Abstract
The mechanical properties of the extracellular matrix play an important role in maintaining cellular function and overall tissue homeostasis. Recently, a number of hydrogel systems have been developed to investigate the role of matrix mechanics in mediating cell behavior within three-dimensional environments. However, many of the techniques used to modify the stiffness of the matrix also alter properties that are important to cellular function including matrix density, porosity and binding site frequency, or rely on amorphous synthetic materials. In a recent publication, we described the fabrication, characterization and utilization of collagen gels that have been non-enzymatically glycated in their unpolymerized form to produce matrices of varying stiffness. Using these scaffolds, we showed that the mechanical properties of the resulting collagen gels could be increased 3-fold without significantly altering the collagen fiber architecture. Using these matrices, we found that endothelial cell spreading and outgrowth from multi-cellular spheroids changes as a function of the stiffness of the matrix. Our results demonstrate that non-enzymatic collagen glycation is a tractable technique that can be used to study the role of 3D stiffness in mediating cellular function. This commentary will review some of the current methods that are being used to modulate matrix mechanics and discuss how our recent work using non-enzymatic collagen glycation can contribute to this field.
Keywords: matrix stiffness, endothelial cell, angiogenesis, glycation, biomaterials, three-dimensional
Introduction
The tissues and organs of the body are comprised of cells and extracellular matrices that are arranged to perform specific biological, chemical and physical functions. Cells within tissues interact with the extracellular matrix and each other to receive and impart both chemical and mechanical cues that influence their behavior. Importantly, these interactions contribute to overall tissue homeostasis and cellular function and, if they are disturbed, can contribute to aberrant cell behavior and disease.
Altered tissue mechanical properties have been correlated with a number of disease states including cancer,1 diabetes,2 cardiovascular disease,3 wound healing4 and asthma.5 Each of these maladies is characterized by a unique set of conditions, but in all cases, the interaction of the tissue cells with their extracellular environment is altered. The composition, density, arrangement and extent of cross-linking have all been shown to influence how cells interact with, move through and remodel their surroundings.6 Since it is very difficult to control all of these parameters independently in an in vivo setting, a number of in vitro hydrogel systems have been designed to study their role in a controlled environment.
Studies using two-dimensional substrates have shown that changes in matrix stiffness are correlated with altered cellular morphology,7,8 traction force generation,9-11 cell-cell connectivity,3,12-14 differentiation,15 chemotactic response16 and matrix deposition.17 However, since most cells in the body reside within a three-dimensional environment, it is important to recapitulate their natural extracellular environment to assess cellular function.
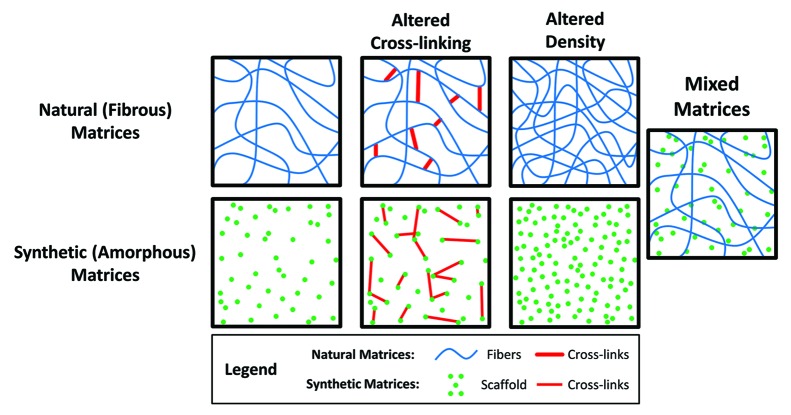
Common methods to create three-dimensional matrices with tunable mechanical properties include altering the density and/or extent of cross-linking of natural or synthetic hydrogels, or creating mixed matrices comprised of multiple synthetic and/or natural hydrogels. Modifying the density of the matrix is a relatively simple way to alter the mechanical properties of a hydrogel system to allow for the investigation of three-dimensional cellular behavior. This technique has commonly been used with native biological proteins such as collagen18,19 and fibrin20 but is also used in other synthetic hydrogel systems such as poly(ethylene glycol).21 Cross-linking approaches to control stiffness are most commonly used in synthetic hydrogel systems, but similar methods have been reported using natural matrices.22,23
While each of these techniques is capable of altering the stiffness of the matrix, they all have inherent advantages and disadvantages for studying the role of mechanical properties within a three-dimensional cell culture system. Specifically, changing the matrix density or cross-linking of a hydrogel can also influence porosity and hydraulic permeability,18,20 fibril arrangement and structure19 and binding domain frequency6 within the hydrogels. Consequently, it can be difficult to decouple the relative effects of the mechanical properties from the other physical and chemical factors that are concurrently affected.
This commentary will provide a brief overview of some of the current approaches to modulate matrix stiffness for three-dimensional studies of cellular behavior and review their general advantages and disadvantages. We will also discuss our recent work using non-enzymatic glycation and provide some further insights into the advantages, disadvantages, and physiological relevance of our approach to modulate the stiffness of in vitro matrices.
Modulating 3D Hydrogel Mechanical Properties
Naturally derived hydrogels
A number of native proteins and polymers have been utilized to make three-dimensional hydrogels for the investigation of cell response to matrix stiffening. Among the many extracellular matrix components that are present within the body, type I collagen, fibrin and hyaluronic acid are among the most commonly used for creating three-dimensional matrices.18,20,24 Importantly, these matrices allow cells to be seeded within a natural environment that is capable of being both degraded and remodeled as is done in vivo by cells.
The most basic approaches utilizing natural matrices are those that increase the stiffness of the matrix by increasing the density (Fig. 1). A number of studies have modified the density of collagen or fibrin hydrogels to study the influence of matrix mechanics on cellular behavior. By increasing the density of collagen matrices from 2 mg/ml to 20 mg/ml the compressive modulus increases approximately 10-fold from approximately 175 Pa to 1800 Pa.18,25 Similarly, by increasing the density of fibrin matrices from 2.5 mg/ml to 10 mg/ml the compressive modulus increases from approximately 1.3 kPa to 9 kPa.20
Figure 1. Methods commonly used to modulate matrix stiffness. The mechanical properties of both synthetic and natural matrices are commonly tuned by altering the number of cross-links and/or the density of the scaffold. Mixed matrices comprised of both synthetic and natural materials can be used to create hybrid in vitro environments that mimic the properties of in vivo tissues.
The stiffness of some natural polymers can be modulated using differential cross-linking to investigate cell behavior (Fig. 1). For example, methacrylated hyaluronic acid is a matrix that is currently being used to dynamically modulate the stiffness of hydrogels via a two-step cross-linking process.26 First, hydrogels are chemically cross-linked and then can be seeded with cells before a secondary photo-cross-linking reaction is initiated. This is an especially interesting procedure because it allows the mechanics of the matrix to be temporally controlled from approximately 1.5 to 7.5 kPa while cellular behavior is simultaneously investigated. Unfortunately, since methacrylated hyaluronic acid is not naturally porous, methods had to be developed to create pores within the matrix. Poly(methyl methacrylate) microspheres are encapsulated during the initial polymerization reaction and subsequently dissolved before cells are seeded within the matrices to create large (~300 um) pores within the hydrogel. This potentially limits the ability of these matrices to truly recapitulate a three-dimensional, in vivo-like environment.
While hydrogels formed from naturally derived materials provide an optimal environment for cell culture, it can be challenging to use natural matrices for studies of the effects of matrix stiffness. Specifically, since the matrix materials are biologically designed for cell adhesion, it is relatively difficult to decouple the role of binding site availability from matrix density. Additionally, the fibrous arrangement and structure of the matrices can be modified with changes to the density or cross-linking which further complicates the analysis of resultant cellular responses. Further, even when both the density and cross-linking of natural hydrogel matrices are modified, the resultant matrix stiffness is only tunable across a relatively narrow range (usually hundreds to thousands of Pa). Taken together, these complexities can make it difficult to pinpoint whether the cellular responses are due to changes in matrix stiffness or other factors.
Synthetic hydrogels
To overcome some of the disadvantages inherent to hydrogels comprised of naturally derived matrices, synthetic hydrogels have been created. The primary advantage of using synthetic hydrogels is that they can be formulated to investigate both mechanical and chemical properties on cellular function. Indeed, many synthetic hydrogel systems offer independent control of mechanical properties, adhesive binding sites and chemical cues. One of the most popular synthetic materials used currently is poly(ethylene glycol) (PEG)21,27-29 although other materials such as poly(caprolactone)30 and poly(methyl methacrylate)31 have also been used. Since these synthetic hydrogels were originally developed for other purposes and do not naturally contain cell binding domains, moieties such as Arg-Gly-Asp peptides (RGD) or laminin-like domains must be incorporated to facilitate cell adhesion. This allows for precise control and modulation of the frequency and availability of adhesive regions within the hydrogel.
Modulating the density and cross-linking of synthetic hydrogels are techniques that are commonly used to alter their mechanical properties (Fig. 1). The density of PEG hydrogels has been used to investigate the role of three-dimensional cellular behaviors.21 Additionally, a number of PEG hydrogel systems have been developed that incorporate matrix metalloproteinase (MMP)-sensitive cross-links so that cells are capable of degrading their surrounding environment.27,28 Systems have also been developed to selectively cross-link PEG gels using multiphoton microscopy resulting in hydrogels with micro-domains similar to those found in tissue structures.32
While synthetic hydrogels provide a highly tunable system for investigating cell behavior, they are generally amorphous and unlike the fibrous extracellular matrices within the body. Thus, while it is feasible to independently tune properties such as the stiffness, binding site availability and degradability, synthetic hydrogels are sub-optimal because the cells are unable to actively remodel them and they do not recapitulate the fibrous nature of the in vivo environment.
Mixed matrices
To overcome some of the disadvantages inherent to investigating matrix stiffness with hydrogels comprised of synthetic or natural materials alone, mixed matrices have been developed (Fig. 1). For example, collagen has been combined with PEG,33 agarose34 or fibrin35 to create matrices with tunable mechanical properties. In each of these cases, the density, extent of cross-linking, or ratio of each component are altered to control the mechanical properties. As such, it is possible that the cellular behavior will be influenced by the matrix composition, which is not completely decoupled from stiffness. While the data generated from cells embedded within these matrices may be complex, these systems lay an important foundation for the development of in vitro hydrogels that more closely mimic the properties of in vivo tissues.
Non-Enzymatic Collagen Glycation
We recently reported the use of non-enzymatic glycation of unpolymerized collagen to investigate the effects of matrix stiffening on endothelial cells.25 Non-enzymatic glycation is a natural process whereby reducing sugars and proteins interact to produce extracellular matrix cross-linking within biological tissues.36 These sugars create chemical alterations within the protein structures that ultimately result in protein-to-protein cross-links that mechanically alter the tissues. Specifically, the interaction of reducing sugars such as glucose or ribose with amino groups on proteins create Schiff bases which are able to rearrange into Amadori products.37 The Amadori products can then form protein-to-protein cross-links that are commonly known as advanced glycation end products (AGE).38 These AGE cross-links slowly accumulate on proteins in vivo during aging, and the rate and extent of accumulation is accelerated in individuals with diabetes.38
In vitro, non-enzymatic collagen glycation can be used to cross-link protein solutions prior to hydrogel polymerization (pre-glycation) or can cross-link polymerized protein matrices (post-glycation).23 Post-glycation is the primary method whereby proteins in vivo are cross-linked. However, the high sugar concentrations necessary to achieve measurable changes in stiffness in vitro limit this technique to seeding cells on the surface of the matrices after the glycation reaction has been completed because cells cannot withstand the osmotic imbalance created by the glycating solutions.39 During pre-glycation, active sites are created within the collagen gels that will later become cross-links during collagen polymerization. Since the collagen solutions are treated with the glycating solutions prior to polymerization, cells can be embedded within the gels during the polymerization process and are not subjected to the osmotic imbalances created by the glycating solutions. Thus, by using the process of pre-glycation, the effects of 3D stiffness on cells embedded within collagen matrices can be investigated.
Many studies have investigated the effects of collagen glycation on endothelial cell behavior. In prior studies, investigators have seeded cells atop of post-glycated matrices or injected cells into matrices following glycation.39-42 While these studies have provided valuable information, post-glycation is limited in its ability to investigate the effects of matrix stiffness on the behavior of cells embedded within the matrix. In our work, we utilized non-enzymatic pre-glycation (henceforth referred to as glycation) to investigate the effects of 3D matrix stiffness on collagen fiber structure and arrangement as well as on endothelial cell spreading and organization.25 To glycate matrices, collagen solutions were incubated with 0–250 mM ribose for 5 d at 4 °C prior to polymerization. At the end of this incubation, the collagen solutions were mixed with sodium hydroxide to neutralize the pH, and complete medium or a suspension of endothelial cells was added to bring the final collagen density to 1.5 mg/ml. Collagen solutions were polymerized at 37 °C and 5% CO2. Using these matrices, we investigated the mechanical properties, polymerization dynamics, collagen fiber distributions and structures, as well as individual and collective endothelial cell responses.
In parsing out the effects of matrix stiffness on cell behavior, it is critical to also control for architecture and fiber arrangement within the collagen matrices. Our study was the first to examine the resultant fiber arrangements within pre-glycated polymerized matrices. Importantly, we found that there exists a range of ribose concentrations (0–100 mM) where the equilibrium compressive modulus of collagen can be increased approximately 3-fold from ~175–515 Pa while the arrangement and size of collagen fibrils is not significantly affected. Increasing the concentration of ribose to 150 mM or greater also results in increased matrix stiffness but the fibrous properties of the matrices change as well. Similarly, we found that the fibril formation rate was similar for collagen solutions that had been glycated with 0–100 mM ribose while the rates for solutions glycated with 150–250 mM ribose were significantly delayed and correlated with larger collagen fibers. Since it is known that the arrangement of fibrous features within matrices can influence cellular function, we focused on gels glycated with 0–100 mM ribose for our studies of endothelial cell behavior.
When individual endothelial cells were embedded within the glycated matrices, we observed that those within stiffer matrices spread to a greater degree than those embedded within softer matrices (Fig. 2). Since endothelial cells do not normally exist as individual cells in vivo, we investigated the effects of stiffness on endothelial outgrowth from embedded multicellular spheroids. As early as the first day after culture, spheroids within the stiffer matrices had significantly more extensions than those in softer matrices. Importantly, even when the interactions of receptors for advanced glycation end products (RAGE) were inhibited with a blocking antibody, spheroids within the stiffer collagen gels maintained their increased sprouting response when compared with those in softer matrices. These initial studies provide the basis for additional studies into the role of matrix stiffening on endothelial cell function.
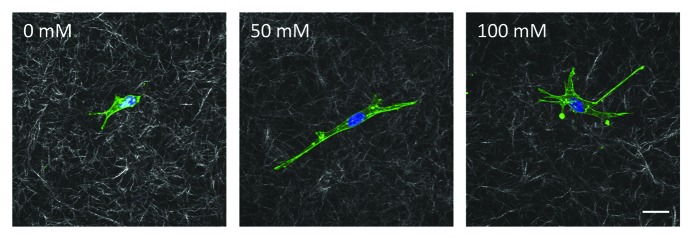
Figure 2. Bovine aortic endothelial cells embedded within glycated collagen gels. Collagen solutions that had been glycated with 0, 50 or 100 mM ribose were neutralized, mixed with endothelial cells and allowed to polymerize. Cells were allowed to spread for 24 h and then were fixed and stained for actin (green) and DAPI (blue). Cells and the surrounding collagen were imaged using confocal microscopy. Scale is 20 μm.
Disadvantages of collagen glycation
Although the non-enzymatic glycation of collagen mimics the stiffening of matrices naturally occurring in vivo, there are several drawbacks to using this method to investigate the effects of matrix stiffness on cell function. Specifically, collagen glycation alters the chemical composition of the matrix by creating AGE cross-links. A variety of cell types, including endothelial cells, are known to have RAGE and the interactions between AGE and RAGE have been shown to influence cell behavior and cell-cell interactions.43 Specifically, AGE/RAGE interactions have been implicated in altering endothelial cell response to shear stress,40 mechanical stretch44 and barrier function.45 A number of methods have been developed to inhibit the interaction of RAGE with AGE including blocking antibodies and pharmaceutical drugs.46-48 However, like other cell-membrane receptors, RAGE can be replenished to the cell surface, making the long-term use of blocking antibodies less effective and very expensive. Additionally, the pharmaceutical drugs that have been used to inhibit the RAGE/AGE interaction are not specific to RAGE and also influence a wide variety of other cellular pathways and behaviors.48-50 Thus, while glycation can recapitulate the mechanical stiffening that occurs naturally within the body, it can also engage RAGE. The respective contributions of these two factors to overall cell behavior can be difficult to decouple.
Advantages collagen glycation and implications for human disease
Although it is challenging to completely decouple the role of matrix stiffness from AGE/RAGE signaling in our hydrogel system, collagen glycation does have many advantages over other current methods of matrix stiffening. Specifically, we demonstrated the ability to increase the stiffness of the collagen gels 3-fold independently of the overall collagen fiber arrangements. Our study was limited to 1.5 mg/ml gels but it is possible that increasing the density of the collagen gels will allow for a wider range of moduli to be achieved.
Since accumulation of AGE cross-links within tissues during aging is universal, using non-enzymatic glycation to investigate the role of matrix stiffening is relevant to understanding conditions in vivo. In fact, the presence of AGEs has been suggested to play a causative role in diseases such as diabetes,51 rheumatoid arthritis,52 atherosclerosis,53 Alzheimer’s54 and cataracts.55 Our data has demonstrated that endothelial cells respond to the changes in matrix stiffness induced by non-enzymatic glycation. Importantly, our lab has also correlated aging with increased vascular stiffness and endothelial monolayer permeability which may contribute the increased prevalence in cardiovascular disease.3 Thus, using non-enzymatic collagen glycation to investigate the impact of altering three-dimensional matrix stiffness on cellular behavior has the potential to inform how we manage and treat different disease conditions.
Conclusions
Researchers today have many options to consider when choosing a hydrogel system to investigate the role of matrix mechanics on cellular behavior. Naturally derived matrices contain many of the essential elements required for cellular culture and can closely mimic the three-dimensional cellular environment. However, synthetic matrices tend to be more customizable so that the intricacies of individual parameters such as cellular adhesion and mechanosensing can be more closely studied. We have presented non-enzymatic collagen glycation as a highly tractable technique that utilizes a cross-linking mechanism that recapitulates in vivo tissue stiffening and is relevant to a number of disease states. Future work should combine the advantages of these techniques to create matrices that allow for independent modulation of hydrogel parameters while mimicking the native extracellular environment.
Acknowledgments
This work was supported by funding from the National Institutes of Health (GM103388 HL097296) and the Cornell Center on the Microenvironment and Metastasis through Award Number U54CA143876 from the National Cancer Institute to C.A.R. and a National Science Foundation Graduate Research Fellowship and a fellowship through the National Science Foundation Graduate STEM Fellows in K–12 Education Program to B.N.M.
Glossary
Abbreviations:
- AGE
advanced glycation end product
- PEG
poly(ethylene glycol)
- RAGE
receptors for advanced glycation end products
- RGD
Arg-Gly-Asp peptide sequence
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/24942
References
- 1.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 2.DeLoach SS, Townsend RR. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008;3:184–92. doi: 10.2215/CJN.03340807. [DOI] [PubMed] [Google Scholar]
- 3.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters CM, Sporn PH, Liu M, Fredberg JJ. Cellular biomechanics in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;283:L503–9. doi: 10.1152/ajplung.00141.2002. [DOI] [PubMed] [Google Scholar]
- 6.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 8.Pelham RJ, Jr., Wang Yl. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng. 2010;3:68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19:1573–9. doi: 10.1021/la026142j. [DOI] [Google Scholar]
- 11.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–16. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Califano JP, Reinhart-King CA. A Balance of Substrate Mechanics and Matrix Chemistry Regulates Endothelial Cell Network Assembly. Cell Mol Bioeng. 2008;1:122–32. doi: 10.1007/s12195-008-0022-x. [DOI] [Google Scholar]
- 13.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–51. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011;300:C146–54. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Jannat RA, Robbins GP, Ricart BG, Dembo M, Hammer DA. Neutrophil adhesion and chemotaxis depend on substrate mechanics. J Phys Condens Matter. 2010;22:194117. doi: 10.1088/0953-8984/22/19/194117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg JL, Safi A, Wei X, Espinosa HD, Budinger GS, Takawira D, et al. Substrate stiffness regulates extracellular matrix deposition by alveolar epithelial cells. Res Rep Biol. 2011;2011:1–12. doi: 10.2147/RRB.S13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–88. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 21.Ehrbar M, Sala A, Lienemann P, Ranga A, Mosiewicz K, Bittermann A, et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys J. 2011;100:284–93. doi: 10.1016/j.bpj.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–29. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy R, Boskey A, Bonassar LJ. Processing of type I collagen gels using nonenzymatic glycation. J Biomed Mater Res A. 2010;93:843–51. doi: 10.1002/jbm.a.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater. 2011;7:2401–9. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013;9:4635–44. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marklein RA, Soranno DE, Burdick JA. Magnitude and presentation of mechanical signals influence adult stem cell behavior in 3-dimensional macroporous hydrogels. Soft Matter. 2012;8:8113–20. doi: 10.1039/c2sm25501d. [DOI] [Google Scholar]
- 27.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–66. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Drinnan CT, Geuss LR, Suggs LJ. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 2010;6:3395–403. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Williamson MR, Woollard KJ, Griffiths HR, Coombes AG. Gravity spun polycaprolactone fibers for applications in vascular tissue engineering: proliferation and function of human vascular endothelial cells. Tissue Eng. 2006;12:45–51. doi: 10.1089/ten.2006.12.45. [DOI] [PubMed] [Google Scholar]
- 31.Quinn TM, Grodzinsky AJ. Longitudinal modulus and hydraulic permeability of poly(methacrylic acid) gels: effects of charge density and solvent content. Macromolecules. 1993;26:4332–8. doi: 10.1021/ma00068a040. [DOI] [Google Scholar]
- 32.Hoffmann JC, West JL. Three-dimensional photolithographic patterning of multiple bioactive ligands in poly(ethylene glycol) hydrogels. Soft Matter. 2010;6:5056. doi: 10.1039/c0sm00140f. [DOI] [Google Scholar]
- 33.Liang Y, Jeong J, DeVolder RJ, Cha C, Wang F, Tong YW, et al. A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials. 2011;32:9308–15. doi: 10.1016/j.biomaterials.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials. 2010;31:1875–84. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 35.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–64. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillard LC. Action des acides amines sur les sucres: formation des melanoides par voie methodique. Comptes Rendus de l’Academie des Sciences. 1912;154:66–8. [Google Scholar]
- 37.Hodge JE. The Amadori rearrangement. Adv Carbohydr Chem. 1955;10:169–205. doi: 10.1016/S0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Roy R, Boskey AL, Bonassar LJ. Non-enzymatic glycation of chondrocyte-seeded collagen gels for cartilage tissue engineering. J Orthop Res. 2008;26:1434–9. doi: 10.1002/jor.20662. [DOI] [PubMed] [Google Scholar]
- 40.Kemeny SF, Figueroa DS, Andrews AM, Barbee KA, Clyne AM. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J Biomech. 2011;44:1927–35. doi: 10.1016/j.jbiomech.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–22. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 42.Francis-Sedlak ME, Moya ML, Huang J-J, Lucas SA, Chandrasekharan N, Larson JC, et al. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. 2010;80:3–9. doi: 10.1016/j.mvr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- 44.Figueroa D, Kemeny S, Clyne A. Glycated Collagen Impairs Endothelial Cell Response to Cyclic Stretch. Cell Mol Bioeng. 2011;4:220–30. doi: 10.1007/s12195-011-0176-9. [DOI] [Google Scholar]
- 45.Hirose A, Tanikawa T, Mori H, Okada Y, Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010;584:61–6. doi: 10.1016/j.febslet.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 46.Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE. Activation of RAGE induces elevated O2- generation by mononuclear phagocytes in diabetes. J Leukoc Biol. 2007;81:520–7. doi: 10.1189/jlb.0406262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–8. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, et al. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865–73. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsui T, Takeuchi M, Yamagishi S. Nifedipine, a calcium channel blocker, inhibits inflammatory and fibrogenic gene expressions in advanced glycation end product (AGE)-exposed fibroblasts via mineralocorticoid receptor antagonistic activity. Biochem Biophys Res Commun. 2010;396:566–70. doi: 10.1016/j.bbrc.2010.04.149. [DOI] [PubMed] [Google Scholar]
- 51.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583–7. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi M, Kushida K, Ohishi T, Kawana K, Hoshino H, Uchiyama A, et al. Quantitative analysis of crosslinks pyridinoline and pentosidine in articular cartilage of patients with bone and joint disorders. Arthritis Rheum. 1994;37:724–8. doi: 10.1002/art.1780370517. [DOI] [PubMed] [Google Scholar]
- 53.Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 54.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:4766–70. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stitt AW. The maillard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043:582–97. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]