Summary
Growing evidence suggests that lower subjective social status (SSS), which reflects where a person positions himself on a social ladder in relation to others, is independently related to poor health. People who rate themselves lower in status also experience more frequent stressors and report higher stress than those who rate themselves higher in status, and chronic stress can enhance an individual's response to subsequent stressors. To address whether SSS predicted stress-induced interleukin-6 (IL-6) changes, we assessed 138 healthy adults at rest and following the Trier Social Stress Test (TSST). Participants completed the TSST at two study visits, separated by 4 months. People who placed themselves lower on the social ladder had larger IL-6 responses from baseline to 45 minutes post-stressor (p = 0.01) and from baseline to 2 hours post-stressor (p = 0.03) than those who placed themselves higher on the social ladder. Based on a ratio of subjective threat and coping ratings of the stress task, participants who viewed themselves as lower in status also tended to rate the speech task as more threatening and less manageable than those who viewed themselves as higher in status (p = 0.05). These data suggest that people with lower perceived status experience greater physiological and psychological burden from brief stressors compared to those with higher perceived status. Accordingly, responses to stressors may be a possible mechanistic link among SSS, stress, and health.
Keywords: subjective social status, inflammation, interleukin-6 (IL-6), laboratory stressor
Introduction
People routinely compare themselves to others; a person's sense of self is grounded in part by his or her perception of where s/he stands in relation to other people (Festinger, 1954; Suls et al., 2002; Dickerson and Kemeny, 2004). A common index of perceived standing, called subjective social status (SSS), is measured by ranking oneself on a “social ladder,” where higher rungs represent higher status in a social group, such as a school, community, or country (Adler et al., 2000). These self-rankings are often based on objective measures of socioeconomic status (SES) such as income and education, as well as current satisfaction and future financial security in relation to others (Singh-Manoux et al., 2003). For example, a man who views a current low-paying job as a stepping stone to a higher position may rate himself as higher in society than someone who feels unhappily “stuck” with the same pay and doubts his capacity to seek other employment. In this way, SSS encompasses more pieces of an individual's life circumstance than a single measure of objective SES (Adler et al., 2000; Singh-Manoux et al., 2003). Accordingly, SSS has generated growing interest in the past decade as an important predictor of health and well-being (Ostrove et al., 2000; Operario et al., 2004).
Viewing oneself as lower than one's peers is chronically stressful. Compared to those who rate themselves higher in status, those who rate themselves lower in status report greater numbers of ongoing social, financial, work, and relationship difficulties, and view situations as more stressful (Adler et al., 2000). In addition, people who perceive themselves as lower in status utilize fewer active coping strategies and report less control over daily life than those with higher perceived status (Adler et al., 2000). These findings remain after controlling for objective measures of SES and general negative affect, suggesting that SSS uniquely relates to stress levels.
Individuals who see themselves as lower in social standing are at greater risk for poor health and disability compared to those who see themselves as higher status in society (Adler et al., 2000; Singh-Manoux et al., 2003; Hu et al., 2005). In cross-sectional studies, people with lower perceived status have higher rates of hypertension (Adler et al., 2008), diabetes (Demakakos et al., 2008), central adiposity (Manuck et al., 2010), and depression (Demakakos et al., 2008) than those higher in SSS. As expected, the relationship between SSS and health variables often decreases in magnitude when controlling for objective SES measures, as they share variance (Singh-Manoux et al., 2003). Typically, however, these objective SES covariates do not fully explain the relationship between SSS and health.
Longitudinal data suggests that the relationship between SSS and health is bidirectional. Those with lower self- and nurse-rated health showed decreases in perceived status over time compared to those with higher health ratings (Nobles et al., 2013). The opposite directional relationship also appears to be true; rating oneself as lower in social status enhances health risks prospectively. For example, lower baseline social status increased risk for adolescent gain in adiposity (Lemeshow et al., 2008), relapse after smoking cessation (Reitzel et al., 2007), and susceptibility to the common cold (Cohen et al., 2008) at follow-up. In light of these results, the relationship between SSS and health appears complex. In addition, the biological mechanisms underlying these associations are poorly understood.
Inflammation is an important part of the body's response to infection or injury. Proinflammatory cytokines, including IL-6 (interleukin-6), activate immune cells and increase immune cell trafficking as needed (Libby, 2007). This process promotes destruction and clearance of pathogens, which is an essential response to acute infection. However, chronically elevated inflammation can be harmful to health. People with higher levels of inflammation have greater prospective risk for all-cause mortality, frailty, and disability compared to those with lower levels of inflammation (Reuben et al., 2002; Krabbe et al., 2004; Cesari et al., 2012). Elevated inflammation is an important risk factor for a variety of age-related diseases, including cardiovascular disease, osteoporosis, periodontal disease, rheumatoid arthritis, Alzheimer's disease, cancer, and Type 2 diabetes (Ershler and Keller, 2000; Maggio et al., 2006). Indeed, heightened levels of inflammation can affect disease onset and prognosis by contributing to processes such as insulin resistance, atherosclerosis, and tumor survival and growth (Coussens and Werb, 2002; Shoelson et al., 2006; Libby, 2007).
Chronic stress accelerates age-related increases in inflammation (Kiecolt-Glaser et al., 2003). Proinflammatory cytokines such as interleukin-6 (IL-6) increase in response to acute stressors, such as academic exams (Marshall et al., 1998), marital conflict (Kiecolt-Glaser et al., 2005), and standardized laboratory tasks (Steptoe et al., 2007). Over time, stress-induced increases in inflammation may contribute to disease processes such as insulin resistance, atherosclerosis (Black, 2003), and increased ambulatory blood pressure (Brydon and Steptoe, 2005). Individuals differ in their inflammatory responses to stressors, and factors that predict these differences can be helpful in describing stress reactivity.
Converging evidence suggests that inflammatory responses to stressful events may be heightened for those experiencing chronic stress or depression (Fagundes et al., 2013b). In an animal model, rats that received prior inescapable tailshocks had higher IL-6 responses to a bacterial immune challenge than those that were not subjected to stress beforehand (Johnson et al., 2002; Johnson et al., 2003). Following an influenza vaccine, serum cytokine levels increased more for individuals with higher levels of depressive symptoms compared to less depressed participants (Glaser et al., 2003). Similarly, a laboratory stressor led to higher IL-6 responses in those with more depressive symptoms compared to people who reported fewer depressive symptoms (Pace et al., 2006; Fagundes et al., 2013a). Additionally, childbirth evoked greater IL-6 increases in pregnant women with a prior history of depression compared to those without a history of depression (Maes et al., 2001).
Based on the evidence that people who see themselves as lower in status report higher levels of chronic stress, depression, and anxiety (Adler et al., 2000), it is possible that their physiological responses to stressors may also be enhanced compared to people who feel higher in status. For example, the body's cortisol response to short stressors dampens inflammation (Barnes, 1998), and initial evidence suggests that SSS may alter cortisol responses. After delivering a brief speech to a panel, college students with lower perceived status on their dormitory floor had smaller increases in cortisol than those who had higher perceived status (Gruenewald et al., 2006). In light of these results, the inflammatory response to stressors may also differ between high and low SSS individuals.
Accordingly, we investigated the impact of SSS on IL-6 responses to a laboratory stressor in healthy middle-aged and older adults. We hypothesized that individuals who viewed themselves as lower in status would have greater stress-induced IL-6 increases compared to those higher in SSS. In addition, we hypothesized that people with lower perceived status would rate the stressful laboratory task as more threatening and less manageable than those with higher perceived status.
Methods
Participants
Participants (N = 138) took part in a randomized clinical trial testing the effects of omega-3 supplementation on inflammation and mood in middle-aged and older adults. The primary results of this trial showed that omega-3 supplementation reduced resting levels of inflammation (Kiecolt-Glaser et al., 2012). Participants were recruited via local community advertisements, brochures, and media announcements. Individuals were eligible for the study if they had a body mass index (BMI) between 22.5 and 40 and engaged in less than two hours per week of vigorous physical activity. Additional screening criteria excluded diabetics, vegetarians, smokers, individuals with a prior history of cancer (except basal or squamous cell), and those with other autoimmune, digestive, and inflammatory diseases. Individuals were also excluded if they were taking medications that alter mood, inflammation, cholesterol, or blood pressure. The study was approved by the university's institutional review board; all participants provided written informed consent prior to beginning the study.
Procedure
Participants attended two study visits in which they completed a speech stressor task, provided blood samples, and completed questionnaires. After a baseline study visit, participants were randomized to placebo, 1.25 g/d omega-3, or 2.496 g/d omega-3 supplementation groups (Kiecolt-Glaser et al., 2012), and attended a final study visit 4 months later. The protocols for blood draws and the stressor task were identical for the two study visits.
An intravenous catheter was inserted into the participant's arm when s/he arrived at the Clinical Research Center (CRC; a hospital research facility) at approximately 7:45 a.m. S/he then ate a standard breakfast, completed a series of questionnaires, and sat quietly for 20 minutes. Following this relaxation period, baseline blood samples were drawn. At approximately 10:00 a.m., the participant began the Trier Social Stress Test (TSST), a well-validated acute laboratory stressor (Kirschbaum et al., 1993). The participant was told that s/he would give a speech addressing why s/he was the best candidate for a job position, and that a panel of behavioral experts would judge the response. In a separate room, the participant briefly viewed the study panel, which consisted of 2 confederates wearing white lab coats. Next, in a different room, s/he answered questions regarding the upcoming speech task, then was given 10 minutes to prepare the speech. The participant returned to the room with the panel, which included a microphone and video camera. Participants gave a 5-minute speech and performed a 5-minute mental arithmetic task in front of the panel. Two blood draws, which occurred 45 minutes and 2 hours after the completion of the TSST, assessed post-stressor IL-6 levels.
Self-report measures
Subjective social status (SSS)
Using the MacArthur Subjective Social Status Scale (Adler et al., 2000; available at www.macses.ucsf.edu/research/psychosocial/usladder.php), participants saw an image of a ladder with 10 rungs and the following description: “At the top of the ladder are the people who are the best off – those who have the most money, the most education and the most respected jobs. At the bottom are the people who are the worst off – who have the least money, least education, and the least respected jobs or no job. The higher up you are on this ladder, the closer you are to the people at the very top; the lower you are, the closer you are to the people at the bottom.” Participants were then asked to mark the rung which best described where they felt they stood relative to other people in the United States. Higher scores corresponded to higher rungs on the ladder, which indicated higher subjective social status. This measure has strong construct validity and adequate 6-month test-retest reliability (Singh-Manoux et al., 2003; Operario et al., 2004).
Depression
We used the Center for Epidemiologic Studies Depression Scale (CES-D) to measure depressive symptoms (Radloff, 1977). Participants rated how often they were bothered by a list of 20 depressive symptoms in the last week. The CES-D has been used extensively in research, and has acceptable test-retest reliability and excellent construct validity (Basco et al., 1997).
Childhood adversity
We used the Child Trauma Questionnaire (Bernstein and Fink, 1998) to assess early childhood abuse and neglect. We followed well-established categorical cut-offs (Walker et al., 1999) for the presence (vs. absence) of physical, emotional, and sexual abuse, and physical and emotional neglect. We then created a categorical variable representing “any maltreatment.”
Objective socioeconomic status (SES)
Participants selected their highest level of education and their pre-tax household income in the past year from the categories listed in Table 1. Data were modeled as continuous variables based on prior research (Winkleby et al., 1992; Adler et al., 1994).
Table 1.
Sample demographic characteristics (n = 138).
| Characteristic | n(%) | M | (SD) |
|---|---|---|---|
| Age (years) | 51.04 | 7.75 | |
| Subjective social status | 6.35 | 1.35 | |
| BMI (kg/m2, baseline visit) | 30.59 | 4.50 | |
| BMI (kg/m2, final visit) | 30.89 | 4.56 | |
| CES-D (baseline visit) | 7.58 | 8.10 | |
| CES-D (final visit) | 6.80 | 8.32 | |
| Threat to coping ratio (baseline visit) | 0.89 | 0.68 | |
| Threat to coping ratio (final visit) | 1.08 | 1.31 | |
| Female | 93 (67.4) | ||
| Supplementation group | |||
| Placebo | 46 (33.3) | ||
| 1.25g/d n-3 | 46 (33.3) | ||
| 2.496 g/d n-3 | 46 (33.3) | ||
| Race | |||
| White | 109 (79.0) | ||
| Black | 22 (15.9) | ||
| Asian | 4 (2.9) | ||
| Native American | 2 (1.4) | ||
| Other | 1 (0.7) | ||
| Education | |||
| High school | 7 (5.1) | ||
| Some college | 32 (23.2) | ||
| College graduate | 52 (37.7) | ||
| Graduate/professional training | 47 (34.0) | ||
| Income | |||
| < 10K | 1 (0.7) | ||
| 10 – 25K | 10 (7.2) | ||
| 25 – 50K | 34 (24.6) | ||
| 50 – 75K | 35 (25.4) | ||
| 75 – 100 K | 26 (18.8) | ||
| >100K | 24 (17.4) | ||
| Prefer not to answer | 8 (5.8) |
Note: Threat to coping ratio scores were available for a subset of participants (n = 90).
Speech task ratings
After hearing the TSST instructions, participants rated two Likert-scale items about the upcoming speech task. Perceived threat was assessed by the item, “On a scale of 1–7, with 1 being the least threatening and 7 being the most threatening, how threatening do you expect the upcoming task to be?” Another question assessed coping: “On a scale of 1–7, with 1 being the least able and 7 being the most able, how well do you expect to be able to cope with the upcoming tasks?” Cognitive appraisal theory (Lazarus and Folkman, 1984) suggests that individuals evaluate threat and coping resources to determine whether an event is primarily a threat (with significant potential for harm or loss) or a challenge (an opportunity to demonstrate mastery). Therefore, in accord with prior research (Tomaka et al., 1993; Heffner et al., 2002; Kiecolt-Glaser et al., 2009), the threat to coping ratio was calculated to summarize participants' interpretations of the stress task (such that higher scores signify threat appraisals, while low scores suggest challenge appraisals).
These supplemental questions were added part-way through the study, and data were available for a subset of participants (N = 90). On average, the 90 people who provided data for the threat to coping ratio were younger (Mage = 49.8 vs 53.4 years respectively, p = 0.02) and had higher BMI (MBMI = 31.2 vs. 29.4 respectively, p = 0.04) than the 48 people who did not provide ratings of the task. The two groups did not differ significantly on any other demographic variables. Importantly, people with and without the threat to coping ratio had similar mean SSS scores (MSSS = 6.3 vs 6.5 respectively, p = 0.47).
IL-6 Assays
Blood samples were stored at −86°C, and each subject's samples for all time points were assayed in the same run. Assays for IL-6 were run in duplicate using MSD 96-well Multispot Custom Cytokine Kits (Meso Scale Discovery, Maryland) according to kit instructions. Samples were read using an MSD Sector Imager 2400. The sensitivity for detecting IL-6 was 0.26 pg/mL; samples that fell below the limit of detection (n = 23, 2.95% of samples) were assigned the value equal to half the detection limit (0.13 pg/mL). The intra-assay coefficients of variation ranged from 5.64 – 6.70%. The inter-assay coefficients of variation had a range of 5.16 – 10.2%.
Analytic Method
Data from both study visits were used in statistical analyses. Prior to the analysis, a natural-log transformation was applied to IL-6 data to normalize the skewed distribution. Continuous variables were mean-centered; predictors that varied across visits (i.e., BMI, CES-D scores) were centered on the mean for the baseline visit.
Linear mixed effect models were used to test the effect of SSS on IL-6 responses to the stressor. Natural log-transformed IL-6 was the dependent variable and SSS was the continuous predictor of primary interest. All models contained the three-way interaction of SSS by time (baseline, 45 min post-stressor, 2 hours post-stressor) by visit plus all lower order interaction terms. The models also adjusted for other important participant characteristics, including age, sex, BMI, visit, and supplementation group. BMI was measured at both visits, and was included as a time-varying covariate. Additionally, to account for the intervention study design, all models included the three-way interaction of supplementation group by time by visit and all lower order interaction terms, though they were not of primary interest. In addition to these fixed effects, participant, study visit, and time point (baseline, 45 minutes post-stressor, and 2 hours post-stressor) were included as random effects in order to properly account for within-subject correlation. The Kenward-Roger method was used to correct the degrees of freedom in the models and control Type I error (Kenward and Roger, 1997). Preplanned contrasts compared the effect of SSS on changes in IL-6 from pre- to post-stressor. Least squares means for IL-6 at each time point were estimated for one standard deviation above and below the mean of centered SSS (M = 0, SD = 1.4). Because several studies have reported a relationship between objective measures of SES and stress reactivity (Gump et al., 1999; Brydon et al., 2004), we ran secondary analyses to test whether income or education predicted IL-6 responses to the stressor. These analyses used models identical to those described above, with income and education (separately) replacing SSS as predictors of interest. All analyses were performed in SAS Version 9.3, and all tests used a two-tailed, α = 0.05 significance level.
Secondary analyses used linear mixed effect models to test whether ratings of the stress task varied by SSS. SSS was entered as a predictor of the natural log-transformed pre-stressor threat to coping ratio, and participant and visit were included as random effects. To correct the degrees of freedom in the model and control Type I error, the Kenward-Roger method was used (Kenward and Roger, 1997). The interaction of SSS by visit was included in the model, and supplementation group was included as a covariate. The interaction of supplementation group by visit was also included to account for the intervention study design. In an additional adjusted model, sex was added as a covariate to the above analysis.
Results
Demographic information for the sample is presented in Table 1. Participants ranged in age from 40 to 85 years. The sample was primarily overweight to obese, and reported a relatively low level of depressive symptoms on average. Zero-order correlations between covariates of interest are presented in Table 2.
Table 2.
Zero-order Pearson correlations between psychosocial variables at the baseline visit (subjects with complete data; n=78).
| Income | Education | CES-D | Age | BMI | Threat to Coping Ratio | |
|---|---|---|---|---|---|---|
| sss | 0.47 | 0.35 | −0.15 | −0.14 | −0.04 | −0.38 |
| Income | 0.34 | −0.17 | −0.08 | −0.02 | −0.20 | |
| Education | −0.08 | −0.09 | −0.14 | −0.26 | ||
| CES-D | −0.03 | 0.12 | 0.03 | |||
| Age | −0.09 | 0.30 | ||||
| BMI | 0.28 |
Note: Bolded correlation estimates indicate statistically significant correlations (p < 0.05).
Table 3 summarizes F-tests from the primary linear mixed effects model for the effect of SSS on IL-6. We addressed our hypothesis by examining the SSS by time interaction, which tested whether SSS predicted stress-induced changes in IL-6 levels. Indeed, IL-6 responses to the stressor differed significantly according to SSS (p = 0.02). Across both visits, IL-6 levels increased in response to the stressor. The three-way interaction of SSS by time by visit was not significant (p = 0.91), so results of the primary analyses (Figure 1; Tables 4 and 5) are presented by collapsing across visits.
Table 3.
F-tests for all predictors of (natural log-transformed) IL-6 levels in the primary linear mixed effects model.
| Effect | F | DF | p |
|---|---|---|---|
| Visit | 3.94 | 1,124 | 0.05 |
| Time | 146.61 | 2,245 | <.0001 |
| Visit × Time | 3.46 | 2,243 | 0.03 |
| SSS | 9.06 | 1,131 | 0.003 |
| SSS × Visit | <0.01 | 1,125 | 0.99 |
| SSS × Time | 3.95 | 2,243 | 0.02 |
| SSS × Visit × Time | 0.09 | 2,242 | 0.91 |
| Group | 3.51 | 2,130 | 0.03 |
| Group × Visit | 1.48 | 2,123 | 0.23 |
| Group × Time | 2.83 | 4,244 | 0.03 |
| Group × Visit × Time | 1.54 | 4,243 | 0.19 |
| BMI | 9.87 | 1,137 | 0.002 |
| Age | 0.34 | 1,131 | 0.56 |
| Sex | 8.63 | 1,130 | 0.004 |
Note: Bolded p-values indicate statistically significant effects (p < 0.05). In ancillary analyses, we controlled for depressive symptoms and child maltreatment separately, and the SSS × Time interaction remained significant (p = 0.05 and p = 0.02, respectively). In addition, depressive symptoms (p = 0.04) and child maltreatment (p = 0.02) significantly predicted IL-6 reactivity to stress (i.e., the interaction with Time was significant).
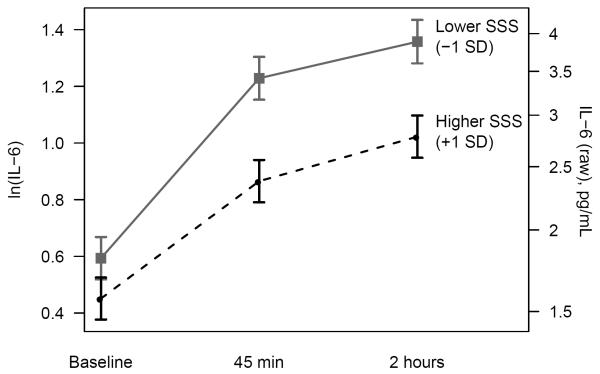
Figure 1.
Mean IL-6 responses (± standard error) for participants with higher and lower SSS. The plot shows least squares means from mixed models adjusting for supplementation group, study visit, age, sex, and BMI.
Table 4.
Results from primary linear mixed model analysis of the effect of SSS on (natural log-transformed) IL-6 levels.
| Time | Effect | Estimate | 95% CI | p |
|---|---|---|---|---|
| Average ln(IL-6) levels | ||||
| Baseline | Average | 0.52 | 0.42 to 0.63 | |
| Difference per unit SSS | −0.051 | −0.12 to 0.023 | 0.17 | |
| 45 min post-stress | Average | 1.0 | 0.9 to 1.2 | |
| Difference per unit SSS | −0.13 | −0.20 to −0.055 | 0.0007 | |
| 2 hr post-stress | Average | 1.2 | 1.08 to 1.296 | |
| Difference per unit SSS | −0.12 | −0.20 to −0.045 | 0.002 | |
| Change in ln(IL-6) in response to stressor | ||||
| Baseline to 45 min post-stress | Average change | 0.52 | 0.44 to 0.61 | |
| Difference in change per unit SSS | −0.079 | −0.14 to −0.019 | 0.01 | |
| 45 min to 2 hr post-stress | Average change | 0.14 | 0.062 to 0.22 | |
| Difference in change per unit SSS | 0.010 | −0.052 to 0.071 | 0.75 |
Note: Bolded p-values indicate statistically significant effects (p < 0.05). Estimates are adjusted for supplementation group, study visit, age, sex, and BMI.
Table 5.
Contrasts comparing changes in (natural log-transformed) IL-6 levels for lower versus higher SSS
| Difference in Changes |
||||||
|---|---|---|---|---|---|---|
| Low SSS | High SSS | Estimate | SE | 95% CI | p | |
| Change, baseline to 45 min post-stressor | 0.64 | 0.41 | 0.22 | 0.086 | 0.05 to 0.39 | 0.01 |
| Change, baseline to 2 hr post-stressor | 0.76 | 0.57 | 0.19 | 0.087 | 0.02 to 0.36 | 0.03 |
| Change, 45 min to 2 hr post-stressor | 0.13 | 0.16 | −0.03 | 0.087 | −0.20 to 0.14 | 0.75 |
Note: Bolded p-values indicate statistically significant effects (p < 0.05). Contrasts are adjusted for supplementation group, study visit, age, sex, and BMI. High and low SSS are estimated at 1 standard deviation above (cSSS = 1.4) and below (cSSS = −1.4) the mean for centered SSS.
Table 4 shows the effect of SSS on average IL-6 levels at each time point and on changes in IL-6 between time points. Higher SSS was significantly associated with lower IL-6 at both 45 minutes post-stressor (p = 0.0007) and 2 hours post-stressor (p = 0.002). SSS was not significantly associated with baseline IL-6 levels (p = 0.17). Higher SSS was associated with smaller increases in IL-6 from baseline to 45 minutes post-stressor (p = 0.01) but not with the change from 45 minutes to 2 hours post-stressor (p = 0.75). Figure 1 shows the average IL-6 levels for those with lower and higher SSS, and Table 5 summarizes contrasts that tested differences in IL-6 responses. Compared to those who rated themselves higher in status, those who reported lower status experienced greater stress-induced IL-6 responses from baseline to 45 minutes post-stressor (p = 0.01) and from baseline to 2 hours post-stressor (p = 0.03; Table 5).
In secondary analyses, we separately adjusted for depressive symptoms, and the SSS by time interaction remained a significant predictor of IL-6 levels (p = 0.05). In a separate model, the SSS by time interaction also remained significant (p = 0.02) when adjusting for child maltreatment. In accord with prior literature, individual contrasts revealed that the average changes in IL-6 from baseline to 45-minutes post-stressor and baseline to 2-hours post-stressor were larger for subjects with a history of maltreatment compared to subjects with no maltreatment (p = 0.08, p = 0.007, respectively).
In separate models, neither income by time nor education by time interactions predicted IL-6 levels (p > 0.54). Although objective SES measures did not predict stress-related changes in IL-6, other studies on SSS have typically included objective SES measures as covariates. Accordingly, we ran secondary analyses to evaluate whether SSS predicted IL-6 changes when adjusting for income and education. When adjusting for education, the SSS by time interaction remained a significant predictor of IL-6 levels (p = 0.03), and education did not predict IL-6 (p = 0.64). When adjusting for current income, the SSS by time interaction was marginally significant (p = 0.12), and income did not predict IL-6 levels (p = 0.91). In this model, contrasts testing differences in IL-6 per unit change in SSS at 45 minutes and 2 hr remained significant (p = 0.01, p = 0.03, respectively), and the change in slope from baseline to 45 minutes per unit change in SSS remained significant (p = 0.05).
Secondary analyses showed that SSS was a significant predictor of the threat to coping ratio, such that lower SSS individuals rated the task as more threatening and less manageable than those with higher SSS (p = 0.05). The interaction of SSS by visit was not significant (p = 0.80). In the adjusted model, SSS remained a marginally significant predictor of the threat to coping ratio scores when controlling for sex (p = 0.06). The interaction between the threat to coping ratio and time was not related to change in IL-6 levels (p = 0.80), suggesting that the threat to coping ratio did not mediate the effects of SSS on IL-6 reactivity.
Discussion
People who placed themselves lower on a social ladder had larger IL-6 responses to a brief laboratory stressor than those who placed themselves higher on the ladder. Participants with lower perceived status tended to report higher threat to coping ratios than those who made higher self-rankings, suggesting that they felt higher levels of threat and less ability to cope with the task. In sum, people who described themselves as lower status were more threatened by the stressor and had greater inflammatory responses to it. Adding to prior literature that links lower SSS to higher levels of ongoing stress (Adler et al., 2000), these results suggest that individuals lower in SSS may react more strongly to a brief stressful situation than those with higher SSS.
The rigorous selection criteria for our study produced an ideal sample for examining stress-induced inflammation. Our sample was overweight and sedentary but otherwise healthy. In addition, we assessed stress reactivity at two time points, adding to the reliability of our findings. Importantly, our results remain significant when controlling for depressive symptoms and child maltreatment, which enhance stress-induced inflammation (Pace et al., 2006; Fagundes et al., 2013a). While lower SSS individuals are more likely to experience depression than people higher in SSS (Adler et al., 2000; Adler et al., 2008; Demakakos et al., 2008), these results suggest that the relationship between SSS and stress-induced inflammation cannot be fully explained by depressive symptoms.
Higher inflammation underlies a number of age-related diseases, including cardiovascular disease, osteoporosis, cancer, and Type 2 diabetes (Ershler and Keller, 2000; Maggio et al., 2006), and increases risk for all-cause mortality (Pedersen and Febbraio, 2008). Other studies indicate that people who describe their status as lower than others' have increased risk for these health problems, including diabetes (Demakakos et al., 2008), hypertension (Adler et al., 2008), and depression (Adler et al., 2008; Demakakos et al., 2008). Repeated or exaggerated responses to transient stressors may exert wear-and-tear on the body over time (McEwen, 1998) or contribute to disease processes like atherosclerosis and increased blood pressure (Black, 2003; Brydon and Steptoe, 2005). Accordingly, exaggerated stress responses may provide one avenue for investigating the link between lower SSS and poor health.
Several studies suggest that those with lower objective SES have heightened basal levels of inflammation compared to those of higher objective SES (Loucks et al., 2006; Friedman and Herd, 2010), but the relationship between SSS and basal inflammation has not been thoroughly explored. In this study, we did not observe an SSS-related difference in basal levels of IL-6, possibly due to restriction in range. Individuals in our study reported relatively high SSS, with 75.3% placing themselves on the highest 5 rungs of the ladder. This is not surprising, as our sample was composed of healthy, primarily college-educated adults. Consequently, these results may underestimate the effect of low SSS, one limitation of our study. Perhaps samples with a broader range of SSS scores would show greater effects of SSS on basal and stress-induced levels of inflammation.
Only a few studies have examined the relationship between objective SES and stress-induced inflammation. In one particularly relevant study, men with lower employment grades had prolonged IL-6 responses to a color-word interference and mirror tracing stress task compared to men of higher employment grades (Brydon et al., 2004). In our sample, neither income nor education predicted stress-induced changes in IL-6. However, our study differs from Brydon and colleagues' study in several ways. Their sample was composed of younger (ages 30 to 59), healthy to overweight males. Our sample included both males and females, and our participants were older and overweight to obese on average. In addition, we used the TSST as the stressor, which is primarily noted for its social evaluative component. In several other studies, objective SES measures did not predict stress-related changes in inflammatory markers (Steptoe et al., 2002; Steptoe et al., 2003).
Our findings contribute to a growing literature that links prior stress exposure (Johnson et al., 2002; Johnson et al., 2003) and depression (Glaser et al., 2003; Pace et al., 2006; Fagundes et al., 2013a) to exaggerated inflammatory responses to novel stressors. Our results suggest that lower perceived status, which is associated with ongoing stressors and dysphoria (Adler et al., 2000), can also contribute to elevated stress-induced inflammation. As such, repeated or daily stressors may be more potent for people who perceive themselves as lower in status, a hypothesis that warrants further study. Further, lower SSS may exacerbate poor health, since the stress of chronic illness may evoke greater distress and inflammation for individuals lower in self-rated status than for those higher in self-rated status.
Because a person's social status is an integral part of his or her sense of self, people react more strongly to situations in which their abilities are being judged by others (i.e., social evaluative threats) than to situations involving performance without evaluation (Dickerson and Kemeny, 2004). Individuals who already perceive themselves as lower in status than others may be particularly threatened by social evaluation. For example, lower and higher SSS individuals differed in their cortisol responses when performing a speech in front of others, but SSS did not affect cortisol reactivity to a speech performed alone (Gruenewald et al., 2006). These social-evaluative studies used the same laboratory task that participants completed in our study (Dickerson and Kemeny, 2004). Accordingly, our study provides additional evidence that SSS affects responses to social-evaluative stress. Future work may investigate whether SSS also affects inflammatory responses to other types of stressors that do not involve social judgment. In addition, further research on mediating variables could address whether SSS affects inflammation by exaggerating perceptions of rejection or negative evaluation, impairing task performance, or heightening autonomic responses. To explore `for whom' these findings are most relevant, individual resources such as social support and optimism may be tested as moderators of this effect (Taylor and Seeman, 1999).
We examined the effect of SSS on stress-induced inflammation in a sample of individuals undergoing an omega-3 supplementation trial, so we statistically controlled for supplementation group in all analyses. However, it would be useful to investigate this effect in a sample that does not include a clinical trial component. In addition, our sample was primarily Caucasian middle-aged and older adults. While there appear to be ethnic differences in basal levels of inflammation (Chapman et al., 2009; Stowe et al., 2010), racial or ethnic differences in stress-induced inflammation have not been reported, and further work with more diverse samples would be helpful.
In sum, individuals who rated themselves as lower status had greater IL-6 responses to a laboratory speech task than those who reported higher SSS. In anticipation of the task, participants with lower perceived status tended to feel more threatened and less able to cope than those with higher perceived status. As SSS emerges as an independent predictor of health (Adler et al., 2000; Singh-Manoux et al., 2003; Singh-Manoux et al., 2005; Adler et al., 2008; Demakakos et al., 2008), this study adds to the literature by introducing stress-induced inflammation as a possible mechanistic link among SSS, stress, and health.
Acknowledgements
We appreciate the helpful assistance of the Stress and Health Study lab staff and the Clinical Research Center nursing staff.
Funding Sources
Work on this project was supported in part by NIH Grants AG029562, AG038621, UL1TR000090, CA16058, the S. Robert Davis endowment, the Kathryn & Gilbert Mitchell endowment, and American Cancer Society Postdoctoral Fellowship Grant PF-11-007-01-CPPB. OmegaBrite (Waltham, MA) supplied the omega-3 supplement and placebo without charge and without restrictions. OmegaBrite did not influence the design, funding, implementation, interpretation, or publication of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no financial interests or relationships that pose potential conflicts of interest with this paper.
Contributors
Heather Derry developed the hypothesis, performed the literature search, was the lead writer of the manuscript, and incorporated all revisions from co-authors. Christopher Fagundes aided in developing the hypothesis and outline of the manuscript, and edited the manuscript. Rebecca Andridge conducted statistical analyses and edited the manuscript. Ronald Glaser supervised the completion of cytokine assays. William Malarkey was the medical supervisor for the study. Janice-Kiecolt Glaser designed the study, wrote the grants that funded the study, developed the protocol, supervised data collection for the study, and edited the manuscript. All authors have contributed to and have approved the final manuscript.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: The challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Adler NE, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot M. Social status and health: A comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc. Sci. Med. 2008;66:1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. American Psychological Association; Washington D. C.: 1997. pp. 207–245. [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. The Psychological Corporation; San Antonio, Texas: 1998. [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain. Behav. Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain. Behav. Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J. Hypertens. 2005;23:1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Nicklas B, Kanaya AM, Patrignani P, Tacconelli S, Tranah GJ, Tognoni G, Harris TB, Incalzi RA, Newman AB, Pahor M. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:671–676. doi: 10.1093/gerona/glr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, Moynihan J. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain. Behav. Immun. 2009;23:636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakakos P, Nazroo J, Breeze E, Marmot M. Socioeconomic status and health: the role of subjective social status. Soc. Sci. Med. 2008;67:330–340. doi: 10.1016/j.socscimed.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive Symptoms Enhance Stress-induced Inflammatory Responses. Brain. Behav. Immun. 2013a;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain. Behav. Immun. 2013b;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L. A Theory of Social Comparison Processes. Human Relations. 1954;7:117–140. [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (The MIDUS study) Psychosom. Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Arch. Gen. Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N. Subjective social status moderates cortisol responses to social threat. Brain. Behav. Immun. 2006;20:410–419. doi: 10.1016/j.bbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Räikkönen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychol. 1999;18:140–150. doi: 10.1037//0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Ginsburg GP, Hartley TR. Appraisals and impression management opportunities: Person and situation influences on cardiovascular reactivity. Int. J. Psychophysiol. 2002;44:165–175. doi: 10.1016/s0167-8760(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Hu P, Adler NE, Goldman N, Weinstein M, Seeman TE. Relationship Between Subjective Social Status and Measures of Health in Older Taiwanese Persons. J. Am. Geriatr. Soc. 2005;53:483–488. doi: 10.1111/j.1532-5415.2005.53169.x. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain. Behav. Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–R432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Heffner KL, Glaser R, Malarkey WB, Porter K, Atkinson C, Laskowski B, Lemeshow S, Marshall GD. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology. 2009;34:670–680. doi: 10.1016/j.psyneuen.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'- A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; New York: 1984. [Google Scholar]
- Lemeshow A, Fisher L, Goodman E, Kawachi I, Berkey C, Colditz G. Subjective social status in the school and change in adiposity in female adolescents: Findings from a prospective cohort study. Arch. Pediatr. Adolesc. Med. 2008;162:23–28. doi: 10.1001/archpediatrics.2007.11. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65:S140–146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, Hayes LJ, D'Agostino RB, Sr., Larson MG, Vasan RS, Benjamin EJ, Berkman LF. Association of educational level with inflammatory markers in the Framingham Offspring Study. Am. J. Epidemiol. 2006;163:622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]
- Maes M, Ombelet W, De J, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J. Affect. Disord. 2001;63:85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Phillips JE, Gianaros PJ, Flory JD, Muldoon MF. Subjective Socioeconomic Status and Presence of the Metabolic Syndrome in Midlife Community Volunteers. Psychosom. Med. 2010;72:35–45. doi: 10.1097/PSY.0b013e3181c484dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GD, Jr., Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain. Behav. Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Nobles J, Weintraub MR, Adler NE. Subjective socioeconomic status and health: relationships reconsidered. Soc. Sci. Med. 2013;82:58–66. doi: 10.1016/j.socscimed.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operario D, Adler NE, Williams DR. Subjective social status: Reliability and predictive utility for global health. Psychol. Health. 2004;19:237–246. [Google Scholar]
- Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and Subjective Assessments of Socioeconomic Status and Their Relationship to Self-Rated Health in an Ethnically Diverse Sample of Pregnant Women. Health Psychol. 2000;19:613–618. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- Pace T, Mletzko T, Alagbe O, Musselman D, Nemeroff C, Miller A, Heim C. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. A. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psych. Meas. 1977;1:385–401. [Google Scholar]
- Reitzel LR, Vidrine JI, Li Y, Mullen PD, Velasquez MM, Cinciripini PM, Cofta-Woerpel L, Greisinger A, Wetter DW. The Influence of Subjective Social Status on Vulnerability to Postpartum Smoking Among Young Pregnant Women. Am. J. Public Health. 2007;97:1476–1482. doi: 10.2105/AJPH.2006.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J. Am. Geriatr. Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc. Sci. Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom. Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behavior and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Rumley A, Lowe GDO, Marmot M. Influence of socioeconomic status and job control on plasma fibrinogen responses to acute mental stress. Psychosom. Med. 2003;65:137–144. doi: 10.1097/01.psy.0000039755.23250.a7. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain. Behav. Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2010;65:429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, Martin R, Wheeler L. Social comparison: Why, with whom, and with what effect? Curr. Dir. Psychol. Sci. 2002;11:159–163. [Google Scholar]
- Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Ann. N. Y. Acad. Sci. 1999;896:210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, Leitten CL. Subjective, physiological, and behavioral effects of threat and challenge appraisal. J. Pers. Soc. Psychol. 1993;65:248–260. [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, Koss MP, Katon W. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch. Gen. Psychiatry. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am. J. Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]