Abstract
Erythropoiesis is the process by which progenitors for red blood cells are produced and terminally differentiate. In all vertebrates, two morphologically distinct erythroid lineages (primitive, embryonic, and definitive, fetal/adult) form successively within the yolk sac, fetal liver, and marrow and are essential for normal development. Red blood cells have evolved highly specialized functions in oxygen transport, defense against oxidation, and vascular remodeling. Here we review key features of the ontogeny of red blood cell development in mammals, highlight similarities and differences revealed by genetic and gene expression profiling studies, and discuss methods for identifying erythroid cells at different stages of development and differentiation.
Keywords: primitive erythropoiesis, transgenic mice, mammalian embryo, yolk sac, fetal liver, progenitor, erythroid differentiation, enucleation
Introduction
Erythroid (red blood) cells play an essential role in oxygen delivery and vascular morphogenesis during embryogenesis and throughout postnatal life. Progenitors of the primitive erythroid (EryP) lineage arise early during postimplantation development in the yolk sac of the mammalian embryo. Large, nucleated EryP emerge in great numbers and are the predominant circulating blood cell until a second wave of definitive, enucleated erythrocytes (EryD) is produced by the fetal liver [1; 2; 3; 4]. EryD then rapidly outnumber EryP in the fetal blood. Cells of the two erythroid lineages differ in size (EryP are larger than EryD) and express distinct sets of α- and β-like globin genes (embryonic/fetal in EryP, adult in EryD) [2; 5; 6]. They also differ in their oxygen-carrying capacity and response to low oxygen tension [7]. It had long been accepted that a key distinguishing feature of circulating primitive and definitive erythroid cells was the presence or absence of a nucleus. It is now known that, like their definitive counterparts, primitive erythroblasts in the mouse embryo also enucleate, but they do so after entering the blood around the time that formation of EryD begins in the fetal liver [8; 9]. Despite their origins from distinct populations of mesodermal progenitors [10], the two erythroid lineages are remarkably similar and both are critical for normal development.
Overview of mammalian hematopoietic development
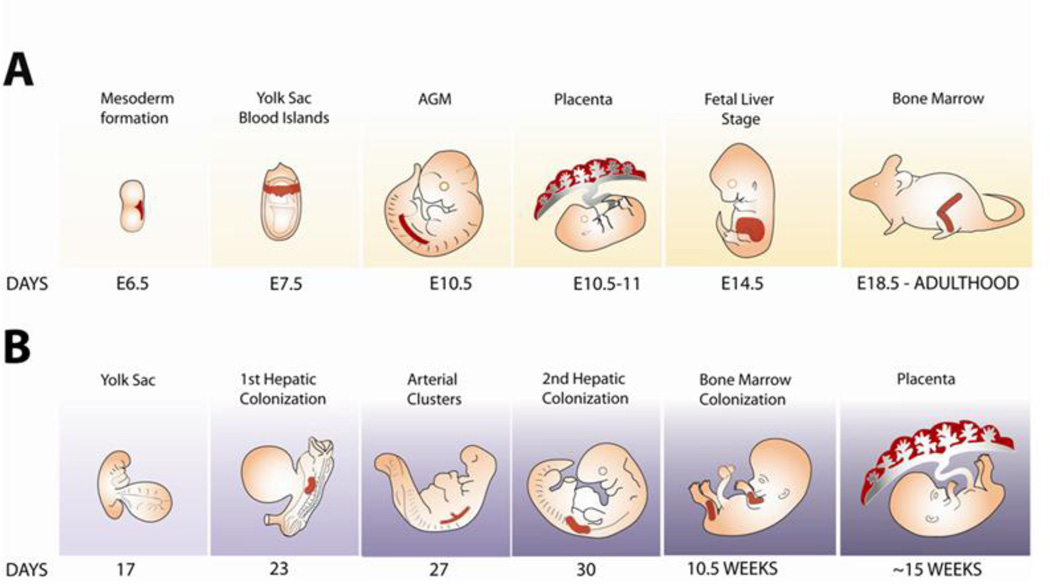
Mammalian hematopoietic development occurs in successive and partially overlapping waves in the embryo and fetus (Fig. 1). In both mouse and humans, the initial wave of hematopoietic activity (primitive) occurs outside the embryo proper, in the yolk sac, and results primarily in the formation of EryP as well as megakaryocytes and macrophages [11; 12]. EryP progenitors can be identified toward the end of gastrulation in the mouse embryo [2; 13]; they arise from bipotential megakaryocyte/erythroid progenitors (MEPs) [12]. The yolk sac is also the site of a second wave of hematopoiesis that produces definitive erythroid, megakaryocyte, and myeloid cells [2; 12; 14], and possibly also lymphoid [15; 16; 17] cells. The third wave arises from hematopoietic stem cells (HSCs) that emerge from multiple sites within the embryo, including the para-aortic splanchnopleure (P-Sp), the aorta-gonad-mesonephros (AGM) region and other large arteries (vitelline and umbilical), and the placenta (for reviews, see refs. [18; 19; 20; 21]). HSC activity identified following transplantation into newborn (but not adult) mice is also found in the E9.0 yolk sac [22].
Figure 1. Ontogeny of mouse and human hematopoiesis.
(A) Hematopoietic development in the mouse. The panels represent, from left to right, formation of mesoderm during gastrulation (E6.5), development of blood islands within the yolk sac (~E7.5), emergence of HSCs in the AGM region (E10.5) and placenta (E10.5–11), active fetal liver hematopoiesis (E14.5), and hematopoiesis in the bone marrow (~E18.5 in the late gestation fetus and throughout postnatal life). Myeloid and definitive erythroid potentials are found in the allantois and chorion (not shown) prior to their fusion to form the placenta [106]. The formation of HSCs is completed by mid-gestation. Lymphopoiesis is not represented in this figure. Cardiac function begins as early as ~E8.25, with active circulation by ~E9.0[107]. For a detailed review of mammalian hematopoiesis, see ref. [19]. (B) Hematopoietic development in the human embryo. The panels represent, from left to right, hematopoiesis at the yolk sac stage (day 17), at the time of the first hepatic colonization by HSCs (day 23), arterial cluster formation (day 27), the second hepatic colonization (day 30), and bone marrow colonization (10.5 weeks). For a review, see ref. [21]. In contrast with the mouse embryo, where HSCs are found in the placenta at around the same time as in the AGM region [108; 109], well before colonization of the bone marrow (~10.5 weeks [110]), human HSC activity is not detected in the placenta until several weeks after bone marrow colonization, at ~15 weeks [111]. Active circulation begins by ~day 21 [4].
The HSCs that form in the P-Sp/AGM do not differentiate there [23] but colonize the fetal liver and, later, the bone marrow, thymus, and (in mouse), the spleen [18; 24; 25; 26].
Primitive erythropoiesis in the yolk sac
EryP form in the yolk sac, in close temporal and spatial association with endothelial cells. For many years the two lineages were thought to arise from a bipotential progenitor termed the hemangioblast. They have also been described as appearing in "blood islands," clusters of erythroblasts surrounded by endothelial cells. The existence of the hemangioblast in vivo and the concept of the yolk sac blood island have both been challenged [reviewed in ref. 27]. EryP progenitor activity, detected using colony-forming assays in vitro, is present from ~E7.5 until early somite stages (~E8.75) and disappears rather abruptly, by ~E9.0 [2; 5; 13]. Thus, they appear as a nearly synchronous cohort and their progression from proerythroblast to orthochromatic erythroblast to enucleate erythrocyte (reticulocyte) can be followed using Giemsa staining. Morphological hallmarks of their maturation include loss of nucleoli, decreased cell diameter and cross-sectional area, nuclear condensation, changes in expression of cell surface molecules, and, eventually, enucleation [8; 9]. Little is known about primitive erythropoiesis in the human yolk sac, owing to the difficulty of obtaining embryonic tissue at this early time in gestation. However, studies using human embryonic stem (ES) cells suggest that human primitive hematopoiesis is broadly similar to that in the mouse embryo [28].
Mammalian embryonic red blood cells have been designated "primitive" for historical reasons. Like the macrocytic erythroblasts of non-mammalian vertebrate embryos, they form in the yolk sac, enter the circulation as nucleated cells, and their progenitors are produced for only a short time in the embryo [29]. It is now known that EryP continue to mature and do eventually enucleate, several days after entering the bloodstream, where they remain at least through birth [8; 9]. Like EryD [30], EryP have been found in erythroblastic islands in the fetal liver [31; 32]. EryP enucleation appears to occur in the circulation and in fetal liver [31; 32], though this process remains to be documented using real-time imaging. The expelled nuclei (also known as "pyrenocytes" [31]) are cleared by macrophages in the fetal liver and perhaps other tissues [31; 32].
Human primitive erythroblasts enucleate within the placenta [33]. Immunostaining of this tissue suggests that expelled human EryP nuclei are engulfed by macrophages whose developmental origin may also be the placenta [33].
Biological significance of primitive erythroid cells
Primitive erythroid cells are crucial for the transition from embryo to fetus in developing mammals. In addition to their function in oxygen delivery to cells within the rapidly growing embryo, EryP may scavenge reactive oxygen species [34] and are thought to play a critical role in vascular remodeling during development. The primary capillary plexus of the early yolk sac is remodeled into mature blood vessels that are aligned along the direction of blood flow as cardiac contraction begins [35]. Real-time confocal imaging of ε-globin-GFP transgenic mouse embryos cultured ex vivo [36] revealed that hemodynamic parameters such as shear force change during embryogenesis and that the pattern of blood flow can be correlated with the stage of cardiac development [37]. Erythroblasts are required to generate shear forces necessary for vascular remodeling [35]. In the absence of a heartbeat, as observed for Ncx1 [38] or Mlc2a mutant embryos [39], shear forces are not generated and the yolk sac vascular plexus fails to remodel. Moreover, in erythroid-tropomodulin (E-Tmod) mutant embryos, the mechanically weakened EryP are unable to support remodeling of the primary capillary plexus into mature vessels [40]. Therefore, circulation between the yolk sac and the embryo proper cannot be established.
Origin of the definitive erythroid lineages in the yolk sac and fetal liver
The fetal liver provides a microenvironment for the robust expansion and differentiation of definitive erythroid cells. The earliest definitive erythroid progenitors that colonize the fetal liver likely originate from erythroid/myeloid progenitors (EMPs) produced during the second wave of hematopoiesis in the (yolk sac E8.25-8.5), rather than from HSCs [2; 41; 42]. EMP-derived definitive erythropoiesis has been proposed to bridge the transition between primitive and HSC-derived erythropoiesis [19; 42]. Definitive yolk sac hematopoiesis produces multipotential, highly proliferative progenitors (HPP-CFC) from E8.25 yolk sac [41] and extensively self-renewing erythroid (ESRE) progenitors from E9.5 mouse yolk sac [43], both identified in vitro. ESREs are also found in the E12.5 fetal liver [43]. These remarkable cells expand dramatically (106-to-1060 fold) upon prolonged culture [43]. Little, if any, ESRE progenitor activity is present in adult bone marrow or spleen under the same conditions [43]. It is possible that the first hepatic colonization described for the human fetus (Figure 1B) corresponds to the second wave of hematopoiesis identified in the mouse embryo [27].
The third wave of hematopoiesis produces HSCs that seed and differentiate within the fetal liver. By ~E14.5 in the mouse [2] and 7–8 weeks in the human embryo [4], definitive erythropoiesis is well underway and EryP are rapidly outnumbered by EryD in the circulation. Throughout postnatal life, definitive erythropoiesis originates from HSCs in the marrow, under normal physiological conditions [19; 20]. Erythroid cells are also produced in the spleen of mice in response to stress or disease [44; 45; 46]. In both mouse and human EryD, the switch to adult β- like globin gene expression is completed after birth [reviewed in ref. 47]. This switch marks the final event in erythroid ontogeny.
Transcriptional regulation of erythroid development
Several transcriptional profiles have been reported for a single erythroid stage, heterogeneous populations, or cells differentiated in vitro, in mouse and human [48; 49; 50; 51; 52; 53]. Chronological global gene expression profiling has been performed for primary mouse EryP at 24 hour intervals from E7.5 through E12.5 [13]. This study took advantage of the essentially synchronous maturation of primitive erythroblasts, in combination with sorting of cells from ε-globin-H2B-GFP transgenic embryos [32], to analyze gene expression at 6 distinct stages of EryP development, including progenitors [13]. The EryP developmental database, which is available online at Gene Expression Omnibus: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24127, provides a transcriptional roadmap for primitive erythropoiesis.
A large number of erythroid genes, including Eklf/Klf1, Gata2, Tal1/Scl, Lmo2, Erythrocyte band 4.1 and Fog-1, are already expressed at high levels in progenitors at E7.5 [13]. Others, such as εY-, ζ -, and α-globin genes (Hbb-y, Hba-x, and Hba-a1), εY-, ζ -, and α-globin genes (Hbb-y, Hba-x, and Hba-a1), the anion transporter band 3/Slc4a1, and Glycophorin A (Gypa), increase significantly in expression during the period of progenitor expansion within the yolk sac (E7.5–8.5) [13].
Transcription in maturing EryP is marked by two discrete waves that correlate with key developmental hallmarks. The first wave (E8.5~E9.5) coincides with the transition from progenitor (yolk sac) to circulation stage development [13]. From E9.5 to E11.5, corresponding to proerythroblast through poly- or orthochromophilic erythroblast, transcription decreases globally. The second wave of transcriptional variation (E11.5~E12.5) corresponds to a period of extensive morphological change, decreased cell division rate, cytoskeletal remodeling, and nuclear condensation and extrusion. Gene ontology functions that are enriched in EryP at this period of their maturation (e.g. nuclear organization, DNA packaging and chromatin assembly) reflect these changes in transcript diversity [13]. A nearly reciprocal pattern of gene expression was observed for the two waves of transcriptional variation that may reflect the need to respond to the distinct microenvironmental niches of the yolk sac and circulation [13].
A study of global gene expression in morphologically comparable stages of primitive, fetal definitive and adult mouse definitive erythroid precursors has been reported [34]. Cells of the primitive and definitive erythroid lineages express different genes within shared functional categories based on gene annotation, (for example, genes within the aquaporin family). The authors created a user-friendly website (http://www.cbil.upenn.edu/ErythronDB) that permits comparative searches.
The zinc finger transcriptional regulators GATA1 and EKLF play key roles in both primitive and definitive erythropoiesis [54; 55; 56; 57; 58; 59; 60; 61; 62]. EryP and EryD differ in their requirements for a number of other transcription factors, including Runx1, Sox6, and c-Myb (Table 1). Transcriptional regulators known to have important functions in erythropoiesis are listed in Table 1.
Table 1. Transcription factors and other regulatory proteins in erythropoiesis.
This table focuses on functions in erythroid cells but many of these genes/proteins are also known to play roles in other lineages. References are primarily for studies on mouse mutant phenotypes
| Gene symbol(s) |
Name(s)/Protein | Mutant Phenotype | Comments | Refs. |
|---|---|---|---|---|
| Gata1 | GATA1 | Maturation block in EryP and EryD | Erythroid progenitors form but fail to mature | [57; 58] |
| Gata2 | GATA2 | Embryonic lethality in knockout at E10.5–E11.5 due to failure in progenitor expansion | Double KO revealed functional overlap between Gata1 and Gata2 in EryP | [61; 67] |
| Fog1 | Friend of GATA1; FOG1 | Fog1 knockout mice die between E10.5 and E12.5 of severe anemia and defective megakaryopoiesis | Acts sequentially with GATA1 to specify definitive erythroid and megakaryocytic progenitors | [68; 69] |
| Klf1/Eklf | Erythroid Kruppel-Like Zinc Finger Protein | Embryonic lethality by ~E13.5 due to defective fetal liver erythropoiesis | Defects in EryP and EryD maturation | [49; 59; 60; 62; 63; 64] |
| Scl/Tal | Basic helix-loop-helix SCL/tal-1 | Embryonic lethality at E9–10.5 due to hematopoietic and vascular defects | Master regulator of hematopoiesis, forms complexes with Gata1 and Lmo2 | [70; 71; 72] |
| Lmo2 | Rbtn2; LIM domain only 2, Cysteine-rich LIM domain protein | Embryonic lethality at E10.5 due to failure in primitive erythropoiesis | [73] | |
| Runx1 | Runt-related transcription factor 1; Core Binding Factor-a2 (Cbfa2), AML1 | Embryonic lethality at E11.5–12.5 due to failure in definitive erythropoiesis and hemorrhaging | Some evidence for a (nonessential) function in primitive erythropoiesis | [74; 75; 76] |
| Ldb1 | LIM domain-binding protein 1 | Embryonic lethality after E9.5 (defective yolk sac development) | Mutant displays various other patterning defects; positive regulator of late steps in erythroid maturation. ES cell studies: decreased hemangioblast numbers and block in differentiation | [77; 78; 79] |
| c-Myb | c-MYB; myeloblastosis oncogene | Absence of EryD progenitors | Required for definitive but not primitive erythropoiesis; reduced megakaryocyte progenitor numbers | [12; 80] |
| c-Myc | c-MYC; myelocytomatosis oncogene | Epiblast-restricted c-Myc-null embryos severely anemic and die before E12, largely due to defects in primitive erythropoiesis | Physiologic levels critical; downregulation required for terminal maturation of fetal liver EryD | [81] |
| Klf2 | Kruppel-Like Zinc Finger Protein 2 | Critical for primitive erythropoiesis | Redundant roles with Klf1/Eklf | [82] |
| Klf6 | Kruppel-Like Zinc Finger Protein 6 | Knockouts embryonic lethal by E12 | May function primarily in mesoderm specification rather than in primitive erythropoiesis | [83] |
| BRG1 | Brahma Related Gene 1, SMARCA4 | Embryonic lethal E10.5–11; severe EryP defect by E9.5 | Tie2-Cre conditional KO | [84] |
| c-Maf | Proto-oncogene | Knockouts die from severe anemia E15–18 | Important function in fetal liver macrophages; decreased EryD in EBIs of fetal liver, effect is non cell-autonomous | [85] |
| ZFAT | Zinc finger and AT hook domain | Embryonic lethal E9.5, required in EryP | Regulates Tal, Lmo2, Gata1 | [86] |
| P400/Do mino | E1A binding protein p400 | Embryonic lethality E11.5 due to defects in primitive erythropoiesis | SWI2/SNF2 family ATPase; Nterminal domain deletion | [87] |
| Zbp89 | ZBP-89; Zinc finger protein 148 (ZFP-148) | Embryonic lethal between E8.5 and E10.5 due to placental insufficiency | Part of complex with GATA1 and FOG1. Required for definitive but not primitive erythropoiesis. | [88] |
| Gfi1b | Growth factor independent 1b; Gfi1b | Embryonic lethal at E14.5 as a result of deficiencies in erythroid and megakaryocyte development | Target of Runx1 and regulator of endothelial to hematopoietic transition | [89; 90] |
| Sox6 | SRY-box containing gene 6 | Necessary for efficient physiological and stress erythropoiesis in mouse EryD; silencer of embryonic and fetal globin genes in EryD | Sox6, Gata1 and Bcl11A co-occupy human β-globin cluster; Sox6 expressed in mouse EryP progenitors but then downregulated | [13; 91; 92] |
| Bcl11A | B cell CLL/lymphoma 11A; Bcl11a | Knockout mice die perinatally from unknown causes. Disruption of embryonic/fetal to adult globin gene switch | Embryonic globin gene expression not silenced in knockout mice; regulated by Eklf/Klf1. Essential for normal lymphoid development | [93; 94; 95; 96] |
| NuRD | Nucleosome remodelling histone deacetylase | Cofactor for Fog1; mutations in Fog1 that disrupt interactions with NuRD results in anemia and macrothrombocytopenia | Context-dependent repressor and activator functions. Complex phenotype with similarities to Gata1 or Fog1 mutations | [97] |
| PIT1 | PIT1/SLC20A1 | Embryonic lethality at E12.5, erythroid maturation defects | Defects in vitro and in vivo; target of Eklf/Klf1 | [98; 99] |
Identification of erythroid cells at different stages of ontogeny and differentiation
Transgenic mouse models for tagging, tracking, and purifying primitive erythroid cells
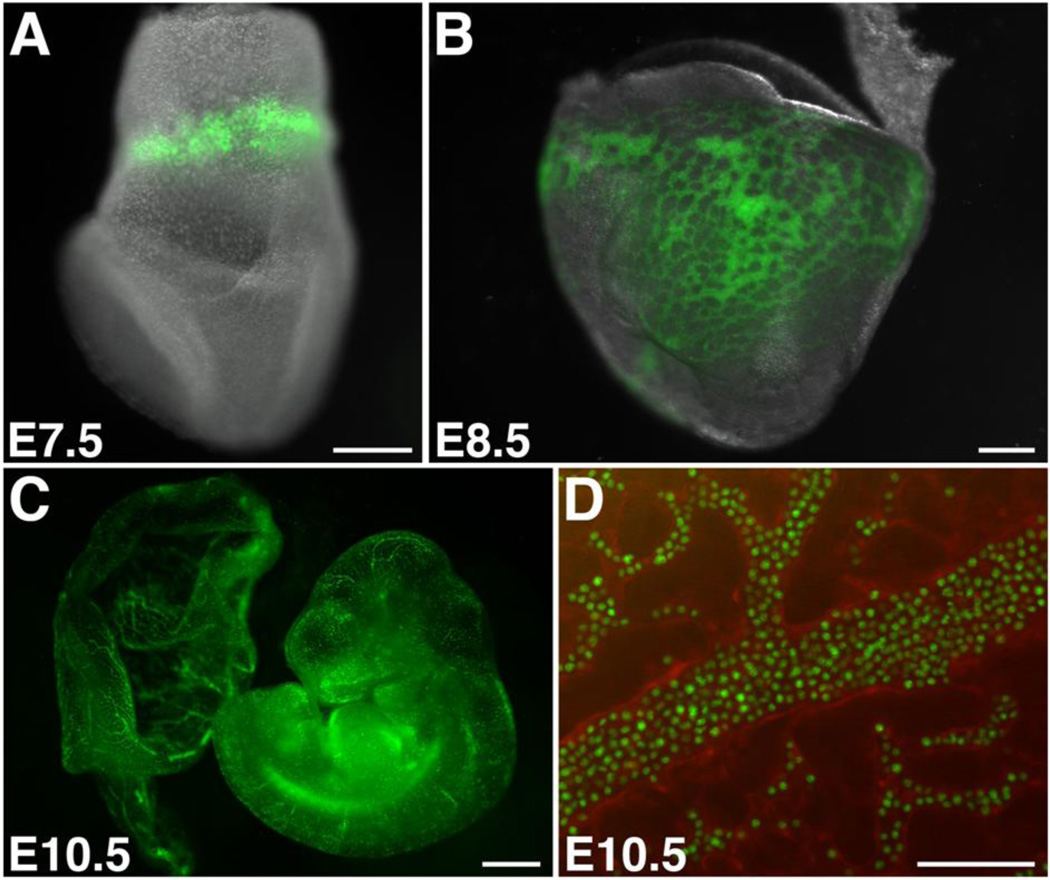
Primitive and definitive erythroid cells can be partially separated by taking advantage of the difference in their size [e.g. see ref. 8]. However, there are presently no cell surface markers known that uniquely distinguish primitive from definitive erythroid cells. Transgenic mouse lines have been created for tagging and tracking EryP during embryogenesis, using a human ε-globin gene promoter and µLCR to target pancellular GFP [63] or nuclear (histone H2B-) GFP [32] (Fig. 2) to these cells. The bright green fluorescence in ε-globin-H2B-GFP transgenic embryos allows the prospective isolation and quantitation of EryP progenitors, which are an abundant cell type at E7.5 (~15% of cells) and E8.5 (40–50%) [13].
Figure 2. Photographs of ε-globin-H2B-GFP transgenic mouse embryos at different developmental stages.
Overlay of bright field and GFP channel of an ~E7.5 (A) and of an ~E8.5 embryo (B). Scale bars, 200µm. (C) GFP expression in an E10.5 embryo. The yolk sac (left) has been opened. Scale bar, 1mm. (D) Detail of yolk sac from an E10.5 ε-globin-H2BGFP; Flk1-Cre; Rosa26-tdTom transgenic embryo. Red fluorescence (tdTomato) is seen in endothelial cells of the yolk sac vasculature and results from the excision of a STOP cassette from the Rosa26-tdTom transgene [112] by Flk1 promoter-driven Cre. Scale bar, 100 µm.
The tetraspanin protein CD9 is expressed on nucleated EryP and EryD but not on enucleated cells of either lineage in the mouse [61]. As nucleated EryP are found in the circulation through~E15.5 [8; 9], CD9 is a useful marker for distinguishing between EryP and EryD in peripheral blood past midgestation [61]. CD9 expression has recently been used for this purpose to study membrane remodeling of EryP [64].
Separation of EryD at distinct stages of maturation using flow cytometry
In contrast with the differentiation of primitive erythroid cells as a cohort, definitive erythropoiesis proceeds continuously in the fetal liver and adult bone marrow. Progressive stages of EryD maturation can be separated based on expression of Ter119 and CD71 [65; 66] or CD44 [67; 68]. A method for isolating human bone marrow erythroblasts at distinct stages has been reported recently, using other markers whose expression changes more dramatically on human than on mouse red cells [69].
Two EryD progenitors have been defined functionally using colony assays: the early erythroid burst-forming unit (BFU-E) and later colony-forming unit (CFU-E). Fetal liver BFU-E and CFU-E can be FACS-purified using a combination of negative selection for 9 cell surface proteins and staining for CD71 [70].
Conclusion
Erythroid cells play essential roles in supporting embryonic and fetal development and throughout postnatal life. Mammalians presumably have evolved to produce two, and arguably at least three distinct erythroid lineages during their ontogeny. Primitive erythroid cells appear rapidly in the yolk sac as a large cohort of cells, maturing only after they enter the circulation. As they differentiate, their hemoglobin production increases, underscoring their need to differentiate further. The segregation of primitive erythropoiesis to an extraembryonic site may allow the embryo proper to devote other resources to early organogenesis. A peculiar feature of primitive red cells is that they do not lose their nuclei until around the time that definitive erythropoiesis begins in the fetal liver, raising the question of what function enucleation serves in this lineage. It has been appreciated for many years that mammalian adult erythrocytes are more deformable than the nucleated erythroid cells of birds [71; 72]. Enucleation may, similarly, confer a hydrodynamic advantage on primitive red cells [64]. The large size of mammalian primitive erythroblasts may result in greater shear forces required for rapid vascular remodeling in the embryo.
Red cells at all stages of development have many common features and, not surprisingly, they share expression of many of the same genes. Primitive and definitive erythroid cells differ in their requirements for certain transcription factors and may express distinct genes within multigene families such as those encoding hemoglobin [47], glucose transporters [13], and aquaporins [34]. The red blood cell has served as a model system for understanding a variety of biological problems and is likely to continue to provide surprises and new insights for many years to come.
Acknowledgements
Work in our lab was supported in part by grants to M.H.B. from the National Institutes of Health (RO1 HL62248 and, DK52191, and EB02209), the Roche Foundation for Anemia Research (grant 9699367999, cycle X) and the New York State Department of Health (NYSTEM grant N08G-024).
Abbreviations
- AGM
aorta-gonad-mesonephros
- BFU-E
burst-forming unit erythroid
- E#
embryonic day post-fertilization
- CFU-E
colony-forming cells erythroid
- EryD
definitive, enucleated erythrocytes
- EMP
erythroid-myeloid progenitor
- EryP
primitive (nucleated) erythrocytes
- ES
embryonic stem
- ESRE
extensively self-renewing erythroid
- E-Tmod
erythroid-tropomodulin
- GFP
green fluorescent protein
- HPP-CFC
high proliferating progenitors-colony forming cell
- MEP
bipotential megakaryocyte/erythroid progenitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haar J, Ackerman GA. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat. Rec. 1971;170:199–224. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- 2.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 3.Palis J, Yoder M. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exper. Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 4.Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 5.Wong PM, Chung SW, Chui DH, Eaves CJ. Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3851–3854. doi: 10.1073/pnas.83.11.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingsley PD, Malik J, Emerson RL, et al. "Maturational" globin switching in primary primitive erythroid cells. Blood. 2006;107:1665–1672. doi: 10.1182/blood-2005-08-3097. First edition paper; prepublished online Nov. 1, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Z, Russell JE. Expression, purification, and characterization of human hemoglobins Gower-1 (zeta(2)epsilon(2)), Gower-2 (alpha(2)epsilon(2)), and Portland-2 (zeta(2)beta(2)) assembled in complex transgenic-knockout mice. Blood. 2001;97:1099–1105. doi: 10.1182/blood.v97.4.1099. [DOI] [PubMed] [Google Scholar]
- 8.Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk Sac-Derived Primitive Erythroblasts Enucleate During Mammalian Embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 9.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts is accompanied by changes in cell surface antigen expression patterns during mouse embryogenesis. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinder SJ, Loebel DA, Tam PP. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc. Med. 2001;11:177–184. doi: 10.1016/s1050-1738(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 11.Tavian M, Peault B. The changing cellular environments of hematopoiesis in human development in utero. Exp. Hematol. 2005;33:1062–1069. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Tober J, Koniski A, McGrath KE, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isern J, He Z, Fraser ST, et al. Single lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood. 2011;117:4924–4934. doi: 10.1182/blood-2010-10-313676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand JY, Jalil A, Klaine M, et al. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 15.Liu C-P, Auerbach R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development. 1991;113:1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- 16.Palacios R, Imhof BA. At day 8–8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 1993;90:6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumano A, Furlonger C, Paige CJ. Differentiation and characterization of B-cell precursors detected in the yolk sac and embryo body of embryos beginning at the 10- to 12-somite stage. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6429–6433. doi: 10.1073/pnas.90.14.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 19.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavian M, Biasch K, Sinka L, Vallet J, Peault B. Embryonic origin of human hematopoiesis. Int J Dev Biol. 2010;54:1061–1065. doi: 10.1387/ijdb.103097mt. [DOI] [PubMed] [Google Scholar]
- 22.Yoder MC, Hiatt K. Engraftment of embryonic hemopoietic cells in conditioned newborn recepients. Blood. 1997;89:2176–2183. [PubMed] [Google Scholar]
- 23.Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J. Exp. Med. 1999;190:43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaravelu P, Hook L, Morrison AM, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 25.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119:4828–4837. doi: 10.1182/blood-2012-01-153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palis J, Malik J, McGrath KE, Kingsley PD. Primitive erythropoiesis in the mammalian embryo. Int J Dev Biol. 2010;54:1011–1018. doi: 10.1387/ijdb.093056jp. [DOI] [PubMed] [Google Scholar]
- 30.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath KE, Kingsley PD, Koniski AD, et al. Enucleation of primitive erythroid cells generates a transient population of "pyrenocytes" in the mammalian fetus. Blood. 2008;111:2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isern J, Fraser ST, He Z, Baron MH. The fetal liver is a niche for maturation of primitive erythroid cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6662–6667. doi: 10.1073/pnas.0802032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–3330. doi: 10.1182/blood-2010-04-279489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsley PD, Greenfest-Allen E, Frame JM, et al. Ontogeny of erythroid gene expression. Blood. 2013;121:e5–e13. doi: 10.1182/blood-2012-04-422394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blatnik JS, Schmid-Schonbein GW, Sung LA. The influence of fluid shear stress on the remodeling of the embryonic primary capillary plexus. Biomech. Model. Mechanobiol. 2005;4:211–220. doi: 10.1007/s10237-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 36.Jones EAV, Crotty D, Kulesa P, Waters CW, Baron MH, Fraser SE, Dickinson ME. Dynamic In Vivo Imaging of Post-Implantation Mammalian Embryos Using Whole Embryo Culture. Genesis. 2002;34:228–235. doi: 10.1002/gene.10162. [DOI] [PubMed] [Google Scholar]
- 37.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am. J. Physiol. Heart. Circ. Physiol. 2004;287:H1561–H1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 38.Lux C, McGrath KE, Conway S, Palis J, Yoder MC. Circulation plays an essential role in distribution of mammalian yolk sac definitive hematopoietic progenitor cells to the embryo proper; using the Ncx1 knockout mouse model to prevent circulation. Blood. 2005;106:154a. abstract 517. [Google Scholar]
- 39.Lucitti JL, Jones EA, Huang C, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu X, Chen J, Reedy MC, et al. E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1827–H1838. doi: 10.1152/ajpheart.00947.2002. [DOI] [PubMed] [Google Scholar]
- 41.Palis J, Chan RJ, Koniski A, et al. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGrath KE, Frame JM, Fromm GJ, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18:139–145. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Zhang J, Ginzburg Y, et al. Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood. 2013;121:e43–e49. doi: 10.1182/blood-2012-09-456079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redmond LC, Haar JL, Giebel ML, et al. Isolation of erythroid cells from the mouse embryonic yolk sac by laser capture microdissection and subsequent microarray hybridization. Blood Cells Mol. Dis. 2006;37:27–32. doi: 10.1016/j.bcmd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Terszowski G, Waskow C, Conradt P, et al. Prospective isolation and global gene expression analysis of the erythrocyte colony-forming unit (CFU-E) Blood. 2005;105:1937–1945. doi: 10.1182/blood-2004-09-3459. [DOI] [PubMed] [Google Scholar]
- 50.Addya S, Keller MA, Delgrosso K, et al. Erythroid-induced commitment of K562 cells results in clusters of differentially expressed genes enriched for specific transcription regulatory elements. Physiol Genomics. 2004;19:117–130. doi: 10.1152/physiolgenomics.00028.2004. [DOI] [PubMed] [Google Scholar]
- 51.Komor M, Guller S, Baldus CD, et al. Transcriptional profiling of human hematopoiesis during in vitro lineage-specific differentiation. Stem Cells. 2005;23:1154–1169. doi: 10.1634/stemcells.2004-0171. [DOI] [PubMed] [Google Scholar]
- 52.Chambers SM, Boles NC, Lin K-YK, et al. Hematopoietic Fingerprints: An Expression Database of Stem Cells and Their Progeny. Cell Stem Cell. 2007;1:578–591. doi: 10.1016/j.stem.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merryweather-Clarke AT, Atzberger A, Soneji S, et al. Global gene expression analysis of human erythroid progenitors. Blood. 2011;117:e96–e108. doi: 10.1182/blood-2010-07-290825. [DOI] [PubMed] [Google Scholar]
- 54.Pevny L, Lin C-S, D'Agati V, et al. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 55.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 57.Perkins AC, Sharpe AH, Orkin SH. Lethal b-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara Y, Chang AN, Williams AM, Orkin SH. Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood. 2004;103:583–585. doi: 10.1182/blood-2003-08-2870. [DOI] [PubMed] [Google Scholar]
- 59.Drissen R, von Lindern M, Kolbus A, et al. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 2005;25:5205–5214. doi: 10.1128/MCB.25.12.5205-5214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodge D, Coghill E, Keys J, et al. A global role for EKLF in definitive and primitive erythropoiesis. Blood. 2006;107:3359–3370. doi: 10.1182/blood-2005-07-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isern J, Fraser ST, He Z, Zhang H, Baron MH. Dose-dependent regulation of primitive erythroid maturation and identity by the transcription factor Eklf. Blood. 2010;116:3972–3980. doi: 10.1182/blood-2010-04-281196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 64.Waugh RE, Huang YS, Arif BJ, Bauserman R, Palis J. Development of membrane mechanical function during terminal stages of primitive erythropoiesis in mice. Exp Hematol. 2012 doi: 10.1016/j.exphem.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 67.Chen K, Liu J, Heck S, et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Mohandas N, An X. Membrane assembly during erythropoiesis. Curr Opin Hematol. 2011;18:133–138. doi: 10.1097/MOH.0b013e32834521f3. [DOI] [PubMed] [Google Scholar]
- 69.Hu J, Liu J, Xue F, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013 doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117:3435–3444. doi: 10.1182/blood-2010-07-295550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaehtgens P, Schmidt F, Will G. Comparative rheology of nucleated and non-nucleated red blood cells. I. Microrheology of avian erythrocytes during capillary flow. Pflugers Arch. 1981;390:278–282. doi: 10.1007/BF00658276. [DOI] [PubMed] [Google Scholar]
- 72.Gaehtgens P, Will G, Schmidt F. Comparative rheology of nucleated and non-nucleated red blood cells. II. Rheological properties of avian red cells suspensions in narrow capillaries. Pflugers Arch. 1981;390:283–287. doi: 10.1007/BF00658277. [DOI] [PubMed] [Google Scholar]
- 73.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 74.Tsang AP, Fujiwara Y, Hom DB, Orkin SH. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12:1176–1188. doi: 10.1101/gad.12.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mancini E, Sanjuan-Pla A, Luciani L, et al. FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. Embo J advance online publication 8 November, 2011. 2011 doi: 10.1038/emboj.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porcher C, Swat W, Rockwell K, et al. The T Cell Leukemia Oncoprotein SCL/tal-1 is Essential for Development of All Hematopoietic Lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 77.Robb L, Elwood NJ, Elefanty AG, et al. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. Embo J. 1996;15:4123–4129. [PMC free article] [PubMed] [Google Scholar]
- 78.Loose M, Swiers G, Patient R. Transcriptional networks regulating hematopoietic cell fate decisions. Curr. Opin. Hematol. 2007;14:307–314. doi: 10.1097/MOH.0b013e3281900eee. [DOI] [PubMed] [Google Scholar]
- 79.Warren AJ, Colledge WH, Carlton MBL, et al. The oncogenic cysteine-rich LIM domain protein Rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 80.Wang Q, Stacy T, Binder M, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the Target of Multiple Chromosomal Translocations in Human Leukemia, Is Essential for Normal Fetal Liver Hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 82.Yokomizo T, Hasegawa K, Ishitobi H, et al. Runx1 is involved in primitive erythropoiesis in the mouse. Blood. 2008;111:4075–4080. doi: 10.1182/blood-2007-05-091637. [DOI] [PubMed] [Google Scholar]
- 83.Mukhopadhyay M, Teufel A, Yamashita T, et al. Functional ablation of the mouse Ldb1 gene results in severe patterning defects during gastrulation. Development. 2003;130:495–505. doi: 10.1242/dev.00225. [DOI] [PubMed] [Google Scholar]
- 84.Soler E, Andrieu-Soler C, de Boer E, et al. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 2011;24:277–289. doi: 10.1101/gad.551810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mylona A, Andrieu-Soler C, Thongjuea S, et al. Genome-wide analysis shows that Ldb1 controls essential hematopoietic genes/pathways in mouse early development and reveals novel players in hematopoiesis. Blood. 2013 doi: 10.1182/blood-2012-11-467654. [DOI] [PubMed] [Google Scholar]
- 86.Tober J, McGrath KE, Palis J. Primitive erythropoiesis and megakaryopoiesis in the yolk sac are independent of c-myb. Blood. 2008;111:2636–2639. doi: 10.1182/blood-2007-11-124685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jayapal SR, Lee KL, Ji P, et al. Down-regulation of Myc is essential for terminal erythroid maturation. J Biol Chem. 2010;285:40252–40265. doi: 10.1074/jbc.M110.181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Basu P, Lung TK, Lemsaddek W, et al. EKLF and KLF2 have compensatory roles in embryonic {beta}-globin gene expression and primitive erythropoiesis. Blood. 2007;110:3417–3425. doi: 10.1182/blood-2006-11-057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsumoto N, Kubo A, Liu H, et al. Developmental regulation of yolk sac hematopoiesis by Kruppel-like factor 6. Blood. 2006;107:1357–1365. doi: 10.1182/blood-2005-05-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500. doi: 10.1242/dev.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kusakabe M, Hasegawa K, Hamada M, et al. c-Maf plays a crucial role for the definitive erythropoiesis that accompanies erythroblastic island formation in the fetal liver. Blood. 2011;118:1374–1385. doi: 10.1182/blood-2010-08-300400. [DOI] [PubMed] [Google Scholar]
- 92.Tsunoda T, Takashima Y, Tanaka Y, et al. Immune-related zinc finger gene ZFAT is an essential transcriptional regulator for hematopoietic differentiation in blood islands. Proc Natl Acad Sci U S A. 2010;107:14199–14204. doi: 10.1073/pnas.1002494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ueda T, Watanabe-Fukunaga R, Ogawa H, et al. Critical role of the p400/mDomino chromatin-remodeling ATPase in embryonic hematopoiesis. Genes Cells. 2007;12:581–592. doi: 10.1111/j.1365-2443.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 94.Woo AJ, Moran TB, Schindler YL, et al. Identification of ZBP-89 as a novel GATA-1- associated transcription factor involved in megakaryocytic and erythroid development. Mol. Cell. Biol. 2008;28:2675–2689. doi: 10.1128/MCB.01945-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson NK, Foster SD, Wang X, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Lancrin C, Mazan M, Stefanska M, et al. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120:314–322. doi: 10.1182/blood-2011-10-386094. [DOI] [PubMed] [Google Scholar]
- 97.Dumitriu B, Bhattaram P, Dy P, et al. Sox6 is necessary for efficient erythropoiesis in adult mice under physiological and anemia-induced stress conditions. PLoS One. 2006;5:e12088. doi: 10.1371/journal.pone.0012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2011;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu P, Keller JR, Ortiz M, et al. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- 100.Sankaran VG, Xu J, Ragoczy T, et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1098. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet. 2010;42:742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 102.Esteghamat F, Gillemans N, Bilic I, et al. Erythropoiesis and globin switching in compound Klf1::Bcl11a mutant mice. Blood. 2013;121:2553–2562. doi: 10.1182/blood-2012-06-434530. [DOI] [PubMed] [Google Scholar]
- 103.Miccio A, Wang Y, Hong W, et al. NuRD mediates activating and repressive functions of GATA-1 and FOG-1 during blood development. EMBO J. 2010;29:442–456. doi: 10.1038/emboj.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beck L, Leroy C, Beck-Cormier S, et al. The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLoS One. 2010;5:e9148. doi: 10.1371/journal.pone.0009148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forand A, Beck L, Leroy C, et al. EKLF-driven PIT1 expression is critical for mouse erythroid maturation in vivo and in vitro. Blood. 2013;121:666–678. doi: 10.1182/blood-2012-05-427302. [DOI] [PubMed] [Google Scholar]
- 106.Zeigler BM, Sugiyama D, Chen M, et al. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 107.McGrath KE, Koniski AD, Malik J, Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 108.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The Placenta Is a Niche for Hematopoietic Stem Cells. Dev. Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 109.Ottersbach K, Dzierzak E. The Murine Placenta Contains Hematopoietic Stem Cells within the Vascular Labyrinth Region. Dev. Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 110.Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996;87:4109–4119. [PubMed] [Google Scholar]
- 111.Ivanovs A, Rybtsov S, Welch L, et al. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]