Abstract
Background
Simian immunodeficiency virus (SIV) infection in macaques chronically receiving ethanol results in significantly higher plasma viral loads and more rapid progression to end-stage disease. We thus hypothesized that the increased plasma viral load in ethanol treated SIV-infected macaques would negatively correlate with antigen-specific immune responses.
Methods
Rhesus macaques were administered ethanol or sucrose (n=12 per group) by indwelling gastric catheters for 3 months, and then intravenously infected with SIVMAC251. Peripheral blood T and B-cells immunophenotyping and quantification was performed. Plasma was examined for viremia, levels of SIV-Env-specific binding, and neutralizing antibodies. Virus-specific IFNγ and TNFα cytokine responses to SIV-Nef, Gag or Env peptide pools were measured in peripheral blood CD8+ T-cells.
Results
Macaques receiving ethanol had both higher plasma viremia and virus-specific cellular immune responses compared to the sucrose-treated group. The emergence of virus-specific cytokine responses temporally correlated with the decline in mean plasma viral load after 14 days post infection in all SIV infected animals. However, neither the breadth and specificity nor the magnitude of virus-specific CD8+ T-cell responses correlated with early post peak reductions in plasma viral loads. In fact, increased cytokine responses against Gag, gp120 and gp41 positively correlated with plasma viremia. Levels of SIV envelope-specific IgG and neutralizing antibodies were similar over the disease course in both groups of macaques.
Conclusions
Persistently higher antigen-specific cytokine responses in animals receiving ethanol are likely an effect of the higher viral loads and antigen persistence, rather than a cause of the increased viremia.
Keywords: Human immunodeficiency virus, Simian immunodeficiency virus, AIDS, viral load, Cytokine, IFNγ, TNFα, SIV-specific antibodies, SIV neutralizing titers, chronic ethanol
INTRODUCTION
Ethanol abuse in HIV infected individuals has a potential impact upon increased HIV transmission and disease progression in the United States and throughout the world [1–5]. Ethanol consumption has also been shown to result in increased plasma viral loads “set points” and accelerated disease progression to end-stage disease through increased expression of TNFα and atrogin-1 in SIV or simian human immunodeficiency virus (SHIV) infected nonhuman primates by our groups and others [6–12]. Alcohol intake in HIV infected patients also induced increased CD4+ T-cells apoptosis and enhanced activation of TNFα [13]. Control of plasma viremia in the acute phase of HIV and SIV infection has been shown to be temporally associated with the emergence of CD8+ cytotoxic T-cell responses [14–16]. However, virus specific cellular immune responses in macaques acutely infected with either SIV or SHIVSF162P3 did not correlate with the post peak decline in plasma viremia [17, 18]. Similar to T-cell responses, B-cell dysfunction represents a central feature in HIV and SIV infection and pathogenesis [19–21]. Further, passive transfer of high-titer SIV-specific gamma globulin or neutralizing anti-HIV antibodies is able to inhibit SIV and SHIV replication and disease progression in macaques [22–28]. Thus early generation of SIV-specific antibody responses might have some impact on the delay in disease progression of SIV infection [29–34].
Despite the high incidence of ethanol use among HIV-infected patients, the consequences of heavy ethanol consumption on HIV progression and its effect on virus-specific cellular and humoral immune responses in humans are poorly defined. It is also not clear whether any association exists between cellular and/or humoral antibody mediated immune responses and plasma viral load in ethanol treated SIV infected rhesus macaques (RMs). The present study was designed to determine whether the emergence or magnitude of SIV-specific CD8+ T-cell cytokine responses and the quantity or quality of SIV-specific antibodies were associated with the outcome of plasma viral loads in both ethanol and sucrose treated macaques. However, these findings suggest that increased plasma viral load in ethanol-treated SIV-infected macaques helps to maintain strong CD8+ antigen-specific cytokine responses.
MATERIALS AND METHODS
Animals, ethanol administration, and virus inoculation
This study was performed on the same cohort of 24 male Indian RMs (Macaca mulatta) between 4–6 years of age as used in a previous publication [35]. Pertinent to the current study, plasma viremia and CD4 counts on these animals were published in the previous report [35]. All animals were housed at the Tulane National Primate Research Center (TNPRC) and under the full care of TNPRC veterinarians in accordance with the standards incorporated in the Guide to the Care and Use of Laboratory Animals (NIH). Approval for all veterinary procedures in this study was obtained from Animal Care and Use Committee of Tulane University and Louisiana State University Health Sciences Center. All macaques were negative for HIV-2, SIV, type-D retrovirus and STLV-1 infection at the beginning of this study.
Animals received ethanol or sucrose via a permanent indwelling intragastric catheter that was attached to a cage mounted swivel via a tether as previously described [35]. Briefly, ethanol was administered to achieve plasma ethanol concentrations of 50–60 mM starting 3 months before SIV infection and continuing for the duration of the study. Some ethanol (n=8) or sucrose (n=8) treated macaques received ethanol or sucrose over a 5h period for 4 consecutive days each week. Others received ethanol or sucrose daily (4 ethanol and 4 sucrose-treated macaques). In both cases, once started ethanol was given for the duration of the study. Blood samples were obtained weekly in order to adjust infusion rates so that plasma ethanol concentrations were between 50 to 60 mM.
Three months after initiating ethanol or sucrose administration, all macaques were inoculated intravenously with 10,000ID50 of SIVMAC251. Heparin or EDTA peripheral blood (PB) was collected at sequential time points for analyses described below.
Lymphocyte isolation
For CFC assays, PB mononuclear cells (PBMC) were isolated from heparinized whole blood by density gradient centrifugation (Lymphocyte Separation Medium, ICN Biomedicals, Ohio) as described elsewhere [17]. Complete blood counts and differentials were also determined using a Bayer Advia-120 instrument optimized for macaque blood.
Analytical procedures
Plasma ethanol was measured using an AM1 Analyzer obtained from Analox Instruments (Lunenburg, MA). Plasma SIV-GagRNA was measured quantitatively by reverse transcription and real time polymerase chain reaction using gene specific primers and FRET probe as previously described [7]. Copy number was determined using a standard curve of previously cloned SIV-Gag cRNA. The lower limit of detection was 10 copies/reaction.
Immunofluorescent staining and cytokine flow cytometry (CFC) assay
For T-cell immunophenotyping, cells were adjusted to 107 cells/ml and 100ul aliquots or 100ul of whole blood samples were stained using anti-CD3 (SP34-2), anti-CD4 (L200), anti-CD28 (CD28.2) and anti-CD95 (DX2) MAbs obtained from BD Biosciences (BD) as reported earlier [18, 36]. At least 20,000 events were gated by gating on lymphocytes and data was acquired on a FACSAria flow cytometer within 24hrs after staining.
A CFC assay was employed to detect SIV specific CD8+ T-cells with proper positive and negative controls as previously described [36–40]. Briefly, fresh heparinized PBMC were resuspended at 1×106 cells/ ml and stimulated with SIV-Gag (cat.6204), Env (cat.6883) and Nef (cat.8762) peptide pools (NIH AIDS Research & Reference Reagent Program) in the presence of 0.5ug/ml of anti-CD28 (CD28.2, BD) and anti-CD49d (9F10, BD) MAbs. Four separate peptide pools were prepared to encompass the entire SIV-Nef (peptide 1–64), SIV-Gag (peptide 1–125), SIV-gp120 (peptide 1–124) and SIV-gp41 (peptide 125–218) protein respectively. Following stimulation, the cells were stained first for live/dead stain (Invitrogen) followed by surface staining with anti-CD3, anti-CD4 and anti-CD8 (3B5, Invitrogen); and intracellular staining with anti-IFNγ (4S.B3, BD), and anti-TNFα (MAb11, BD) MAbs (6 color flow cytometry). Data were acquired using a FACSAria flow cytometer (BD Biosciences, CA) within 24hrs after staining. At least 50,000 events were collected from each sample by gating on CD3+CD8+ T-cells and data were analyzed using FlowJo software (TreeStar Inc.) version 9.5.3. (supplementary figure S1). The criterion for a positive cytokine response was a two-fold increase in frequency for that specific antigen and cytokine above the medium control culture. All values were subtracted from medium control before the analysis.
Measurement of total and SIV-envelope specific IgG levels
Measurement of total IgG levels from frozen EDTA-plasma samples were performed by ELISA using microtiter plates coated with goat anti-monkey IgG (MP Biochemicals, OH) and blocked with 0.05% Tween-20 and 2% goat serum (Equitech-Bio Inc., TX) in PBS as described previously [41, 42]. Serial dilutions of RM plasma were evaluated along with serial dilutions of pooled normal RM serum containing known amounts of IgG (for standard curve generation). Plates were developed by consecutive treatment with biotin conjugated goat anti-human IgG (Southern Biotech, AL), streptavidin-peroxidase (Sigma) and tetramethylbenzidine substrate (Sigma) with 1M H2SO4 stop solution. Absorbance at 450nm was measured and standard curves were generated using SoftMax Pro (Molecular Devices) to calculate total IgG levels in samples. SIVEnv-specific IgG concentrations in monkey plasma were determined similarly using recombinant SIV-gp130 (ImmunoDiagnostics, MA) as the coating antigen. SIV-gp130 IgG standard curves were generated using a calibrated and standardized pool of plasma from SIVMAC251-infected macaques at 18 months post infection (p.i.).
SIV neutralization titers
To evaluate the functionality of the SIV-specific antibodies in blood, we measured the capacity of plasma samples to inhibit in vitro infection of SIV using a well-characterized neutralization assay with Tzm-bl cells, which produce luciferase upon HIV/SIV infection, and a neutralization sensitive reference isolate of SIV (SIVMAC239-cl3) as previously described [42, 43]. Heat-inactivated plasma samples were serially diluted. Plasma dilutions were mixed with 100TCID50 of cell-free virus stock, SIVMAC239-Cl3Env, and incubated for 1h prior to the addition of TZM-bl cells. After incubation at 37°C for 48h, luminescence was assessed using the Bright-Glo Luciferase Assay System (Promega), and Hidex Oy CHAMELEON V plate reader. Reduction in relative light units (RLU) in plasma-containing samples of greater than or equal to 70% of levels in virus control wells (n=8 controls per assay) was recorded as neutralization positive. SIV neutralization titers (NT-70) were identified as the reciprocal of the highest plasma dilution which reduced RLU >70% of average control wells. All plasma samples were assayed in three, replicate experiments, and consensus titers recorded. Samples negative for neutralization at a starting plasma dilution of 1:100 were assigned a value of 50 for statistical comparisons.
Statistical Analysis
Graphical presentation and statistical analysis of the antigen-specific cytokine responses were performed using GraphPad Prism 5.0d (GraphPad Software Inc., CA). Differences in cytokine responses between groups of animals were compared by Student’s t test. Statistical comparisons of antibody levels were performed by Mann-Whitney U test. For all analysis, results were considered significant if p<0.05.
RESULTS
Enhanced plasma SIV load in ethanol treated macaques
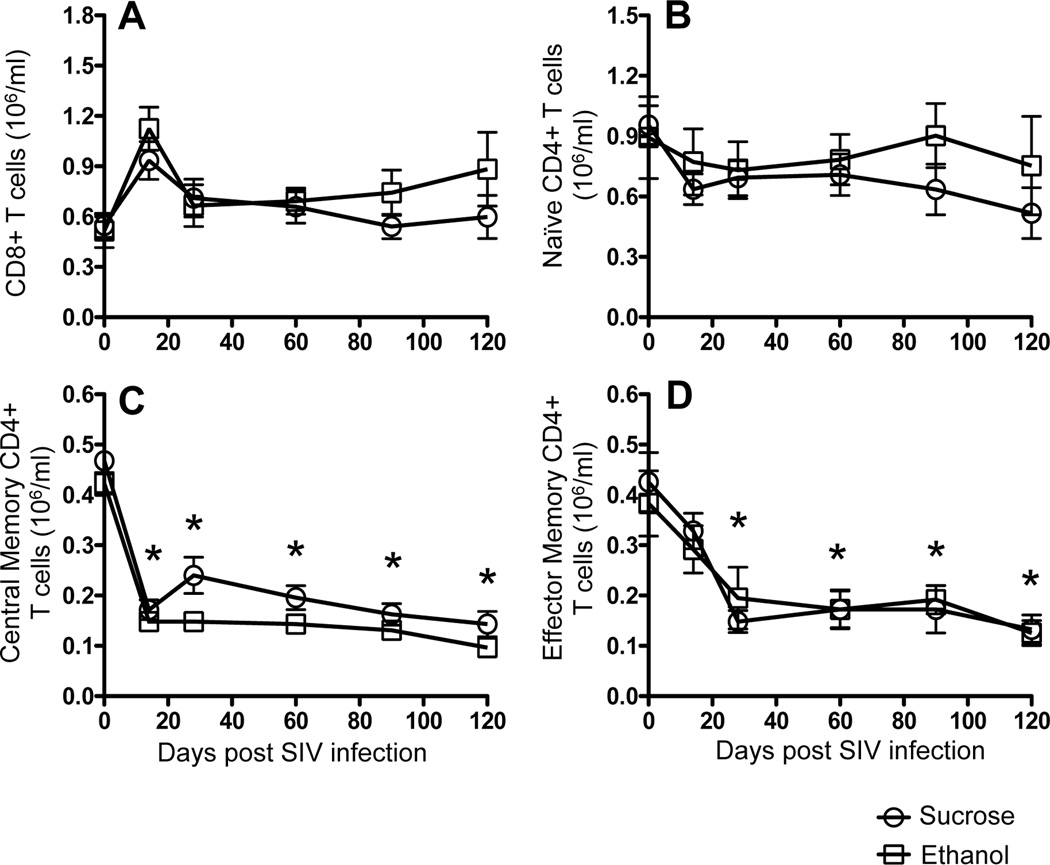
Plasma viral loads were measured from 0 to 120 days after SIV inoculation. As reported in a previously published paper [35], alcohol treated SIV-infected macaques used in this study had higher plasma viral loads compared to sucrose-treated, SIV-infected animals throughout the study period [35]. Total leukocyte and polymorphonuclear leukocyte counts remained within the normal range during the time period of this study, and did not significantly differ between sucrose and ethanol treatment macaques [35]. Similar to our previous study [9], peripheral blood CD4+ T-cells also decreased during the first 120 days p.i. but this decrease did not differ between alcohol and sucrose treated animals [35]. In addition, there were no significant differences in absolute CD4+ and CD8+ T-cell counts between ethanol and sucrose-treated infected macaques (Fig. 1A).
Figure 1.
T-cell populations in peripheral blood in SIV infected macaques. Absolute numbers of CD8+ T-cells (A), naïve (CD28+CD95-) CD4+ T-cells (B), central memory (CD28+CD95+) CD4+ T-cells (C), and effector memory (CD28-CD95+) CD4+ T-cells (D) in peripheral blood in both sucrose treated and ethanol treated SIV infected macaques. Data are expressed as mean ± standard errors. Asterick (*) indicates significant differences (p<0.05) in respective cell populations when compared to preinfection timepoints.
Increased naïve CD4+ T-cell population in ethanol treated SIV infected macaques
Impact of SIV infection on the distribution of peripheral naïve and memory CD4+ T-cell populations was measured in both sucrose and ethanol-treated SIV infected macaques. In all macaques there was a modest, selective depletion of absolute numbers of central memory (CD28+CD95+) and effector memory (CD28-CD95+) CD4+ T-cells from 0 to 14 days p.i. and remained lower throughout the 120 days of this study. However naïve (CD28+CD95-) CD4+ T-cells remained stable. Mean naïve CD4+ T-cells were higher in ethanol-treated macaques compared to the sucrose-treated macaques, however the differences were not statistically significant (Fig. 1B–D). Mean values of naïve, central memory and effector memory CD4+ T-cells between sucrose and ethanol treated macaques at pre SIV infection (day 0) time point was not statistically significant.
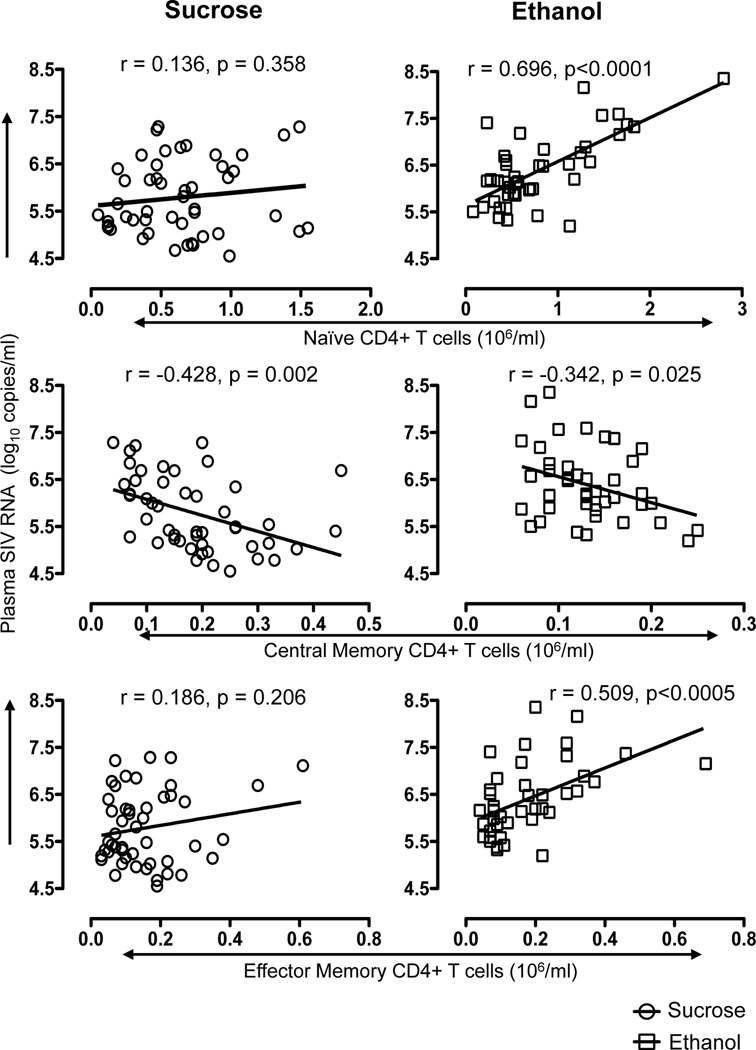
Because CD4+ T-cells are recognized as targets of SIV and likely are responsible for most SIV replication, we sought a correlation between CD4+ T-cell subpopulations and plasma viral load during viral set points points (from 28 to 120 days p.i.). Increased plasma viral load in ethanol-treated macaques significantly correlated with increased peripheral naïve CD4+ T-cells (r=0.696, p<0.0001), decreased central memory CD4+ T-cells (r=−0.342, p=0.025) and increased effector memory CD4+ T-cells (r=0.509, p<0.005, Fig. 2). Interestingly, most comparisons did not significantly correlate within the sucrose-treated animals, but absolute central memory CD4+ T-cell counts negatively correlated with plasma viral load in sucrose-treated macaques (r=−0.428, p=0.002, Fig. 2). Combined, these data indicate that higher naïve and effector memory CD4+ T-cell counts in PB are associated with increased plasma viral load in ethanol-treated macaques.
Figure 2.
Correlation between plasma viral load and different blood CD4 T-cell subsets in sucrose and ethanol treated macaques determined between 28–120 days post SIV infection. A significant positive correlation was detected between either naïve (CD28+CD95-) or effector memory (CD28-CD95+) CD4+ T-cells and plasma viral load in ethanol treated SIV infected macaques, but not sucrose treated animals. In contrast, a significant but weaker inverse correlation was observed between central memory (CD28+CD95+) CD4+ T-cells and plasma viral load in both sucrose and ethanol treated SIV infected macaques. Spearman’s nonparametric correlation was used to determine the degree of correlation in all comparisons.
Increased antigen-specific cytokine responses in ethanol-treated SIV infected macaques
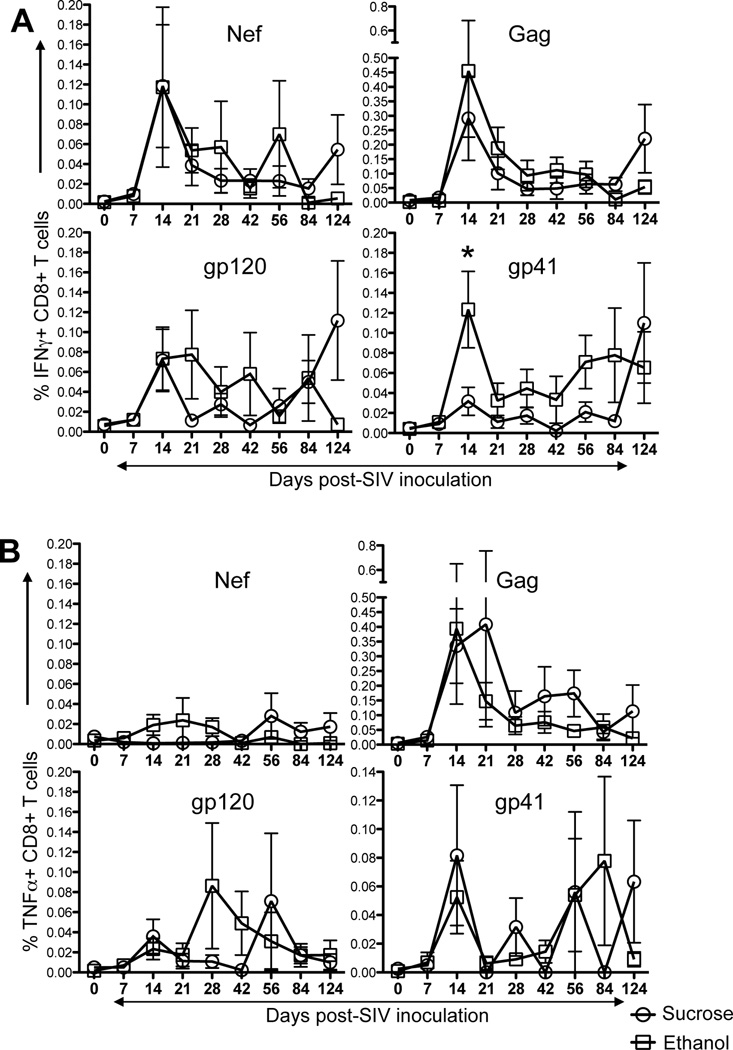
Antigen specific T-cell responses were measured using CFC assays in all SIV-macaques (Fig. 3, Supplementary Fig. 1). Overall, virus-specific cytokine responses were detected in all animals at some time points. Interestingly, positive responses were consistently detected in all ethanol-treated macaques at 14 days p.i. as compared to sucrose-treated animals, where only 4 out of 12 macaques had early detectable cytokine responses at 21–28 days p.i. In all macaques, SIV-Gag specific IFNγ responses were higher than all other responses tested. The percentage of IFNγ responses ranged from 0.00–0.89%, 0.00–2.68%, 0.00–0.53% to 0.00–0.73% for Nef, Gag, gp120 and gp41 peptide pools respectively (Fig. 3A). Increased plasma viremia at 14 day p.i. is consistent with the early increase of mean IFNγ responses in both groups. However, the magnitude of virus-specific cytokine responses decreased during the course of infection. Interestingly ethanol-treated animals had higher percentages of Gag (0.46% vs. 0.29% for ethanol and sucrose-treated animals respectively) and gp41-specific (0.12% vs. 0.03% for ethanol and sucrose-treated animals respectively; P<0.05) responses at 14 days p.i. (Fig. 3A). Overall, SIV-gp41 specific IFNγ responses were higher in ethanol-treated macaques compared to sucrose treated animals during acute infection (Fig. 3A).
Figure 3.
SIV specific T-cell responses in either ethanol or sucrose treated SIV infected macaques. Intracellular IFNγ responses (A) and TNFα responses (B) were measured against SIV-Nef, SIV-Gag, SIV-gp120, and SIV-gp41 antigens at 9 different time points post SIV infection. Data presented as means ± standard errors of either CD8+IFNγ+ T-cells or CD8+TNFα+ T-cells at different time points of infection. In general, increased IFNγ/TNFα responses were detected in ethanol treated macaques compared to sucrose treated controls in peripheral blood CD8+ T-cells. Asterick (*) indicates statically significant difference between sucrose and ethanol treated SIV infected macaques for the specified time points. Criteria for a positive cytokine response was a two-fold increase in frequency for that specific antigen and cytokine above the medium control culture. All values were subtracted from medium control before analysis.
TNFα responses in all ethanol-treated animals ranged from 0.00–0.18%, 0.00–3.14%, 0.00–0.70% to 0.00–0.54% for Nef, Gag, gp120 and gp41 peptide pools respectively (Fig. 3B). Three out of 12 ethanol-treated macaques had detectable Nef-specific TNFα responses; whereas none of the sucrose-treated animals had detectable TNFα responses at any time point tested. However, mean Nef-specific TNFα responses were low to undetectable in all infected animals throughout the time points examined. Mean Gag-specific TNFα responses peaked early in ethanol-treated animals compared to sucrose-treated animals (Fig. 3B). In general, TNFα responses remained higher in sucrose compared to ethanol-treated animals. The mean TNFα responses were 0.01% vs. 0.02%, 0.34 vs. 0.39%, 0.04 vs. 0.02%, and 0.08 vs. 0.05% in sucrose and ethanol-treated animals for Nef, Gag, gp120 and gp41 respectively at 14 days p.i. However, there were no statistically significant differences in TNFα responses at any time point between sucrose and ethanol-treated animals.
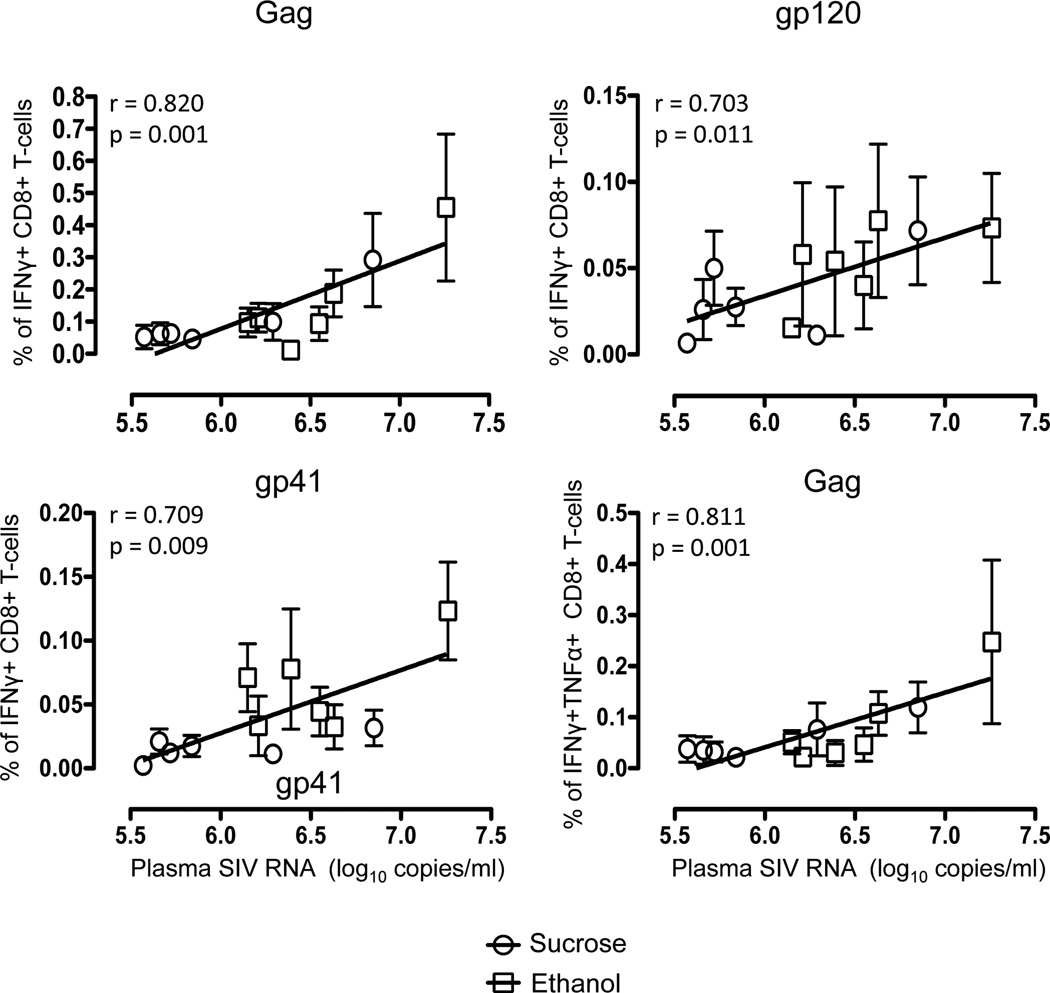
We also compared Gag, gp120 and gp41 specific double-positive (DP) IFNγ and TNFα responses in both ethanol and sucrose-treated animals (Supplementary Fig. 2). The dynamics of the DP responses were similar as those observed for total IFNγ responses where SIV-Gag specific responses were higher in ethanol-treated animals compared to sucrose-treated animals (0.12% vs. 0.25% in sucrose and ethanol groups at 14 days p.i.). However, unlike the total IFNγ or TNFα responses, DP responses to gp41 were significantly different between ethanol and sucrose groups at day 14, but both gp120 and gp41-specific responses were undetectable to very low throughout infection (Supplementary Fig. 2). Interestingly however, all sucrose-treated macaques had higher mean IFNγ and/or TNFα responses at the end point (120 days) of the study (Fig. 3, Supplementary Fig. 2). Statistical analyses comparing breadth and magnitude for responses to each peptide pool, as well as Nef, Gag and Env responses combined, demonstrated a possible correlation of increased cytokine responses with increased plasma viremia compared to sucrose–treated macaques in early infection. This is supported by an association between the magnitude of CD8+ responses and high plasma viral loads, as macaques with higher levels of CD8+ responses had higher levels of viremia during 14–90 day p.i. (Fig. 4). Spearman’s correlation analysis of these independent measures showed highly significant correlations between antigen-specific cytokine-producing CD8+ T-cells and plasma viremia for Gag, gp120 and gp41 antigens regardless of sucrose or ethanol treatment or timepoints (correlations ranged from 0.703 to 0.820; Fig. 4). Interestingly there was no significant correlation between Nef-specific cytokine responses in CD8+ T-cells and plasma viremia in any SIV infected macaques.
Figure 4.
Positive correlations are shown between the anti-SIV immune responses in peripheral blood and mean plasma viral load in sucrose (circle, mean ± standard error) and ethanol (square, mean ± standard error) treated macaques from day14 to day90 post SIV infection timepoints. Note higher viral loads in ethanol treated macaques showed higher percentages of either CD8+IFNγ+ or CD8+IFNγ+TNFα+ T-cell responses against Gag, gp120 and gp41 antigens. Spearman’s nonparametric correlation was used to determine correlations between anti-SIV cytokine responses of CD8+ T-cells and mean plasma viral load from all macaques using GraphPad Prism (version 5.0d, GraphPad software, CA).
The magnitude and functional antibody response is similar in ethanol and sucrose-treated animals
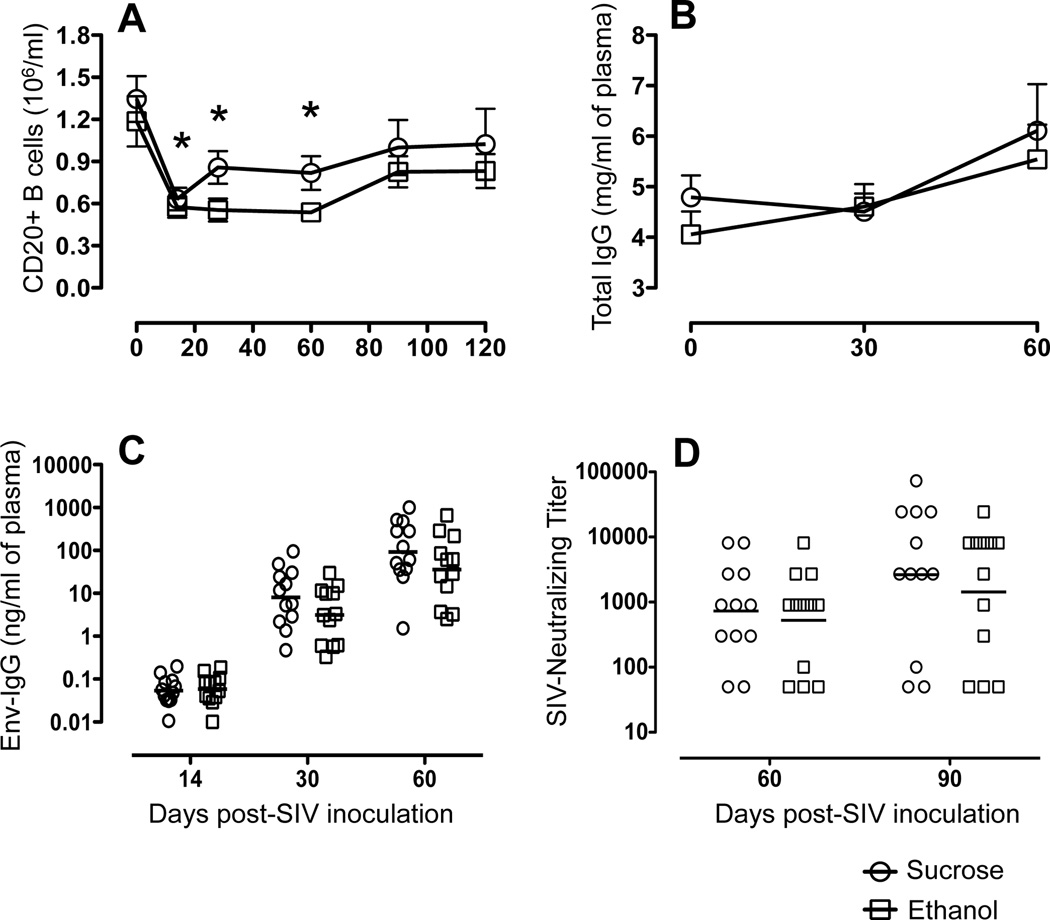
CD20+ B-cell depletion was detected in both sucrose and ethanol-treated macaques very early after SIV infection. There was a significant drop in peripheral B-cell populations from day 0 (an average 1346/µl and 1186/µl for sucrose and ethanol-treated macaques respectively) to day 14 (633/µl and 575/µl for sucrose and ethanol-treated macaques respectively) (Fig. 5A). After the initial depletion, B-cell populations slightly rebounded, however the total counts were significantly lower than baseline until 90 day p.i., when CD20+ B-cell counts recovered to preinfection levels (Fig. 5A). The overall effect that chronic ethanol consumption had on the development of humoral immune responses to SIV infection was evaluated. Levels of total IgG measured in both groups were similar throughout the SIV disease course (Fig. 5B). Mean levels of total IgG increased at 30 weeks post-SIV infection in both groups, however these values were within the normal range of plasma IgG levels previously observed [44]. All SIV infected animals had levels of SIVEnv-specific IgG above background by 4 weeks p.i. (≥0.5 ng/ml), which increased over time (Fig. 5C). The development and magnitude of the SIVEnv-specific antibody responses were similar in ethanol and sucrose-treated animals (Fig. 5C).
Figure 5.
Humoral immune measures over the first 90 days of SIV infection in sucrose (n=12) and ethanol (n=12) treated macaques. (A) Mean ± standard errors for total CD20+ B cell numbers following SIV infection in ethanol and sucrose treated animals are shown. Asterisk (*) indicates significant differences (p<0.05) in CD20+ B-cell population when compared to preinfection timepoints (B) Total plasma IgG levels. Mean and standard error values of total IgG levels measured at 0, 30 and 60 days post SIV infection are shown. (C) Levels of SIV-Envelope-specific IgG in plasma of sucrose and ethanol treated macaques measured over time. Values for individual animals are designated by circles and the geometric mean for each group shown by the line. (D) Plasma neutralization titers. The reciprocal value of the highest plasma sample dilution that reduced infection of SIVMAC239−Cl3env by ≥70% of controls was identified as the neutralizing titer. Values for individual animals are designated by circles and the geometric mean of each group by the line.
Plasma neutralizing titers were compared between ethanol and sucrose-treated SIV-infected macaques. Over the course of infection, two ethanol-treated animals failed to develop measurable neutralizing titers against the reference virus; of these, one animal was euthanized after 12 weeks, while the second survived through 30 weeks p.i. (Fig. 5D). Similarly, two sucrose-treated animals failed to develop neutralizing titers until 30 weeks p.i., and at that time, only low-level titers (1:100) were detected. Although a wide range of neutralizing titers were developed in the remaining animals, similar levels of neutralization were observed between ethanol and sucrose-treated macaques at each time point in the disease course. In general, neutralizing titers were inversely correlated with plasma viral load, with animals displaying the highest viral loads at 8 and 12 weeks p.i., having the lowest neutralizing titers. Despite higher viral levels in ethanol-treated macaques, there was no statistical differences in neutralizing antibody titers between ethanol and sucrose groups. Neither the quantity nor functional neutralizing capability of the SIVEnv-specific antibody responses correlated with the increased viral load observed in the ethanol-treated macaques.
DISCUSSION
Here we utilized the well-characterized and highly relevant SIV-infected rhesus macaque model of HIV infection to understand the effects of ethanol consumption on antigen-specific cellular and humoral immune responses, and its impact on disease progression and pathogenesis. The present data is consistent with prior results in that SIV viral load set-points were significantly higher in ethanol-treated than sucrose-treated animals. Also similar to previous studies, ethanol animals showed similar declines in peripheral CD4+ T-cells, and CD4+ T-cell subsets (central and effector memory T-cells) compared to sucrose controls [6, 45]. However, the data here show this was not associated with more potent virus-specific CD8+ T cell responses, nor neutralizing antibody responses, thus the mechanisms by which viral loads are increased due to ethanol consumption remain elusive.
Our hypothesis was that the increased plasma viral load in ethanol-treated macaques would correlate with an absence, or lower levels of virus specific cellular and humoral immune responses due to impairments of immune responses attributed to chronic alcohol ingestion. Surprisingly however, we observed significantly higher levels of virus-specific cellular responses in macaques receiving ethanol compared to the sucrose-treated group. As expected from prior studies, the emergence of virus-specific cytokine responses temporally correlated with the decline in mean plasma viremia after 14 days p.i. in both groups of SIV infected animals, suggestive of at least a partial role of virus-specific cellular responses in regulating early plasma viremia. However, neither the breadth, specificity nor the magnitude of virus-specific CD8+ T-cell responses correlated with the early post-peak reductions in plasma viral loads.
We also hypothesized the quantity or functionality of SIV-specific antibody responses would be decreased in ethanol-treated macaques. However, we did not observe differences in the quantity of total or viral neutralizing titers of SIVEnv-specific antibodies between groups. Despite the higher viral loads observed in the ethanol-treated animals at set point, these macaques developed antibody responses which were comparable to sucrose-treated controls. However, B-cell depletion in both sucrose and ethanol-treated SIV infected macaques was consistent with prior observations where SIV infected macaques have shown substantial drops in CD20+ B-cells in blood following infection [46, 47].
SIV-infected animals demonstrated a remarkable breadth and magnitude of antigen-specific T-cell responses. These data also clearly emphasize that the response to a single epitope is not predictive of the total T-cell response in an infected macaque consistent with previous reports [17]. A similar lack of correlation has also been described between control of viremia in HIV-1 infected patients and HIV-specific CD4+ and CD8+ T-cell responses [48, 49]. However, our observation of increased or similar levels of virus-specific cytokine responses in ethanol-treated macaques contrasts with prior reports where ethanol treatment was associated with T-cell dysfunction [50–52]. Since the present data showed a higher magnitude of functional CD8+ T-cell responses in macaques with higher plasma viral loads (Fig. 4), this suggests that the increased levels of persistent antigenemia is associated with stronger CD8+ T-cell responses. These findings support prior observations where the frequencies of HIV-specific CD8+ T-cells positively correlated with plasma HIV RNA levels in humans [53]. In summary, our data suggest ethanol use results in higher plasma viral loads in SIV-infected macaques, which in turn, results in more robust and persistent antigen-specific cytokine responses. A limitation of this study is that we only examined virus-specific IFNγ and TNFα responses in CD8+ T-cells, and results may have differed if more polyfunctional CD8+, or virus-specific CD4+ T-cell response assays been used. However, these were standard cytokine assays for detecting cellular immune responses in most SIV/HIV studies at the time these studies were initiated, and were thus believed to be the most useful for detecting potent virus specific CD8+ T-cell responses.
An interesting observation in this study was detection of a positive correlation between set point viral loads and levels of naïve CD4+ cells in the blood of ethanol-treated animals. A similar positive correlation was observed for circulating effector memory CD4+ T-cells in ethanol-treated animals. Although naïve cells are not considered targets for infection, they may become infected as they become activated and express CCR5. Thus one possible explanation is that increased turnover of naïve CD4+ T cells and transition to effector memory cells is associated with the higher viremia in alcohol fed animals. This is supported by our recent findings of increased targeting of proliferating CD4+ T-cells (viral specific and non-specific) in both pediatric and adult SIV infection [54, 55]. However, we did not examine CCR5 expression on memory cells in this study.
We have previously shown alcohol ingestion results in increased central memory cells in the intestine, but not the blood, suggesting alterations in inflammatory profiles and CD4+ T-cell turnover specifically in the intestine [45]. Previous studies have demonstrated that chronic ethanol consumption is pro-inflammatory, leading to conditions such as alcoholic liver disease in patients with prolonged alcohol dependence. Conceivably, this immune activation is also associated with increased CD4+ T-cell turnover in the intestine, and higher viral loads in early infection, such as the “set point” stages described here. However, caution is needed because correlation analyses do not imply a cause and effect, and circulating cells represent a small portion of the total CD4+ T-cell population in the body. However, since most of the viral target cells (memory CD4+CCR5+ T-cells) reside in mucosal tissues rather than blood, it is difficult to calculate T-cell turnover in mucosal tissues. Nonetheless, higher levels of immune activation, directly or indirectly resulting in the higher viral loads consistently detected in macaques receiving alcohol, are also consistent with the higher levels of CD8+ T-cell responses described here. Further, these data do not support the hypothesis that alcohol ingestion results in dysfunctional or impaired cellular or humoral immune responses. Combined, these data are more consistent with the hypothesis that alcohol use increases CD4+ T-cell turnover leading to increased viral target cells that may result in higher viremia, concomitant with CD8+ T-cell responses, and accelerated exhaustion of CD4+ target cells leading to more rapid disease progression.
Supplementary Material
Intracellular cytokine flow cytometry for IFNγ and TNFα responses from a representative alcohol treated SIV-infected rhesus macaque. Cells were gated first on singlets, lymphocytes, followed by live cells and then on CD3+ T-cells and subsequently on CD3+CD4+ and CD3+CD8+ T-cell subsets. The percentages of total IFNγ, total TNFα, and double positive IFNγ+TNFα+ CD8+ T-cells are shown in each upper box of each plot. Note that this macaque had increased Gag and gp120 specific IFNγ responses and double positive IFNγ and TNFα responses detected 14 days post SIV infection.
SIV specific T-cell responses in either ethanol or sucrose treated SIV infected macaques. Intracellular double-positive (DP) IFNγ+TNFα+ responses against SIV-Gag, SIV-gp120, and SIV-gp41 peptides were measured at 9 different time points post SIV infection. Data presented are means ± standard errors of CD8+DP T-cells at different time points of infection. Higher levels of SIV-Gag and gp41 specific DP responses were detected in ethanol treated macaques compared to sucrose treated controls in peripheral CD8+ T-cells (*p=0.02). However, SIV-gp120 and gp41 specific DP responses were low to undetectable in all animals. Criteria for a positive cytokine response was a two-fold increase in frequency for that specific antigen and cytokine above the medium control culture. All values were subtracted from medium control before analysis.
ACKNOWLEDGEMENTS
From the TNPRC (Covington, LA) we thank Dr. Preston Marx for providing SIVMAC251, Larissa Devlin, Wayne A. Cyprian and Nancy Dillman for excellent care of study animals including the delivery of ethanol and sucrose, and Lynn F. Fresh and Gail B. Plauche for laboratory assistance. From the Ethanol and Drug Abuse Center at LSUHSC (New Orleans, LA) we acknowledge the contributions of Nedra Lacour, Jane A. Schexnayder, Constance Porretta, Amy B. Weinberg, Rhonda R. Martinez and Jean W. Carnal for analytical assistance, and Howard L. Blakesley for data management and statistical analysis. This study was supported by Public Health Service Grant AA09803, AA007577 and the National Center for Research Resources, and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant no. OD011104-51.
Source of Support: This study was supported by NIH grants AA009803, AA007577, AA007555, the National Center for Research Resources, and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant no. OD011104-51.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have declared that no competing interests exist.
REFERENCES
- 1.Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC, Veterans Aging Cohort 3-Site S. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr. 2003;33:521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 3.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 4.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7:226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conigliaro J, Justice AC, Gordon AJ, Bryant K, Alcohol V, Behavior Change Research G. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44:S1–S6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- 6.Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2006;22:589–594. doi: 10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- 7.Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr., et al. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27:495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Perez-Casanova AE, Tirado G, Noel RJ, Torres C, Rodriguez I, et al. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- 9.Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 10.Staprans SI, Dailey PJ, Rosenthal A, Horton C, Grant RM, Lerche N, et al. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol. 1999;73:4829–4839. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, et al. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008;32:138–147. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barve SS, Kelkar SV, Gobejishvilli L, Joshi-Barve S, McClain CJ. Mechanisms of alcohol-mediated CD4+ T lymphocyte death: relevance to HIV and HCV pathogenesis. Front Biosci. 2002;7:d1689–d1696. doi: 10.2741/A872. [DOI] [PubMed] [Google Scholar]
- 14.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimann KA, Tenner-Racz K, Racz P, Montefiori DC, Yasutomi Y, Lin W, et al. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahar B, Wang X, Dufour J, Lackner AA, Veazey RS. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3) Virology. 2007;363:36–47. doi: 10.1016/j.virol.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pahar B, Lackner AA, Piatak M, Jr., Lifson JD, Wang X, Das A, et al. Control of viremia and maintenance of intestinal CD4(+) memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge. Virology. 2009;387:273–284. doi: 10.1016/j.virol.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Veazey RS, Wang X, Lackner AA, Xu H, Pahar B. Simian immunodeficiency virus infection in rhesus macaques induces selective tissue specific B cell defects in double positive CD21+CD27+ memory B cells. Clin Immunol. 2011;140:223–228. doi: 10.1016/j.clim.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 22.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 23.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 24.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka RJ, Buckler-White A, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haigwood NL, Watson A, Sutton WF, McClure J, Lewis A, Ranchalis J, et al. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 28.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 29.Baskin GB, Murphey-Corb M, Watson EA, Martin LN. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch VM, Fuerst TR, Sutter G, Carroll MW, Yang LC, Goldstein S, et al. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lifson JD, Nowak MA, Goldstein S, Rossio JL, Kinter A, Vasquez G, et al. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller CJ, Genesca M, Abel K, Montefiori D, Forthal D, Bost K, et al. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J Virol. 2007;81:5024–5035. doi: 10.1128/JVI.02444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dykhuizen M, Mitchen JL, Montefiori DC, Thomson J, Acker L, Lardy H, et al. Determinants of disease in the simian immunodeficiency virus-infected rhesus macaque: characterizing animals with low antibody responses and rapid progression. J Gen Virol. 1998;79(Pt 10):2461–2467. doi: 10.1099/0022-1317-79-10-2461. [DOI] [PubMed] [Google Scholar]
- 34.Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Gettie A, Hendrickx AG, et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson S, Happel KI, Zhang P, Myers L, Dufour JP, Bagby GJ. Effect of Bacterial Pneumonia on Lung Simian Immunodeficiency Virus (SIV) Replication in Alcohol Consuming SIV-Infected Rhesus Macaques. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahar B, Gray WL, Phelps K, Didier ES, Deharo E, Marx PA, et al. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus - simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virol J. 2012;9:160. doi: 10.1186/1743-422X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003;282:103–115. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pahar B, Li J, McChesney MB. Detection of T cell memory to measles virus in experimentally infected rhesus macaques by cytokine flow cytometry. J Immunol Methods. 2005;304:174–183. doi: 10.1016/j.jim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 41.Kozlowski PA, Chen D, Eldridge JH, Jackson S. Contrasting IgA and IgG neutralization capacities and responses to HIV type 1 gp120 V3 loop in HIV-infected individuals. AIDS Res Hum Retroviruses. 1994;10:813–822. doi: 10.1089/aid.1994.10.813. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JS, Lacour N, Kozlowski PA, Nelson S, Bagby GJ, Amedee AM. Characterization of SIV in the oral cavity and in vitro inhibition of SIV by rhesus macaque saliva. AIDS Res Hum Retroviruses. 2010;26:901–911. doi: 10.1089/aid.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im1211s64. Chapter 12:Unit 12 11. [DOI] [PubMed] [Google Scholar]
- 44.Rychert J, Amedee AM. The antibody response to SIV in lactating rhesus macaques. J Acquir Immune Defic Syndr. 2005;38:135–141. doi: 10.1097/01.qai.0000148947.03416.b5. [DOI] [PubMed] [Google Scholar]
- 45.Poonia B, Nelson S, Bagby GJ, Veazey RS. Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: implications for SIV/HIV infection. J Acquir Immune Defic Syndr. 2006;41:537–547. doi: 10.1097/01.qai.0000209907.43244.ee. [DOI] [PubMed] [Google Scholar]
- 46.Mattapallil JJ, Letvin NL, Roederer M. T-cell dynamics during acute SIV infection. AIDS. 2004;18:13–23. doi: 10.1097/00002030-200401020-00002. [DOI] [PubMed] [Google Scholar]
- 47.Kuhrt D, Faith SA, Leone A, Rohankedkar M, Sodora DL, Picker LJ, et al. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: rapid depletion of naive and memory B-cell subsets with delayed reconstitution of the naive B-cell population. J Virol. 2010;84:2466–2476. doi: 10.1128/JVI.01966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalod M, Dupuis M, Deschemin JC, Sicard D, Salmon D, Delfraissy JF, et al. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Watson RR. Chronic ethanol consumption before retrovirus infection is a cofactor in the development of immune dysfunction during murine AIDS. Alcohol Clin Exp Res. 1994;18:976–981. doi: 10.1111/j.1530-0277.1994.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O'Brien CP, et al. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin Exp Res. 2002;26:1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Huang DS, Giger PT, Watson RR. Ethanol-induced modulation of cytokine production by splenocytes during murine retrovirus infection causing murine AIDS. Alcohol Clin Exp Res. 1993;17:1035–1039. doi: 10.1111/j.1530-0277.1993.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 53.Buseyne F, Scott-Algara D, Porrot F, Corre B, Bellal N, Burgard M, et al. Frequencies of ex vivo-activated human immunodeficiency virus type 1-specific gamma-interferon-producing CD8+ T cells in infected children correlate positively with plasma viral load. J Virol. 2002;76:12414–12422. doi: 10.1128/JVI.76.24.12414-12422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Xu H, Pahar B, Lackner AA, Veazey RS. Divergent kinetics of proliferating T cell subsets in SIV Infection: SIV eliminates the “first responder” CD4+ T cells in primary infection. J Virol. 2013 doi: 10.1128/JVI.00027-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, et al. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood. 2010;116:4168–4174. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular cytokine flow cytometry for IFNγ and TNFα responses from a representative alcohol treated SIV-infected rhesus macaque. Cells were gated first on singlets, lymphocytes, followed by live cells and then on CD3+ T-cells and subsequently on CD3+CD4+ and CD3+CD8+ T-cell subsets. The percentages of total IFNγ, total TNFα, and double positive IFNγ+TNFα+ CD8+ T-cells are shown in each upper box of each plot. Note that this macaque had increased Gag and gp120 specific IFNγ responses and double positive IFNγ and TNFα responses detected 14 days post SIV infection.
SIV specific T-cell responses in either ethanol or sucrose treated SIV infected macaques. Intracellular double-positive (DP) IFNγ+TNFα+ responses against SIV-Gag, SIV-gp120, and SIV-gp41 peptides were measured at 9 different time points post SIV infection. Data presented are means ± standard errors of CD8+DP T-cells at different time points of infection. Higher levels of SIV-Gag and gp41 specific DP responses were detected in ethanol treated macaques compared to sucrose treated controls in peripheral CD8+ T-cells (*p=0.02). However, SIV-gp120 and gp41 specific DP responses were low to undetectable in all animals. Criteria for a positive cytokine response was a two-fold increase in frequency for that specific antigen and cytokine above the medium control culture. All values were subtracted from medium control before analysis.