Abstract
Diaphragm pacing is a clinically useful modality providing artificial ventilatory support in patients with ventilator dependent spinal cord injury. Since this technique is successful in providing full-time ventilatory support in only ~50% of patients, better methods are needed. In this paper, we review a novel method of inspiratory muscle activation involving the application of electrical stimulation applied to the ventral surface of the upper thoracic spinal cord at high stimulus frequencies (300 Hz). In an animal model, high frequency spinal cord stimulation (HF-SCS) results in synchronous activation of both the diaphragm and inspiratory intercostal muscles. Since this method results in an asynchronous pattern of EMG activity and mean peak firing frequencies similar to those observed during spontaneous breathing, HF-SCS is a more physiologic form of inspiratory muscle activation. Further, ventilation can be maintained on a long-term basis with repetitive stimulation at low stimulus amplitudes (<1 mA). These preliminary results suggest that HF-SCS holds promise as a more successful method of inspiratory muscle pacing.
Keywords: Spinal cord injury, Spinal cord stimulation, Inspiratory muscles, Diaphragm pacing
1. Introduction
Cervical spinal cord injury (SCI) resulting in chronic respiratory failure is a common clinical problem. Of the 5,000 to 6,000 new cervical spinal cord injuries reported each year, nearly 20% will require mechanical ventilation (National Spinal Cord Injury Statistical Center, 2012). While most patients can eventually breathe spontaneously, approximately 5% (200–400 per year) will require chronic mechanical ventilatory support (Carter et al., 1987). These patients are subject to discomfort associated with attachment to a mechanical device, higher risk of infections, interference with speech, need for greater caregiver assistance and high costs of clinical support.
Respiratory failure occurs in patients with cervical SCI as a consequence of disruption of the bulbospinal pathways innervating the phrenic and intercostal motoneuron pools. Since the neuromuscular apparatus below the level of injury is often intact, the peripheral nerves innervating the inspiratory muscles can be electrically stimulated to restore inspiratory muscle function (Hamid and Hayek, 2008; Pancrazio and Peckham, 2009). Phrenic nerve stimulation can be applied to activate the diaphragm while upper thoracic ventral root stimulation can be applied to activate the inspiratory intercostal muscles. In fact, for the past several decades, bilateral phrenic nerve stimulation to restore diaphragm function has been a clinically accepted modality in the management of ventilator dependent tetraplegics (Adler et al., 2009; Brown et al., 2006; Carter et al., 1987; Creasey et al., 1996; DiMarco, 1999, 2001, 2004, 2009; DiMarco et al., 2002, 2005a; Dobelle et al., 1994; Elefteriades et al., 2002; Glenn et al., 1980, 1984, 1985a, 1985b, 1986b, 1988; Hirschfeld et al., 2008; Moxham and Shneerson, 1993; Onders et al., 2007; Peterson et al., 1994). Intercostal muscle pacing, however, remains experimental (DiMarco et al., 1987, 1989, 1994, 2005b).
While diaphragm pacing (DP) is successful in many patients in terms of eliminating or reducing the need for mechanical ventilation, current methods to provide respiratory support have the capacity to sustain full-time ventilatory support in only ~50% of patients (DiMarco et al., 1994, 2005a, 2005b; Onders et al., 2007; Weese-Mayer et al., 1996). There is a clinical need, therefore, to develop better methods of inspiratory muscle stimulation with the capacity to generate larger inspired volumes on a repetitive basis without the development of fatigue.
In this paper, we review recent studies of a novel method of electrical activation of the inspiratory muscle activation via the application of high frequency (300 Hz) electrical stimulation on the ventral surface of the upper thoracic spinal cord. Unlike all previous methods, this technique involves activation of the inspiratory motoneuron pools resulting in more physiologic activation of the inspiratory muscles and coincident activation of both the diaphragm and inspiratory intercostal muscles. In theory, this technique should provide greater success in providing artificial ventilatory support to ventilator dependent tetraplegics, compared to current DP technology.
2. Brief Historical Perspective
Diaphragm pacing to achieve artificial ventilation has a long and rich history dating back more than 2 centuries. In the 18th century, Caldani (1786) first demonstrated in animal studies that diaphragm movement could be achieved by electrical stimulation of the phrenic nerve. Immediately following execution, Ure (1818) first demonstrated that breathing could be restored in a human by stimulation of the phrenic nerve. In the latter 19th century, Duchenne (1872) found that ventilation in humans could be restored by stimulation of moistened sponges placed over the outer borders of the sternocleidomastoid muscles. Subsequently, Sarnoff et al. (1948; Whittenberger et al., 1949) in the 1940s demonstrated that ventilation could be maintained in polio victims with percutaneous electrodes placed in the cervical region.
Major technological issues were resolved by Glenn and colleagues (1964; Van Heeckeren and Glenn, 1966) in the 1960s leading to the development and implementation of fully implantable modern-day phrenic nerve pacing systems. Other investigators have since developed systems with improved electrode design (Talonen et al., 1990; Thoma et al., 1987) and less-invasive methods of electrode placement (DiMarco et al., 2002, 2005a; Onders et al., 2004; Shaul et al., 2007). DP has been commercially available for several decades and is considered a safe and practical method of providing respiratory support in ventilator dependent patients with cervical spinal cord injury (DiMarco, 1999, 2001; DiMarco et al., 1994, 2002, 2005a; Glenn et al., 1980, 1984, 1985a, 1985b, 1986b, 1988; Onders et al., 2004; Thoma et al., 1987) and also central hypoventilation syndrome (Ali and Flageole, 2008; Brouillette et al., 1983; Hunt et al., 1988; Ilbawi et al., 1985; Tibballs, 1991; Weese-Mayer et al., 1992, 1996).
Using motor root stimulation techniques, previous studies (DiMarco et al., 1987, 1989, 1994, 2004) have also shown that the inspiratory intercostal muscles can be activated to support breathing in patients with only a single functional phrenic nerve who are, therefore, not candidates for phrenic nerve pacing.
3. Clinical Relevance of Diaphragm Pacing
Modern day mechanical ventilators can provide reliable, portable and efficient means of respiratory support. The impact of DP, compared to mechanical ventilation, on long-term survival is unknown. Carter et al (1987), however, compared survival rates in a retrospective analysis. Overall survival rates were similar between groups, but the patients on mechanical ventilation expired earlier than did patients maintained on DP. In a more recent clinical trial of 64 patients over a 20 year period, there was no significant difference in longevity between use of DP vs. mechanical ventilation (Hirschfeld et al., 2008). However, respiratory tract infections were a more common cause of death in the mechanical ventilation group. Additionally, there are important subjective parameters which make DP preferable to mechanical ventilation in most individuals (DiMarco, 1999, 2001, 2004, 2009; Hunt et al., 1988; Dobelle et al., 1994; Elefteriades et al., 2002; Glenn et al., 1986; Ilbawi et al., 1985; Tibballs, 1991). Perhaps since patients are using their own breathing muscles generating negative airway pressures (rather than the positive pressures generated by mechanical ventilations), they describe the sensation of more normal breathing (Brown et al., 2006; Creasey et al., 1996; DiMarco, 1999, 2001; Elefteriades and Quin, 1998; Glenn et al., 1985b; Ilbawi et al., 1985; Moxham and Shneerson, 1993). Negative pressure ventilation may also reduce the incidence of barotrauma and have beneficial cardiovascular effects (Langou et al., 1978). Since ventilator tubing is not necessary, tension on the tracheostomy tube is eliminated, improving patient comfort. Other potential benefits include improved speech, elimination of fear of ventilator disconnection, restoration of olfactory sensation contributing to an improvement in quality of life, improvement in patient mobility, easier patient transport, elimination of ventilator noise and associated social embarrassment, and reduction in overall cost (Adler et al., 2009; DiMarco, 1999, 2001, 2004, 2009; Dobelle et al., 1994; Elefteriades et al., 2002; Fodstad 1987, 1989). Given these advantages, most ventilator dependent SCI patients seek out DP and once they begin using these devices, have no interest in returning to mechanical ventilation.
4. Limitations of Diaphragm Pacing
The fact that DP is not successful in providing full-time ventilatory support in a substantial number of patients is generally due to inadequate inspired volume production (DiMarco et al., 1994, 2005a, 2005b; Onders et al., 2007; Weese-Mayer et al., 1996). Low inspired volumes may occur for several reasons. With current methodology, electrical stimulation of the phrenic nerve is applied directly either to peripheral nerves, motor roots or functional motor points. This results in synchronous activation of all motor units within the field of stimulation and reversal of the normal recruitment order of motoneurons. Motor axons with larger diameters which innervate more fatigueable fibers are activated initially and smaller axons innervating fatigue-resistant muscle fibers are activated only at higher stimulus levels (Levy et al., 1990). Further, given their higher thresholds (Enoka, 2002; Grandjean and Mortimer, 1986; Lertmanorat and Durand, 2004; Prochazka, 1993), some axons are not activated at all resulting in incomplete diaphragm activation. With the application of chronic DP, however, the diaphragm becomes reconditioned from a muscle with a population of mixed fiber types to one with predominantly slow fibers (Acker et al., 1987; Glenn et al., 1980; Peterson et al., 1986, 1994). A benefit of this fiber type conversion is that the endurance capacity of the diaphragm isen hanced. Slow fibers, however, have relatively low maximum force-generating capacities resulting in reduced inspired volume generation.
Sub-optimal inspired volume generation during DP also occurs as a consequence of the lack of co-incident inspiratory intercostal muscle activation. While the diaphragm is clearly the primary inspiratory muscle, the intercostal muscles generate 35–40% of the inspiratory capacity (Agostini et al., 1965). Contraction of the intercostal muscles also acts to stabilize the chest wall preventing paradoxical inward motion of the rib cage during diaphragm contraction alone.
5. High Frequency Spinal Cord Stimulation (HF-SCS) to Activate the Inspiratory Muscles
5.1 General observations
We have recently shown in animal studies that HF-SCS results in synchronous activation of the diaphragm and intercostal muscles (DiMarco et al., 2009, 2010, 2011, in press). All of the studies referenced in this paper related to the technique of HF-SCS have been performed in dogs anesthetized with pentobarbital sodium. Initial descriptive studies of this method indicated that both the diaphragm and inspiratory intercostal muscles can be activated in concert with the application of electrical stimulation on the epidural surface of the upper thoracic spinal cord at high stimulus frequencies (300 Hz) (DiMarco and Kowalski, 2009, 2010). Mean changes in inspired volume and negative airway pressure during electrical stimulation over a wide range of stimulus frequencies and amplitudes, is shown in Fig. 1. With increasing stimulus frequency from 50 to 300 Hz at any given stimulus current, there were progressive increases in the magnitude of inspired volume and negative airway pressure. While values at 200 Hz were significantly smaller than those at 300 Hz, there were no significant differences between 300 and 400 Hz. Another important finding is the leftward shift of these curves indicating that maximum inspired volumes and airway pressures were achieved at lower levels of stimulus current. The magnitude of inspired volume generation during HF-SCS at 300 Hz, 0.5 mA (0.93 ± 0.01 liter) approximates the inspiratory capacity of these animals (1.04 ± 0.02 liter) suggesting that HF-SCS results in near-maximum diaphragm and intercostal muscle activation.
Figure 1.
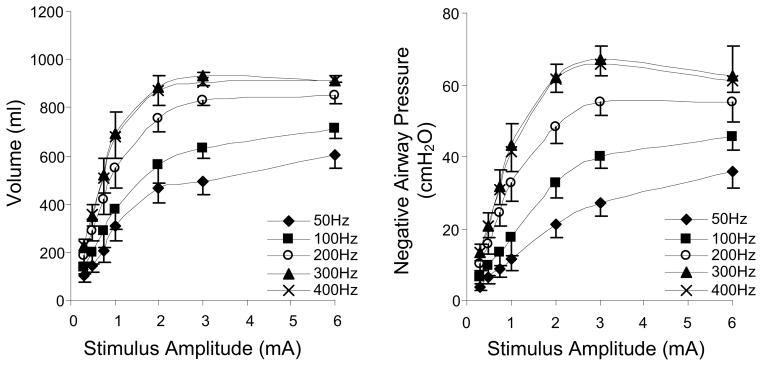
Relationships between stimulus amplitude and inspired volume (left panel) and negative airway pressure generation (right panel) at different stimulus frequencies. With increasing stimulus frequencies between 50 and 300, there were significant increases in the magnitude of these parameters. The 300 and 400 Hz curves, however, were not statistically different. With HF-SCS, large inspired volumes and airway pressures were generated with small stimulus amplitudes. Peak values were achieved with 2–3 mA.
5.2 Multiunit EMG and single motor unit (SMU) activities
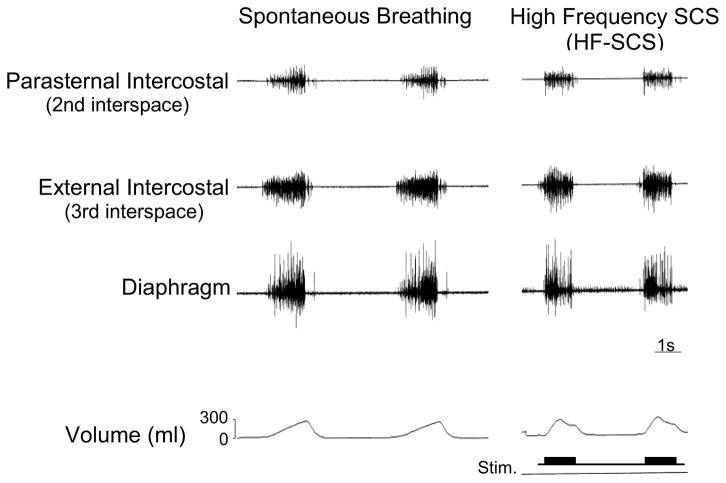
Multiunit EMGs of the parasternal (2nd interspace) and external intercostal (3rd interspace) muscles and costal diaphragm are displayed in a representative animal in Fig. 2. EMGs recorded during spontaneous breathing are shown in the left panel and those obtained at similar inspired volumes during HF-SCS are presented in the right panel. Of note, inspired volume achieved during HF-SCS could be modulated by adjustments in stimulus current while maintaining stimulus frequency at 300 Hz. Remarkably, the EMG activities during HF-SCS and spontaneous breathing are very similar demonstrating an asynchronous pattern of activation. This stands in marked contrast to direct phrenic nerve stimulation, which results in synchronous activation of all axons and consequent development of a single action potential following each stimulus spike.
Figure 2.
Multiunit EMGs of the parasternal intercostal muscle, external intercostal muscle and diaphragm during spontaneous breathing (left panel) and during HF-SCS (right panel) at comparable inspired volumes. As with spontaneous breathing, HF-SCS results in an asynchronous EMG pattern of activation. This pattern is in marked contrast to the synchronous EMG pattern observed during phrenic nerve stimulation.
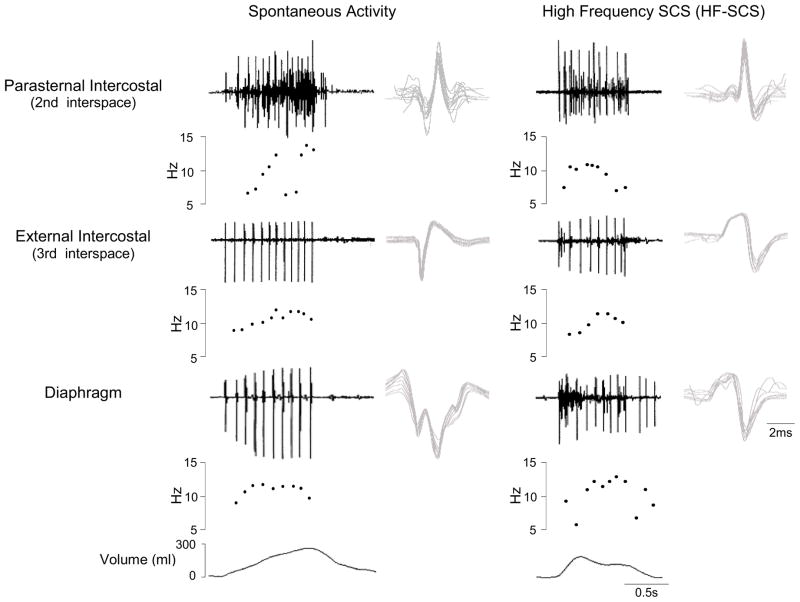
Single motor unit activity during HF-SCS (300 Hz, 0.5 mA) is shown in a representative animal in Fig. 3. All SMUs were analyzed as described previously (De Troyer et al., 1997; Gandevia et al., 1996) using a commercially available software package (Spike 2, Cambridge Electronic Design, Cambridge, UK). SMUs were sorted into ‘templates’ based on their size and detailed morphology. Moreover, the interactive software provides a review of the frequency plot of each SMU and allows superimposition of spikes from each particular SMU. One to three SMUs could usually be distinguished at each recorded site. Fig. 3 shows an example of three different SMUs that were recorded simultaneously and subsequently sorted. All spikes from each SMU are shown superimposed in the right hand portion of the figure confirming their similar morphology. Firing frequencies ranged between 7.8 and 14.9 Hz. Examples of SMU recordings from the parasternal and external intercostal muscles and costal diaphragm during spontaneous breathing and HF-SCS are shown in Fig. 4. A plot of the instantaneous firing frequencies is shown just below the raw tracings from each muscle. As in the previous example, the SMU recordings are superimposed to the right of each tracing. During both spontaneous breathing and HF-SCS, firing frequencies gradually increase during the breath until a plateau is reached in the mid to latter portion of the breath. During spontaneous breathing, mean firing frequencies of the parasternal and external intercostal muscles and diaphragm were 12.3 ± 0.6, 12.1 ± 0.5 and 10.8 ± 0.3 Hz, respectively. During HF-SCS, mean firing frequencies were 10.6 ± 0.4, 11.7 ± 0.4 and 10.4 ± 0.3 Hz, respectively. Importantly, there were no significant differences in SMU firing frequencies between spontaneous breathing and HF-SCS. Similar values of intercostal and diaphragm SMU firing frequencies have been observed in cats (Iscoe et al., 1976; Sieck et al., 1984) and also normal human subjects (Saboisky et al., 2007).
Figure 3.
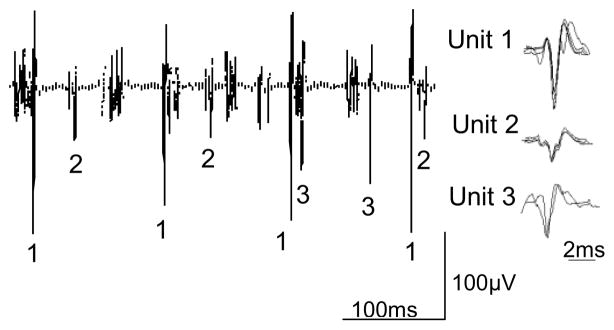
Examples of single motor unit recordings from the diaphragm during HF-SCS in one animal. Three separate motor units are readily identified. Each of the action potentials from the three SMUs is shown superimposed to the right, confirming their similar morphology. See text for further explanation.
Figure 4.
SMU activity recorded from the parasternal intercostal muscle, external intercostal muscle and diaphragm during spontaneous breathing (left panel) and during HF-SCS (right panel) in one animal. EMG, instantaneous motor unit discharge frequency and the corresponding volume are plotted in each panel. All the action potentials from each SMU are superimposed on the right. Note that during both spontaneous breathing and HF-SCS, discharge frequencies of single motor units gradually increase during the first part of inspiration until a plateau is reached and then gradually decline. Note also that there were no significant differences in SMU firing frequencies between spontaneous breathing and HF-SCS.
The fact that HF-SCS results in an asynchronous pattern of inspiratory EMG activity resembling spontaneous breathing and SMU firing frequencies are not significantly different than those observed during spontaneous breathing are strong evidence that this method results in physiologic activation of the inspiratory muscles.
5.3 Distribution of inspiratory drive to the external intercostal muscles
During normal breathing, inspiratory drive is distributed to the inspiratory intercostal muscles in a stereotypical pattern: inspiratory drive is greater in those regions of the muscles in which the mechanical advantage is greatest. This occurs in the most rostral and dorsal portions of the external intercostal muscles and most rostral and ventral portions of the parasternal muscles (De Troyer et al., 2003, 2005). This matching pattern would be expected to minimize oxygen consumption. Moreover, this effect is magnified by the fact that the topographical distribution of external intercostal muscle mass, in both dogs (De Troyer et al., 1999) and humans (De Troyer et al., 2005), is greatest in the dorsal half of the rostral intercostal spaces and decreases both caudally and ventrally. The distribution of inspiratory drive to the intercostal muscles during HF-SCS, therefore, is of particular interest.
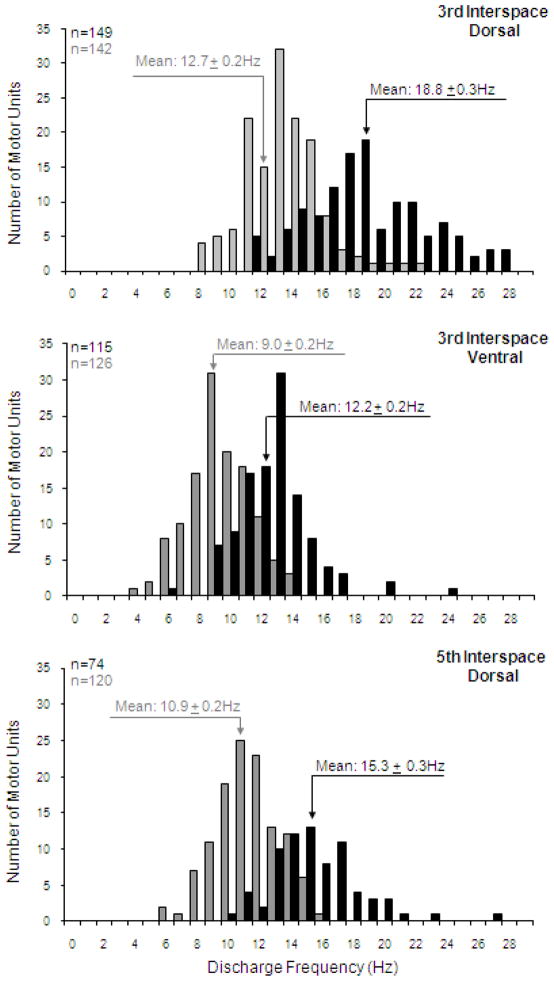
In these studies, stimulus parameters during HF-SCS were adjusted to provide inspired volumes that matched those occurring during spontaneous breathing. Mean peak SMU discharge frequencies were used to assess the relative distribution of inspiratory drive to the various intercostal muscles. Histograms of the discharge frequencies of the SMU in the dorsal portion of the 3rd interspace, ventral portion of the 3rd interspace and dorsal portion of the 5th interspace during spontaneous breathing and during HF-SCS are shown in Fig 5. Although mean peak firing frequencies were significantly higher during HF-SCS compared to spontaneous breathing, the relative spatial distribution of inspiratory activity, i.e. electrical activation of the dorsal portion of the 3rd interspace and dorsal portion of the 5th interspace, was preserved in the same proportion during HF-SCS. When stimulus amplitude was adjusted such that the absolute rib cage contribution to inspired volume during HF-SCS matched that occurring during spontaneous breathing, the spatial distribution of electrical activation was also maintained in the same proportion. The physiologic implication of these results is that the neural circuitry responsible for the unique distribution of neural drive to the external intercostal muscles is present at a spinal cord level and does not depend on descending synaptic input from supraspinal centers. The clinical significance of these findings is that the external intercostal muscles are activated during HF-SCS in the same efficient manner as that which occurs during spontaneous breathing. Since breathing is a highly repetitive task, this physiologic pattern of intercostal muscle recruitment serves to minimize the potential for muscle fatigue.
Figure 5.
Histograms of the discharge frequencies of all single motor units identified in the dorsal portion of the 3rd interspace (upper panel), ventral portion of the 3rd interspace (middle panel) and dorsal portion of the 5th interspace (lower panel) during spontaneous breathing (grey bars) and during HF-SCS (dark bars). Bin width, 1 Hz. Recordings were obtained under conditions in which inspired volume during HF-SCS was matched to inspired volumes recorded during spontaneous breathing by adjustment of stimulus amplitude. Note that mean peak firing frequencies were significantly higher during HF-SCS compared to spontaneous breathing due to the higher absolute ribcage contribution to inspired volume. However, dorsoventral and rostrocaudal gradients of distribution of inspiratory drive observed during spontaneous activity were similar to those occurring during HF-SCS.
5.4 Chronic HF-SCS to provide ventilatory support
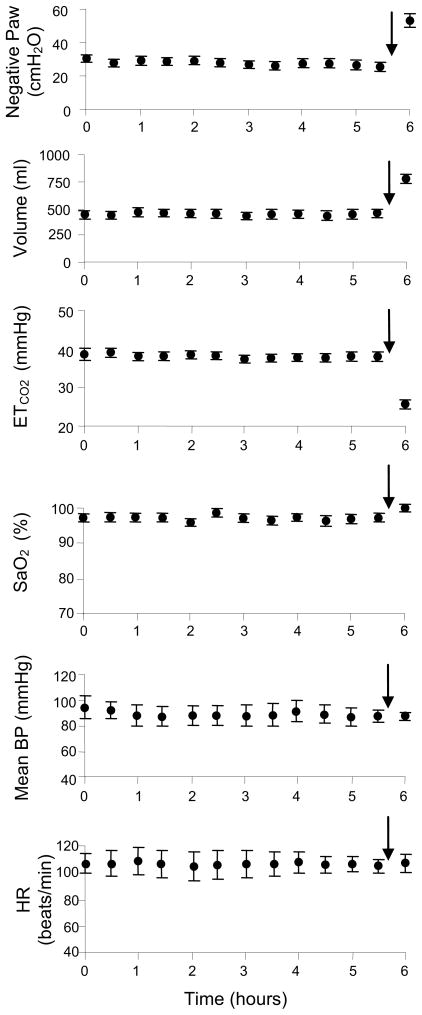
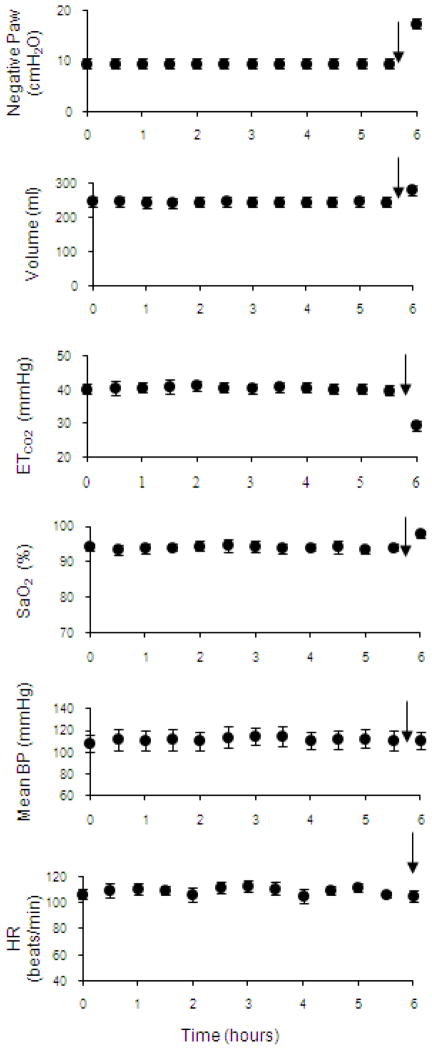
A crucial requirement of this technique as a potential method of clinical DP is the demonstration that HF-SCS can be applied repetitively for long periods without the development of fatigue. To this end, the capacity of chronic stimulation to sustain artificial ventilation was evaluated by applying a fixed stimulus paradigm (300 Hz, 0.5 mA, 0.2 pulse width) over a prolonged time period. This stimulus paradigm was selected as it resulted in inspired volumes in the range that occurred while the animals breathed spontaneously. HF-SCS was applied intermittently in pulsatile fashion at fixed intervals. Respiratory rate (stimulus trains/min) was adjusted to maintain end-tidal PCO2 between 35 and 40 mmHg over a 10–15 min period. Inspiratory time was fixed at 1.2 s. Inspiratory muscle pacing was maintained continuously over an arbitrary period of 6 hours. During prolonged HF-SCS, mean airway pressure, inspired volume, end-tidal PCO2, oxygen saturation, mean blood pressure and heart rate remained unchanged during the initial 5.5 hours (Fig. 6, Table 1). As an additional test of the viability of this system, stimulus amplitude was increased to 1.5 mA to induce hyperventilation during the final 30 minutes of stimulation. The increased stimulus parameters resulted in a sustained fall in end-tidal PCO2 to 25.7 ± 0.7 mmHg. Importantly, the development of reduced inspired volume and/or hypercapnia, which would have indicated the development of system fatigue, did not occur. No significant limb movement was observed during HF-SCS. These experiments suggest, therefore, that HF-SCS can be applied for prolonged periods without the development of fatigue.
Figure 6.
Negative airway pressure generation (Paw) (following airway occlusion), inspired volume, end-tidal PCO2, oxygen saturation, mean blood pressure and heart rate are plotted every 30 min during continuous inspiratory muscle pacing with HF-SCS. Results are means ± SE. Airway pressure and inspired volume generation remained constant throughout the initial 5.5 hours of stimulation. End-tidal PCO2 and oxygen saturation were also maintained between 35–40 mmHg and 95–99%, respectively. Mean blood pressure and heart rate also remained stable throughout this period. After 5.5 hour of continuous pacing, stimulus parameters were increased (indicated by arrows) resulting in an increase in inspired volume and consequent fall in PCO2, which was sustained over a 30 min period. These results indicate that adequate levels of ventilation can be maintained over a prolonged period with HF-SCS.
Table 1.
Mean gas values during 6 hours of continuous HF-SCS. Values are means ± SE.
| Time (hour) | ETCO2 (mmHg) | SaO2 (%) |
|---|---|---|
| 0 | 38.7 ± 1.7 | 97.5 ± 0.5 |
| 2 | 38.3 ± 0.9 | 96.0 ± 1.0 |
| 4 | 38.0 ± 0.6 | 97.5 ± 0.5 |
| 5.5 | 37.7 ± 0.7 | 97.5 ± 0.5 |
| 6 | 25.7 ± 0.7 | 100 ± 0.0 |
ETCO2, End-tidal PCO2; SaO2, arterial oxygen saturation
Unfortunately, a substantial number of patients with ventilator dependent spinal cord injury have suffered damage to their phrenic nerves directly and/or phrenic motoneurons and, therefore, are not candidates for DP. In this regard, the utility of HF-SCS of the intercostal muscles alone to provide complete ventilatory support has also been studied. It is noteworthy that this method would require the repetitive activation of a greater portion of the intercostal motoneuron pool compared to that which occurs during spontaneous breathing (Sieck and Fournier, 1989). To mimic the patient population of interest, HF-SCS was provided following bilateral cervical phrenicotomy. As in the previously described study, a stimulus paradigm was selected that resulted in inspired volumes in the range that was observed during spontaneous breathing (300 Hz, 0.2 pulse width, 0.9–1.2 mA). Likewise, respiratory rate was adjusted to maintain end-tidal PCO2 between 35 and 40 mmHg; inspiratory time was fixed at 1.2 s. The mean effects of chronic HF-SCS applied over a 6 h period on airway pressure, inspired volume, end-tidal PCO2, oxygen saturation, mean blood pressure and heart rate recorded every 30 min, is shown in Fig. 7 and Table 2. Throughout the initial 5.5 h of chronic stimulation, airway pressure and inspired volume generation were unchanged. There were also no significant changes in end-tidal PCO2 or oxygen saturation. As in the previous experiment, stimulus parameters were increased during the final 30 min of pacing. This resulted in a sustained decrease in end-tidal PCO2 to 29.3 ± 0.4 mmHg. Cardiovascular parameters also remained stable throughout the period of stimulation. Importantly, despite the higher stimulus parameters employed with intercostal muscle pacing alone compared to combined diaphragm and intercostal pacing, there was no observable limb motion and upper limb EMG recordings were electrically silent. Of interest, the mean peak SMU firing frequencies of the scalene parasternal intercostal (2nd interspace) and external intercostal (3rd interspace) at the beginning and end of the stimulus period were not significantly different. Moreover, these values were not significantly different compared to those which occur during spontaneous breathing. These results lend further support to the concept that HF-SCS results in physiologic activation of the intercostal muscles and suggest that HF-SCS may be successful in sustaining artificial ventilation in ventilator dependent patients with SCI who are not candidates for DP.
Figure 7.
Negative airway pressure generation (Paw) (following airway occlusion), inspired volume, end-tidal PCO2, oxygen saturation, mean blood pressure and heart rate are plotted every 30 min during continuous inspiratory muscle pacing with HF-SCS after bilateral phrenicotomy. Results are means ± SE. Airway pressure and inspired volume generation remained constant throughout the initial 5.5 hours of stimulation. End-tidal PCO2 and oxygen saturation were also maintained around 40 mmHg and 94%, respectively. Mean blood pressure and heart rate also remained stable throughout this period. After 5.5 hour of continuous pacing, stimulus parameters were increased (indicated by arrows) resulting in an increase in inspired volume and consequent fall in PCO2, which was sustained over a 30 min period. These results indicate that adequate levels of ventilation can be maintained over a prolonged period with HF-SCS of the intercostal muscles alone.
Table 2.
Mean gas values during 6 hours of continuous HF-SCS following bilateral cervical phrenicotomy. Values are means ± SE.
| Time (hour) | ETCO2 (mmHg) | SaO2 (%) |
|---|---|---|
| 0 | 40.3 ± 0.3 | 94.3 ± 0.6 |
| 2 | 41.3 ± 0.6 | 94.5 ± 1.1 |
| 4 | 40.6 ± 0.6 | 94.0 ± 0.9 |
| 5.5 | 39.9 ± 0.8 | 94.2 ± 0.9 |
| 6 | 29.3 ± 0.4 | 97.8 ± 0.7 |
ETCO2, End-tidal PCO2; SaO2, arterial oxygen saturation
5.5 Mechanism of inspiratory muscle activation with HF-SCS
Based upon the observation of a physiological pattern of inspiratory muscle activation, these results suggest that electrical stimulation of spinal cord tracts in the upper thoracic spinal cord synapse with the diaphragm and inspiratory intercostal motoneuron pools. Consequently, HF-SCS allows for processing of the stimulus within these pools resulting in a more physiologic pattern of inspiratory muscle activation. Rather than passive integrators of synaptic input, motoneurons possess intrinsic membrane properties that allow processing of afferent signals in a specific manner, according to Hennemans’s size principle (Berger, 1979; Dick et al., 1987a; Donnelly et al., 1985; Henneman et al., 1965; Hilaire et al., 1987; Jodkowski et al., 1987; Sieck, 1988). Smaller motoneurons that have lower membrane surface area and higher input resistance are more excitable. Smaller motoneurons, therefore, have a lower recruitment threshold than larger ones and are recruited first. Accordingly, motoneuron activation and, therefore, motor unit recruitment proceed in an orderly fashion from slow to fast units. As a result, slow fibers, which are fatigue resistant and generate relatively low-twitch and maximum forces, are recruited first. Subsequently, there is progressive recruitment of faster fibers, which are more fatigueable but capable of generating much larger forces (Dick et al., 1987a, 1987b; Mantilla and Sieck, 2003). While it is likely that this same orderly recruitment pattern occurs during HF-SCS, this phenomenon has not yet been specifically investigated.
5.6 Potential spinal pathways mediating phrenic motoneuron activation
Specific studies were performed to evaluate two separate but related aspects of the spinal circuits mediating transmission of the electrical signal between the electrode, which is optimally positioned on the ventral surface of the spinal cord at the T2 level, and the phrenic motoneurons (DiMarco and Kowalski, 2013). The first relates to the potential role of pre-phrenic interneurons located in the upper cervical spinal cord and the second relates to the spinal pathways in the vicinity of the stimulating electrode that are activated by electrical stimulation.
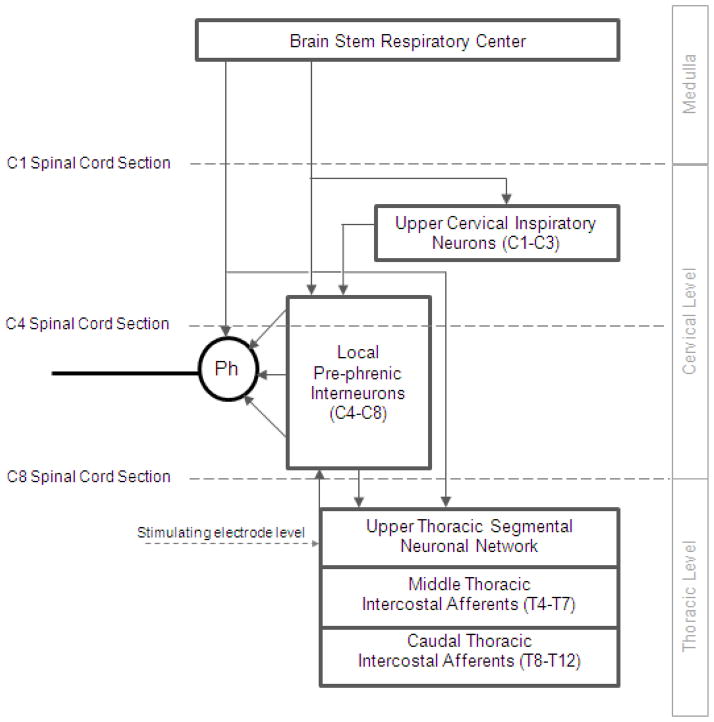
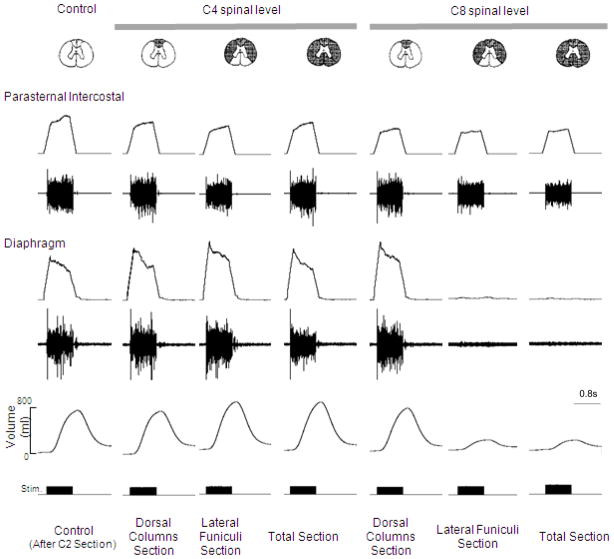
The specific neuronal circuits mediating phrenic motoneuron activation in the cervical spinal cord are also unknown. Potential spinal cord tracts that may be involved are provided in Fig. 8. As a first approach, we evaluated the potential involvement of pre-phrenic interneurons in the upper cervical spinal cord (C1-C3) which synapse with the phrenic and upper thoracic inspiratory motoneuron pools. A population of pre-phrenic upper cervical inspiratory interneurons with projections to the phrenic motoneuron pools has been identified (Bellingham, 1999; Dobbins and Feldman, 1994; Duffin and Iscoe, 1996; Hayashi et al., 2003; Lane et al., 2008b, 2009; Lipski and Duffin, 1986; Lois et al., 2009; Nakazono and Aoki, 1994; Palisses and Viala, 1987). Previous investigators have shown that these neurons display inspiratory-related phasic bursting and have direct input from the rostral ventral respiratory group (Duffin and Iscoe, 1996; Hayashi et al., 2003; Hilaire et al., 1986; Lane at al., 2009; Palisses and Viala, 1987). Their functional role during spontaneous breathing, however, is unclear. Other studies suggest that the pre-phrenic cervical interneurons may serve as a synaptic relay between the brainstem and the phrenic and intercostal motoneurons (Bellingham, 1999; Decima et al., 1967; Lane et al., 2008a, 2008b, 2009; Lee and Fuller, 2011; Sandhu et al., 2009). It is possible that they may have greater significance in pathological states such as cervical SCI (Sandhu et al., 2009). The potential role of interneurons in this region of the spinal cord has important clinical relevance: if HF-SCS is dependent upon functional neuronal structures in the upper cervical spinal cord, this method may not be successful in providing inspiratory muscle pacing in ventilator dependent patients with SCI, as this region of the spinal cord is often significantly damaged. Therefore, we investigated the potential role of neurons in this region of the spinal cord by evaluating the degree of phrenic activation, before and after sequential section of the spinal cord at the upper C4 level, over a wide range of applied stimulation amplitudes. Following sequential section of the dorsal columns, lateral funiculi and complete spinal cord at this level, there were no changes in the magnitude of inspiratory muscle EMG (Fig. 9), inspired volume (Fig. 10) or negative airway pressure (Fig. 11) compared to control values. Based upon these results, the presence of pre-phrenic upper cervical interneuronal networks are not required in the generation of the observed responses to HF-SCS. Rather, stimulated neuronal tracts in the lateral funiculi synapse with interneurons in the vicinity of or directly with the phrenic motoneurons.
Figure 8.
A simplified diagram of potential spinal cord tracts mediating activation of the phrenic motoneuron pools during upper thoracic HF-SCS in the dog model. To mimic spinal cord injury and localized potential involvement of different groups of neurons, HF-SCS was performed under control conditions and following sequential section of the spinal cord: at the C1 and C4 (high tetraplegia with injury above the phrenic pool of motoneurons) and C8 (low tetraplegia with injury below the phrenic pool of motoneurons) spinal levels. The dotted horizontal lines indicate the areas of spinal cord section. See text for further explanation.
Figure 9.
The effects of sequential section of the spinal cord at the C4 and C8 spinal levels on parasternal intercostal and diaphragm EMG activation and inspired volume generation during HF-SCS (2 mA, 300 Hz, 0.2ms pulse width) in a representative animal. Following sequential section at the C4 level and dorsal columns at the C8 level, there were no significant changes in peak integrated diaphragm EMG activity or inspired volume compared to control values. However, following section of the lateral funiculi at the C8 level, there was a marked reduction in the degree of diaphragm activation and associated marked reduction in inspired volume generation. There were no further changes following complete section. In contrast, there were no changes in peak integrated parasternal EMG following all spinal sections. See text for further explanation.
Figure 10.
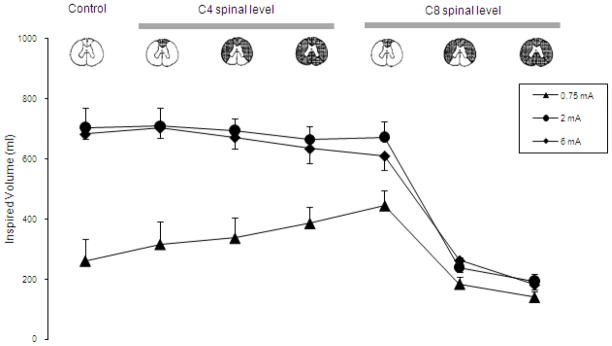
Mean inspired volume during HF-SCS at different levels of stimulation under control conditions and following sequential section of the spinal cord. Note that following sequential section at the C4 level and dorsal columns at the C8 level, there were no significant changes in mean inspired volume compared to control values. However, section of the lateral funiculi at the C8 level resulted in a marked reduction in mean inspired volume generation. There were no further changes following complete section. See text for further explanation.
Figure 11.
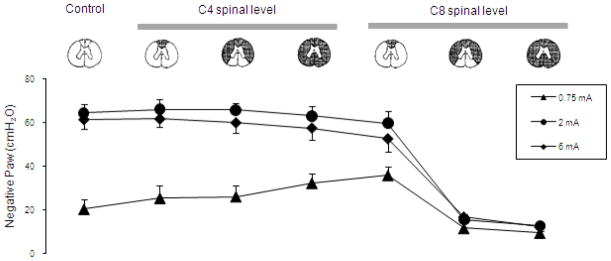
Mean airway pressure generation during HF-SCS at different levels of stimulation under control conditions and following sequential section of the spinal cord. Note that following sequential section at the C4 level and dorsal columns at the C8 level, there were no signficant changes in mean airway pressure generation compared to control values. However, section of the lateral funiculi at the C8 level resulted in a marked reduction in mean airway pressure generation. There were no further changes following complete section. See text for further explanation.
With regard to spinal pathways in the vicinity of the stimulating electrode, the fact that tidal volume can be restored with very low current levels (< 1 mA), suggests that these afferents are superficially located near the ventral surface of the upper thoracic spinal cord. To further isolate the location of these pathways activated during HF-SCS, inspiratory muscle EMG recordings of the parasternal (2nd interspace) and costal diaphragm (via the 7th interspace), inspired volume and negative airway pressure generation were assessed before and after sequential section of the spinal cord at the C8 level at different stimulus amplitudes (0.75, 2 and 6 mA at 300 Hz). As shown in Fig. 9 in a representative animal, there were negligible changes in EMG activities following C8 section of the dorsal columns bilaterally (mean 93 ± 12 and 118 ± 9% of control for the parasternal and diaphragm, respectively). However, following section of the lateral funiculi bilaterally, parasternal EMG fell marginally to 71 ± 12% while diaphragm EMG was virtually eliminated. These changes were corroborated with changes in inspired volume and negative airway pressure generation (Figs. 10, 11). There were no significant changes in inspired volume or airway pressure generation at each stimulus amplitude following dorsal column section at the C8 level. However, following bilateral section of the lateral funiculi, there were marked decreases in these parameters. For example at 2 mA, inspired volume and airway pressure fell from 703 ± 37 ml and 64 ± 4 cm H2O to 238 ± 40 ml and 16 ± 3 cm H2O (p < 0.05 for each comparison). There were no further significant changes in these parameters following complete spinal cord section. Relevant to these observations, previous investigators have described afferent inputs from the lower thoracic intercostal and abdominal muscles that reflexly facilitate phrenic motoneuron discharge via spinal pathways, i.e intercostal to phrenic reflex (Decima et al., 1967, 1969a, 1969b). Moreover, Decima and colleagues also performed successive lesioning of the spinal cord and found that the ascending pathways mediating the intercostal to phrenic reflex ran bilaterally in the ventrolateral funiculi. It is reasonable to speculate, therefore, that the responses to HF-SCS are mediated by these same pathways.
It is noteworthy that there is an extensive distribution of pre-phrenic interneurons throughout the cervical spinal cord which project to both the phrenic and intercostal motoneuron pools (Billing et al., 2000; Lane et al., 2008b). Recent studies also suggest that there may be a network of interneurons comprising a central pattern generator at the level of the phrenic motoneuron pools (Alilain et al., 2008). Stimulation of axons of these interneurons may also mediate the observed responses to HF-SCS.
Most patients with ventilator dependent tetraplegia and who are candidates for DP have spinal cord damage cephalad to the phrenic motoneuron pools. The fact that activation of the phrenic motoneuron pools via HF-SCS does not involve cervical pre-motor neurons and interneuronal networks in the upper portion of the cervical spinal cord suggests that damage to the upper portion of the spinal cord would not preclude the success of this technique as a method of DP.
6. Summary
Diaphragm pacing is a clinically useful modality, providing a more advantageous method of respiratory support compared to mechanical ventilation in ventilator dependent tetraplegics. Unfortunately, this method has significant limitations as only ~50% of subjects are able to be supported on a full-time basis (DiMarco et al., 1994, 2005a, 2005b; Onders et al., 2007; Weese-Mayer et al., 1996). Recent studies demonstrate that the inspiratory muscles can also be activated by a novel method of electrical stimulation involving the application of high stimulus frequencies (300 Hz) on the ventral surface of the upper thoracic spinal cord (DiMarco and Kowalski, 2009, 2010, 2011, in press). Unlike conventional phrenic nerve stimulation, this method results in activation of the inspiratory motoneuron pools via spinal cord pathways resulting in a) an asynchronous pattern of EMG activity, b) single motoneuron firing frequencies in the same range as those occurring during spontaneous breathing, and c) synchronous activation of both the intercostal muscles and diaphragm. Presumably, motoneuron recruitment occurs in an orderly fashion from slow to fast units allowing fatigue resistant slow fibers to be activated first, maintaining the range of diaphragm force modulation and preserving the mixed fiber population of the diaphragm. Consequently, inspiratory muscle pacing by this method should result in larger inspired volumes and greater fatigue resistance. In theory, therefore, high frequency spinal cord stimulation should provide a more successful method of providing respiratory support compared to conventional diaphragm pacing. Further animal testing and ultimately clinical trials, however, will be necessary to determine the applicability of this method in the SCI population.
Highlights.
Diaphragm pacing is a useful modality for ventilatory support in spinal cord injury
HF-SCS results in synchronous activation of the diaphragm and intercostal muscles
HF-SCS results in a more physiological activation of the inspiratory muscles
Acknowledgments
We wish to thank Dana Hromyak for her assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony F. DiMarco, Email: afd3@case.edu.
Krzysztof E. Kowalski, Email: kek5@case.edu.
References
- Acker MA, Mannion JD, Brown WE, Salmons S, Henriksson J, Bitto T, Gale DR, Hammond R, Stephenson LW. Canine diaphragm muscle after 1 yr of continuous electrical stimulation: its potential as a myocardial substitute. J Appl Physiol. 1987;62:1264–1270. doi: 10.1152/jappl.1987.62.3.1264. [DOI] [PubMed] [Google Scholar]
- Adler D, Gonzalez-Bermejo J, Duguet A, Demoule A, Le Pimpec-Barthes F, Hurbault A, Morélot-Panzini C, Similowski T. Diaphragm pacing restores olfaction in tetraplegia. Eur Respir J. 2009;34:365–370. doi: 10.1183/09031936.00177708. [DOI] [PubMed] [Google Scholar]
- Agostoni E, Mognoni P, Torri G, Agostoni AF. Static features of the passive rib cage and abdomen-diaphragm. J Appl Physiol. 1965;20:1187–1193. [Google Scholar]
- Ali A, Flageole H. Diaphragmatic pacing for the treatment of congenital central alveolar hypoventilation syndrome. J Pedatr Surg. 2008;43:792–796. doi: 10.1016/j.jpedsurg.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28:11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82:1224–1232. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Billing I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains fo pseudorabies virus. J Neuroscience. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette RT, Ilbawi MN, Hunt CE. Phrenic nerve pacing in infants and children: A review of experience and report on the usefulness of phrenic nerve stimulation studies. J Pediatr. 1983;102:32–39. doi: 10.1016/s0022-3476(83)80282-0. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–870. [PMC free article] [PubMed] [Google Scholar]
- Caldani LMA. Institutiones physiologicae, Venice, Pezzana, 1786. Cited by Schechter DC: Application of electrotherapy to noncardiac thoracic disorders (40) Bull NY Acad Med 1970. 1786;46:932–51. [PMC free article] [PubMed] [Google Scholar]
- Carter RE, Dono WH, Halstead L, Wilkerson MA. Comparative study of electrophrenic nerve stimulation and mechanical ventilatory support in traumatic spinal cord injury. Paraplegia. 1987;25:86–91. doi: 10.1038/sc.1987.16. [DOI] [PubMed] [Google Scholar]
- Creasey G, Elefteriades J, DiMarco A, Talonen P, Bijak M, Girsch W, Kantor C. Electrical stimulation to restore respiration. J Rehabil Res Dev. 1996;33:123–132. [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature. 1967;214:312–313. doi: 10.1038/214312a0. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand. 1969a;75:580–591. doi: 10.1111/j.1748-1716.1969.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand. 1969b;75:568–579. [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol. 2003;546:943–954. doi: 10.1113/jphysiol.2002.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. J Physiol. 1999;518:283–289. doi: 10.1111/j.1469-7793.1999.0283r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman’s size principle. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Alan R Liss Inc; New York: 1987a. pp. 239–247. [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity of supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987b;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Diaphragm pacing in patients with spinal cord injury. Top Spinal Cord Inj Rehabil. 1999;5:6, 20. [Google Scholar]
- DiMarco AF. Neural prostheses in the respiratory system. J Rehabil Res Dev. 2001;38:601–607. [PubMed] [Google Scholar]
- DiMarco AF. Respiratory muscle stimulation in patients with spinal cord injury. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. World Scientific; New Jersey: 2004. p. 951.p. 978. [Google Scholar]
- DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol. 2009;169:200–209. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Altose MD, Cropp A, Durand D. Activation of intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis. 1987;136:1385–1390. doi: 10.1164/ajrccm/136.6.1385. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Budzinska K, Supinski GS. Artificial ventilation by means of electrical activation of intercostal/accessory muscles alone in anesthetized dogs. Am Rev Respir Dis. 1989;139:961–967. doi: 10.1164/ajrccm/139.4.961. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Supinski GS, Petro J, Takaoka Y. Evaluation of intercostal pacing to provide artificial ventilation in quadriplegics. Am J Respir Crit Care Med. 1994;150:934–940. doi: 10.1164/ajrccm.150.4.7921466. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002;166:1604–1606. doi: 10.1164/rccm.200203-175CR. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Connors AF, Kowalski KE. Gas exchange during separate diaphragm and intercostal muscle breathing. J Appl Physiol. 2004;96:2120–2124. doi: 10.1152/japplphysiol.00628.2003. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Stefan SL, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005a;127:671–678. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Takaoka Y, Kowalski KE. Evaluation of intercostal and diaphragm pacing to provide artificial ventilation in tetraplegics. Arch Phys Med Rehabil. 2005b;86:1200–1207. doi: 10.1016/j.apmr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol. 2009;107:662–669. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol. 2010;171:218–224. doi: 10.1016/j.resp.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol. 2011;589:1383–1395. doi: 10.1113/jphysiol.2010.199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Spinal cord pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol. 2013;186:1–6. doi: 10.1016/j.resp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobelle WH, D’Angelo MS, Goetz BF, Kiefer DG, Lallier TJ, Lamb JI, Yazwinsky JS. 200 cases with a new breathing pacemaker dispel myths about diaphragm pacing. ASAIO J. 1994;40:M244–252. doi: 10.1097/00002480-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Cohen MI, Sica AL, Zhang H. Responses of early and late onset phrenic motoneurons to lung inflation. Respir Physiol. 1985;61:69–83. doi: 10.1016/0034-5687(85)90029-5. [DOI] [PubMed] [Google Scholar]
- Duchenne G. De l’electrisation localisee et de son application a la pathologie et a la therapeutique par courants induits et par courants galvaniques interompus et continus. Paris: Librairie J.B. Bailliere; 1872. [Google Scholar]
- Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res. 1996;112:35–40. doi: 10.1007/BF00227175. [DOI] [PubMed] [Google Scholar]
- Elefteriades JA, Quin JA. Diaphragm pacing. Chest Surg Clin N Am. 1998;8:331–357. [PubMed] [Google Scholar]
- Elefteriades JA, Quin JA, Hogan JF, Holcomb WG, Letsou GV, Chlosta WF, Glenn WW. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. Pacing Clin Electrophysiol. 2002;25:897–906. doi: 10.1046/j.1460-9592.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve. 2002;25:763–764. doi: 10.1002/mus.10117. [DOI] [PubMed] [Google Scholar]
- Fodstad H. The Swedish experience in phrenic nerve stimulation. Pacing Clin Electrophysiol. 1987;10:246–251. doi: 10.1111/j.1540-8159.1987.tb05957.x. [DOI] [PubMed] [Google Scholar]
- Fodstad H. Pacing of the diaphragm to control breathing in patients with paralysis of central nervous system origin. Stereotact Funct Neurosurg. 1989;53:209–222. doi: 10.1159/000099537. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Leeper JB, McKenzie DK, De Troyer A. Discharge frequencies of parasternal intercostal and scalene motor units during breathing in normal and COPD subjects. Am J Respir Crit Care Med. 1996;153:622–628. doi: 10.1164/ajrccm.153.2.8564108. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Hageman JH, Mauro A, Eisenber L, Flanigan S, Harvard BM. Electrical stimulation of excitable tissue by radiofrequency transmission. Ann Surg. 1964;160:338–350. [PMC free article] [PubMed] [Google Scholar]
- Glenn WW, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am. 1980;60:1055–1078. doi: 10.1016/s0039-6109(16)42233-4. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Hogan JF, Loke JS, Ciesielski TE, Phelps ML, Rowedder R. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. N Engl J Med. 1984;310:1150–1155. doi: 10.1056/NEJM198405033101804. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Phelps ML. Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery. 1985a;17:974–984. doi: 10.1227/00006123-198512000-00021. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Sairenji H. Diaphragm pacing in the treatment of chronic ventilatory insufficiency. In: Roussos C, Macklem PT, editors. The Thorax: Lung Biology in Health and Disease. Marcel Dekker; New York: 1985b. pp. 1407–1440. [Google Scholar]
- Glenn WW, Phelps ML, Elefteriades JA, Dentz B, Hogan JF. Twenty years of experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol. 1986;9:780–784. doi: 10.1111/j.1540-8159.1986.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Brouillette RT, Dentz B, Fodstad H, Hunt CE, Keens TG, Marsh HM, Pande S, Piepgras DG, Vanderlinden RG. Fundamental considerations in pacing of the diaphragm for chronic ventilatory insufficiency: A multi-center study. Pacing Clin Electrophysiol. 1988;11:2121–2127. doi: 10.1111/j.1540-8159.1988.tb06360.x. [DOI] [PubMed] [Google Scholar]
- Grandjean PA, Mortimer JT. Recruitment properties of monopolar and bipolar epimysial electrodes. Ann Biomed Eng. 1986;14:53–66. doi: 10.1007/BF02364648. [DOI] [PubMed] [Google Scholar]
- Hamid S, Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur Spine J. 2008;17:1256–1269. doi: 10.1007/s00586-008-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Khatib M, Monteau R. Central drive on Renshaw cells coupled with phrenic motoneurons. Brain Res. 1986;376:133–139. doi: 10.1016/0006-8993(86)90907-8. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Khatib M. Determination of recruitment order of phrenic motoneurons. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Alan R Liss Inc; New York: 1987. pp. 249–261. [Google Scholar]
- Hirschfeld S, Exner G, Luukkaala T, Baer GA. Mechanical ventilation or phrenic nerve stimulation for treatment of spinal cord injury-induced respiratory insufficiency. Spinal Cord. 2008;46:738–742. doi: 10.1038/sc.2008.43. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Brouillette RT, Weese-Mayer DE, Morrow A, Ilbawi MN. Diaphragm pacing in infants and children. Pacing Clin Electrophysiol. 1988;11:2135–2141. doi: 10.1111/j.1540-8159.1988.tb06362.x. [DOI] [PubMed] [Google Scholar]
- Ilbawi MN, Idriss FS, Hunt CE, Brouillette RT, DeLeon SY. Diaphragmatic pacing in infants: techniques and results. Ann Thorac Surg. 1985;40:323–329. doi: 10.1016/s0003-4975(10)60061-6. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langou RA, Cohen LS, Sheps D, Wolfson S, Glenn WW. Ondine’s curse: hemodynamic response to diaphragm pacing (electrophrenic respiration) Am Heart J. 1978;95:295–300. doi: 10.1016/0002-8703(78)90359-9. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179:71–79. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertmanorat Z, Durand DM. Extracellular voltage profile for reversing the recruitment order of peripheral nerve stimulation: a simulation study. J Neural Eng. 2004;1:202–211. doi: 10.1088/1741-2560/1/4/003. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Moxham J, Shneerson JM. Diaphragmatic pacing. Am Rev Respir Dis. 1993;148:533–536. doi: 10.1164/ajrccm/148.2.533. [DOI] [PubMed] [Google Scholar]
- Nakazono Y, Aoki M. Excitatory connections between upper cervical inspiratory neurons and phrenic motoneurons in cats. J Appl Physiol. 1994;77:679–683. doi: 10.1152/jappl.1994.77.2.679. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center, University of Alabama at Birmingham. Annual Statistical Report 2012. Birmingham: University of Alabama; [Google Scholar]
- Onders RP, DiMarco AF, Ignagni AR, Aiyar H, Mortimer JT. Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery. 2004;136:819–826. doi: 10.1016/j.surg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Onders RP, Elmo MJ, Ignagni AR. Diaphragm pacing stimulation system for tetraplegia in individuals injured during childhood or adolescence. J Spinal Cord Med. 2007;30:S25–S29. doi: 10.1080/10790268.2007.11753965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisses R, Viala D. Existence of respiratory interneurons in the cervical spinal cord of the rabbit. C R Acad Sci III. 1987;305:321–324. [PubMed] [Google Scholar]
- Pancrazio JJ, Peckham PH. Neuroprosthetic devices: how far are we from recovering movement in paralyzed patinets? Expert Rev Neurother. 2009;9:427–430. doi: 10.1586/ern.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DK, Nochomovitz M, DiMarco AF, Mortimer JT. Intramuscular electrical activation of the phrenic nerve. IEEE Trans Biomed Eng. 1986;33:342–351. doi: 10.1109/TBME.1986.325720. [DOI] [PubMed] [Google Scholar]
- Peterson DK, Nochomovitz ML, Stellato TA, Mortimer JT. Long-term intramuscular electrical activation of the phrenic nerve: efficacy as a ventilatory prosthesis. IEEE Trans Biomed Eng. 1994;41:1127–1135. doi: 10.1109/10.335861. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Comparison of natural and artificial control of movement. IEEE Trans Rehabil Eng. 1993;1:7–17. [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnoff SJ, Hardenberg E, Whittenberger JL. Electrophrenic respiration. Am J Physiol. 1948;155:1–9. doi: 10.1152/ajplegacy.1948.155.1.1. [DOI] [PubMed] [Google Scholar]
- Shaul DB, Danielson PD, McComb JG, Keens TG. Throacoscopic placement of phrenic nerve electrodes for diaphragmatic pacing in children. J Pediatr Surg. 2002;37:974–978. doi: 10.1053/jpsu.2002.33821. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Expir Neurol. 1984;85:316–335. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med. 1988;9:195–210. [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Talonen PP, Baer GA, Hakkinen V, Ojala JK. Neurophysiological and technical considerations for the design of an implantable phrenic nerve stimulator. Med Biol Eng Comput. 1990;28:31–37. doi: 10.1007/BF02441674. [DOI] [PubMed] [Google Scholar]
- Thoma H, Gerner H, Holle J, Kluger P, Mayr W, Meister B, Schwander G, Stohr H. The phrenic pacemaker: substitution of paralyzed functions in tetraplegia. ASAIO Trans. 1987;33:472–479. [PubMed] [Google Scholar]
- Tibballs J. Diaphragmatic pacing: An alternative to long-term mechanical ventilation. Anaesth Intensive Care. 1991;19:597–601. doi: 10.1177/0310057X9101900424. [DOI] [PubMed] [Google Scholar]
- Ure A. Experiments made on the body of a criminal immediately after execution, with physiological and philosophical observations. J Sciences Arts. 1818;12:1. [Google Scholar]
- Van Heeckeren DW, Glenn WWL. Electrophrenic respiration by radiofrequency induction. J Thorac Cardiovasc Surg. 1966;52:655–665. [PubMed] [Google Scholar]
- Weese-Mayer DE, Hunt CE, Brouillette RT, Silvestri JM. Diaphragm pacing in infants and children. J Pediatr. 1992;120:1–8. doi: 10.1016/s0022-3476(05)80588-8. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Silvestri JM, Kenny AS, Ilbawi MN, Hauptman SA, Lipton JW, Talonen PP, Garcia HG, Watt JW, Exner G, Baer GA, Elefteriades JA, Peruzzi WT, Alex CG, Harlid R, Vincken W, Davis GM, Decramer M, Kuenzle C, Saeterhaug A, Schöber JG. Diaphragm pacing with a quadripolar phrenic nerve electrode: an international study. Pacing Clin Electrophysiol. 1996;19:1311–1319. doi: 10.1111/j.1540-8159.1996.tb04209.x. [DOI] [PubMed] [Google Scholar]
- Whittenberger JL, Sarnoff SJ, Hardenberg E. Electrophrenic respiration II. Its use in man. J Clin Invest. 1949;28:124–128. [PubMed] [Google Scholar]