Abstract
The physical environment of myocardium, featuring excitation-contraction coupling, constant and efficient provision of nutrient/oxygen and delicate integration of cardiomyocytes and supporting cell population (fibroblasts, endothelial cells), is one of the most complex systems in human body. Numerous studies have demonstrated the significance of physical stimulation in cardiac cell physiology, including the maintenance of contractile function in cardiomyocytes,1 cell alignment and extracellular matrix secretion in fibroblasts and endothelial cells.2,3 In effort to reconstruct the physical environment found in the cardiac niche for routine cell culture use, we have devised a bioreactor system to account for three major forms of physical stimuli, namely, cyclic stretch, electrical stimulation and fluid perfusion.4
Keywords: cardiac bioreactor, human atrial fibroblast, cyclic stretch, electrical stimulation, cardiac tissue engineering scaffold
Many previous studies on cardiac bioreactors were targeted at tissue engineering purposes; cardiac progenitors and myocytes were the most often used cell population. Another significant supporting cell population, cardiac fibroblasts, were under-explored as a candidate for bioreactor studies. Over the past decade, a growing body of evidence pointed to the influential role that cardiac fibroblasts play in conditions like adverse remodeling, myocardial repair and atrial fibrillation. Fibroblasts regulate the production and degradation of ECM proteins by fine-tuning the secretion of collagen and matrix metalloproteinases. Cardiac fibroblasts also secrete biochemical signals, such as angiotensin, TGF-β, and endothelin-1, that act as autocrine and paracrine factors to regulate the homeostasis of the myocardium.5 Under pathological conditions, fibroblasts undergo the phenotype transition to “myofibroblasts,” marked by the upregulation of α-smooth muscle actin (αSMA). Myofibroblasts are considered more active compared with normal fibroblasts, manifested by elevated rate of proliferation, migration and secretion of pro-fibrotic proteins.6 However, most previous findings on human cardiac fibroblasts were conducted with conventional cell culture environments, and myofibroblasts were obtained by either biochemical challenge7 or prolonged culture time/repeated passaging.8
In this commentary, we first present our exploratory study on the structural and functional changes in human atrial fibroblasts due to cyclic stretch delivered through our bioreactor system, and briefly discuss its implications in cardiac (patho)physiology, then we would like to share our thoughts on engineering optimization of our bioreactor system and possibilities for further improvement of this novel device.
Fibroblast Isolation
Human atrial fibroblast cells were isolated as described.4 Preliminary experiments on characterizing human atrial outgrowth fibroblasts indicated that patient source (such as age, sinus rhythm vs. atrial fibrillation), seeding density, passage number and time in culture can influence their phenotype and functional properties. Cyclic stretch experiments were therefore designed to account for all these variables. In this study, fibroblasts were derived from sinus rhythm patients only. After a 2-week initial outgrowth period, tissue biopsies were removed, outgrowth fibroblasts were harvested by standard trypsin treatment, plated at 200 cells/mm2 in a T-25 flask for a “priming period” of 3 to 6 d to exclude any influence brought forth by co-culture with tissue biopsies.
Cyclic Stretch Experimental Setup
As shown in the experimental setup scheme (Fig. 1), after the priming period, human atrial outgrowth fibroblasts (2 × 104 per substrate) were seeded on fibronectin/collagen mixture-coated PDMS substrates and subjected to 10% stretch at 1 Hz for 24 h or placed in bioreactor without stretch as negative control. Each bioreactor chamber can house 2 PDMS substrates at a time, thus all experiments were performed in duplicates. Upon termination of this 24-h stimulation period, cells were transferred out of the bioreactor along with PDMS substrates for either immunocytochemistry or transwell migration analysis.
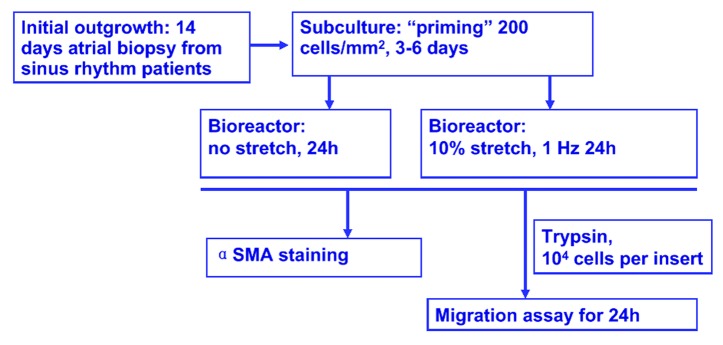
Figure 1. Experimental set up. Human atrial fibroblasts were obtained from minced right atrial biopsies derived from sinus rhythm patients, and outgrowth culture was performed for two weeks. Cells were then harvested by trypsinization and seeded in a tissue culture flask at 200 cells/mm2 for 3–6 d. For cyclic stretch experiments, cells were allowed to attach to collagen/fibronectin coated PDMS substrate, and 10% cyclic stretch were applied for 24 h at 1 Hz, fibroblast cells seeded in the bioreactor without stretch served as negative control. At the end of this 24-h stimulation period, stretched and control cells were harvested for analysis. To assess the phenotype transition to myofibroblasts, cell-seeded PDMS substrates were removed from bioreactor, stained for myofibroblast marker α-smooth muscle actin (α-SMA). For cell motility measurement, cells were trypsinized, removed from PDMS substrate and transferred into the top chamber of transwell migration assay, cell migration during a subsequent 24-h period was assessed.
Immunocytochemistry and Myofibroblast Assessment
Cardiac fibroblasts are known to undergo a phenotype transition into myofibroblasts when challenged by biochemical signals or after prolonged culture. To assess the influence of cyclic stretch on such phenotype transition, cells were stained for myofibroblast marker α-smooth muscle actin (α-SMA). Briefly, PDMS substrates with stretched or control cells were removed from the bioreactor chamber, washed three times with PBS and fixed with 4% formalin. After another wash in PBS and blocking with horse serum for an hour, cells were incubated with primary antibody (A 5228, Sigma, 1:200 dilution in blocking buffer) for an hour, followed by another wash in PBS and one hour incubation in secondary antibody (115-166-008, Dianova, 1:200 dilution in blocking buffer) in a moisturized, dark room environment. Images of stained cells were acquired with an immunofluorescent microscope, and standard cell count was performed by counting the number of α-SMA-positive cells in a particular image. At least six images were taken from each PDMS substrate.
Transwell Migration Assay
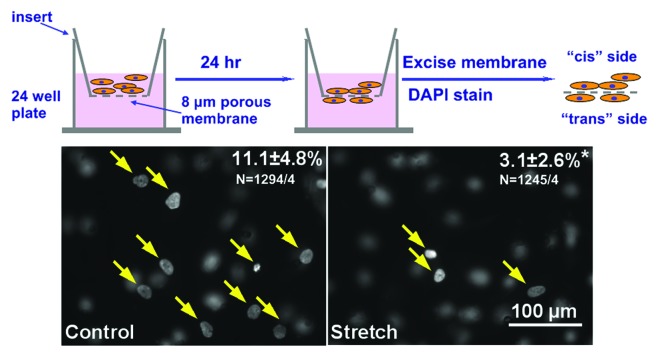
Cardiac fibroblast cell motility is critically related to myocardial repair and adverse remodeling. To investigate how cyclic stretch affects fibroblast cell motility, transwell migration assay was performed. Cells were trypsinized from the PDMS substrates and counted in a standard hemacytometer. Ten thousand cells were resuspended in 100 μl of fibroblast culture medium [Dulbecco's modified Eagle medium (DMEM); Gibco 22320 + 10% fetal bovine serum (FBS) + 1% pen./strep.] and seeded in a Transwell insert (polycarbonate membrane with 8 μm pores, 10 μm thick, Costar 3422). Each experiment was performed in duplicates. Transwell inserts were placed on 24-well plates, with 600 μl of fibroblast culture medium in each well. No attractants were applied in the bottom chamber, because this assay is intended to evaluate the steady-state migration capability without introducing other factors that might confound cell motility.9
During a subsequent 24 h period, cells settle down and attach to the bottom of the inserts, and a fraction of the cells migrate and adhere to the bottom side of the membrane. Cells were fixed in 4% formaldehyde, stained with DAPI to visualize the nuclei. Polycarbonate membranes were then excised with a scalpel, mounted onto a standard microscope glass slide for image analysis. The top side of the polycarbonate membrane, i.e., the side where the fibroblasts were loaded on is arbitrarily termed the “cis” side, and the bottom side of the membrane to which fibroblasts migrate and adhere is termed the “trans” side.
When focused on the trans side of the membrane, nuclei of migrated cells appear as filled white signals with sharp edges (Fig. 2, yellow arrows), whereas cells that stayed on the cis side of the membrane exhibited faint signals with blurred borders. The opposite observation could be made when focusing on the cis side of the membrane. From each pair (i.e., cis and trans from the same position) of images, the number of nuclei on both sides of the membrane was be recorded. For each membrane, at least six pairs of images were taken, and percentage of migrated cells could be calculated by the formula: trans nuclei/(trans + cis nuclei) × 100.
Figure 2. Transwell migration assay. Top, Experimental procedure. Bottom, Representative images from migration assay, filled circles (yellow arrows) correspond to nuclei of migrated cells, blurred are “cis” nuclei. Upper right numbers indicated percent of cells migrated, stretched cells showed a significant decrease in cell motility. (p < 0.05, n = ~1200 cells from four experiments).
As shown in Figure 3, original images of control (left) showed low levels of α-SMA signal with a diffusive distribution and a granular texture. In contrast, cells subjected to 10% stretch at 1 Hz for 24 h exhibited larger areas of organized, interwoven fibrous signals of α-SMA. Cell orientation, as outlined by α-SMA signals, is mostly perpendicular to the direction of stretch (white arrow), whereas cells in control group aligned randomly. Standard cell count analysis indicated less than a quarter of the cells in control group were positive for α SMA signal, whereas the myofibroblasts population tripled after stretch treatment (Fig. 3). Transwell migration assay revealed over 10% of the cells in control group migrated to the trans side of the porous membrane, but cell motility is significantly reduced to less than 5% in stretched cells (n = ~1200 cells from four independent experiments).
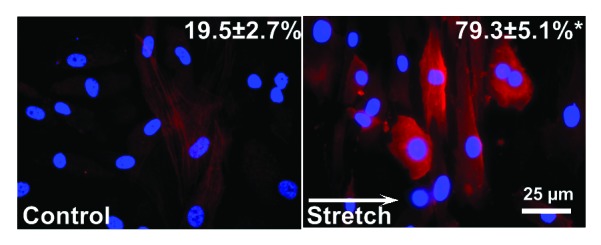
Figure 3. Myofibroblast assessment. Representative images from immunocytochemical staining experiments from no stretch control (left) and stretched cells (right) Red signal: α-smooth muscle actin (α-SMA), blue: DAPI. Upper right numbers indicate percentage of myofibroblasts determined by standard cell count, which revealed a significantly higher percentage of α-SMA in stretched cells (p < 0.05). Data obtained from 3 independent experiments (n = ~600 cells). White arrow indicates direction of stretch.
Transition to Myofibroblasts Due to Mechanical Stimulation
Cardiac fibrosis often leads to abnormalities in the electrical conduction and mechanical functions, thereby contributing to the pathophysiology of a variety of cardiac conditions, including hypertrophy, heart failure and arrhythmias.10 Fibrosis is characterized by the excessive deposition of extracellular matrix (ECM) proteins, particularly by myofibroblasts.11 The presence of myofibroblasts is rare in normal cardiac tissue,5 but in response to pathological conditions, such as mechanical stretch, inflammation and autocrine/paracrine production, fibroblasts undergo the phenotype transition to myofibroblasts, which supposedly exhibit enhanced levels of proliferation and migration.12
Human atrial outgrowth fibroblasts subjected to 10% cyclic stretch at 1 Hz for 24 h have a significantly higher proportion of myofibroblasts compared with control group (Fig. 2). Such observation is consistent with previous study on the stretch-induced (8% at 0.5 Hz for 72 h) phenotype transition to myofibroblast in human patellar tendon fibroblasts.13 However, in a more recent study with primary human lung fibroblasts, cyclic stretch treatment (10% at 0.2 Hz for 48 h) had been shown to reduce the myofibroblasts population.14 Similar controversy also exists in animal studies. In neonatal rat cardiac fibroblasts, static stretch has been shown to induce an 1.5-fold increase in αSMA protein expression within 4 h;15 however, in immortalized mouse cardiac myofibroblasts with high preexisting levels of αSMA, static stretch can reduce the expression of αSMA.16 This disparity can be attributed to the differences in starting cell population (lung, tendon and heart, primary culture vs. cell line), stimulation parameters (amplitude, frequency and duration) and stretch regimes (static vs. cyclic). Additional studies on how these physical stimuli can influence fibroblast phenotype will provide a more thorough understanding of fibroblast physiology.
Substrate Modification
In our current prototype, flat, unpatterned PDMS was used as an elastic cell culture substrate. Several techniques have been developed to manipulate cell morphology and alignment on PDMS substrates, including microgroove patterns17 and collagen fibril deposition.18 Contact guidance has been shown to play a more prominent role in dictating cell alignment than stretch; cells align along the direction of surface collagen fibers on a PDMS surface, regardless the direction of stretch.19 Furthermore, spin coating techniques are available to generate cardiac tissue thin films from stem cells seeded on PDMS substrates.20 Neonatal rat ventricular myocytes grown on micropatterned elastic cell culture substrate indeed exhibited structural improvement after stretch treatment.21 For future studies on this bioreactor, incorporating these surface modification techniques of the PDMS substrate will greatly expand its applications for various fields of study, including mechanobiology, stem cell biology and cardiac tissue engineering.
Another potential application for this bioreactor system, in addition to investigating the influence of physical stimulation on cell physiology, is cardiac tissue engineering. Our study has been limited to cell monolayers, but the unique design of this bioreactor’s clamp-and-motor stretch system permits the use of multi-layered tissue constructs without major modification. For example, previous studies have proposed that accordion-like honeycombs constructs, made of poly(glycerol sebacate), by virtue of resembling the anisotropic mechanical properties of the native heart, can facilitate the formation of cardiac tissue microstructure and cell alignment.22 Besides poly(glycerol sebacate), other biomaterials, including fibrin,23 collagen,24 matrigel25 and alginate,26 can be used to make 3-D constructs by a transfer molding process similar to the one utilized in making PDMS substrates.
Potential Upgrades to Perfusion System
In our study, we performed short experiments (less than 10 min) to demonstrate that, the fluid inlet and outlet of the bioreactor chamber, along with rubber tubing, vacuum suction and peristaltic pump, can form a functional fluid exchange system.4 However, for long-term cell culture and tissue engineering studies, a reservoir system must be built and incorporated to the bioreactor system to sustain continuous flow. Given the volumetric capacity of the bioreactor, which typically holds about 7 ml of solution in each chamber, such reservoir system must be large enough to provide sufficient cell culture medium, especially for cardiac tissue engineering studies where high flow rate (25 ml/min) is desired.27
In addition, the fluidic flow profile in our device has not been systemically characterized, therefore, it is unclear what kind of microenvironment (i.e., shear stress, pressure gradient, mass transport, gas/nutrient permeability) is being imposed to cultured cells/constructs in this bioreactor system. Since one important aspect for the biomimetic approach of cardiac tissue engineering is the replication of the body’s natural hydrodynamic environment, i.e., flow velocity, shear stress and pressure drop,28 another possible direction for future improvement is to integrate microfluid devices into our bioreactor system to provide a more defined physical environment.
Possibilities to Incorporate Analytic Devices
The design and construction of the bioreactor chamber took the ease of optical inspection into account, i.e., the PDMS substrate is transparent, and the top and the bottom of the bioreactor chamber are covered with microscope slides. This configuration allows optical instruments to be coupled to the bioreactor system. For example, fluorescent indicators are available for simultaneous measurement of action potentials and calcium transients,29 and GFP-based monitoring can also be integrated into a bioreactor system for non-invasive assessment on cell growth.30 More recently, a PDMS-based microelectrode array has been proposed to allow surface stimulation and electrical signal detection in soft tissue,31 incorporating such functional assessment devices would allow real-time analysis of biological systems stimulated in the bioreactor chamber, thereby greatly enhance the prowess of this novel system.
Acknowledgment
The authors gratefully acknowledge generous funding by Fondation Leducq (07 CVD 03, “Leducq European-North American Arial Fibrillation Research Alliance”).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25014
References
- 1.Berger HJ, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, et al. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–9. doi: 10.1152/ajpheart.1994.266.1.H341. [DOI] [PubMed] [Google Scholar]
- 2.Ruwhof C, van Wamel AET, Egas JM, van der Laarse A. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem. 2000;208:89–98. doi: 10.1023/A:1007046105745. [DOI] [PubMed] [Google Scholar]
- 3.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532–8. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Mende M, Yang X, Körber HF, Schnittler H-J, Weinert S, et al. Design and validation of a bioreactor for simulating the cardiac niche: a system incorporating cyclic stretch, electrical stimulation, and constant perfusion. Tissue Eng Part A. 2013;19:403–14. doi: 10.1089/ten.tea.2012.0135. [DOI] [PubMed] [Google Scholar]
- 5.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Yue LX, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–53. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du JY, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter KE, Turner NA, O’Regan DJ, Ball SG. Tumor necrosis factor alpha induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: inhibition by simvastatin. Cardiovasc Res. 2004;64:507–15. doi: 10.1016/j.cardiores.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Gallucci RM, Sloan DK, Heck JM, Murray AR, O’Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004;122:764–72. doi: 10.1111/j.0022-202X.2004.22323.x. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 11.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–13. doi: 10.1161/01.RES.0000046452.67724.B8. [DOI] [PubMed] [Google Scholar]
- 12.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–62. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 13.Wang JHC, Yang GG, Li ZZ, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J Biomech. 2004;37:573–6. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Blaauboer ME, Smit TH, Hanemaaijer R, Stoop R, Everts V. Cyclic mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts. Biochem Biophys Res Commun. 2011;404:23–7. doi: 10.1016/j.bbrc.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–81. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Lukse E, Seth A, McCulloch CAG. Use of conditionally immortalized mouse cardiac fibroblasts to examine the effect of mechanical stretch on alpha-smooth muscle actin. Tissue Cell. 2001;33:86–96. doi: 10.1054/tice.2000.0160. [DOI] [PubMed] [Google Scholar]
- 17.Wang JHC, Yang GG, Li ZZ. Controlling cell responses to cyclic mechanical stretching. Ann Biomed Eng. 2005;33:337–42. doi: 10.1007/s10439-005-1736-8. [DOI] [PubMed] [Google Scholar]
- 18.Lanfer B, Hermann A, Kirsch M, Freudenberg U, Reuner U, Werner C, et al. Directed growth of adult human white matter stem cell-derived neurons on aligned fibrillar collagen. Tissue Eng Part A. 2010;16:1103–13. doi: 10.1089/ten.tea.2009.0282. [DOI] [PubMed] [Google Scholar]
- 19.Wang JHC, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect Tissue Res. 2000;41:29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- 20.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–9. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camelliti P, Gallagher JO, Kohl P, McCulloch AD. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc. 2006;1:1379–91. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 22.Engelmayr GC, Jr., Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Hu QS, Braunlin EA, Suggs LJ, Zhang JY. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–36. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 24.Dai WD, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 25.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112(Suppl):I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 26.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 27.Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods. 2010;16:1417–26. doi: 10.1089/ten.tec.2010.0068. [DOI] [PubMed] [Google Scholar]
- 28.Freed LE, Engelmayr GC, Jr., Borenstein JT, Moutos FT, Guilak F. Advanced material strategies for tissue engineering scaffolds. Adv Mater. 2009;21:3410–8. doi: 10.1002/adma.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurita KR, Singal A. Mapping action potentials and calcium transients simultaneously from the intact heart. Am J Physiol Heart Circ Physiol. 2001;280:H2053–60. doi: 10.1152/ajpheart.2001.280.5.H2053. [DOI] [PubMed] [Google Scholar]
- 30.Kluge JA, Leisk GG, Cardwell RD, Fernandes AP, House M, Ward A, et al. Bioreactor system using noninvasive imaging and mechanical stretch for biomaterial screening. Ann Biomed Eng. 2011;39:1390–402. doi: 10.1007/s10439-010-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Deweerth SP. PDMS-based conformable microelectrode arrays with selectable novel 3-D microelectrode geometries for surface stimulation and recording. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1623–6. doi: 10.1109/IEMBS.2009.5333446. [DOI] [PubMed] [Google Scholar]