Abstract
Background
Prenatal alcohol exposure has been shown to increase offspring susceptibility to some chemical carcinogens. Whether prenatal exposure to alcohol makes the offspring more susceptible to the development of prostate cancer is not known. Therefore, we determined if any functional abnormalities and increased cancer susceptibility exist in the prostate of fetal alcohol exposed male rats during the adult period.
Methods
Pregnant rats were fed with a liquid diet containing alcohol (alcohol-fed), pair-fed with isocaloric liquid diet (pair-fed), or ad libitum fed with rat chow (ad lib-fed). Male offspring of these rats were given N-Nitroso-N-methylurea and testosterone to induce prostate neoplasia or left untreated. Around 6 to 8 months of age, the prostate of these animals were processed for determination of biochemical changes and histopathologies.
Results
Prostates of non-carcinogen treated animals which were alcohol exposed during the prenatal period demonstrated inflammatory cell infiltration and epithelial atypia and increased number of proliferative cells in the ventral lobe of this gland, but the prostate of control animal showed normal cytoarchitecture. In addition, prenatally alcohol-exposed rats showed decreased levels of cell-cell adhesion marker and increased estrogenic activity in the ventral prostate. Prenatally ethanol-exposed rats, when treated with carcinogen and testosterone, showed histological evidence for high-grade prostatic intraepithelial neoplasia primarily in the ventral prostate, whereas control animals showed only low-grade prostatic intraepithelial neoplasia. Prenatally ethanol-exposed rats treated with carcinogen and testosterone also showed increased number of proliferative cells and androgen receptor with concomitant decreased levels of tumor suppressor proteins in the ventral prostate.
Conclusions
These results suggest for the first time that prenatal ethanol exposures induces histophysiological changes in the prostate as well as it increases the susceptibility of the prostate to develop neoplasia during adulthood.
Keywords: Aromatase, estrogen receptor, fetal alcohol, prostate cancers
INTRODUCTION
Prostate cancer is the second leading cause of cancer death in men (Hsing and Chokkalingam, 2006). The National Cancer Institute, a part of the US National Institute of Health, notes that there are 240,890 new cases of prostate cancer with 33,720 deaths in the United States in 2011. It is predicted that prostate cancer incidence will increase by more than 60% by 2015 in the United States due to various endogenous (genetic, hormones, aging and oxidative stress) and exogenous factors (environmental agents, infection, diet and lifestyle) (Bostwick et al., 2004). Although the factors associated with prostate cancer remain poorly understood, intensive research endeavors described that chronic process such as infection and inflammation could be accounted in part, for the development of cancer in the gland. It is well established that there is a positive association between excessive alcohol consumption and increased risk of cancers of various organs (Adami et al., 1992; Bagnardi et al., 2001; Rothman, 1980), including the breast (Singletary and Gapstur, 2001). Many studies have provided the evidence about deleterious effects of repetitive alcohol drinking on prostate epithelial cell in humans and animal models (Crispo et al., 2004; Gómez et al., 2007; Velicer and Kristal, 2006). Clinically, increased risk of prostate cancer has been seen in a large cohort study of heavy alcohol abusers from Denmark (Tønnesen et al., 1994) and among alcoholics of Sweden who are less than 65 years of age (Adami et al., 1992). Similar association of heavy alcohol drinking and increased prostate cancer risk was observed in a recent study conducted in San Francisco with 2,129 participants in the Prostate Cancer Prevention Trial (PCPT) (Gong et al., 2009).
Prenatal alcohol exposure has also been shown to cause increased susceptibility of offspring to some chemical carcinogens (Gottesfeld et al., 1992). A growing body of evidence from animal models shows that maternal alcohol consumption during pregnancy increased breast cancer risk (Polanco et al., 2010) and advanced the occurrence of malignant tumor phenotype to the offspring (Hilakivi-Clarke et al., 2004). Interestingly, breast and prostate cancer share many similarities, in terms of geographical distribution, risk factors, biomolecular determinants, and natural history of disease (Coffey, 2001). Hence, we tested whether an analogous circumstance occurs in the prostate because diet and hormones, especially estrogen and androgen, are crucial and interactive players in many biological and pathological processes of tumorigenesis in breast and prostate.
Prostate gland development from urogenital sinus (UGS) during fetal life occurs at around 10 to 12 weeks of gestation in humans and 18.5 embryonic days in rats (Prins and Putz, 2008). The proper development of prostate is largely depends on the constant supply and binding of circulating testosterone or its more potent metabolite, 5α-dihydrotestosterone (DHT), to androgen receptors in the UGS mesenchyme and further activation of UGS epithelium by mesenchyme. A variation in androgen synthesis and secretion and/or delivery to the UGS has the potential to perturbate normal development (Prins and Putz, 2008). Studies have shown that estrogens are equally involved in the normal and abnormal growth of prostate in human and many rodents (Berges, 2009; Nicholson et al., 2011). Studies with rat models show that alcohol consumption during pregnancy increases maternal circulating estradiol levels on gestation day 19 (Hilakivi-Clarke et al., 2004) and also in their prenatal alcohol-exposed female offspring at their adult age (Polanco et al., 2010). Whether or not prenatal exposure to alcohol has any effects on prostate histophysiology and its susceptibility to carcinogens in the offspring have not been previously determined. However, it is known that exposure to estrogenic compounds during the neonatal period leads to permanent alterations in prostatic growth and also produced lobe-specific histological changes later in life (Cunha et al., 2001). Neonatal exposure of rodents to high doses of estrogen permanently imprints the growth and function of the prostate and predisposes the gland to hyperplasia and severe dysplasia analogous to prostatic intraepithelial neoplasia (PIN) during aging (Cunha et al., 2001). Thus, the aim of this study was to analyze the histophysiological alterations that may occur in the prostate of rat offspring in response to prenatal exposure to alcohol, as well as to associate these changes with pathogenesis of the prostatic diseases, especially prostatic cancer in their adult life.
MATERIALS AND METHODS
Maternal alcohol exposure
Male and female Sprague Dawley rats were purchased from Charles River (Wilmington, MA) and housed in a controlled environment. These rats were bred at 2 months of age and the pregnant dams were individually housed in 12-h light/12-h dark cycles at a constant temperature (22°C). On gestational days 7 to 21, pregnant rats were either fed a liquid diet containing ethanol (alcohol-fed, AF) (Bio-Serv, Frenchtown, NJ), or an isocaloric liquid control diet (pair-fed, PF) (Bio-Serv) in which the alcohol calories were replaced by maltose-dextrin or rat chow ad libitum (ad lib-fed, AD) (Purina Mills Lab Diet, St. Louis, MO). To habituate the dams with an alcohol diet, they were fed a liquid diet containing 1.7% (v/v) ethanol on day 7 and 8 and 5.0% (v/v) ethanol on day 9 and 10. From days 11 to 21, animals were fed with ethanol 6.7% (v/v) providing about 35% of the total dietary calories. Previous studies have shown that the peak blood ethanol concentration is achieved in the range of 120–150 mg/dl in pregnant dams fed with this liquid diet (Miller, 1992). The mothers were given experimental diets (AF or PF) only during gestational days 7 to 21. After birth, AF and PF pups were cross fostered with the rat chow fed (AD) fostering dams until postnatal day 22 to avoid the behavior or physiological changes of the original mom on pups during alcohol withdrawal after birth. Pups were then weaned and subsequently housed by treatment and sex and provided with rat chow and water ad libitum for the duration of the study. Animal care was performed in accordance with institutional guidelines and complied with the National Institutes of Health policy.
Study 1
At 8 months of age after birth, ten offspring from each treatment group were sacrificed. Individual prostatic lobes (anterior, lateral, dorsal and ventral) were dissected quickly from adhering connective tissue; one side was fixed in 10% neutral buffered formalin for histologic analyses and other side was snap frozen in liquid nitrogen for western blotting studies. Fixed prostatic tissues were processed, paraffin embedded, and sectioned (5 µm) along the longitudinal axis and used for hematoxylin and eosin (H & E) and immunohistochemical analysis.
Study 2
In order to determine the effects of maternal exposure to ethanol during pregnancy on offspring’s susceptibility to prostate cancer at their adult life, a second study was conducted with N-Nitroso-N-methylurea (NMU) and androgen-induced prostate carcinogenesis model (Arunkumar et al., 2006; Sarkar et al., 2011). Beginning at postnatal day 90, a group of AF, PF and AD offspring were injected interperitoneally (i.p.) with cyproterone acetate (50 mg/0.3 ml DMSO/kg; Sigma Chemical, St. Louis, MO) for 21 consecutive days followed by a daily i.p. injection of 100 mg/kg testosterone propionate (TP) in propylene glycol for 3 days. One day after the last TP injections, all rats received a single i.p. dose (50 mg/kg body wt.) of NMU (Sigma). Immediately prior to injection, NMU was dissolved in isotonic sterile saline at 10 mg/ml and acidified with 3% acetic acid to a final pH of 5.5. One week after NMU administration, rats received continuous exposure to testosterone via a subcutaneous implant of testosterone pellet (IRA; Innovative Research of America; 5 mg pellets for 60 days release). After 60 days of TP pellets, rats (AF = 15, PF = 11 and AD = 9) were sacrificed, and prostate lobes were processed as described above for histopathology, immunohistochemistry and Immunoblot analysis.
Histopathological evaluation
Prostate tissues from the experimental animals were paraffin-embedded, sectioned into 5 µm thick slices and placed on slides. Three serial sections were placed on each slide representing a total of 15 µm of tissue thickness. Every tenth slide was stained with H & E and examined for histopathological lesions. The serial sections of each prostatic lobes were examined. The experimenters were blind to the experimental treatment group of the section during counting. Prostatic lesions were identified histologically using criteria described previously (Bosland et al., 1992; Gleson, 1992). Epithelial focal hyperplasia was defined as an accumulation (piling) of cells facing the lumen without invasion or pleomorphism. Inflammation was scored if there was luminal abscess or if there was evident of periepithelial or intraepithelial infiltration of any inflammatory cells. Often, this was accompanied by an increased surrounding stromal thickness. Areas of atypia were defined as foci of cells with irregular acinar shape and increased nuclear size, nuclear stratification and epithelial evagination in the absence of inflammation. The diagnoses of areas with PIN were distinguished from typical hyperplasia, and defined as multilayers of dysplastic epithelial cells, with loss of cellular polarity and with evidence of focality and proliferation. The frequency of mitosis figures in the epithelial cell layers were also frequently observed in PIN. Low-grade PIN or high-grade PIN were distinguished by the degree of cellular atypia in epithelium in the pre-existing ducts or acini. PIN-lesions consisted of focal areas of hyperplastic epithelium displaying distinct variation in size, shape, and staining properties of cells and nuclei. High-grade PIN-lesions had more evident cytologic atypia, nuclear enlargement and higher frequency of cytological abnormality in most nuclei, prominent eosinophillic nucleoli positioned peripherally, noticeable nuclear enlargement, and degree of nuclear crowding, some areas was as severe as they form bridges of epithelial cells extending across the glandular lumina.
Immunohistochemical localization of various proteins
Immunohistochemistry was performed according to methods published previously (Sarkar et al., 2011) with slight modifications. Briefly, thin paraffin sections (5 µm) were mounted on Superfrost Plus glass slides (Fisher Scientific, Itasca, IL) and heated at 37°C overnight. The sections were deparaffinized in xylene, gradually hydrated with decreasing concentrations of ethanol and water, and endogenous peroxidases were removed with 3% H2O2 in methanol for 30 min. Then the slides were subjected to antigen retrieval by microwave irradiation for 10 min at 98°C in 0.1 M citrate buffer (pH 6.0). After cooling, the slides were incubated with a blocking reagent (2 % BSA in PBS) at 25°C for 30 min and subsequently incubated overnight at 4°C with primary antibodies. Primary antibodies for immunohistochemistry were used as follows: polyclonal rabbit antibodies against homeobox protein Nkx3.1 (1:50), estrogen receptor (ER)-α (1:100), androgen receptor (AR; 1:100), E-cadherin (1:100) and mouse monoclonal anti-phosphatase and tensin homolog (PTEN) (1:100; all from Santa Cruz Biotechnology Inc., Santa Cruz, CA), and polyclonal rabbit antibody for aromatase (1:200; from Abcam, Cambridge, MA) and a mouse monoclonal antibody for Ki-67 (1:50; from Abcam, Cambridge, MA). After the primary antibody incubation and PBS wash, sections were incubated with peroxidase-coupled anti-rabbit or anti-mouse IgG as secondary antibodies (ImmPRESS reagent, Vector Laboratories, Inc., Burlingame, CA). Antigen localization was achieved by using the 3,3′-diaminobenzidine-peroxidase reaction (resulted brown staining) and sections were stained with Gill’#3 hematoxylin (1:4) as a blue nuclear counterstain, dehydrated, and cover slipped with Permount (Fisher Scientific, Fair Lawn, NJ). For ER-α immunostaining we employed sections known to be positive for ER-α (rat uterus and NMU-induced mammary tissue) and confirmed the presence of ER-α immunostaining. Additionally, we were able to block the ER-α immunostaining in prostate following co-incubation with the blocking peptide (10 µg/ml, sc-542P, Santa Cruz Biotechnology) and ER-α antibody. For, aromatase immunostaining we used a positive control sample, rat liver section, and found positive for this enzyme. In all staining conditions, the omission of the primary antibody and substitution with the diluting solution alone served as a negative control. To evaluate the immunohistochemical staining, five animals in each experimental group and five microscopic fields per animal were photographed using Nikon-TE 2000 inverted microscope. Intensity of staining was categorized as negative (−), weak (+), moderate (++) and strongly positive (+++).
Western blotting
To confirm the immunohistochemical results, the levels of ER-α, aromatase, E-cadherin, AR, PTEN and Nkx3.1 proteins were evaluated by Western blot analysis. Ventral prostate tissue extracts equivalent to 50 µg total protein were separated by 4% to 20% SDS-PAGE and transferred overnight to immobilon-P polyvinylidene difluoride membranes. Membranes were incubated with a primary antibody overnight at 4°C in blocking buffer (5% not-fat dry milk in TBS). Membranes were then washed and incubated with peroxidase-conjugated secondary antibody (1: 5,000) for 1 hour. Finally, membranes were washed and signals visualized by Pierce enhanced chemiluminescence Western Blotting substrate (Thermo Fisher Scientific Inc., Rockford, IL) and were exposed to X-ray films and developed using an X-Ray developer. β-Actin served as an internal control for loading and transfer of equal amounts of protein samples. Details of all primary antibodies used are described above. The immunoblot bands were quantified through analyzing the optical densities of scanned bands using the NIH Image software (Image J).
Measurement of aromatase concentration in prostate tissue
Aromatase concentrations in prostate tissues samples were measured using by enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc, Houston, TX). Briefly, prostate tissues were rinsed in ice-cold PBS (0.02 mol/L, pH 7.2) to remove excess blood and weighed before homogenization. Minced the tissues to small pieces and homogenized in PBS and the resulting suspension was subjected to two freeze-thaw cycles to further break the cell membranes. After that, the homogenates were centrifuged for 5 minutes at 5000×g and the supernatants were used for aromatase assay as described in the manufacturer’s instructions. Rat ovarian tissue (1:10 diluted) was used as a positive control. The protein content of supernatant was measured using Bio-Rad Protein Assay reagent (Bio-Rad, Hercules, CA) and aromatase concentration was expressed as ng/mg protein.
Quantification of estradiol in prostate tissues
Tissue estradiol (E2) levels were quantitated by using commercially available E2 competitive enzyme-linked immunoassay (EIA) (Cayman Chemical, Ann Arbor, MI) (sensitivity =20 pg/ ml). Briefly, tissue lysates from each sample (in triplicates) were diluted (1:50) with EIA buffer to obtain a concentration appropriate for the range of the E2 (6.6–4000 pg/ml). Rat ovarian tissue (1:100 diluted) was used as a positive control. The average of the triplicates (picograms E2 per milliliter) obtained through the EIA was corrected for the dilution factor and standardized for protein concentration (milligrams protein per milliliter giving values in units of picograms E2 per milligram protein).
Statistical Analysis
The changes in numbers of Ki-67 cells between groups and Western blot results were analyzed by one-way ANOVA followed by Newman-Keuls post hoc test. Differences in histopathological changes and cancer incidences between groups were evaluated using a Fischer Exact test. A P value less than 0.05 were considered significant.
RESULTS
Pathological changes in the prostate gland of adult animals prenatally exposed to alcohol
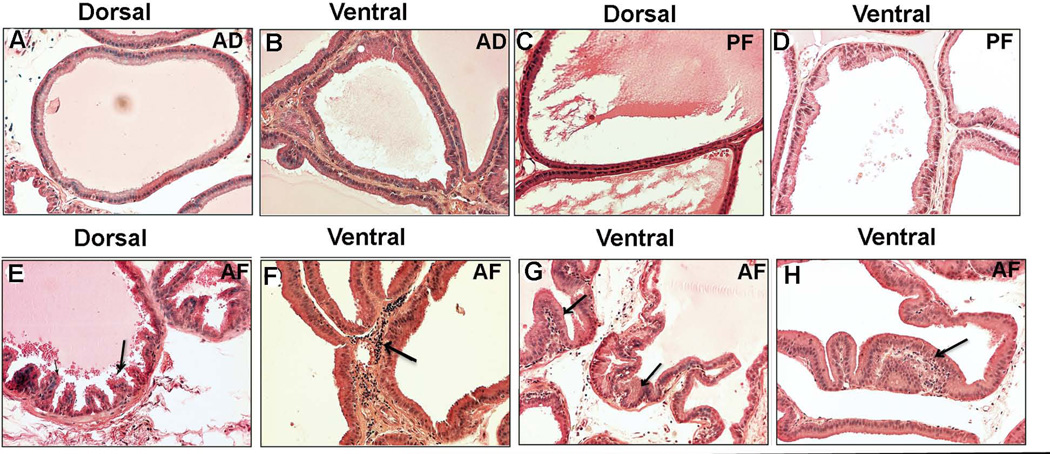
To investigate whether rats that were exposed to alcohol in utero displayed any histological abnormalities in the prostate during adulthood, AD, PF and AF offspring rats at eight months of age were sacrificed and analyzed for histopathological lesions. The anterior, lateral, dorsal and ventral lobes of the prostate were examined to determine histopathologies. The appearance and architecture of the epithelial cells in the anterior and lateral lobes of any treatment groups was normal (data not shown). Both dorsal and ventral lobes of AD and PF rats also showed normal architecture of glandular epithelium (Fig. 1A–D). Dorsal prostate of AF animals showed a few mild focal hyperplasia but mostly normal architecture of glandular epithelium (Fig. 1E). However, the ventral prostate of AF animals showed abundant infiltration of inflammatory cells in stromal compartment accompanied by increased stromal thickness (Fig. 1F). Also AF animals demonstrated severe focal hyperplasia (Fig. 1G) and varying degrees of atypia (dysplasia) characterized by nuclear stratification and epithelial evagination (Fig. 1H) in ventral epithelium of the prostate. When the incidence of prostate with abnormalities in each group was compared, only the prenatal alcohol exposed AF group had a significantly higher number of preneoplastic lesions in the prostates (Table 1).
Figure 1.
Histopathological changes in ventral and dorsal prostatic lobes of fetal alcohol exposed offspring at 8 month after birth. Photomicrographs of representative H&E stained sections of dorsal and ventral prostatic lobes of ad lib-fed (AD), pair-fed (PF) and alcohol-fed (AF) rat offspring are shown. Dorsal and ventral prostatatic lobes of AD (A, B) and PF (C, D) animals showed normal architecture of glandular epithelium. The dorsal lobe of AF animals showed a few mild focal hyperplasia (E), while the ventral lobe of AF animals showed many inflammatory cells in the stromal compartment accompanied by stromal thickness (F; shown by an arrow), focal hyperplasia (G; shown by an arrows), and epithelial evagination (H; atypia-dysplasia, shown by an arrow). All the images were captured at 40X magnification.
Table 1.
Prostate histology of fetal alcohol exposed and control offspring at adult age
| Treatment | % Normal (N) | % Atypia + hyperplasia (N) |
|---|---|---|
| Ad lib-fed | 100 (10) | 0 |
| Pair-fed | 100 (10) | 0 |
| Alcohol-feda | 23 (3)a | 70 (7)a |
P<0.001, alcohol-fed vs. the rest as determined by the Fisher’s Exact Test
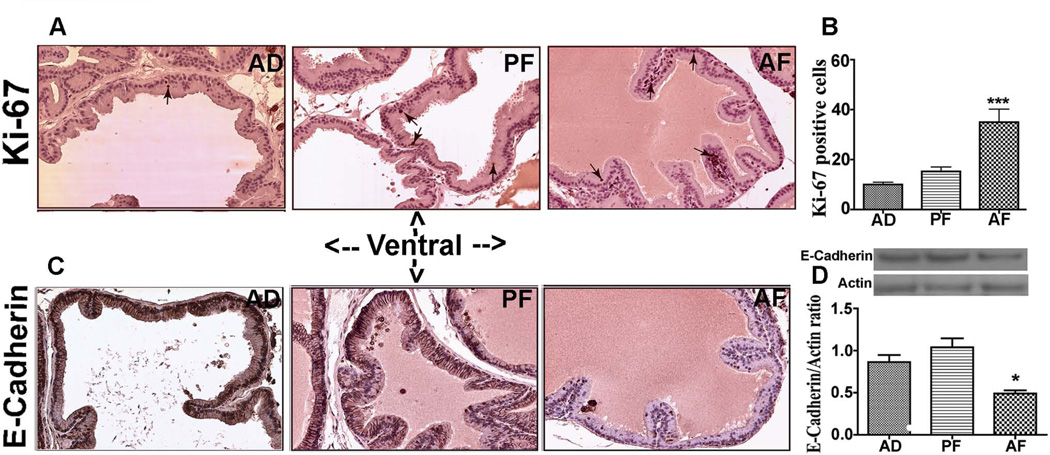
Since we observed severe focal hyperplasia and varying degrees of atypia in the ventral prostate of AF animals, we assessed the cell proliferation by determining Ki-67 positive cell numbers in this prostatic lobe. The cell number of Ki-67-positive cells was generally low in all the lobes in alcohol non-exposed AD and PF rats. Whereas, AF rats showed increased number of Ki-67 positive cells throughout the epithelial layer of the ventral prostate (Fig. 2A, B), suggesting that the cell proliferation rate throughout this tissue was increased in prenatally ethanol exposed rats.
Figure 2.
Changes in levels of Ki-67 and E-cadherin in the ventral prostate of fetal alcohol exposed offspring at 8 month after birth. Representative Ki-67 immunostained images of ventral prostates from AD, PF and AF exposed animals at adult age (A). Only few Ki-67 immuno positive cells (shown by arrows) were found in AD and PF animals. The ventral prostate of AF rats showed a marked increase of Ki-67 positive cells. The average number of Ki-67 from 5 representative slides containing 3 serial sections from each part of the ventral lobe in each group was presented in histograms (B). N = 5 rats. ***, P< 0.001 vs. AD and AF. Immunohistochemical analysis of E-cadherin in ventral prostatic lobes (C). Strong membrane and nuclear staining is seen in ventral lobe of AD and PF rats, but weaker staining was noted in the ventral lobe of AF rats. 40X magnification. The level of E-Cadherin in the extract of ventral prostate was measured by western blot using actin as a control for protein loading. E-Cadherine and actin values were presented in represented gel blots and mean ± SEM values as histograms (D). N = 5–6 rats. *P< 0.05 vs. AD and AF.
We also determined the changes in the level of cell adhesion protein E-cadherin using immunohistochemistry and immunoblotting in the ventral prostate, since this adhesion protein is critical for retaining the integrity of epithelial cells and preventing cell transformation (Bussemakers et al., 1992). Results show that ventral epithelial lobe of the AD and PF rats had strong staining for E-cadherin, whereas this prostatic lobe of AF rats showed lower E-cadherin immunostaining (Fig. 2C). Western blot analysis of prostatic tissue samples from each group confirmed that the E-cadherin level was lower in the AF group compared to AD and PF controls (Fig. 2D).
Changes in the steroidal environment in the prostate of adult animals exposed to prenatal ethanol
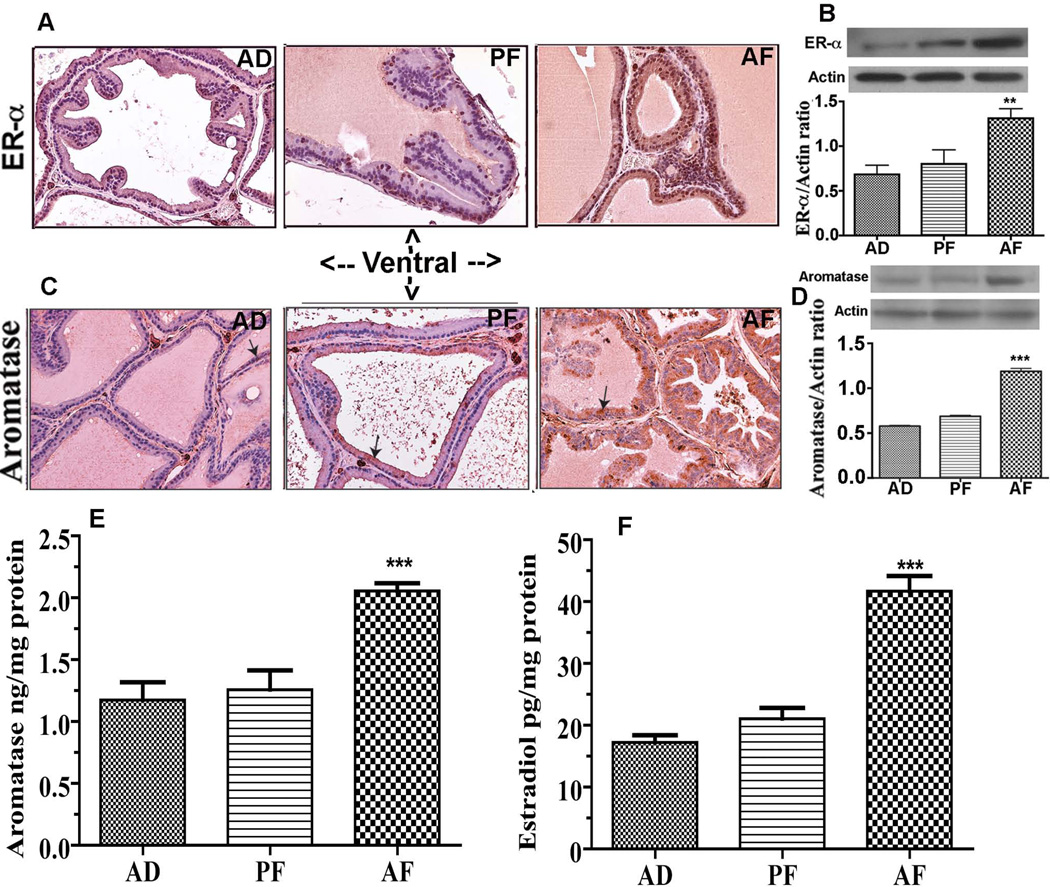
Because prostatic levels of steroid hormones and their receptors are known to be critically involved in prostate maintenance and pathogenesis (Ellem et al., 2010), we measured the levels of sex steroid hormone receptor (ER-α), aromatase, an enzyme essential for production of estradiol from testosterone, and tissue levels of estradiol (E2) in ventral lobe of the prostate of AF, AD and PF rats. The ER-α immunoreactivity was observed in both stromal and epithelial areas of the ventral lobe of the prostate in all treatment groups (Fig. 3A). The ER-α immunoreactivity was higher in the ventral prostate of AF rats as compared to AD and PF rats. Consistent with these, western blot measurement of ER-α protein levels also revealed increased levels of ER-α protein in the ventral prostate of AF rats as compared to those in AD and PF rats (Fig. 3B).
Figure 3.
Changes in levels of estrogen receptor-α (ER-α) and aromatase in the ventral prostate of fetal alcohol exposed offspring at 8 month after birth. (A) Representative ER-α immunostained images of ventral prostatic lobe from AD, PF and AF exposed animals at adult age. Only occasional ER-α positivity in dorsal lobe of the prostate was observed in AD and PF offspring rats. In contrast, strong ER-α positivity throughout stromal and luminal epithelial cells of ventral lobe was observed in AF rats. 40X magnification. ER-α and actin values were presented in represented gel blots and mean ± SEM values as histograms (B). N = 5–6 rats. **P< 0.01 vs. AD and AF. Immunohistochemical analysis of aromatase in ventral prostatic lobe from AD, PF and AF exposed animals at adult age (C). Aromatase staining was observed in the cytoplasm of the ventral tissue and was more dense in AF rats-derived tissue than in AD and PF rats-derived tissues. Immunoblot of aromatase and actin were presented in represented gel blots and mean ± SEM values as histograms (D). N = 5–6 rats. ***P< 0.001 vs. AD and AF. The mean ± SEM tissue concentration of aromatase in the ventral prostate measured using ELISA are shown as histograms (E). N = 6–8 rats. ***P< 0.001 vs. AD and AF. The mean ± SEM tissue concentration of estradiol (E2) in the ventral prostate measured using ELISA are shown as histograms (F). N = 6–7 rats. ***P< 0.001 vs. AD and AF.
Immunohistochemical analysis of aromatase in prostatic tissues revealed that this enzyme is present in the cytoplasm of prostatic epithelial cells of the ventral prostate of all the groups. The immunohistochemical level of aromatase protein was greatly increased in the ventral prostate of prenatal alcohol exposed animals (AF) compared to control (AD and PF) rats (Fig. 3C). Western blot measurement of aromatase protein also revealed an increased level of this protein in the ventral prostate of AF rats as compared to AD and PF rats (Fig. 3D). Quantitative measurement of tissue aromatase levels using ELISA also indicated a significant increase in the tissue level of this protein in the ventral prostate of AF rats as compared to those in AD and PF rats (Fig. 3E). The increased in the aromatase level in the ventral prostate positively correlated with the E2 levels in this tissue in AF rats (Fig. 3F).
Changes in the tumorigenic response of prostatic lobe to carcinogen in animals exposed to prenatal ethanol
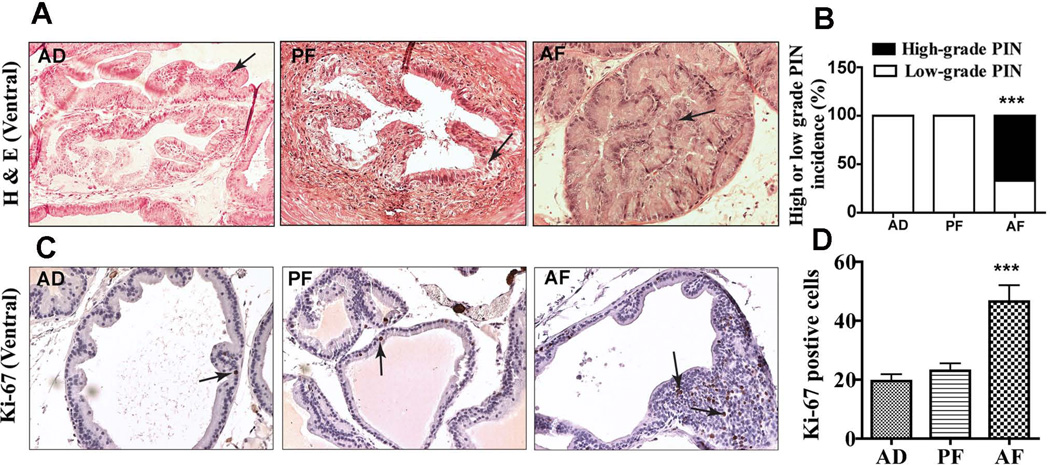
In order to determine whether the preneoplastic prostatic lesion we observed in AF rats make their prostate more susceptible to carcinogen, we studied the effect of a combined carcinogen (NMU) and hormonal (testosterone) treatment on prostate carcinogenesis in adult AF, PF and AD male rats. These rats were treated with NMU and testosterone and their prostate glands were processed and analyzed for histopathological lesions. In non-alcohol exposed animals, NMU and testosterone treatment did not change the architecture of the epithelial cells in the dorsal lobes (not shown) but induced lower-grade PIN lesions in the ventral lobes (Fig. 4A). However, in AF rats, NMU and testosterone treatment induced low-grade PIN in dorsal (not shown) but mostly high-grade PIN in the ventral prostate (Fig. 4A; B).
Figure 4.
Changes in histopathology and Ki-67 immunostaining in ventral prostatic lobes of adult (7–8 months of age) fetal alcohol exposed offspring treated with NMU and testosterone. Representative H&E stained photomicrographs of ventral prostatic lobes of AD, PF and AF rat offspring (A). The ventral lobe of AD and PF rats showed low-grade prostatic intraepithelial neoplasia (PIN; arrows), while similar prostatic lobe of AF rats showed high-grade PIN. All the images were captured at 40X magnification. Mean ± SEM values of percentage ratio of low grade and high grade PIN in animals after carcinogen treatment in AD, PF and AF groups are shown as histograms (B). N = 9–14 rats. ***P< 0.001 vs. AD and AF. Showing representative Ki-67 immunostained images of ventral prostates from AD, PF and AF exposed animals at adult age (C). Ki-67 immuno positive cells (shown by arrows) are sparsely distributed in the ventral prostate of AD and PF animals but heavily concentrated in the epithelia of the ventral prostate of AF rats. The average number of Ki-67 from 5 representative slides containing 3 serial sections from each part of the ventral lobe in each group was presented in histograms (D). N = 9–14 rats. ***P< 0.001 vs. AD and AF.
We have also measured several biochemical parameters to characterize the tumor phenotypes in the prostate of AF, AD and PF rats. First, we determined the changes in cell proliferation rate using Ki-67 staining procedures. Immunostaining for Ki-67 indicated that only a few Ki-67 positive cells were present in the ventral prostate of AD and PF animals. Whereas, prenatal alcohol exposed AF animals that are treated with carcinogen and hormone had a large number of Ki-67 positive cells throughout the dysplastic epithelial cells of the ventral lobe (Fig. 4C, D).
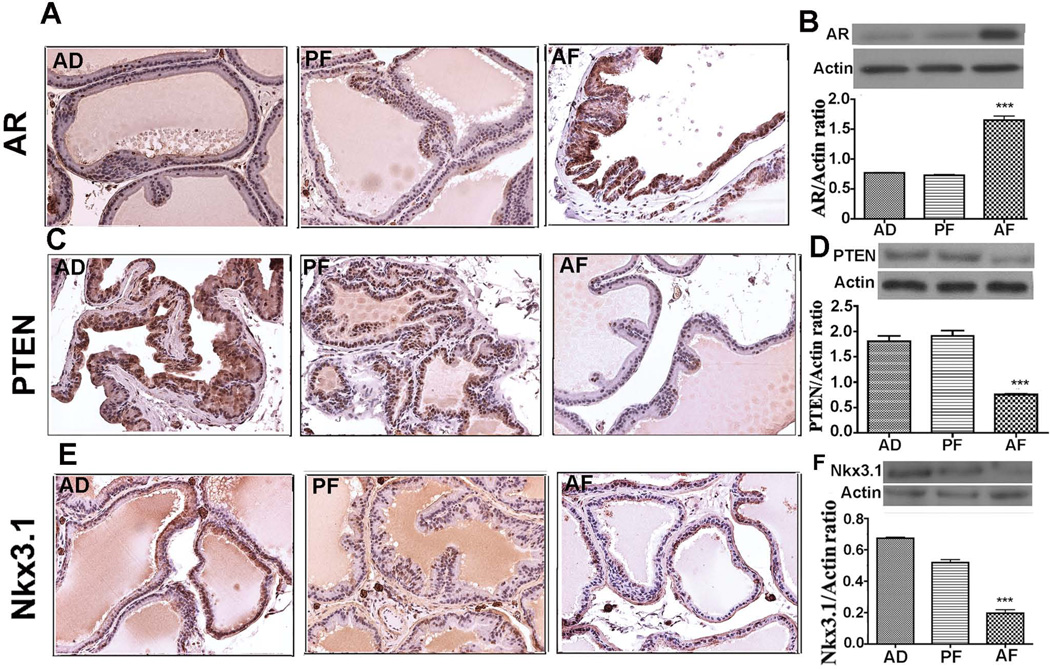
We have also measured the cellular levels of AR, PTEN and Nkx3.1 proteins in the ventral prostate of AF, AD and PF rats following NMU and testosterone treatments, since an alteration of these proteins are known to be strongly associated with prostate cancer development (Lei et al., 2006). Immunostaining of the prostate for AR demonstrated a uniform and intense expression of AR in ventral lobes of AF animals, but low intensity AR expression is observed in the ventral lobes of control animals (Fig. 5A). In contrast, strongly positive PTEN- or Nkx3.1 staining was detected throughout the ventral lobe of control animals and weakly stained PTEN or Nkx3.1 cells were detected in the similar lobe of the prostate of AF animals (Fig. 5C, E). Western blot analysis of these proteins in the ventral prostate identified similar results with those of immunohistochemical data and showed an increase of AR (Fig. 5B) and a decrease of PTEN (Fig. 5D) and Nkx3.1 (Fig. 5F) levels in AF rats than those in AD and PF animals.
Figure 5.
Changes in levels of androgen receptor (AR), phosphatase and tensin homolog (PTEN) and homeobox protein Nkx3.1 in the ventral prostate of adult (7–8 months of age) fetal alcohol exposed exposed offspring treated with NMU and testosterone. Showing representative immunostaining and immunoblot measurements of AR (A, B), PTEN (C, D) and Nkx3.1 (E, F) in prostatic lobes of AD, PF and AF animals. Uniform and intense staining of AR was observed in ventral lobes of AF animals but not in AD and PF rats. Strongly positive PTEN cells as well as Nkx3.1 cells were also detected throughout the ventral lobes of AD and PF, while cell positive for these proteins were sparsely distributed in ventral lobes of AF rats. Western blots data of AR, PTEN and Nkx3.1 in ventral prostate extracts were presented in represented gel blots and mean ± SEM values as histograms. N = 5–6 rats. **P< 0.01 vs. AD and AF.
DISCUSSION
Prostate cancer is the most common visceral cancer of men, with few established risk factors. Prostate cancer susceptibility may be determined during fetal development (Driscoll and Taylor, 1980; Ekbom et al., 2000; Narayanan et al., 2009; Troisi et al., 2003). While a connection between the fetal alcohol and breast cancer risk has been proposed (Hilakivi-Clarke et al., 2004; Polanco et al., 2010), no study has addressed alcohol as the fetal insult for prostate cancer. Our work provides the first evidence that alcohol exposure in utero leads to an increase in epithelial hyperplasia of the prostate and promotes high-grade PIN development in response to NMU and testosterone.
We provide in this study histomorphological evidence for the presence of early epithelial hyperplasia in the ventral prostate of fetal ethanol-exposed rats at the age of about 8 months who had no carcinogen or testosterone treatment. Control rats showed no microscopic evidence of epithelial hyperplasia and atypia at this age, suggesting a significant lesion in the prostate is caused by the fetal alcohol effect. Biochemical evidence with increased Ki-67 in the ventral prostate of AF rats further supported hyperplasia in this tissue.
We showed here that majority of the alcohol exposed animals demonstrated varying degrees of atypia in ventral epithelium of the prostate. In addition, the ventral prostate of AF animals showed abundant infiltration of inflammatory cells in stromal compartment accompanied by increased stromal thickness. Several experimental studies have shown that the appearance of acute and chronic inflammation and associated mediators of inflammation, increases cell proliferation, hyperplasia and dysplastic lesions which precedes the development of prostate cancer (Bernoulli et al., 2008; Narayanan et al., 2009). The observed finding in this study are also in agreement with data showing that chronic ethanol feeding in adult rats induces biochemical and ultrastructural changes to the epithelial cells of the prostate, especially in the ventral prostate (Gómez et al., 2007; Tønnesen et al., 1994). A recent study shows that exposure of rat prostate to alcohol through repetitive drinking, increased oxidative stress and caused prostate epithelial cell injury by producing acetaldehyde, 1-hydroxyethyl free radical and decreased antioxidant defenses (Castro et al., 2009). These toxic metabolites ultimately lead to the biochemical and ultrastructural changes to the epithelial cells of the prostate. This might be of interest, because oxidative stress has been increasingly considered as an etiological factor in the development and progression of prostate cancer (Khandrika et al., 2009). Supporting this possibility is the finding that highly reactive chemical molecules such as hydrogen peroxide and nitric oxide released by activated inflammatory cells (phagocytes) can cause oxidative/nitrosative damage to DNA in the epithelial cells and can contribute directly to neoplastic transformation (Khandrika et al., 2009). Thus, it could be hypothesized that prenatal alcohol exposure during the development of prostate that induces inflammation and several histological changes within ventral prostatic lobes of the offspring may significantly facilitate vulnerability of the gland to toxicities and/or hormonal changes for neoplasm.
Our results also show that in prenatally alcohol-exposed animals there is a significant loss of E-cadherin immunostaining in ventral prostatic at their adulthood. These data are consistent with available data showing that adult and aged prostate of estrogenized rats had larger foci of epithelial cells that were negative for E-cadherins and this is highly significant, because loss of E-cadherin is associated with malignancy and progression of prostatic diseases (Bernoulli et al., 2008). Thus, absence or weak reactivity for E-cadherin in prenatally alcohol-exposed animals suggest that these changes may be one of the key mechanisms through which changes towards a dysplastic state are mediated.
Alterations in the physiological levels of steroid hormone have been implicated in the prostate maintenance and also in development of benign and malignant prostate disease (Ellem et al., 2010). Local production of estrogens from androgens is mediated through the aromatase enzyme, which often changes during the development of prostate malignancy. We found that both aromatase, E2 and ER-α levels in prostate were greatly increased in the ventral prostate of prenatal alcohol exposure animals. These data are in agreement with the previous data showing that exposure of alcohol during pregnancy elevate the serum estradiol levels in mothers and up regulates the levels of aromatase and ER-α in the mammary glands of their female offspring during adult life (Polanco et al., 2010; 2011). It could be hypothesized that alcohol’s alteration in maternal steroid (Hilakivi-Clarke et al., 2004), activates (imprints) aromatase activity and the ER-α expression which might promotes aberrant cell growth and other cellular and molecular changes in the prostatic epithelium leading to prostatic hyperplasia/atypia during the adult life. Further studies are needed to establish this hypothesis.
We show here that the prostate of fetal alcohol exposed offspring is more sensitive to NMU and testosterone. Unlike control animals, fetal alcohol exposed offspring developed high grade PIN. Although there are a number of animal carcinogenesis models, they are based on single sex hormone, testosterone, or a combination of testosterone and estrogen (Leav et al., 1988; Wong et al., 1998). The combination of testosterone and carcinogen NMU has the advantage of inducing higher incidence of prostate carcinogenesis in rats (Arunkumar et al., 2006; Liao et al., 2002; McCormic and Rao, 1999). General findings suggest that the NMU model is relevant to human disease (Liao et al, 2002). PIN is a lesion that is now widely accepted as a pre-malignant condition for prostate cancer development (Brawer, 1992; Lipski et al., 1996). In NMU and testosterone treated alcohol-fed animals, higher-grade PINs were observed, which shows the induction of prostate tumor.
Previous studies have identified several dysregulated genes and their protein products that may contribute to the development of prostate cancer. Of these, increased AR is associated with tumor progression while loss of PTEN and NKX3.1 are strongly correlated in prostate cancer development (Eng, 2003; Lei et al., 2006). Also, AR negatively controls PTEN expression in the prostate (Lei et al., 2006). We found lower levels of PTEN and NKX3.1, and higher levels of AR in the prostate of prenatal alcohol exposed rats. So it appears that the AR expression in prostate glands of AF rats is no longer under the control of NKX3.1. In the absence of NKX3.1, increased AR level and activity is known to lead an increased cell proliferation, decreased cell death, and prostate cancer initiation (Troisi et al., 2003). Thus AR, PTEN and NKX3.1 interaction might be a potential mechanism for the increased malignancy in the prostate of AF animals.
In summary, we show here for the first time that exposure of alcohol during the early life induces molecular and cellular changes that predispose the prostate to the neoplastic state later in life. However, further studies are required to identify the molecular mechanism of fetal alcohol promotion of prostate carcinogenesis.
ACKNOWLEDGEMENTS
We thank Nicole Rafferty and Kathleen Roberts for technical assistance. We also thank Dr. K.R. Reuhl for his advice on tissue pathological assessments. This work is partly supported by a National Institute of Health grant R37AA08757.
REFERENCES
- Adami HO, McLaughlin JK, Hsing AW, Wolk A, Ekbom A, Holmberg L, Persson I. Alcoholism and cancer risk: a population-based cohort study. Cancer Causes Control. 1992;3:419–425. doi: 10.1007/BF00051354. [DOI] [PubMed] [Google Scholar]
- Arunkumar A, Vijayababu MR, Venkataraman P, Senthilkumar K, Arunakaran J. Chemoprevention of rat prostate carcinogenesis by diallyl disulfide, an organosulfur compound of garlic Biol Pharm Bull. 2006;29:375–379. doi: 10.1248/bpb.29.375. [DOI] [PubMed] [Google Scholar]
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernoulli J, Yatkin E, Laakso A, Anttinen M, Bosland M, Vega K, Kallajoki M, Santti R, Pylkkänen L. Histopathological evidence for an association of inflammation with ductal pin-like lesions but not with ductal adenocarcinoma in the prostate of the noble rat. Prostate. 2008;68:728–739. doi: 10.1002/pros.20719. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55:533–542. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Bosland MC, Prinsen MK, Rivenson A, Silverman J, Fiala E, Williams GM, Kroes R, Weisburger JH. Induction of proliferative lesions of ventral prostate, seminal vesicle, and other accessory sex glands in rats by N-methyl-N-nitrosourea: effect of castration, pretreatment with cyproterone acetate and testosterone propionate and rat strain. Prostate. 1992;20:339–353. doi: 10.1002/pros.2990200408. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters DJ, Timms B. Human prostate cancer risk factors. Cancer. 2004;101:2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- Brawer MK. Prostatic intraepithelial neoplasia: a premalignant lesion. Hum Pathol. 1992;23:242–248. doi: 10.1016/0046-8177(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Bussemakers MJ, van Moorselaar RJ, Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM, Schalken JA. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992;52:2916–2922. [PubMed] [Google Scholar]
- Castro GD, Costantini MH, Castro JA. Rat ventral prostate xanthine oxidase-mediated metabolism of acetaldehyde to acetyl radical. Hum Exp Toxicol. 2009;28:203–208. doi: 10.1177/0960327109105406. [DOI] [PubMed] [Google Scholar]
- Coffey DS. Similarities of prostate and breast cancer: Evolution, diet, and estrogens. Urology. 2001;57:31–38. doi: 10.1016/s0090-4295(00)00938-9. [DOI] [PubMed] [Google Scholar]
- Crispo A, Talamini R, Gallus S, Negri E, Gallo A, Bosetti C, La Vecchia C, Dal Maso L, Montella M. Alcohol and the risk of prostate cancer and benign prostatic hyperplasia. Urology. 2004;64:717–722. doi: 10.1016/j.urology.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wang YZ, Hayward SW, Risbridger GP. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev. 2001;13:285–296. doi: 10.1071/rd01010. [DOI] [PubMed] [Google Scholar]
- Driscoll SG, Taylor SH. Effects of prenatal maternal estrogen on the male urogenital system. Obstet Gynecol. 1980;56:537–542. [PubMed] [Google Scholar]
- Ekbom A, Wuu J, Adami HO, Lu CM, Lagiou P, Trichopoulos D, Hsieh C. Duration of gestation and prostate cancer risk in offspring. Cancer Epidemiol Biomarkers Prev. 2000;9:221–223. [PubMed] [Google Scholar]
- Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118:246–251. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. 1992;23:273–279. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- Gómez MI, de Castro CR, Fanelli SL, Quintans LN, Costantini MH, Castro JA, Castro GD. Biochemical and ultrastructural alterations in the rat ventral prostate due to repetitive alcohol drinking. J Appl Toxicol. 2007;27:391–398. doi: 10.1002/jat.1219. [DOI] [PubMed] [Google Scholar]
- Gong Z, Kristal AR, Schenk JM, Tangen CM, Goodman PJ, Thompson IM. Alcohol consumption, finasteride, and prostate cancer risk: results from the Prostate Cancer Prevention Trial Cancer. 2009;115:3661–3669. doi: 10.1002/cncr.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z, Trippe K, Wargovich MJ. Berkowitz AS Fetal alcohol exposure and adult tumorigenesis. Alcohol. 1992;9:465–471. doi: 10.1016/0741-8329(92)90082-l. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cabanes A, de Assis S, Wang M, Khan G, Shoemaker WJ, Stevens RG. In utero alcohol exposure increases mammary tumorigenesis in rats. Br J Cancer. 2004;90:2225–2231. doi: 10.1038/sj.bjc.6601793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst. 1988;80:1045–1053. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–378. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Liao Z, Boileau TW, Erdman JW, Jr, Clinton SK. Interrelationships among angiogenesis, proliferation, and apoptosis in the tumor microenvironment during N-methyl-N-nitrosourea androgen-induced prostate carcinogenesis in rats. Carcinogenesis. 2002;23:1701–1711. doi: 10.1093/carcin/23.10.1701. [DOI] [PubMed] [Google Scholar]
- Lipski BA, Garcia RL, Brawer MK. Prostatic intraepithelial neoplasia: significance and management. Semin Urol Oncol. 1996;14:149–155. [PubMed] [Google Scholar]
- McCormick DL, Rao KV. Chemoprevention of hormone-dependent prostate cancer in the Wistar-Unilever rat. Eur Urol. 1999;35:464–467. doi: 10.1159/000019880. [DOI] [PubMed] [Google Scholar]
- Miller MW. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: effect of in utero exposure to ethanol. Brain Res. 1992;595:17–24. doi: 10.1016/0006-8993(92)91447-m. [DOI] [PubMed] [Google Scholar]
- Narayanan NK, Nargi D, Horton L, Reddy BS, Bosland MC, Narayanan BA. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinoge nesis: preventive effects of celecoxib. Prostate. 2009;69:133–141. doi: 10.1002/pros.20862. [DOI] [PubMed] [Google Scholar]
- Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco TA, Crismale-Gann C, Cohick WS. Alcohol exposure in utero leads to enhanced prepubertal mammary development and alterations in mammary IGF and estradiol systems. Horm Cancer. 2010;2:239–248. doi: 10.1007/s12672-011-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco TA, Crismale-Gann C, Reuhl KR, Sarkar DK, Cohick WS. Fetal alcohol exposure increases mammary tumor susceptibility and alters tumor phenotype in rats. Alcohol Clin Exp Res. 2011;34:1879–1887. doi: 10.1111/j.1530-0277.2010.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. The proportion of cancer attributable to alcohol consumption. Prev Med. 1980;9:174–179. doi: 10.1016/0091-7435(80)90072-9. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Zhang C, Murugan S, Dokur M, Boyadjieva NI, Ortigüela M, Reuhl KR, Mojtehedzadeh S. Transplantation of β-endorphin neurons into the hypothalamus promotes immune function and restricts the growth and metastasis of mammary carcinoma. Cancer Res. 2011;71:6282–6291. doi: 10.1158/0008-5472.CAN-11-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary KW, Gapstur SM. Alcohol and breast cancer: Review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- Tønnesen H, Møller H, Andersen JR, Jensen E, Juel K. Cancer morbidity in alcohol abusers. Br J Cancer. 1994;69:327–332. doi: 10.1038/bjc.1994.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, Sims C, Hoover RN. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States) Cancer Causes Control. 2003;14:347–355. doi: 10.1023/a:1023934518975. [DOI] [PubMed] [Google Scholar]
- Velicer CM, Kristal A, White E. Alcohol use and the risk of prostate cancer: results from the VITAL cohort study. Nutr Cancer. 2006;56:50–56. doi: 10.1207/s15327914nc5601_7. [DOI] [PubMed] [Google Scholar]
- Wong YC, Wang YZ, Tam NN. The prostate gland and prostate carcinogenesis. Ital J Anat Embryol. 1998;103:237–252. [PubMed] [Google Scholar]