Abstract
There is a growing need to understand muscle cell behaviors and to engineer muscle tissues to replace defective tissues in the body. Despite a long history of the clinical use of electric fields for muscle tissues in vivo, electrical stimulation (ES) has recently gained significant attention as a powerful tool for regulating muscle cell behaviors in vitro. ES aims to mimic the electrical environment of electroactive muscle cells (e.g., cardiac or skeletal muscle cells) by helping to regulate cell-cell and cell-extracellular matrix (ECM) interactions. As a result, it can be used to enhance the alignment and differentiation of skeletal or cardiac muscle cells and to aid in engineering of functional muscle tissues. Additionally, ES can be used to control and monitor force generation and electrophysiological activity of muscle tissues for bio-actuation and drug-screening applications in a simple, high-throughput, and reproducible manner. In this review paper, we briefly describe the importance of ES in regulating muscle cell behaviors in vitro, as well as the major challenges and prospective potential associated with ES in the context of muscle tissue engineering.
Keywords: Electrical stimulation, muscle cells, alignment, differentiation, muscle tissue engineering, bio-actuators, drug-screening models
Introduction
There is a growing need to understand muscle cell biology and to fabricate muscle tissues in vitro. Our current knowledge of the molecular biology, normal physiology, and pathology of muscle tissues is incomplete and must be expanded to confront the associated healthcare problems and improve the quality of life. Engineered muscle tissues are promising candidates with which to study these phenomena.1 Such muscle tissues can also help to replace severely damaged muscle tissues caused by injury, congenital defects, trauma, neuromuscular disorders or tumor ablation. In addition, common clinical treatments of damaged muscle tissues, such as grafting host and healthy muscle tissues to the damaged area or intramuscular injection of myogenic cells, often fail due to volume deficiency and functional loss of healthy muscles.2,3 In this respect, the engineering of muscle tissues has been proposed as a promising approach to regenerate, replace or recover damaged muscle tissues.4,5 Moreover, engineered muscle tissues could have other important applications in drug- and gene-screening models6 and as bio-actuators.7 Engineered muscle tissues can potentially replace animal studies with the advantages of further mimicking human physiological and pathological conditions for the purpose of testing drug candidates and gene therapies. Such an approach may dramatically reduce the time and cost involved in the drug discovery process. Fabricated muscle tissues can also serve as bio-actuators powered by the activation of actin-myosin molecular motors that convert chemical energy into mechanical force. Such mechanical actuators can be used to drive hybrid bio-devices and bio-robots.8
The proper design and fabrication of muscle tissues in vitro require the ability to engineer the components, architecture and function of muscle tissues, which is accomplished through tissue engineering using cells, scaffold materials and growth factors.9 However, the coordination of external and biomimetic stimuli, such as mechanical or electrical stimuli, is important for the fabrication of functional tissues. In particular, ES is an efficient tool for regulating the behaviors of electroactive cells, such as skeletal muscle or cardiac cells, and consequently, for fabricating and controlling the corresponding tissues.10 One of the earliest uses of ES dates back to 1942, when ES was proposed as a useful technique to replace the nervous stimulation in denervated skeletal muscles to preserve muscle tissue functions.11 ES was able to maintain and improve the mass and contractility of denervated muscle tissues. Since then, many studies have been performed to employ this technique to restore lost functions of skeletal muscle or cardiac tissues both in vivo and in vitro.12 Here, we review the importance of ES associated with the regulation of muscle cell behavior and controlling of engineered muscle tissues in vitro. Potential applications and limitations of this technique are also addressed.
Use of ES for Muscle Cell Manipulation
Contractility is an essential electrophysiological feature of muscle cells. Muscle cell contraction at the cellular level is regulated through the so-called excitation-contraction (EC) coupling process.13,14 First, an action potential (AP) is activated in the cell membrane followed by a series of cellular events that relate the AP-mediated excitation to contractility of muscle cell. The most important step in the EC coupling process is Ca2+ ion balance throughout the cell membrane. The APs can be sensed by voltage-gated L-type Ca2+ ion channels of sarcolemma [i.e., dihydropyridine receptors (DHPRs)]. DHPRs interact with Ca2+ release channels [i.e., ryanodine receptors (RyR1)] localized on internal calcium stores [i.e., sarcoplasmic reticulum (SR)] to release Ca2+ ions from the SR lumen into the cytoplasm through RyR1. The chemical gradient of Ca2+ ions within the cell membrane is crucial for the propagation of AP. APs spontaneously propagate throughout cardiac cells, while a nervous stimulus via neuromuscular junctions is required to activate skeletal muscle cells.15 ES aims to recreate such electrical signals for skeletal muscle or cardiac cells in vitro as to generate APs.
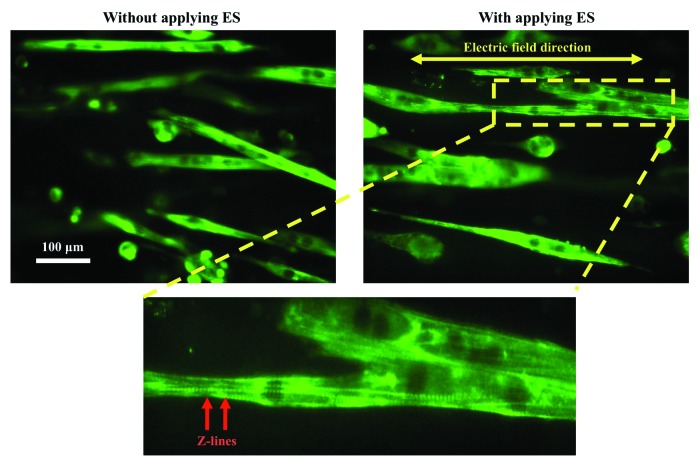
Chronic low frequency ES regimes affect various behaviors of skeletal muscle or cardiac cells, such as alignment,16 differentiation,17 metabolic activity18 and protein synthesis.19 Most importantly, the effect of ES on muscle cell alignment and differentiation is crucial because they are important factors for the fabrication of functional muscle myofibers. For instance, ES has been shown to have a significant effect on C2C12 myotube alignment, causing the myotubes to align themselves parallel to the direction of the applied electric field (Fig. 1).20,21 Even though, microgrooved methacrylated gelatin (GelMA) hydrogel was used to provide the topological cue for muscle cell alignment, the cells were not able to recognize this cue upon excessive growth on the GelMA hydrogel. Here, the ES was used to align C2C12 myotubes deviated from the direction of GelMA micropattern. ES is able to propagate through thick muscle cell structures or muscle myofibers due to intrinsic electrophysiological conductivity of muscle cells and therefore it has an advantage for the muscle cell alignment over commonly used topological cues.
Figure 1. ES of muscle cells on a microgrooved GelMA hydrogel. C2C12 myotubes were immunostained against the myosin heavy chain protein (green). Z-lines within the electrically stimulated myotubes were obvious after ES, indicating highly mature myotubes compared with the non-stimulated myotubes. Reproduced with permission of The Royal Society of Chemistry.20
A recent study also revealed that cardiac cells effectively responded to electric fields, which led to the optimal cardiac cell morphology and function.22 In particular, ES was required to generate excitability and force within muscle constructs. Interestingly, there was an optimum substrate stiffness for muscle cells in which the effect of ES was profound. This study demonstrates that it is required to simultaneously provide suitable mechanical and electrical stimulations for muscle cells to fabricate biomimetic muscle constructs.
ES has also been shown to be able to reverse the effect of phosphatidyl-inositol 3 kinase (PI3K) pathway inhibitors on orientation and elongation of muscle cells.23 Furthermore, ES can induce differentiation of both skeletal muscle and cardiac cells and consequently tissue formation through intracellular Ca+2 oscillations that cause the development of mature sarcomeric structures. For example, Flaibani et al. employed ES to differentiate skeletal muscle cells24 that were cultured on micropatterned poly-(L-lactic acid) membranes. Interestingly, micropatterned membranes increased the effect of ES on the muscle cell differentiation showing the synergistic effect of ES and muscle cell alignment to differentiate muscle cells and to maximize the force generation within contractile muscle myofibers.
The potential application of ES to fabricate functional cardiac tissue constructs has also been demonstrated in which ES was employed to induce synchronous contraction of cardiac myocytes after over only 8 days of culture.25 In these experiments, ES was shown to induce cell alignment and organization mimicking that of native cardiac tissues. As the native myocardium involves multiple cell types, such as fibroblasts and endothelial cells, it would be interesting to examine the effect of ES on co-cultures of such cell types in enhancing the functionality of the resulting tissue construct. The optimization of ES parameters (e.g., electrode material, amplitude and frequency) is important to induce muscle tissue development and maturation as well as to avoid damaging muscle tissues.26 In particular, electrochemical damage to muscle tissues may occur for those stimulated at currents or voltages six times greater than the rheobase, which is the minimum current or voltage required for muscle contraction.27 Taken together, ES has multiple effects on both skeletal muscle and cardiac cells, notably on cell alignment and differentiation. ES is a useful tool in regenerative medicine because it provides effective, safe, inexpensive, and easy to implement stimulus for muscle tissues and does not require exogenous chemicals, such as soluble factors and hormones.28,29
Integration of ES for Engineering and Employing Muscle Tissues
ES has been extensively employed to control and maintain the functionality of engineered muscle tissues. For example, Donnelly et al.30 recently proposed an inexpensive bioreactor for the ES of skeletal muscle tissues. They demonstrated that physiological function of muscle tissues was improved in vitro using their designed bioreactor that allowed the control of ES parameters, such as amplitude, width, frequency and work-to-rest ratio. The designed bioreactor was able to stimulate both two-dimensional (2D) and three-dimensional (3D) muscle tissue cultures. Interestingly, it was feasible to change ES parameters (i.e., voltage, frequency and duration) over a wide range with high accuracy. Therefore, it was possible to precisely examine the effect of ES parameters on muscle cell functions and maturation of the tissue constructs. It was found that voltage had the most important impact on 3D engineered muscle tissues; whereas, frequency and duration were dominant parameters in the response of 2D muscle cell cultures. In the future, it could be interesting to relate such findings with the stimulation of native muscle tissues in vivo.
In another study, rat myoblasts were cultured onto fibrin gels to construct 3D muscle tissues in vitro. Interestingly, ES was able to integrate with nerve constructs attached to muscle tissues and to generate the contractility of muscle tissues through the nerve constructs. The measured contraction forces for stimulated tissues were five times higher than those for non-stimulated muscle tissues.31 This study highlights the importance of fabricating neuromuscular junctions within muscle tissues to have proper muscle tissue integrity and function.
ES has been also used to electrically activate and control cardiac tissues. For instance, we recently demonstrated that ES activated electrophysiological functions of cardiac patches of cardiomyocytes cultured onto hybrid carbon nanotube (CNT)-GelMA hydrogels.32 Most importantly, CNTs increased the conductivity of GelMA hydrogels, and therefore hybrid CNT-GelMA hydrogels enhanced the electroactivity of cardiac patches compared with the pristine GelMA hydrogels. In addition, CNTs could increase the viscoelastic behavior of hydrogels due to the viscoelastic behavior of CNTs,33 which increase the mechanical integrity and robustness of cardiac tissues in response to electric fields. It would be interesting to synthesize other hybrid CNT-scaffold materials to effectively increase the efficiency of ES for cardiac tissues because commonly used scaffolds for cardiac tissue engineering are not conductive at biologically relevant potentials with low frequencies. Anisotropically conductive hybrid CNT-scaffold materials also hold great promise to further increase the efficiency of ES for muscle tissue engineering to obtain highly aligned and functional muscle myofibers. It is worthy to note that there have been some attempts to fabricate such materials by using electrospinning34 or other fabrication35 techniques.
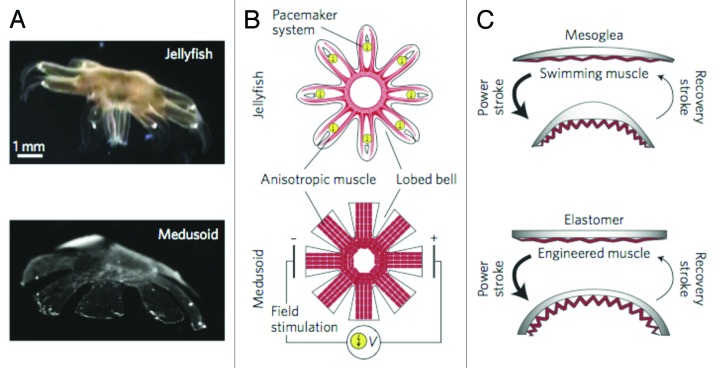
As mentioned earlier, muscle tissues are natural bio-actuators. Upon integration with suitable microsystems, these engineered tissues also can be used for hybrid bio-robotic applications. ES is a powerful tool with which to control the contractility and force generation within muscle tissues or associated bio-actuators. For example, ES has been used to precisely control the biomimetic movement of artificial jellyfish comprised of a single layer of heart muscle cells cultured on a micropatterned polydimethylsiloxane (PDMS) sheet36 (Fig. 2). The synchronized contraction of cardiac cells due to ES led to the bending of PDMS-cell sheet and therefore creating its desirable conformations. Here, optimized and time-dependent ES parameters are required to achieve the desirable movement of artificial jellyfish. Locomotive bio-bots were also recently fabricated using cardiomyocytes and poly(ethylene glycol) hydrogels assembled using a 3D printer.38 Here, ES could involve into fabricated bio-robots to improve their functionality. It is envisioned that neural cells can be involved in such bio-bots to further control muscle cell contraction by the aid of neuromuscular junctions.39 Indeed, the movement of engineered muscle tissues as bioactuators can be controlled by applying and controlling the parameters of ES. An interesting example was demonstrated by controlling the movement of cardiomyocytes cultured on thin PDMS films.40 These hybrid structures could perform many physical activities, such as walking, swimming and pumping, according to different ES protocols. Other influencing parameters to control mechanical performance of such structures were muscle tissue architecture and thickness of PDMS substrates. However, PDMS substrates are often more stiff than native muscle tissues.41 Therefore, researchers have attempted to fabricate muscle cell-based hybrid actuators by using softer materials. For example, in a recent study, Chan et al.42 used the mixture of poly(ethylene glycol)diacrylate and acrylic-poly(ethylene glycol)-collagen hydrogels to fabricate muscle cell-based actuators. In principle, engineered muscle tissues can imitate various aspects of living organisms for various applications, including as bio-robots capable of working in aqueous environments.
Figure 2. Structural characteristics of a natural jellyfish and its synthetic medusoid counterpart. (A) Body structure of the jellyfish (top) and medusoid (bottom). (B) Schematics of shape and anisotropic muscle tissues in the jellyfish (top) and medusoid (bottom). Here, ES was used to control the movement of the medusoid. (C) Stroke kinematics of the jellyfish (top) and medusoid (bottom). Reproduced from ref. 37 with permission.
Fabricated muscle tissues that exhibit physiologically relevant functions are promising candidates for drug-screening applications.43,44 For example, since muscle tissues are a major regulator of blood glucose levels they can be used to test drug candidate against diabetes. The contractility of engineered muscle tissues is required to measure their biological activity and metabolism. ES is capable of inducing the controllable contraction, and, therefore, metabolic activity, of fabricated muscle tissues. For example, we recently demonstrated that ES can be used to selectively induce the contractility of C2C12 myotubes.45 Electrically stimulated myofibers exhibited higher glucose consumption compared with non-stimulated tissues; therefore, they are promising tissue models with which to examine drug candidates and exercise-based therapies for diabetes.
Future Directions
As briefly reviewed, many studies have used ES to regulate muscle cell behavior and functions. However, the development of more sophisticated biomimetic ES devices and the optimization of ES parameters depend on a deeper understanding of cell response mechanisms to ES and associated molecular pathways.46 Based on this knowledge, one can design and implement highly efficient and biomimetic ES setups for muscle cells in vitro, thus increasing the efficiency of ES regimes on muscle cell behaviors and controlling muscle tissue constructs.
Muscle cells sense different types of stimuli in vivo, such as chemical, mechanical, and electrical stimuli. Therefore, it is desirable to mimic such a native and multi-stimuli sensitive environment for muscle cells and tissues in vitro. Multi-mode stimulation approaches can fulfill this requirement by providing several physical and chemical stimuli, simultaneously. For instance, Lu et al.47 recently reported the successful use of combined electrical and mechanical stimulations with a perfusion system to culture cardiac cells. Furthermore, it is expected that future stimulation tools would be able to make intelligent decisions and regulate themselves in a time-dependent manner. Thus, the type and quantity of applied electric fields, as well as the stimulation stop time and rest periods, can be automatically controlled. Such parameters are important to engineer muscle tissues, while maintaining their phenotype and survival for a long culture time. For example, the ES efficiency to induce the differentiation and contraction of muscle cells strongly depends on the time of its initiation because muscle cells require a certain amount of time to develop their excitation-contraction coupling structure before ES is applied to reinforce this structure.48
Micro- and nanofabrication techniques have recently gained much attention in biology and tissue engineering.49 They are powerful tools with which to make novel and efficient devices for ES and to monitor the response of muscle cells at the micro- or nanoscale resolution. Therefore, one can precisely define and control ES parameters even for a single muscle cell or myofiber. For instance, we recently proposed the use of an interdigitated array of Pt (IDA-Pt) electrodes as a novel and microscale ES device.20,50 This device is comprised of Pt microelectrodes fabricated on a glass slide using conventional lithography techniques. Muscle cells can be directly cultured on the IDA-Pt electrodes and therefore this device can produce a more efficient, homogeneous, and reproducible ES regimes for muscle cells compared with conventional stimulator devices. C2C12 myotubes stimulated using this device exhibited higher maturation and contractility compared with those stimulated using conventional ES devices and non-stimulated myotubes.20 We further improved the device performance by making it contactless with the muscle tissues by using a thin coverslip (thickness 50 μm). This device avoids detrimental effects of the direct contact of electrodes with culture medium, such as water electrolysis and electrode corrosion and therefore it provides safe and non-invasive ES to muscle tissues.51
ES is crucial to improve and maintain physiological functions (e.g., contractility and metabolism) of engineered muscle tissues for regenerative medicine applications. However, to increase the electrical response of muscle tissues advanced in materials engineering such as using electrically conductive scaffolds or co-culture with neural cells may be useful. ES also plays a central role in manipulating engineered muscle tissues to mimic biological features. There may be some technical difficulties and a lack of resources that hinder the precise mimicking of such natural features. The contractile ability of muscle tissues triggered and restored by ES can be also employed as a propelling force to drive bio-devices for various applications, such as biohybrid pumps mimicking the performance of the heart or heart pacemakers.37 However, methods to control and monitor the contractility of muscle tissues need to be developed or improved, particularly for 3D muscle tissue constructs that better represent the complexity of in vivo muscle tissues compared with 2D muscle structures. Other interesting applications of ES to manipulate fabricated muscle tissues may be as drug delivery pumps in muscle tissues for long-term and controlled drugs loaded within muscle tissues.
Finally, a greater degree of collaboration among biology, medical, engineering, and materials disciplines may further reveal potential applications of ES and improve the use of this technique for muscle cell and tissue engineering applications. For example, ES has been used for muscle tissue stimulation in vivo (e.g., for clinical applications) more than it has been used in vitro. Therefore, the transfer of knowledge and experience from different specialized domains could lead to advances in using ES in regulating muscle cell behavior.
Acknowledgments
This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Glossary
Abbreviations:
- 2D
two-dimensional
- 3D
three-dimensional
- AP
action potential
- CNT
carbon nanotube
- DHPRs
dihydropyridine receptors
- ECM
extracellular matrix
- ES
electrical stimulation
- GelMA
methacrylated gelatin
- IDA-Pt
interdigitated array of Pt
- PDMS
polydimethylsiloxane
- PI3K
phosphatidyl-inositol 3 kinase
- RyR1
ryanodine receptors
- SR
sarcoplasmic reticulum
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25121
References
- 1.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–7. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 2.Gilmore KJ, Kita M, Han Y, Gelmi A, Higgins MJ, Moulton SE, et al. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials. 2009;30:5292–304. doi: 10.1016/j.biomaterials.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Rossi CA, Pozzobon M, De Coppi P. Advances in musculoskeletal tissue engineering: moving towards therapy. Organogenesis. 2010;6:167–72. doi: 10.4161/org.6.3.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–22. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao H, Zhou G-Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng Part B Rev. 2009;15:319–31. doi: 10.1089/ten.teb.2009.0092. [DOI] [PubMed] [Google Scholar]
- 6.Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17:173–81. doi: 10.1016/j.drudis.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricotti L, Menciassi A. Bio-hybrid muscle cell-based actuators. Biomed Microdevices. 2012;14:987–98. doi: 10.1007/s10544-012-9697-9. [DOI] [PubMed] [Google Scholar]
- 8.Dennis RG, Herr H. Engineered muscle actuators: Cells and tissues. In: Bar Cohen Y, ed. Biomimetics: Biologically Inspired Technologies. New York: CRC Press; 2005:234. [Google Scholar]
- 9.Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009;300:64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 10.Markx GH. The use of electric fields in tissue engineering: A review. Organogenesis. 2008;4:11–7. doi: 10.4161/org.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutmann E, Guttmann L. Effect of electrotherapy on denervated muscles in rabbits. Lancet. 1942;239:169–70. doi: 10.1016/S0140-6736(00)79433-5. [DOI] [Google Scholar]
- 12.Dennis RG, Smith B, Philp A, Donnelly K, Baar K. Bioreactors for guiding muscle tissue growth and development. In: Kasper C, Griensven M, Pörtner R, eds. Bioreactor Systems for Tissue Engineering. Heidelberg: Springer Berlin Heidelberg; 2009:39-79. [DOI] [PubMed] [Google Scholar]
- 13.Andersson DC, Betzenhauser MJ, Marks AR. Excitation-contraction coupling in the heart. In: Hill JA, Olson EN, eds. Muscle: Fundamental Biology and Mechanisms of Disease. San Diego, CA: Academic Press, 2012:153-60. [Google Scholar]
- 14.Schneider MF, Hernández-Ochoa EO. Skeletal muscle excitation-contraction coupling. In: Hill JA, Olson EN, eds. Muscle: Fundamental Biology and Mechanisms of Disease. San Diego, CA: Academic Press, 2012:811-21. [Google Scholar]
- 15.Ríos E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- 16.Wehrle U, Düsterhöft S, Pette D. Effects of chronic electrical stimulation on myosin heavy chain expression in satellite cell cultures derived from rat muscles of different fiber-type composition. Differentiation. 1994;58:37–46. doi: 10.1046/j.1432-0436.1994.5810037.x. [DOI] [PubMed] [Google Scholar]
- 17.Stern-Straeter J, Bach AD, Stangenberg L, Foerster VT, Horch RE, Stark GB, et al. Impact of electrical stimulation on three-dimensional myoblast cultures - a real-time RT-PCR study. J Cell Mol Med. 2005;9:883–92. doi: 10.1111/j.1582-4934.2005.tb00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor MK, Irrcher I, Hood DA. Contractile activity-induced transcriptional activation of cytochrome C involves Sp1 and is proportional to mitochondrial ATP synthesis in C2C12 muscle cells. J Biol Chem. 2001;276:15898–904. doi: 10.1074/jbc.M100272200. [DOI] [PubMed] [Google Scholar]
- 19.Brevet A, Pinto E, Peacock J, Stockdale FE. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science. 1976;193:1152–4. doi: 10.1126/science.959833. [DOI] [PubMed] [Google Scholar]
- 20.Ahadian S, Ramón-Azcón J, Ostrovidov S, Camci-Unal G, Hosseini V, Kaji H, et al. Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab Chip. 2012;12:3491–503. doi: 10.1039/c2lc40479f. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, et al. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng Part A. 2012;18:2453–65. doi: 10.1089/ten.tea.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhana B, Iyer RK, Chen WLK, Zhao R, Sider KL, Likhitpanichkul M, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–60. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 23.Au HTH, Cheng I, Chowdhury MF, Radisic M. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 2007;28:4277–93. doi: 10.1016/j.biomaterials.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaibani M, Boldrin L, Cimetta E, Piccoli M, De Coppi P, Elvassore N. Muscle differentiation and myotubes alignment is influenced by micropatterned surfaces and exogenous electrical stimulation. Tissue Eng Part A. 2009;15:2447–57. doi: 10.1089/ten.tea.2008.0301. [DOI] [PubMed] [Google Scholar]
- 25.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med. 2011;5:e115–25. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khodabukus A, Baar K. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle. Tissue Eng Part C Methods. 2012;18:349–57. doi: 10.1089/ten.tec.2011.0364. [DOI] [PubMed] [Google Scholar]
- 28.Balint R, Cassidy NJ, Cartmell SH. Electrical stimulation: a novel tool for tissue engineering. Tissue Eng Part B Rev. 2013;19:48–57. doi: 10.1089/ten.teb.2012.0183. [DOI] [PubMed] [Google Scholar]
- 29.Hronik-Tupaj M, Kaplan DL. A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng Part B Rev. 2012;18:167–80. doi: 10.1089/ten.teb.2011.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16:711–8. doi: 10.1089/ten.tec.2009.0125. [DOI] [PubMed] [Google Scholar]
- 31.Dhawan V, Lytle IF, Dow DE, Huang Y-C, Brown DL. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 2007;13:2813–21. doi: 10.1089/ten.2007.0003. [DOI] [PubMed] [Google Scholar]
- 32.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–80. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suhr J, Koratkar N, Keblinski P, Ajayan P. Viscoelasticity in carbon nanotube composites. Nat Mater. 2005;4:134–7. doi: 10.1038/nmat1293. [DOI] [PubMed] [Google Scholar]
- 34.Sung JH, Kim HS, Jin H-J, Choi HJ, Chin I-J. Nanofibrous membranes prepared by multiwalled carbon nanotube/poly(methyl methacrylate) composites. Macromolecules. 2004;37:9899–902. doi: 10.1021/ma048355g. [DOI] [Google Scholar]
- 35.Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5:7383–90. doi: 10.1021/nn2023057. [DOI] [PubMed] [Google Scholar]
- 36.Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012;30:792–7. doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel V. Soft robotics: Bionic jellyfish. Nat Mater. 2012;11:841–2. doi: 10.1038/nmat3438. [DOI] [PubMed] [Google Scholar]
- 38.Chan V, Park K, Collens MB, Kong H, Saif TA, Bashir R. Development of miniaturized walking biological machines. Sci Rep. 2012;2:857. doi: 10.1038/srep00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umbach JA, Adams KL, Gundersen CB, Novitch BG. Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS One. 2012;7:e36049. doi: 10.1371/journal.pone.0036049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Muscular thin films for building actuators and powering devices. Science. 2007;317:1366–70. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 41.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan V, Jeong JH, Bajaj P, Collens M, Saif T, Kong H, et al. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip. 2012;12:88–98. doi: 10.1039/c1lc20688e. [DOI] [PubMed] [Google Scholar]
- 43.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve. 2008;37:438–47. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 44.Vandenburgh H. High-content drug screening with engineered musculoskeletal tissues. Tissue Eng Part B Rev. 2010;16:55–64. doi: 10.1089/ten.teb.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaji H, Ishibashi T, Nagamine K, Kanzaki M, Nishizawa M. Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials. 2010;31:6981–6. doi: 10.1016/j.biomaterials.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 46.Meng S, Rouabhia M, Zhang Z. Electrical stimulation in tissue regeneration. In: Gargiulo GD, McEwan A, eds. Applied Biomedical Engineering. InTech, 2011:37-62. [Google Scholar]
- 47.Lu L, Mende M, Yang X, Körber H-F, Schnittler H-J, Weinert S, et al. Design and validation of a bioreactor for simulating the cardiac niche: a system incorporating cyclic stretch, electrical stimulation, and constant perfusion. Tissue Eng Part A. 2013;19:403–14. doi: 10.1089/ten.tea.2012.0135. [DOI] [PubMed] [Google Scholar]
- 48.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169–87. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramón-Azcón J, Ahadian S, Obregón R, Camci-Unal G, Ostrovidov S, Hosseini V, et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip. 2012;12:2959–69. doi: 10.1039/c2lc40213k. [DOI] [PubMed] [Google Scholar]
- 51.Ahadian S, Ramón-Azcón J, Ostrovidov S, Camci-Unal G, Kaji H, Ino K, et al. A contactless electrical stimulator: application to fabricate functional skeletal muscle tissue. Biomed Microdevices. 2013;15:109–15. doi: 10.1007/s10544-012-9692-1. [DOI] [PubMed] [Google Scholar]