Abstract
Cell sheet engineering has been progressing rapidly during the past few years and has emerged as a novel approach for cell based therapy. Cell sheet harvest technology enables fabrication of viable, transplantable cell sheets for various tissue engineering applications. Currently, the majority of cell sheet studies use thermo-responsive systems for cell sheet detachment. However, other responsive systems began showing their potentials for cell sheet harvest. This review provides an overview of current techniques in creating cell sheets using different types of responsive systems including thermo-responsive, electro-responsive, photo-responsive, pH-responsive and magnetic systems. Their mechanism, approach, as well as applications for cell detachment have been introduced. Further development of these responsive systems will allow efficient cell sheet harvesting and patterning of cells to reconstruct complex tissue for broad clinical applications.
Keywords: cell sheet engineering, poly(N-isopropylacrylamide), responsive systems, cell sheet detachment
Introduction
Cell sheet technology enables a sheet of interconnected cells to be obtained and further layered to form tissue-like and organ-like structures. The rapid progression of the technology has emerged as a novel approach for cell based therapy. The most profound advantage of cell sheet technology is that it could achieve high cell density as well as retain cell-cell junctions and deposited extracellular matrix (ECM). In a series of studies, Okano’s group has proven the superiority of cell sheet transplantation compared with cell injection for cardiac repair.1-5 Using thermo-responsive culture dishes, confluent cardiac cell sheets were harvested without any enzymatic treatment. The stacked cardiac cell sheets developed sheet-to-sheet communication via cell-cell junctions and could pulsate synchronously.2 After cardiac cell sheet transplantation, increased cell survival rate compared with cell injection was observed and cardiac function was significantly improved. Importantly, successful engraftment of cell sheets to the host tissue was demonstrated.3 In addition to cardiac applications, cell sheet technology has been used to treat many other diseases and have generated promising results. For patients with bilateral total limbal stem-cell deficiency, autologous epithelial cells were harvested from oral mucosa and grew into cell sheets for transplantation onto the ocular surface. The presence of ECM in the cell sheet provided sufficient adhesion to stabilize it to the host eye without sutures. Visual acuity was dramatically improved 2–8 weeks after the epithelial cell sheet transplantation.6 Layered fibroblast cell sheets have been proven to be a novel lung air leak sealant.7 The feasibility of using cell sheet technology to treat bone fractures, Periodontitis, esophageal cancer, diabetes and liver disease have all been explored. The results, both experimental and clinical, have demonstrated the efficacy of cell sheet technology as an effective approach for regenerative medicine.
Cell sheet harvest technology enables fabrication of viable, transplantable cell sheets for various tissue engineering applications. Currently, the majority of cell sheet studies use thermo-responsive systems for cell sheet detachment. However, other responsive systems began showing their potentials for cell sheet harvest. This review provides an overview of the current techniques in creating cell sheets using different types of responsive systems. Their mechanism, approach and applications for cell detachment have been included.
Thermo-responsive Systems
The use of thermo-responsive systems has created a novel way to harvest cell sheets and greatly accelerated the progress of cell sheet engineering. The most studied thermo-responsive system is a poly(N-isopropylacrylamide) (pNIPAAm) modified surface. pNIPAAm is a well characterized thermo-responsive polymer that undergoes a sharp coil-globule transition at its lower critical solution temperature (LCST) of 32°C in aqueous solution, changing from a hydrophilic state to a hydrophobic state.8 Therefore, a surface retaining insoluble pNIPAAm is hydrophobic, allowing cells to attach, when the temperature is higher than 32°C. The surface becomes hydrophilic to release cells once the temperature falls below 32°C. The critical temperature of pNIPAAm can be further altered by copolymerization using hydrophilic or hydrophobic monomers for broad biomedical applications.9
Different methods have been developed to fabricate pNIPAAm modified thermo-responsive surfaces for cell sheet engineering (Table 1). Electron beam (EB) polymerization is the most widely used method to graft N-isopropylacrylamide (NIPAAm) onto tissue culture polystyrene (TCPS) for cell sheet engineering.10 Briefly, TCPS dishes are uniformly coated by NIPAAm monomer solution (in 2-propanol) and irradiated with 0.3 MGy EB. The non-grafted NIPAAm monomers are rinsed off with deionized (DI) water after irradiation and the resulting pNIPAAm-modified dishes are used to culture cells. Okano’s group, the pioneers of cell sheet engineering, has extensively studied the surface properties of the pNIPAAm-modified dishes fabricated by EB polymerization and reported that cell adhesion is greatly affected by the thickness of the grafted NIPAAm layer. A thin layer, ~15.5 ± 7.2 nm, with a low density of pNIPAAm allows for cell attachment at 37°C and detachment from the surface at 20°C.11 However, a thick layer, 29.3 ± 8.4 nm, with a high density of pNIPAAm was found to have no cell adhesion at 37°C although both graft thicknesses show a change in wettability between temperatures above and below LCST. According to their studies, an effective thickness of a grafted pNIPAAm layer should be between 15 nm and 20 nm to obtain optimal cell attachment and detachment in response to temperature changes. This electron beam polymerization of NIPAAm has been used in cell sheet technology for many different cell types including keratinocytes, corneal and oral mucosal epithelia cells, myocardial cells, hepatocytes, and skeletal myoblasts.11-15
Table 1. Comparison of different types of pNIPAAm modified thermoresponsive surfaces for cell sheet engineering.
| Fabrication Method | Underlying Substrate | Detached Cell Type | Detachment time | Refs. |
|---|---|---|---|---|
| Electron Beam Polymerization |
Tissue Culture Polystyrene (TCPS) |
Keratinocytes, Corneal and Oral mucosal epithelia cells, Myocardial cells, Hepatocytes, Skeletal myoblasts |
1 h at 20°C |
11–15 |
| Plasma Polymerization |
Silica Wafer, Glass, TCPS |
Bovine artery carotid endothelial cells, Retinal cells |
2 h at 20°C |
16,20–23 |
| UV irradiation |
Polydimethylsiloxane, Poly (ethylene terephthalate) |
Smooth muscle cells, Retinal pigment epithelial cells |
30 min |
24,25 |
| Solvent Cast |
Not Grafted; solid support underneath: TCPS, Glass |
3T3 Fibroblasts, HUVEC |
20 min at 4°C |
27–27 |
| Spin-Coated w/APTES | Si Wafer, Glass | Fibroblasts, MSCs | 2 min at 20°C | 31 |
Another tested method to graft pNIPAAm is through vapor phase plasma polymerization. A plasma glow discharge of NIPAAm monomer vapor was used to deposit pNIPAAm onto solid surfaces such as silicon, glass, and TCPS.16 The deposition process includes an 80W methane plasma deposition followed by NIPAAm plasma deposition with stepwise decreasing powers from 80 to 1 W for 30 min. The pNIPAAm-grafted surfaces are then rinsed with DI water to remove non-cross-linked molecules.17 The plasma glow discharge power is slowly reduced to form an adhesion promoting layer on the substrate and to deposit a functional coating at the outer surface.18 The advantage of plasma polymerization is that it is a one step, solvent free vapor phase coating technique. A major concern over the use of this technique is the loss of chemical functional groups and material functionality in the coating due to possible monomer fragmentation. High power and high temperature conditions during the plasma polymerization process yields the most stable films. However, high temperature also reduces the chemical functionality of the grafted monomer.19 Pan et al.16 were the first group to overcome the fragmentation problem by finding an optimal balance between power and temperature conditions during the polymerization of NIPAAm monomer. Contrary to EB polymerized pNIPAAm, the thickness of pNIPAAm film fabricated by plasma polymerization does not have an impact on cell attachment. Cell studies showed no difference between different batches of plasma polymerized films when the batches varied in thickness from one another.20,21 Thus, cell adhesion and proliferation are independent of plasma deposited film thickness. Grafting of pNIPAAm through plasma polymerization has been used to detach bovine artery carotid endothelial cells and retinal cells.20-23
An alternative approach to graft pNIPAAm is through UV (UV) irradiation. Plasma-induced UV or heat polymerization of NIPAAm monomers has been successfully immobilized on polydimethylsiloxane (PDMS) surfaces and applied to smooth muscle cell (SMC) sheet detachment.24 The PDMS surface is activated by Argon plasma to generate peroxides on the surface. These peroxides will decompose by UV light or heat to initiate the polymerization of NIPAAm solution. UV polymerization can also be used to graft pNIPAAm to TCPS surfaces as well. One such method involves the entrapment of copolymers NIPAAm and 4-(N-cinnamoylcarbamide)methylstyrene (CCMS) onto a poly(ethylene terephthalate) (PET) surface. The surface is irradiated with UV light to cross-link the copolymer through dimerization of the cinnamoyl groups.25 Similar to plasma polymerized pNIPAAm, the thickness of the film does not affect cell attachment and proliferation.
Solvent casting methods have also been employed to create bulk pNIPAAm films. These films are not grafted to a substrate. Instead, the pNIPAAm films are conjugated with collagen and deposited on a solid support such as TCPS or glass. Here, cells attach due to the collagen in the film and detach because of the dissolution of pNIPAAm under cooling conditions.26 Solvent casting methods have also been used to deposit thick pNIPAAm films which are coated with adhesion molecules such as collagen, fibronectin or laminin. The addition of the overlaying adhesion coating does not affect the detachment rates of cells, as evidenced by culture and detachment of 3T3 fibroblasts and human umbilical vein endothelial cells (HUVEC).27,28
More recently, spin-coating techniques have been developed to deposit pNIPAAm thin films on substrates without the need for expensive equipment such as EB or plasma polymerization equipment. Reed et al.29 diluted pNIPAAm in hydrochloric acid (HCl) and mixed it with tetraethyl orthosilicate (TEOS). They deposited this solution on glass coverslips and spun the substrate at 2000 rpm for 60 sec to yield a thin pNIPAAm film on glass coverslips. Cells grew to confluence on the surfaces but detached in clumps instead of as whole cell sheets. Another disadvantage to the system is the dissolution of pNIPAAm, leading to possible cytotoxic effects on the cells. A study by Nash et al.30 spin-coated pNIPAAm/ethanol mixtures onto thin films on Thermanox disks. The resulting films exhibited a rapid cell sheet detachment for various cell types, ranging from detachment times of 5–10 min, and in some cases ~60 min when 4°C media was placed over the film. However, the authors could not provide a convincing explanation for the mechanism of their system since many others reported that bulk films with similar properties were incapable of attaching and growing cells due to the inability of pNIPAAm to provide anchor points for cell attachment. The study, nevertheless, indicated the potential of spin-coating pNIPAAm films for cell attachment/growth and rapid cell sheet detachment without the use of expensive equipment.
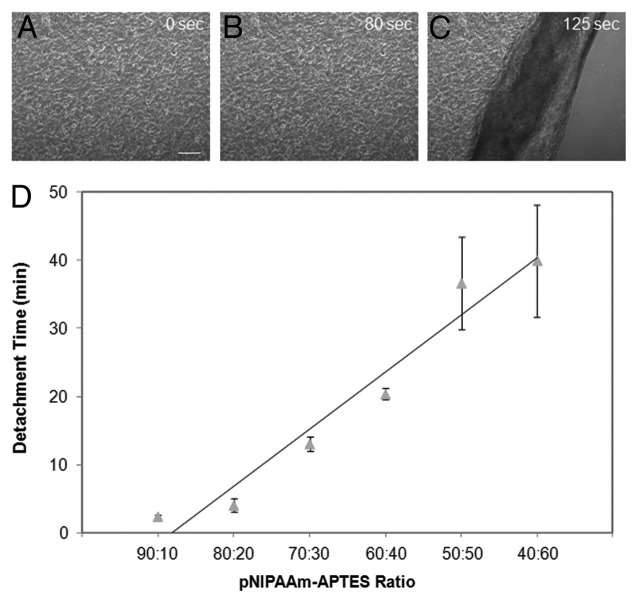
Our laboratory has developed a novel approach to graft pNIPAAm films onto silica based surfaces.31 Utilizing a spin-coating technique, thermo-responsive films were deposited on glass slides using pNIPAAm blended with 3-aminopropyltriethoxysilane (APTES). APTES was used to enhance the retention of pNIPAAm on the surface while providing the anchor points needed for cell attachment and proliferation. Additionally, changing the concentration of the adhesion promoting agent allowed for tunable detachment rates. When the surface was cooled, the pNIPAAm chains extended, pushing the cells away from the APTES anchor points, thereby releasing the cells. By changing the ratio of pNIPAAm to APTES, the detachment times of the cell sheets ranged from 2.5 min to 40 min (Fig. 1). The spin coating technique used in our research provides a straightforward and economical approach for creating cell sheets in comparison to the traditional techniques used to create other thermo-responsive surfaces.
Figure 1. (A–C) Cell detachment from 90:10 pNIPAAm/APTES films cured at 160{degree sign}C. Time indicated is after cold medium induction. Scale bar denotes 200µm. (D) Cell sheet detachment time can be tuned using films containing different pNIPAAm/APTES ratios. The cell detachment time shows a rough linear relationship vs. pNIPAAm/APTES ratios. 90:10 pNIPAAm to APTES ratio substrates had cell detachment times of ~2 min. With decreased pNIPAAm to APTES ratio, the cell detachment time increases to ~40 min with the 40:60 ratio.
Electro-responsive Systems
Electro-responsive systems, which enable cell attachment and release upon an electrical trigger, represent a second common platform for cell sheet engineering. One example of an electro-responsive system was developed by the Mrksich group.32 Electroactive self-assembled monolayers (SAMs) on gold were utilized in their approach to immobilize ligands. The electroactive molecules tethered to the monolayer can be oxidized by applying electrical potential to the gold film releasing the immobilized ligands. By selecting the peptide ligand that mediates cell attachment, the system can be electrically switched to permit cell adhesion or detachment. In particular, the peptide Cys-Gly-Arg-Gly-Asp-Ser (CGRGDS) containing RGD as a cell adhesive ligand was tethered to monolayers of alkanethiolates via electroactive O-silyl hydroquinone. Fibroblast cells were cultured on the RGD-presenting monolayers. When an electrical potential of 550 mV was applied to the monolayers for 5 min, the O-silyl hydroquinone oxidized to benzoquinone and the silyl ether was hydrolyzed, releasing the RGD containing peptide from the monolayer with the attached cells. This approach has been used to control cell adhesion, pattern cells and activate cell migration.32,33
Fukuda et al.34 have exploited a similar electro-responsive system for efficient cell sheet detachment. The RGD-containing oligopeptide CCRRGDWLC was designed to form a gold-thiolate bond on a gold-coated substrate. Fibroblasts were cultured on the substrate for 7 d and grown into a confluent cell sheet. The viable cell sheet was detached within 10 min after application of -1.0V electrical potential to the surface. The cell sheet detachment was caused by the peptide desorption from the gold substrate by electrical stimulus.
Another example of an electro-responsive system for controlling release of a cell sheet is polyelectrolyte-modified surfaces. Polyelectrolytes adsorb to oppositely charged surfaces due to electrostatic interaction and desorbs from the conducting substrates upon electrochemical polarization. Based on this mechanism, an electro-responsive system was developed for harvesting cell sheets. The Voros group had shown that a poly(l-lysine) (PLL) grafted poly(ethylene glycol) (PEG) monolayer on metal oxide can be desorbed by electrochemical polarization of the substrate.35 By exploiting this concept, they were able to desorb a PLL-g-PEG/PEG-RGD monolayer from indium tin oxide (ITO) by applying a short positive potential.36 More specifically, the adsorbed PLL-g-PEG/PEG-RGD monolayer allows cells to attach to the metal oxide substrate. When the monolayer desorbs, the cells lose their attachment points and detach. The thickness of the polyelectrolyte layer has been shown to impact cell adhesiveness. Multiple layers lead to thicker, softer films with weaker adhesive properties. This can be overcome by increasing the pH at which polyelectrolyte films are assembled, leading to stiffer films and better adhesive properties. Several different cell types have been grown to confluence and detached as cell sheets from these surfaces including hepatocytes, fibroblasts, endothelial cells, and HeLa cells.
In order to create more complex cell sheets with multiple cell types, Voros et al.37 used an electrochemically responsive platform for micro-patterning and release of cell sheets. Biointerfacial properties of the micro-patterned regions can be switched electrochemically by controlling the dissolution and adsorption of polyelectrolyte coatings. Micro-patterns were created by insulating SU-8 on transparent ITO in the desired formations through photolithography methods. A layer of a polyelectrolyte was placed over the platform while the polyelectrolyte was shielded from the electrochemical treatment by a photoresist stencil. Cells grown on the ITO detached upon electrochemical dissolution of the polyelectrolyte substrate. Cells grown on the neighboring weakly adhesive substrate detached through the contractile forces generated by the cells detaching from the ITO domains. This approach allows high precision in cell patterning for cell sheet formation. A drawback of using polyelectrolyte-modified surfaces to detach cell sheets is that electrochemical dissolution of polyelectrolyte coatings could cause local pH change, which may be harmful to sensitive cells.
Photo-responsive Systems
Light is an ideal stimulus for responsive surfaces because it can be controlled with high spatial and temporal resolution. One strategy of developing photo-responsive surfaces is to use light to change the wettability of a surface. Metal oxides, mainly Zinc oxide (ZnO) and Titanium dioxide (TiO2), are the most studied for this application since their wettability can be switched between hydrophilicity and hydrophobicity by light illumination. In a recent published article, a light-induced cell detachment approach has been investigated using TiO2 due to its biocompatibility and wettability variation when illuminated at a cell safe wavelength of 365 nm.38 Mouse calvaria-derived, pre-osteoblastic MC3T3-E1 cells were seeded on TiO2 nanodot films that were coated on a quartz substrate. The data demonstrated detachment of cell sheets after 20 min of UV365 illumination. The exact mechanism of cell detachment is still under investigation; however, it is probably caused by the release of adsorbed adhesive protein when the surface becomes more hydrophilic under illumination.
Photo-responsive molecules have been explored to control adhesion and detachment of cells. Spiropyran is a photosensitive molecule that isomerizes when exposed to UV light from a hydrophobic spiro conformation to the polar hydrophilic zwitterionic merocyanine conformation.39 Higuchi et al.39 have used this concept to graft spiropyran to poly(methyl methacyrlate) (PMMA) and use the developed copolymer to realize UV-regulated detachment of fibrinogen, platelets, and mesenchymal stem cells.
Photo-responsive surfaces can also be fabricated by incorporating spiropyran into the side chains of pNIPAAm. Because irradiating spiropyran with UV light enhances cell adhesion, incorporating spiropyran into pNIPAAM can prevent cell detachment when the surface is cooled. The advantage to this system is the ability to spatially control detachment of cells for patterning. Edahiro et al.40 used this concept by attaching cells at 37°C to a pNIPAAm-spiropyran surface. They UV irradiated a region and then cooled and washed the entire surface. Cells remained adhered to the UV irradiated region while cells in the other region detached. By irradiating the unattached portion with visible light (400–440 nm), the spiropyran isomerizes back into the nonionic structure and the cells can be detached by cooling the surface. The researchers demonstrated the ability to spatially control cell attachment through the use of a photomask. This system can be used to control the location of a particular cell type in a co-culture and harvest the cell sheet with the intended arrangement.
Another photosensitive molecule being explored is azobenzene which isomerizes by UV light from trans to cis form. The isomerization is fully reversible and extremely fast. RGD peptides were coupled with azobenzene derivatives and coated on PMMA disks.41 The azobenzene is responsible for controlling the spacing between RGD peptides and PMMA disks leading to either attachment or detachment of cells. Cell adhesion was achieved when the azobenzene derivative was in its trans form, while cells detached when azobenzene isomerized into its cis form after irradiating with 366 nm light. The light irradiation caused the azobenzene to shorten, moving RGD peptides closer to the surface, resulting in cell detachment. Although cell sheet detachment was not shown, the photo-responsiveness of azobenzene can be utilized to control cell adhesion.
RGD peptides have also been used with a photocleavable linker to provide a photo-responsive surface for cell adhesion and release. Wirkner et al.42 used a photolabile 4,5-dialkoxy 1-(2-nitrophenyl)-ethyl group to attach to the free amine groups of a surface. A tetraethyleneglycol (TEG) spacer was included in the structure enabling the photolabile group to also bind to RGD peptides. The RGD peptides provide a surface for cells to attach, while the photolabile group links the RGD to the substrate. Upon light exposure, the chromophore was photocleaved, removing the linker along with the RGD and the bound cells from the substrate. Using this method, about 85% of adhered HUVEC were detached from the surface, while the remaining cells displayed a rounded morphology. The inability of some cells to attach can be attributed to the incomplete cleavage of the photolinker. Although all the cells did not release, this technology has the ability to be applied to any substrate as long as the linker is properly designed.
A vastly different approach to releasing cells from surfaces using light was taken by the Mohwald group.43 Fibroblasts were cultured on gold nanoparticle based surfaces. Gold nanoparticles have strong photoabsorpotion in the green spectral range. They irradiated the surface with a green laser (532 nm), causing a photochemical mechanism to produce reactive oxygen species (ROS). The ROS caused damage to cell membranes at the cell-surface interface, releasing the cells from the substrate. The cells did not immediately detach from the surface, but rather take up to 24 h to fully detach. One advantage to the system is that the surface is able to recover, allowing the reattachment of cells in the irradiated areas within 72 h. This property can be used to spatially pattern cells, and create co-culture cell sheets by controlling the areas the green laser is irradiated and seeding different cell types once the surface has recovered to allow reattachment.
Another photo-responsive material explored for cell attachment and detachment is a methacrylate with a photocleavable unit, 4-[4-(1-methacryloyloxyethyl)-2-methoxy-5-nitrophenoxy]butyric acid (PL).44 These PL groups were placed in the side chains of poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate) (PMB). PMB is a bioinert material preventing the adhesion of cells to its surface. The PL groups provide adhesion points for cell attachment. Under irradiation with 365 nm UV light, the 2-nitrobenzyl group is cleaved off, causing loss of the adhesion points on PL groups. Cells were seeded on the PMB-PL surface and allowed to attach for 4 h. The surface was irradiated with UV light for 60 sec, resulting in detachment of 50% of adhered cells. Cells seeded on previously irradiated regions showed no attachment to the surface, indicating the loss of the adhesion points on the PL groups. The photo-responsive surface used here shows the ability to rapidly detach cells although PL group density needs to be optimized to allow for complete detachment.
pH-responsive Systems
pH-responsive systems have been extensively studied as drug delivery carriers because of the varying pH in the human body that can be used to direct response. For example, tumor tissue has an extracellular pH of 6.5–7.2, which is lower than the normal pH of 7.4. Thus acid triggered pH-responsive systems can be used to specifically deliver anti-cancer drugs. The remarkable change in pH along the gastrointestinal tract (from acid in stomach to basic in the intestine) has also served as potent stimuli for pH-responsive systems of oral drug delivery. Ionisable polymers containing either acidic or basic pendant groups in their structure that can accept or release protons in response to pH changes in the surrounding environment are candidates for pH-responsive systems. Examples of common polymers used in pH-responsive systems are: polyacrylamide (PAAm), poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA), poly(2-diethylaminoethyl methacrylate) (PDEAEMA) and poly(N,N-dimethylaminoethylmetha crylate) (PDMAEMA).9,45
Only a few studies, however, have been performed using pH-responsive systems for cell-based applications due to the limited range of pH (6.8~7.4) for normal cell functions. A recent publication by Ehrbar et al.46 has shown the feasibility of controlling cell sheet detachment by either local or global pH lowering. The pH responsive substrates were formed by alternate layering of cationic poly(allylamine hydrochloride) (PAH) layers and anionic poly(styrene sulfonate) (PSS) layers on a conductive ITO surface. Placenta derived mesenchymal stem cells (PD-MSCs) easily adhered and proliferated to confluence on polyelectrolyte surfaces. Because this system is similar to an electro-responsive system, an electrical trigger with a current density of 30uA/cm2 was found to detach the cells with their ECM intact within 10–20 min. The Ehrbar group hypothesized that one of the mechanisms driving cell sheet detachment was the drop in local pH at the cell-substrate interface. Thus, instead of using an electrical trigger to detach cell sheets, they tried inducing the cells to detach by decreasing the bulk pH through change of culture media pH. A range of pH 5.0 to 7.4 resulted in no change in cell adhesion, while a pH of 4.0 resulted in complete cell sheet detachment within 2–3 min. After conducting viability assays and mesodermal plasticity experiments, the detached PD-MSCs retained their viability and differential potential after exposure to acidic media. Thus, the pH responsive substrate used in this study provided another method to release cell sheets from a surface, although caution must be taken for cells sensitive to pH change.
Chen et al.47 use chitosan, a natural polymer, to attach/detach cells with a change in pH. The primary amine of the glucosamine residue on chitosan makes the polymer a pH responsive cation.48 When the cells are cultured on the chitosan in a medium with a pH of 7.2, the fibronectin excreted from the cells is adsorbed on the chitosan substrate. The adsorption allows the cells to attach to the chitosan surface. Chitosan has an isoelectric point at pH 7.4.49 At a pH of 7.2, the chitosan surface becomes positively charged, allowing for the adsorption of the negatively charged fibronectin. When the pH of the medium is raised to 7.65, the chitosan surface deprotonates and exhibits a positive charge. The fibronectin desorbs from the surface resulting in the detachment of cells. Although Chen et al.47 did not demonstrate the detachment of an intact cell sheet, they have shown promise for creating cell sheet modules using pH responsive chitosan at physiological conditions.
Magnetic Systems
The use of magnetic force to aid in attachment and release of cells have been studied through ferrous based nanoparticle labeling of cells. Magnetite (Fe3O4) nanoparticles were mixed with N-(a-trimethylammonioacetyl)-didodecyl-D-glutamate chloride (TMAG) to create magnetite cationic liposomes (MCLs). The electrostatic interaction between positively charged MCL and negatively charged cell membrane resulted in a high adsorption rate of magnetite nanoparticles in target cells.50 Cells labeled with magnetite nanoparticles are easily influenced by an external magnetic field, resulting in an accumulation of cells toward the source magnet. Ito et al.51 exploited this property to attach and release keratinocytes using magnetic force. Magnetite labeled keratinocytes were cultured on ultra low attachment plates that had a covalently bound hydrophilic and neutral hydrogel on its surface. Upon placement of a neodymium magnet under the plate, cells evenly spread on the surface, while no cell spreading was observed in the absence of a magnet. In order to detach the cell sheet, the magnet was removed from underneath the plate and a polyvinylidene fluoride (PVDF) membrane was placed on the surface of the magnet. The magnet was moved on top of the cells and the magnetic force resulted in the keratinocyte sheet sticking to the PVDF membrane on the magnet surface. With the use of the magnet, the cells could be transferred to a new location, and the PVDF membrane could subsequently be removed from the magnet to detach the cells from the magnet. No cell toxicity has been reported by the use of the magnetite nanoparticle technology and has been applied to create and transfer cell sheets of many different cell types including keratinocytes, cardiomyocytes, hepatocytes, endothelial cells, mesenchymal stem cells, and retinal pigment epithelial cells.51-55 Although the system is effective in releasing and transferring cells, the detached sheets are not cell monolayers, but aggregates of the detached cells clumped together forming several layer thick sheets.
Key Challenges and Future Directions
Responsive systems triggered by different stimuli have been developed to enable the detachment of confluent cell sheets, which is one of the most critical steps in cell tissue engineering. While mounting evidence has demonstrated the potential of these systems, more cell studies are needed for further optimization. Current commercially available responsive surfaces for cell sheet detachment are very expensive especially in consideration for the number of cell sheets required to layer them into 3D tissue constructs. In addition, most of the approaches involve non-easily accessible facilities and complicated procedures, which will hinder tissue culture laboratories in custom designing their own surfaces for specific downstream applications. Therefore, one of the major challenges to overcome in the future is developing simple and economical methods for responsive surface fabrication, which will greatly encourage researchers to exploit cell sheet engineering. Another challenge is to accelerate the cell sheet detachment time. It takes upwards of 40 min for a single cell sheet to detach using the most popular commercial available thermo-responsive surface (UpCell®).56 The exposure to prolonged cooling will affect cell function and dramatically increase the time needed for cell sheet stacking. There are several aforementioned technologies discussed in this review article that strive to address improving cell detachment times. But, these technologies are yet to be fully adapted in the field. With further improvement in techniques and incorporation with other microfabrication methods (e.g., micropatterning), cell sheet engineering will bring tissue engineering constructs one step closer to clinical applications.
Conclusions
Cell sheet engineering based on a thermo-responsive surface has been proposed by Okano as a promising strategy for fabrication of viable tissue equivalents. It has sparked great interests in the field of tissue engineering and regenerative medicine during the past two decades. However, it usually takes more than 30 min for a confluent cell sheet to detach from pNIPAAm modified thermo-responsive surfaces, which hinders future clinical applications when multiple layers of cell sheets are needed. This review summarizes recent advances in improving pNIPAAm modified thermo-responsive surfaces toward rapid cell-sheet recovery. In addition, other stimuli-responsive systems with potential use for cell sheet harvest are also introduced here. Further development of these responsive systems will allow efficient cell sheet harvesting and patterning of cells to reconstruct complex tissue for broad clinical applications.
Glossary
Abbreviations:
- pNIPAAm
poly(N-isopropylacrylamide)
- ECM
extracellular matrix
- LCST
lower critical solution temperature
- EB
electron beam
- TCPS
tissue culture polystyrene
- DI
deionized
- UV
ultraviolet
- PDMS
polydimethylsiloxane
- SMC
smooth muscle cell
- CCMS
4-(N-cinnamoylcarbamide)methylstyrene
- PET
poly(ethylene terephthalate)
- HUVEC
human umbilical vein endothelial cell
- HCl
hydrochloric acid
- TEOS
tetraethyl orthosilicate
- APTES
3-aminopropyltriethoxysilane
- SAMs
self-assembled monolayers
- PLL
poly(L-lysine)
- PEG
poly(ethylene glycol)
- ITO
indium tin oxide
- ZnO
zinc oxide
- TiO2
Titanium dioxide
- PMMA
poly(methyl methacyrlate)
- TEG
tetraethyleneglycol
- ROS
reactive oxygen species
- PL
4-[4-(1-methacryloyloxyethyl)-2-methoxy-5-nitrophenoxy]butyric acid
- PMB
poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate)
- PD-MSCs
placenta derived mesenchymal stem cells
- MCLs
magnetite cationic liposomes
- PVDF
polyvinylidene fluoride
05/22/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25149
References
- 1.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–10. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. Tissue Eng. 2006;12:499–507. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- 3.Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973–80. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 4.Sekine H, Shimizu T, Yang J, Kobayashi E, Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114(Suppl):I87–93. doi: 10.1161/CIRCULATIONAHA.105.000273. [DOI] [PubMed] [Google Scholar]
- 5.Haraguchi Y, Shimizu T, Yamato M, Kikuchi A, Okano T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials. 2006;27:4765–74. doi: 10.1016/j.biomaterials.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Okano T. [Cell sheet engineering] Rinsho Shinkeigaku. 2006;46:795–8. [PubMed] [Google Scholar]
- 7.Kanzaki M, Yamato M, Yang J, Sekine H, Kohno C, Takagi R, et al. Dynamic sealing of lung air leaks by the transplantation of tissue engineered cell sheets. Biomaterials. 2007;28:4294–302. doi: 10.1016/j.biomaterials.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Mendes PM. Stimuli-responsive surfaces for bio-applications. Chem Soc Rev. 2008;37:2512–29. doi: 10.1039/b714635n. [DOI] [PubMed] [Google Scholar]
- 9.de Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–85. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 10.Tekin H, Sanchez JG, Tsinman T, Langer R, Khademhosseini A. Thermoresponsive platforms for tissue engineering and regenerative medicine. AIChE J. 2011;57:3249–58. doi: 10.1002/aic.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–11. doi: 10.1021/la036139f. [DOI] [PubMed] [Google Scholar]
- 12.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chem-Rapid. 1990;11:571–6. doi: 10.1002/marc.1990.030111109. [DOI] [Google Scholar]
- 13.Ide T, Nishida K, Yamato M, Sumide T, Utsumi M, Nozaki T, et al. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials. 2006;27:607–14. doi: 10.1016/j.biomaterials.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima K, Honda S, Nakamura Y, López-Redondo F, Kohsaka S, Yamato M, et al. Intact microglia are cultured and non-invasively harvested without pathological activation using a novel cultured cell recovery method. Biomaterials. 2001;22:1213–23. doi: 10.1016/S0142-9612(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–80. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 16.Pan YV, Wesley RA, Luginbuhl R, Denton DD, Ratner BD. Plasma polymerized N-isopropylacrylamide: synthesis and characterization of a smart thermally responsive coating. Biomacromolecules. 2001;2:32–6. doi: 10.1021/bm0000642. [DOI] [PubMed] [Google Scholar]
- 17.Cheng X, Canavan HE, Stein MJ, Hull JR, Kweskin SJ, Wagner MS, et al. Surface chemical and mechanical properties of plasma-polymerized N-isopropylacrylamide. Langmuir. 2005;21:7833–41. doi: 10.1021/la050417o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva RM, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol. 2007;25:577–83. doi: 10.1016/j.tibtech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Nash ME, Healy D, Carroll WM, Elvira C, Rochev YA. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. J Mater Chem. 2012;22:19376–89. doi: 10.1039/c2jm31748f. [DOI] [Google Scholar]
- 20.Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J Biomed Mater Res A. 2005;75:1–13. doi: 10.1002/jbm.a.30297. [DOI] [PubMed] [Google Scholar]
- 21.Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir. 2005;21:1949–55. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- 22.Canavan HE, Graham DJ, Cheng X, Ratner BD, Castner DG. Comparison of native extracellular matrix with adsorbed protein films using secondary ion mass spectrometry. Langmuir. 2007;23:50–6. doi: 10.1021/la062330o. [DOI] [PubMed] [Google Scholar]
- 23.Tunc M, Humayun M, Cheng X, Ratner BD. A reversible thermosensitive adhesive for retinal implants: in vivo experience with plasma-deposited poly(N-isopropyl acrylamide) Retina. 2008;28:1338–43. doi: 10.1097/IAE.0b013e31817b6b42. [DOI] [PubMed] [Google Scholar]
- 24.Rayatpisheh S, Li P, Chan-Park MB. Argon-plasma-induced ultrathin thermal grafting of thermoresponsive pNIPAm coating for contractile patterned human SMC sheet engineering. Macromol Biosci. 2012;12:937–45. doi: 10.1002/mabi.201100477. [DOI] [PubMed] [Google Scholar]
- 25.von Recum HA, Okano T, Kim SW, Bernstein PS. Maintenance of retinoid metabolism in human retinal pigment epithelium cell culture. Exp Eye Res. 1999;69:97–107. doi: 10.1006/exer.1999.0682. [DOI] [PubMed] [Google Scholar]
- 26.Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology (N Y) 1990;8:854–6. doi: 10.1038/nbt0990-854. [DOI] [PubMed] [Google Scholar]
- 27.Moran MT, Carroll WM, Selezneva I, Gorelov A, Rochev Y. Cell growth and detachment from protein-coated PNIPAAm-based copolymers. J Biomed Mater Res A. 2007;81:870–6. doi: 10.1002/jbm.a.31089. [DOI] [PubMed] [Google Scholar]
- 28.Moran MT, Carroll WM, Gorelov A, Rochev Y. Intact endothelial cell sheet harvesting from thermoresponsive surfaces coated with cell adhesion promoters. J R Soc Interface. 2007;4:1151–7. doi: 10.1098/rsif.2007.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JA, Lucero AE, Hu S, Ista LK, Bore MT, López GP, et al. A low-cost, rapid deposition method for “smart” films: applications in mammalian cell release. ACS Appl Mater Interfaces. 2010;2:1048–51. doi: 10.1021/am900821t. [DOI] [PubMed] [Google Scholar]
- 30.Nash ME, Carroll WM, Nikoloskya N, Yang R, O’Connell C, Gorelov AV, et al. Straightforward, one-step fabrication of ultrathin thermoresponsive films from commercially available pNIPAm for cell culture and recovery. ACS Appl Mater Interfaces. 2011;3:1980–90. doi: 10.1021/am200204j. [DOI] [PubMed] [Google Scholar]
- 31.Patel NG, Cavicchia JP, Zhang G, Zhang Newby BM. Rapid cell sheet detachment using spin-coated pNIPAAm films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012;8:2559–67. doi: 10.1016/j.actbio.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Yeo WS, Hodneland CD, Mrksich M. Electroactive monolayer substrates that selectively release adherent cells. Chembiochem. 2001;2:590–3. doi: 10.1002/1439-7633(20010803)2:7/8<590::AID-CBIC590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Yousaf MN, Houseman BT, Mrksich M. Turning on cell migration with electroactive substrates. Angew Chem Int Ed Engl. 2001;40:1093–6. doi: 10.1002/1521-3773(20010316)40:6<1093::AID-ANIE10930>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Seto Y, Inaba R, Okuyama T, Sassa F, Suzuki H, Fukuda J. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials. 2010;31:2209–15. doi: 10.1016/j.biomaterials.2009.11.104. [DOI] [PubMed] [Google Scholar]
- 35.Tang CS, Schmutz P, Petronis S, Textor M, Keller B, Vörös J. Locally Addressable Electrochemical Patterning Technique (LAEPT) applied to poly(L-lysine)-graft-poly(ethylene glycol) adlayers on titanium and silicon oxide surfaces. Biotechnol Bioeng. 2005;91:285–95. doi: 10.1002/bit.20395. [DOI] [PubMed] [Google Scholar]
- 36.Guillaume-Gentil O, Akiyama Y, Schuler M, Tang C, Textor M, Yamato M, et al. Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv Mater. 2008;20:560–5. doi: 10.1002/adma.200700758. [DOI] [Google Scholar]
- 37.Guillaume-Gentil O, Gabi M, Zenobi-Wong M, Vörös J. Electrochemically switchable platform for the micro-patterning and release of heterotypic cell sheets. Biomed Microdevices. 2011;13:221–30. doi: 10.1007/s10544-010-9487-1. [DOI] [PubMed] [Google Scholar]
- 38.Hong Y, Yu M, Weng W, Cheng K, Wang H, Lin J. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34:11–8. doi: 10.1016/j.biomaterials.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi A, Hamamura A, Shindo Y, Kitamura H, Yoon BO, Mori T, et al. Photon-modulated changes of cell attachments on poly(spiropyran-co-methyl methacrylate) membranes. Biomacromolecules. 2004;5:1770–4. doi: 10.1021/bm049737x. [DOI] [PubMed] [Google Scholar]
- 40.Edahiro J, Sumaru K, Tada Y, Ohi K, Takagi T, Kameda M, et al. In situ control of cell adhesion using photoresponsive culture surface. Biomacromolecules. 2005;6:970–4. doi: 10.1021/bm0493382. [DOI] [PubMed] [Google Scholar]
- 41.Auernheimer J, Dahmen C, Hersel U, Bausch A, Kessler H. Photoswitched cell adhesion on surfaces with RGD peptides. J Am Chem Soc. 2005;127:16107–10. doi: 10.1021/ja053648q. [DOI] [PubMed] [Google Scholar]
- 42.Wirkner M, Alonso JM, Maus V, Salierno M, Lee TT, García AJ, et al. Triggered cell release from materials using bioadhesive photocleavable linkers. Adv Mater. 2011;23:3907–10. doi: 10.1002/adma.201100925. [DOI] [PubMed] [Google Scholar]
- 43.Kolesnikova TA, Kohler D, Skirtach AG, Möhwald H. Laser-induced cell detachment, patterning, and regrowth on gold nanoparticle functionalized surfaces. ACS Nano. 2012;6:9585–95. doi: 10.1021/nn302891u. [DOI] [PubMed] [Google Scholar]
- 44.Byambaa B, Konno T, Ishihara K. Detachment of cells adhered on the photoreactive phospholipid polymer surface by photoirradiation and their functionality. Colloids Surf B Biointerfaces. 2013;103:489–95. doi: 10.1016/j.colsurfb.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/S0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 46.Guillaume-Gentil O, Semenov OV, Zisch AH, Zimmermann R, Vörös J, Ehrbar M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials. 2011;32:4376–84. doi: 10.1016/j.biomaterials.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 47.Chen YH, Chung YC, Wang IJ, Young TH. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials. 2012;33:1336–42. doi: 10.1016/j.biomaterials.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 48.Jahren SL, Butler MF, Adams S, Cameron RE. Swelling and viscoelastic characterisation of pH-responsive chitosan hydrogels for targeted drug delivery. Macromol Chem Phys. 2010;211:644–50. doi: 10.1002/macp.200900560. [DOI] [Google Scholar]
- 49.Yeh HY, Lin JC. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. J Biomater Sci Polym Ed. 2008;19:291–310. doi: 10.1163/156856208783720985. [DOI] [PubMed] [Google Scholar]
- 50.Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn J Cancer Res. 1996;87:1179–83. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito A, Hayashida M, Honda H, Hata K, Kagami H, Ueda M, et al. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004;10:873–80. doi: 10.1089/1076327041348446. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu K, Ito A, Lee JK, Yoshida T, Miwa K, Ishiguro H, et al. Construction of multi-layered cardiomyocyte sheets using magnetite nanoparticles and magnetic force. Biotechnol Bioeng. 2007;96:803–9. doi: 10.1002/bit.21094. [DOI] [PubMed] [Google Scholar]
- 53.Ito A, Takizawa Y, Honda H, Hata K, Kagami H, Ueda M, et al. Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10:833–40. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- 54.Ito A, Hibino E, Kobayashi C, Terasaki H, Kagami H, Ueda M, et al. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;11:489–96. doi: 10.1089/ten.2005.11.489. [DOI] [PubMed] [Google Scholar]
- 55.Ishii M, Shibata R, Numaguchi Y, Kito T, Suzuki H, Shimizu K, et al. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler Thromb Vasc Biol. 2011;31:2210–5. doi: 10.1161/ATVBAHA.111.231100. [DOI] [PubMed] [Google Scholar]
- 56.Kwon OH, Kikuchi A, Yamato M, Sakurai Y, Okano T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J Biomed Mater Res. 2000;50:82–9. doi: 10.1002/(SICI)1097-4636(200004)50:1<82::AID-JBM12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]