1074/jbc.M803355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 2010;17:318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizo J, Rosen MK, Gardner KH. Enlightening molecular mechanisms through study of protein interactions. J. Mol. Cell Biol. 2012;4:270–283. doi: 10.1093/jmcb/mjs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arac D, Murphy T, Rizo J. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy. Biochemistry. 2003;42:2774–2780. doi: 10.1021/bi0272050. [DOI] [PubMed] [Google Scholar]
- 49.Ubach J, Lao Y, Fernandez I, Arac D, Sudhof TC, Rizo J. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry. 2001;40:5854–5860. doi: 10.1021/bi010340c. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 51.Shao X, Davletov BA, Sutton RB, Sudhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Tang J, Sudhof TC, Rizo J. Are neuronal SNARE proteins Ca2+ sensors? J. Mol. Biol. 2005;347:145–158. doi: 10.1016/j.jmb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Pang ZP, Shin OH, Meyer AC, Rosenmund C, Sudhof TC. A gainof-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao X, Sudhof TC, Rizo J. Assignment of the 1H, 15N and 13C resonances of the calcium-free and calcium-bound forms of the first C2-domain of synaptotagmin I. J. Biomol. NMR. 1997;10:307–308. doi: 10.1023/a:1018349601965. [DOI] [PubMed] [Google Scholar]
- 55.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and RSNAREs. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]