Abstract
Objective
To describe the off-label use of antithrombin concentrate in tertiary care pediatric hospitals across the United States.
Study design
This is a retrospective, multicenter, cohort study of 4,210 admissions of children less than 18 years of age who received antithrombin concentrate between 2002 and 2011 within the Pediatric Health Information System administrative database. An on-label admission was defined as an admission with an International Classification of Diseases diagnostic code for a primary hypercoagulable state; admissions without this code were classified as off-label.
Results
Over the 10 year study period, off-label use of antithrombin concentrate increased 5-fold. Overall, 97% of study subjects received antithrombin off-label. Neonates < 30 days old was the largest age group (45.7%) of use; 87% of patients had at least one complex chronic condition with congenital heart/lung defects being the most prevalent primary diagnosis (36.3%). Extracorporeal membrane oxygenation (ECMO) was the most common procedure associated with antithrombin use (43.7%).
Conclusions
The off-label use of antithrombin concentrate is increasing rapidly, particularly in critically-ill children receiving ECMO, with few parallel studies to substantiate its safety or efficacy. Further pre-clinical and controlled clinical studies are critical to expanding our knowledge of this drug. In the meantime, antithrombin concentrate should be used judiciously by clinicians and following guidelines instated by hospitals.
Keywords: Pediatric Health Information System, extracorporeal membrane oxygenation, extracorporeal life support, anticoagulation therapy, critical care
Antithrombin, a 58-kD serine protease inhibitor synthesized in the liver, irreversibly inactivates several endogenous active clotting factors, including factor Xa and thrombin.1,2 Heparin, the most commonly used parenteral anticoagulant, functions by potentiating antithrombin’s anticoagulant activity 1000- to 5000-fold.3,4 Antithrombin also possesses anti-inflammatory properties mediated primarily through its interaction with the endothelium.1 Congenital antithrombin deficiency is uncommon, with an estimated prevalence of 1:500–20,000, and results from either quantitative or qualitative defects in the circulating antithrombin protein.1,5,6 Acquired antithrombin deficiency is more common resulting from low production (e.g., liver disease), consumption (e.g., disseminated intravascular coagulation), increased losses (e.g., protein-losing enteropathy, nephrotic syndrome, chylothoraces), or by drug-induced mechanisms (eg, L-asparaginase, heparin). Term neonates typically do not reach adult antithrombin levels until 6 months of age.7
Two formulations of antithrombin concentrate are available commercially. The U.S. Food and Drug Administration (FDA) approved a plasma-derived concentrate (Thrombate III®, Grifols, Los Angeles, CA) in 1991 and a recombinant concentrate (ATryn ®, GTC Biotherapeutics, Framingham, MA) in 2006. The two formulations share an identical amino acid sequence, but the glycosylation pattern differs so that the recombinant form has a higher heparin affinity and a significantly shorter half-life and is therefore given as a continuous infusion.8 Both formulations are FDA-approved for the prevention of peri-operative and peripartum thromboembolic events in adults with congenital antithrombin deficiency.9,10 The safety and efficacy of antithrombin concentrates in pediatrics has not been established; therefore, antithrombin is not FDA-approved in this population.
Three decades of literature abound with the use of antithrombin concentrate in children to treat either congenital or acquired antithrombin deficiency. At one tertiary-level pediatric hospital, antithrombin concentrate use increased from 3 patients per year in 1999 to 29 patients per year in 2009 with the most common indication being acquired antithrombin deficiency associated with major cardiac surgery, heparin infusion, sepsis, or severe thromboembolism.11 Other significant associations were extracorporeal membrane oxygenation (ECMO) and orthotopic liver transplantation.11 However, rigorous safety and efficacy studies have not paralleled the increase in the off-label use of antithrombin concentrate. This multicenter cohort study aims to describe antithrombin concentrate use in U.S. children’s hospitals.
Methods
This retrospective, multi-institutional cohort study was deemed to be exempt by the institutional review boards of Cincinnati Children’s Hospital and Medical Center, Children’s Hospital of Philadelphia, and Seattle Children’s Hospital.
Data were obtained from Pediatric Health Information System (PHIS), which contains resource utilization data from 43 U.S. free-standing children’s hospitals. Participating hospitals are located in non-competing markets of 27 states plus the District of Columbia. PHIS contains comprehensive discharge data from all participating hospitals including patient demographics, diagnosis and procedure codes, and utilization data for radiologic imaging, laboratory studies, and medication use. PHIS also contains data on the admitting hospital and service. Data were de-identified prior to inclusion in the study dataset. Encrypted medical record numbers allowed for tracking of individuals across multiple hospitalizations. The Children’s Hospital Association (formerly the Child Health Corporation of America; Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.12
Eligible subjects included children who were less than 18 years of age at time of admission, admitted to a PHIS participating hospital between January 1, 2002 and December 31, 2011, and had a pharmacy billing code for antithrombin concentrate. Subjects were excluded if the date of service was not available for all antithrombin concentrate doses or if subjects were not traceable across PHIS data files.
Although antithrombin’s efficacy and safety have not been studied in the pediatric population, for the purpose of this study, adult indications for antithrombin concentrate use were used to define on-label and off-label use. A subject was considered to have received antithrombin concentrate on-label if the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic code for a primary hypercoagulable state (289.81) was present. This ICD-9-CM code for primary hypercoagulable state encompasses multiple congenital prothrombotic disorders but, most importantly includes congenital antithrombin deficiency. All subjects without this ICD-9-CM code were defined as having received antithrombin concentrate off-label.
A previously published approach was used to classify subjects into complex chronic conditions (CCC) with the following categories: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematology/immunodeficiency, metabolic, congenital/genetic defect, and malignancy.13 Patients with arterial or venous thrombosis, sepsis, hemorrhage, chylothoraces, subjects undergoing liver transplant, and subjects receiving ECMO or cardiopulmonary bypass (CPB) were identified by ICD-9-CM codes (Appendix; available at www.jpeds.com).
Statistical Analyses
Categorical variables were summarized using frequencies and percentages and analyzed using Pearson’s chi-squared test. Continuous variables were summarized by the median and range. The rate of antithrombin concentrate use was calculated by dividing the number of admissions with antithrombin use by the total number of hospital admissions in PHIS for each year during the study period. To calculate rate of antithrombin concentrate use in ECMO patients, the number of admissions billed for both antithrombin and ECMO was divided by the total number of PHIS hospital admissions billed for ECMO for each year during the study period. To determine whether the rate for on- vs. off-label use significantly differed over time in the overall study population and for the ECMO subpopulation, a likelihood ratio test compared the linear regression model which included an interaction term for study year and on- versus off-label cohort to the model without the interaction term. For those subjects with multiple hospitalizations in which antithrombin was given, only the first hospitalization was included in the analysis. A value of P ≤ .05 was considered statistically significant. Statistical analyses were performed using Stata 12 software (Statacorp LP, College Station, TX).
Results
During the 10-year study period between January 2002 and December 2011, 4,040 unique subjects received antithrombin concentrate over 4,210 admissions. Based on the inclusion and exclusion criteria, subjects in the final study cohort corresponded to only 39 of the 43 PHIS participating hospitals. Three percent of subjects received antithrombin concentrate as on-label use and the remaining 97% were off-label uses (Table).
Table 1.
Characteristics of patients who received antithrombin concentrate
| Off-label (n=3914) | On-label (n=126) | Total (n=4040) | |
|---|---|---|---|
| Age at Admission | |||
| Median (range) | 56.5 days (1–6551) | 79 days (1–6376) | 57 days (1–6376) |
| ≤30 days old | 1789 (45.7) | 58 (46.0) | 1847 (45.7) |
| 31–364 days old | 845 (21.6) | 19 (15.1) | 864 (21.4) |
| 1 – 2.9 years old | 369 (9.4) | 8 (6.4) | 377 (9.3) |
| 3 – 9.9 years old | 463 (11.8) | 13 (10.3) | 476 (11.8) |
| 10 – 17.9 years old | 448 (11.5) | 28 (22.2) | 476 (11.8) |
| Sex | |||
| Female | 1689 (43.2) | 66 (52.4) | 1755 (43.4) |
| Race/Ethnicity | |||
| White | 1573 (40.2) | 63 (50) | 1636 (40.5) |
| Hispanic | 728 (18.6) | 22 (17.5) | 750 (18.6) |
| Black | 370 (9.5) | 9 (7.1) | 379 (9.4) |
| Other | 425 (10.9) | 9 (7.1) | 434 (10.8) |
| Length of Stay | |||
| Median (range) | 35 (1–585) | 40.5(2–328) | 35 (1–585) |
| 0–14 days | 722 (18.5) | 18 (14.3) | 740 (18.3) |
| 15–30 days | 1,018 (26) | 34 (27) | 1,052 (26) |
| 31–60 days | 1,063 (27.2) | 36 (28.6) | 1,099 (27.2) |
| 61–90 days | 478 (12.2) | 19 (15) | 497 (12.3) |
| >90 days | 633 (16.2) | 19 (15) | 652 (16.1) |
| Mortality | |||
| In-hospital death | 1190 (30.4) | 27 (21.4) | 1217 (30.1) |
| Number of days receiving AT per admission | |||
| Median (range) | 2 (1–108) | 3 (1–44) | 2 (1–108) |
| 1 | 1611 (41.2) | 30 (23.8) | 1641 (40.6) |
| 2 – 3 | 1152 (29.4) | 38 (30.2) | 1190 (29.5) |
| 4 – 5 | 467 (11.9) | 17 (13.5) | 484 (12) |
| >6 | 684 (17.5) | 41 (32.5) | 725 (18) |
| Top 5 Principal Diagnosis Categories | |||
| Congenital heart/lung defect | 1433(36.6) | 34 (27) | 1467 (36.3) |
| Perinatal diseases | 576 (14.7) | 23 (18.3) | 599 (14.8) |
| Circulatory disease | 413 (10.6) | 15 (11.9) | 428 (10.6) |
| Congenital defect, non-heart/lung | 292 (7.5) | 13 (10.3) | 305 (7.6) |
| Respiratory, incl pneumonia | 292 (7.5) | 2 (1.6) | 294 (7.3) |
| Associated Diagnoses/Procedures | |||
| Sepsis | 1322 (33.8) | 35 (27.8) | 1357 (33.6) |
| Chylothorax | 294 (7.5) | 25 (19.8) | 319 (7.9) |
| Liver transplant | 121 (3.1) | 6 (4.8) | 127 (3.1) |
| ECMO | 1734 (44.3) | 33 (26.2) | 1767 (43.7) |
| Cardiopulmonary bypass | 1459 (37.3) | 28 (22.2) | 1487 (36.8) |
| Hemorrhage and Thrombotic Events | |||
| Hemorrhage | 1317 (33.7) | 42 (33.3) | 1359 (33.6) |
| Venous thrombosis | 681 (17.4) | 52 (41.3) | 733 (18.1) |
| Arterial thrombosis | 527 (13.5) | 32 (25.4) | 559 (13.8) |
| Any thrombosis | 1109 (28.3) | 72 (57.1) | 1181 (29.2) |
Neonates less than one month old at admission comprised the largest proportion of the overall sample and in each cohort. Congenital malformations were the most common principal diagnoses in both cohorts (Table). Of these, heart and lung malformations were the most common. At least one CCC flag was identified for 87% of all subjects who received antithrombin concentrate. Forty-nine percent had one CCC, 25% had two CCCs and 13% had at least three CCCs. The most common CCCs were cardiovascular (66%) and congenital/genetic (17%) abnormalities. Other associated diagnoses include sepsis (33%), venous thrombosis (18%), and arterial thrombosis (14%; Table). An additional 8% had a chylothorax and 3% underwent liver transplantation during their hospitalization. The most common admitting services were cardiology (19%), neonatology (17%), critical care medicine (17%), and cardiovascular surgery (14%).
Overall, subjects received antithrombin concentrate for a median of two days per admission (range, 1–108 days), with the on-label cohort receiving a median of 3 days (range 1–44 days) and the off-label cohort receiving a median of 2 days per admission (range, 1–108 days; Table). The first dose of antithrombin was given on a median of six days from the time of admission (range 1–32 days).
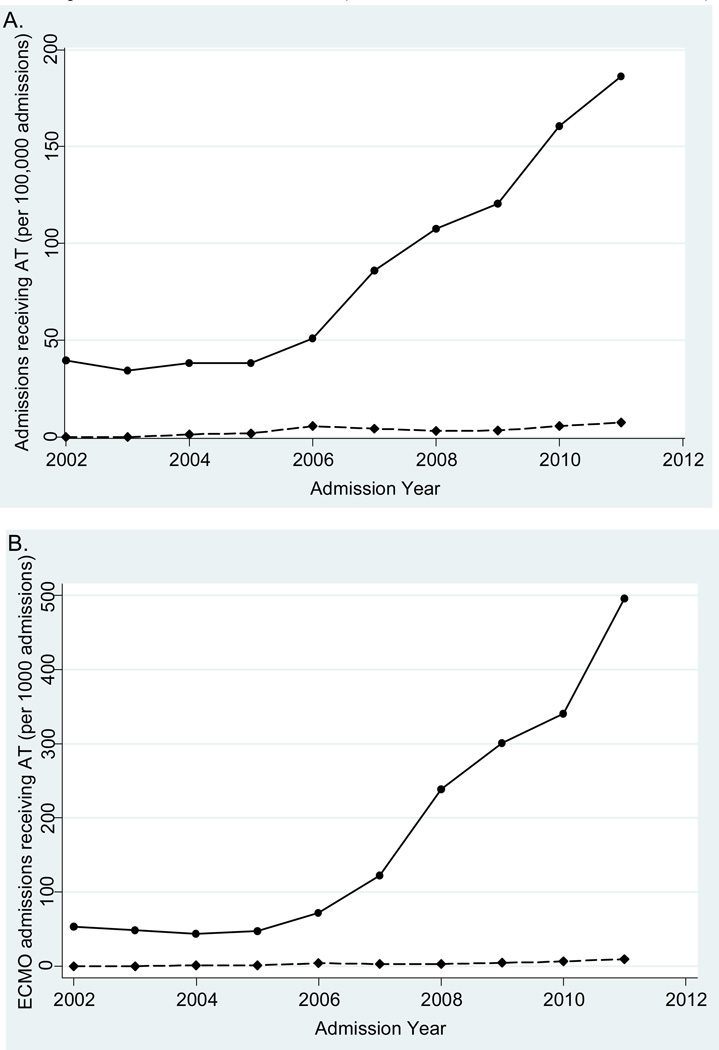
The number of PHIS admissions with off-label antithrombin concentrate use steadily increased over the 10-year study period. In 2002, 0.04% of all PHIS admissions received antithrombin concentrate, whereas in 2011, 0.19% received antithrombin concentrate, demonstrating an almost 5-fold increase. The rate of increase was significantly steeper in the off-label cohort compared with the on-label cohort over time (p<0.001; Figure).
Figure 1.
AT use over 10-year study period. (A) AT use within PHIS over time; (B) AT use in ECMO patients within PHIS over time (Off-label =  ; On-label =
; On-label =  )
)
ECMO was the most common procedure associated with antithrombin concentrate use. Of the total number of admissions, 43% underwent ECMO: 43% of off-label admissions and 25% of on-label admissions (p<0.001; Table). The proportion of ECMO patients who received antithrombin concentrate steadily increased over the study period. Twenty-nine of the 544 (5.3%) PHIS admissions in which ECMO was billed in 2002 received antithrombin concentrate compared with 475 of the 939 (50.6%) PHIS admissions in which ECMO was billed in 2011, a 9.5-fold increase (Figure). The rate of increase of off-label use in ECMO was significantly greater than increase of on-label use over time (p<0.001).
Discussion
This multicenter cohort study of antithrombin concentrate use in pediatric patients in tertiary care centers across the US sought to compare antithrombin concentrate use in children with congenital antithrombin deficiency with children without the disorder. Most subjects (97%) received antithrombin off-label regardless of the fact that inherited antithrombin deficiency, a rare congenital disorder, is the only approved indication in adults. Most antithrombin used in neonates and in patients with congenital heart and lung defects. Only 30% of subjects had a thrombosis during their admission as determined by ICD-9-CM coding. The two most common, major procedures were ECMO (44%) and CPB (37.5%). Over the 10-year study period, we demonstrated an almost 5-fold overall increase in off-label antithrombin use. Antithrombin use among all PHIS ECMO admissions during the study period increased 9.5 fold, from 5.3% to 50.6%.
Medical diagnoses associated with antithrombin administration in the pediatric literature includes respiratory distress syndrome,14,15 disseminated intravascular coagulation from sepsis,16,17 clot prevention associated with L-asparaginase use in leukemia,18,19 nephrotic syndrome,20 liver failure,21 thermal injuries,22 veno-occlusive disease,23 and necrotizing enterocolitis.24 Patients received antithrombin either to treat or prevent acquired antithrombin deficiency, but in the case of sepsis and disseminated intravascular coagulation (DIC), antithrombin also appeared to be given for its anti-inflammatory properties. The two randomized controlled clinical trials of antithrombin replacement in preterm infants with respiratory distress syndrome (RDS) did not demonstrate a benefit and one of these studies reported increased mortality in the antithrombin treatment group.15 Currently, antithrombin is not recommend in sepsis following adult studies that demonstrated increased hemorrhagic complications and no mortality benefit in patients who received antithrombin concurrently with heparin.25,26 Off-label antithrombin has been administered for heparin resistance, a state in which unusually high doses of unfractionated heparin are needed to achieve therapeutic activated partial thromboplastin times, anti-factor Xa activity, or activated clotting times.27–31
The overall impact of off-label antithrombin use on clinical outcomes in critically-ill patients is unclear. A meta-analysis of 20 randomized controlled trials that enrolled 3,458 critically-ill patients, including 267 children, with various diagnoses but mainly with sepsis, concluded that administration of antithrombin concentrate did not decrease mortality, respiratory failure, days on mechanical ventilation, length of stay in the hospital or intensive care unit, or improve quality of life.26 This lack of efficacy could be due to two reasons: (1) thrombin inhibition by antithrombin does not directly or sufficiently mitigate the mechanisms leading to organ failure and poor clinical outcomes; or (2) antithrombin does indeed play a role, but the ideal dosing schedule in this heterogeneous population has not been defined in order to observe consistent clinical improvement. The meta-analysis also demonstrated that subjects who received antithrombin concentrate had a statistically significant 1.5-fold greater risk of bleeding compared with controls; this increase is likely attributable to higher circulating levels of this anticoagulant.26
Our study demonstrates that antithrombin use during ECMO therapy has clearly increased over time and currently is used in over half of the patients receiving ECMO. The increase in on-label use in ECMO is intriguing and, because a congenital hypercoagulable state should not predispose someone to need ECMO, likely reflects the misclassification of ICD-9 diagnosis codes. Few studies have evaluated the effect of antithrombin concentrate in ECMO. The non-biologic surfaces of the extracorporeal circuit place patients at high risk of thrombosis, leading some centers to administer antithrombin standardly in order to optimize heparin’s effect. The administration of heparin and exposure to non-biologic materials in these critically-ill patients result in hemorrhagic complications, with rates of intracranial hemorrhage reaching as high as 52% of patients in certain ECMO populations.32–35 Small case series including 7 to 34 children who received antithrombin concentrate while on ECMO did not report excessive bleeding complications;34,36–39 however, efficacy studies and well-powered safety studies are lacking.
The only prior publication describing antithrombin concentrate use in pediatrics is from a single pediatric institution.11 Similarly to our multicenter study, they reported a significant increase in use over time, predominantly in neonates with complex medical conditions. The majority of their 37 subjects received antithrombin for suspected heparin resistance (78%) and acquired antithrombin deficiency (57%). ECMO was the most common procedure associated with antithrombin use in 78% of their subjects. Authors did not report hemorrhagic complications. Our multicenter study confirms the finding that antithrombin use is increasing, particularly for off-label use in critically-ill patients receiving ECMO.
PHIS is a large database with stringent quality-control measures, but limitations are inherent to research using administrative data. We were unable to determine the temporal relationship between antithrombin concentrate dosing and the diagnosis of associated medical conditions. Resistance to unfractionated heparin is a growing justification for antithrombin use11; unfortunately, we were unable to determine whether patients received antithrombin for this particular indication as PHIS pharmacy data do not differentiate between unfractionated heparin used for flushing indwelling catheters from doses administered for treatment or prevention of thrombosis. In addition, we are unable to confirm whether our on-label vs. off-label differentiation using ICD-9-CM code 289.91 is a true representation of congenital antithrombin deficiency. As ICD-9-CM code 289.91 can be used for any primary hypercoagulable state and the prevalence of ICD-9-CM code 289.91 in our study sample is higher than the expected prevalence of congenital antithrombin deficiency in the general population, we suspect that patients without antithrombin deficiency may have been misclassified as on-label. Thus, our study would underestimate the prevalence of off-label antithrombin use. Regardless, our findings highlight the need for prospective studies to determine whether antithrombin treatment is associated with improved outcomes of children with acquired antithrombin deficiency.
Acknowledgment
Thanks to Doug Bolgiano, MS (Puget Sound Blood Center), for his additional statistical support, and David Bertoch (Children’s Hospital Association) and the Children’s Hospital Association for their general help and support with this study.
T.W. was supported by the National Institutes of Health (NIH; K12-HL087165 Benign Hematology). C.W. is supported by NIH (K23-HL107455) and The Hemostasis and Thrombosis Research Society Mentored Research Award. S.S. receives research support from the Children's Hospital Association for his role as an Executive Council member of the Pediatric Research in Inpatient Settings Network.
List of Abbreviations
- CCC
complex chronic conditions
- CPB
Cardiopulmonary bypass
- DIC
Disseminated intravascular coagulation
- ECMO
Extracorporeal membrane oxygenation
- ICD-9
International Classification of Diseases 9th Revision Clinical Modification
- PHIS
Pediatric Health Information System
- RDS
Respiratory distress syndrome
Appendix
Diagnostic codes used to indicate conditions/treatment
| Condition | Codes used to define condition |
|---|---|
| Arterial thrombosis | ICD codes: 362.30, 433.0, 433.1, 433.2, 433.8, 433.9, 434.0, 434.1, 434.9, 435.9, 444.0, 444.1, 444.2, 444.21, 444.22, 444.81, 444.89, 444.9, 593.81, 410.0, 410.1, 410.2, 410.3, 410.4, 410.5, 410.6, 410.7, 410.8, and 410.9. |
| Venous thrombosis | ICD codes: 325, 362.35, 415.1, 415.11, 415.12, 415.19, 451.1, 451.11, 451.19, 451.2, 451.8, 451.81, 451.83, 451.84, 451.89, 451.9, 452, 453.0, 453.1, 453.2, 453.3, 453.40, 453.41, 453.42, 453.8, 453.9, and 572.1. |
| Hemorrhage | ICD codes: 423.0, 423.3, 430, 431, 432, 432.0, 432.1, 432.9, 459.0, 511.89, 530.82, 568.81, 569.3, 578, 578.0, 578.1, 578.9, 595.0, 770.3, 772, 772.1, 772.11, 772.12, 772.13, 772.14, 772.2, 772.4, 772.5, 772.8, 772.9, 784.7, 784.8, 786.3, 786.30, 786.31, 786.39, and 998.11. |
| Sepsis | ICD codes: 038, 038.0, 038.1, 038.10, 038.11, 038.12, 038.19, 038.2, 038.3, 038.4, 038.40, 038.41, 038.42, 038.43, 038.44, 038.49, 038.8, 038.9, 771.81 |
| ECMO |
ICD code: 39.65 CTC code: 521181 |
| CPB | ICD code: 39.61 |
| Liver transplant | ICD code: 50.5 |
| Chylothorax | ICD code: 457.8 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Rodgers GM. Role of antithrombin concentrate in treatment of hereditary antithrombin deficiency. An update. Thrombosis and haemostasis. 2009 May;101(5):806–812. [PubMed] [Google Scholar]
- 2.Franzen LE, Svensson S, Larm O. Structural studies on the carbohydrate portion of human antithrombin III. The Journal of biological chemistry. 1980 Jun 10;255(11):5090–5093. [PubMed] [Google Scholar]
- 3.Edmunds T, Van Patten SM, Pollock J, et al. Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood. 1998 Jun 15;91(12):4561–4571. [PubMed] [Google Scholar]
- 4.Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. The Journal of biological chemistry. 1992 Jun 25;267(18):12528–12538. [PubMed] [Google Scholar]
- 5.Tait RC, Walker ID, Perry DJ, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994 May;87(1):106–112. doi: 10.1111/j.1365-2141.1994.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 6.Wells PS, Blajchman MA, Henderson P, et al. Prevalence of antithrombin deficiency in healthy blood donors: a cross-sectional study. American journal of hematology. 1994 Apr;45(4):321–324. doi: 10.1002/ajh.2830450409. [DOI] [PubMed] [Google Scholar]
- 7.Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE. Hematology of Infancy and Childhood. Vol 2. Philadelphia, PA: WB. Saunders Company; 2003. [Google Scholar]
- 8.ATryn for Injections: U.S Package Insert. Framingham, MA: GTC Biotherapeutics, Inc.; 2010. Nov, [Google Scholar]
- 9.U.S. Food and Drug Administration. Cumulative List of Orphan Products Designations and Approvals. [Accessed May 22, 2013];1999 http://www.fda.gov/ohrms/dockets/dailys/00/mar00/030100/lst0094.pdf.
- 10.U.S. Food and Drug Administration. Approval Letter - ATryn. [Accessed May 22, 2013];2009 Feb 6; Available at: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm134046.htm.
- 11.Kozul C, Newall F, Monagle P, Mertyn E, Ignjatovic V. A clinical audit of antithrombin concentrate use in a tertiary paediatric centre. Journal of paediatrics and child health. 2012 Apr 19; doi: 10.1111/j.1440-1754.2012.02451.x. [DOI] [PubMed] [Google Scholar]
- 12.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008 May 7;299(17):2048–2055. doi: 10.1001/jama.299.17.2048. [DOI] [PubMed] [Google Scholar]
- 13.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001 Jun;107(6):E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt B, Gillie P, Mitchell L, Andrew M, Caco C, Roberts R. A placebo-controlled randomized trial of antithrombin therapy in neonatal respiratory distress syndrome. American journal of respiratory and critical care medicine. 1998 Aug;158(2):470–476. doi: 10.1164/ajrccm.158.2.9712116. [DOI] [PubMed] [Google Scholar]
- 15.Bassler D, Millar D, Schmidt B. Antithrombin for respiratory distress syndrome in preterm infants. Cochrane database of systematic reviews. 2006;(4):CD005383. doi: 10.1002/14651858.CD005383.pub2. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreuz WD, Schneider W, Nowak-Gottl U. Treatment of consumption coagulopathy with antithrombin concentrate in children with acquired antithrombin deficiency--a feasibility pilot study. European journal of pediatrics. 1999 Dec;158(Suppl 3):S187–S191. doi: 10.1007/pl00014353. [DOI] [PubMed] [Google Scholar]
- 17.von Kries R, Stannigel H, Gobel U. Anticoagulant therapy by continuous heparin-antithrombin III infusion in newborns with disseminated intravascular coagulation. European journal of pediatrics. 1985 Jul;144(2):191–194. doi: 10.1007/BF00451912. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thrombosis and haemostasis. 2003 Aug;90(2):235–244. doi: 10.1160/TH02-11-0283. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Gottl U, Kuhn N, Wolff JE, et al. Inhibition of hypercoagulation by antithrombin substitution in E. coli L-asparaginase-treated children. European journal of haematology. 1996 Jan-Feb;56(1–2):35–38. doi: 10.1111/j.1600-0609.1996.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 20.Storti S, Pagano L, Marra R, et al. Urokinase and AT-III concentrate treatment in inferior vena cava thrombosis associated with nephrotic syndrome. Blood Coagul Fibrinolysis. 1990 Dec;1(6):743–745. [PubMed] [Google Scholar]
- 21.Ribeiro AA, Lourenco DM, Toledo CF, Noguti MA, Borges DR. Antithrombin III concentrate use in patients with cirrhosis with coagulation disorders. Revista da Associacao Medica Brasileira(1992) 1997 Jul-Sep;43(3):189–194. doi: 10.1590/s0104-42301997000300004. [DOI] [PubMed] [Google Scholar]
- 22.Kowal-Vern A, McGill V, Walenga JM, Gamelli RL. Antithrombin(H) concentrate infusions are safe and effective in patients with thermal injuries. The Journal of burn care & rehabilitation. 2000 Mar-Apr;21(2):115–127. doi: 10.1097/00004630-200021020-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shin-Nakai N, Ishida H, Yoshihara T, et al. Control of hepatic veno-occlusive disease with an antithrombin-III concentrate-based therapy. Pediatrics international : official journal of the Japan Pediatric Society. 2006 Feb;48(1):85–87. doi: 10.1111/j.1442-200X.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 24.St Peter SD, Little DC, Calkins CM, Holcomb GW, 3rd, Snyder CL, Ostlie DJ. The initial experience of antithrombin III in the management of neonates with necrotizing enterocolitis. Journal of pediatric surgery. 2007 Apr;42(4):704–708. doi: 10.1016/j.jpedsurg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock:2008. Critical care medicine. 2008 Jan;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 26.Afshari A, Wetterslev J, Brok J, Moller AM. Antithrombin III for critically ill patients. Cochrane database of systematic reviews. 2008;(3):CD005370. doi: 10.1002/14651858.CD005370.pub2. (Online) [DOI] [PubMed] [Google Scholar]
- 27.Kanbak M, Oc B, Salman MA, Ocal T, Oc M. Peroperative effects of fresh frozen plasma and antithrombin III on heparin sensitivity and coagulation during nitroglycerine infusion in coronary artery bypass surgery. Blood Coagul Fibrinolysis. 2011 Oct;22(7):593–599. doi: 10.1097/MBC.0b013e32834a0478. [DOI] [PubMed] [Google Scholar]
- 28.Lemmer JH, Jr, Despotis GJ. Antithrombin III concentrate to treat heparin resistance in patients undergoing cardiac surgery. The Journal of thoracic and cardiovascular surgery. 2002 Feb;123(2):213–217. doi: 10.1067/mtc.2002.119060. [DOI] [PubMed] [Google Scholar]
- 29.Avidan MS, Levy JH, van Aken H, et al. Recombinant human antithrombin III restores heparin responsiveness and decreases activation of coagulation in heparin-resistant patients during cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2005 Jul;130(1):107–113. doi: 10.1016/j.jtcvs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Koster A, Fischer T, Gruendel M, et al. Management of heparin resistance during cardiopulmonary bypass: the effect of five different anticoagulation strategies on hemostatic activation. Journal of cardiothoracic and vascular anesthesia. 2003 Apr;17(2):171–175. doi: 10.1053/jcan.2003.42. [DOI] [PubMed] [Google Scholar]
- 31.Williams MR, D'Ambra AB, Beck JR, et al. A randomized trial of antithrombin concentrate for treatment of heparin resistance. The Annals of thoracic surgery. 2000 Sep;70(3):873–877. doi: 10.1016/s0003-4975(00)01550-2. [DOI] [PubMed] [Google Scholar]
- 32.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J. 2009 Jan-Feb;55(1):111–116. doi: 10.1097/MAT.0b013e318190b6f7. (1538-943X (Electronic); 1058–2916 (Linking)). [DOI] [PubMed] [Google Scholar]
- 33.Sell LL, Cullen ML, Whittlesey GC, et al. Hemorrhagic complications during extracorporeal membrane oxygenation: prevention and treatment. Journal of pediatric surgery. 1986 Dec;21(12):1087–1091. doi: 10.1016/0022-3468(86)90015-1. [DOI] [PubMed] [Google Scholar]
- 34.Stiller B, Lemmer J, Merkle F, et al. Consumption of blood products during mechanical circulatory support in children: comparison between ECMO and a pulsatile ventricular assist device. Intensive care medicine. 2004 Sep;30(9):1814–1820. doi: 10.1007/s00134-004-2352-z. [DOI] [PubMed] [Google Scholar]
- 35.Extracorporeal Life Support Organization. ECMO Registry. Michigan: Ann Arbor; 2013. Jan, International Summary. [Google Scholar]
- 36.Niebler RA, Christensen M, Berens R, Wellner H, Mikhailov T, Tweddell JS. Antithrombin replacement during extracorporeal membrane oxygenation. Artificial organs. 2011 Nov;35(11):1024–1028. doi: 10.1111/j.1525-1594.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 37.Pollock ME, Owings JT, Gosselin RC. ATIII replacement during infant extracorporeal support. Thrombosis and haemostasis. 1995;(73):936. [Google Scholar]
- 38.Urlesberger B, Zobel G, Rodl S, et al. Activation of the clotting system: heparin-coated versus non coated systems for extracorporeal circulation. The International journal of artificial organs. 1997 Dec;20(12):708–712. [PubMed] [Google Scholar]
- 39.Agati S, Ciccarello G, Salvo D, Turla G, Undar A, Mignosa C. Use of a novel anticoagulation strategy during ECMO in a pediatric population: single-center experience. ASAIO journal (American Society for Artificial Internal Organs(1992) 2006 Sep-Oct;52(5):513–516. doi: 10.1097/01.mat.0000242596.92625.a0. [DOI] [PubMed] [Google Scholar]